Abstract
The development of a microwave-assisted, intermolecular SNAr protocol for the synthesis of a 126-member benzothiaoxazepine-1,1-dioxide library is reported. Diversification of 12 benzothiaoxazepine-1,1-dioxides was achieved in rapid fashion utilizing a variety of 2° amines and amino alcohols to generate an 80-member library. A second 48-member library was subsequently generated via a two-step alkylation, intermolecular SNAr diversification protocol.
Keywords: Benzothiaoxazepine-1,1-dioxides; Sultams; MACOS; SNAr
1. Introduction
A key area of modern-day combinatorial science is the development and advancement of methodologies, protocols and reaction platforms to enable the discovery of small-molecule probes in order to advance our understanding of chemical biology.1 One aspect of this effort is the development of efficient methods to access collections of heterocycles for high-throughput screening (HTS). In this regard, the synthesis of core scaffolds on multi-gram scale, followed by efficient parallel diversification is one approach to generate diverse compound collections for HTS.
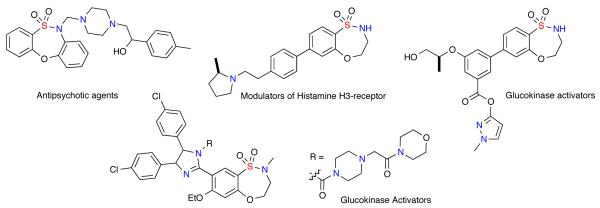
Sultams (cyclic sulfonamide analogues) have emerged as important targets in drug discovery due to their chemical and biological profiles.2 Though not found in nature, sultams display activity across a wide variety of biological targets.3,4 In particular, benzothiaoxazepine-1,1-dioxide-containing sultams, possessing a rich content of sp3 amine functionality, have shown biological activity as antipsychotic agents,5 modulators of histamine H3-recceptor,6 and glucokinase activators (Figure 1).7
Figure 1.
Biologically active benzothiaoxazepine-1,1-dioxide-containing sultams
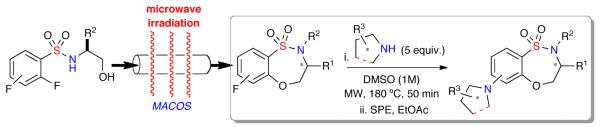
Despite these reports, methods to generate collections of benzofused sultams for HTS screening are limited.8 In this regard, efforts have focused on the development of a variety of methodologies and protocols for the generation of diverse sultam collections.9 These methodologies include the development of a variety of protocols namely, “Click-Click-Cyclize”,10 complementary ambiphile pairing (CAP),11 and reagent-based diversity-oriented synthesis (DOS).12 Building on these methods, we herein report the design and synthesis of a 126-member library of amino-benzothiaoxazepine-1,1-dioxides via a microwave-assisted, intermolecular SNAr diversification of core benzothiaoxazepine-1,1-dioxides scaffolds (Scheme 1).13
Scheme 1.
Proposed library generation via microwave assisted-SNAr diversification to access benzofused Sultam library.
2. Results and Discussion
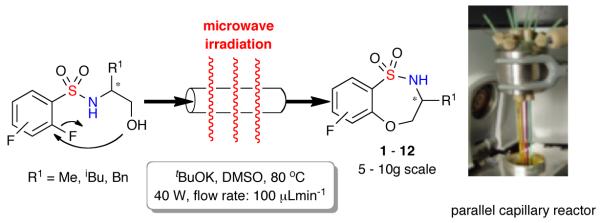
The facilitated, scale-out synthesis of small molecules via microwave-assisted, continuous-flow organic synthesis (MACOS)14 was previously reported for the generation of a number of core sultam scaffolds.15 Utilizing this enabling technology, the generation of benzofused sultams via an intramolecular SNAr cyclization was achieved on multigram scale.15a With this protocol in hand, the re-synthesis of benzothiaoxazepine-1,1-dioxide scaffolds 1–12 possessing both functional and stereochemical diversity, was achieved.
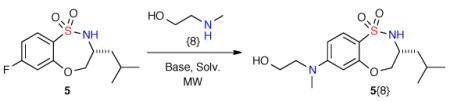
Initial investigation focused on the development and optimization of the corresponding intermolecular SNAr reaction for the diversification of benzothiaoxazepine-1,1-dioxide 5 with amino alcohol {8}, chosen to probe chemoselectivity when utilizing amino alcohols (Table 1). We screened a variety of bases using 3 equivalents of amino alcohol {8}, along with a control reaction without base (Table 1, entries, 1–4).13b It was observed that after heating under microwave irradiation at 150 °C for 30 min. a 56% yield could be achieved when no base was present. Additional optimization of solvent (DMF, THF, DMSO), equivalents of {8}, temperature, and reaction time (Table 1, entries 6–9) revealed that 5 equivalents of amino alcohol {8} heated at 150 °C for 50 min. provided the desired sultam 5{8} now in 90% yield. It is of note that when only 1 equivalent of {8} was used under optimized conditions, only a 40% yield was achieved. Further, it was found that DBU (10 mol%) not only increased the yield to 95%, but also resulted in greater reproducibility (Table 1, entry 11).
Table 1.
Optimization of microwave-assisted, SNAr reaction conditions utilizing sultam E.
| entry | {8} (equiv.) | base (equiv.) | solv. | temp (°C) | time (min) | yield (%)a |
|---|---|---|---|---|---|---|
| 1 | 3 | Cs2CO3 (3) | DMF | 150 | 30 | 20 |
| 2 | 3 | NaOtBu (3) | DMF | 150 | 30 | 46 |
| 3 | 3 | LiHMDS (3) | DMF | 150 | 30 | 42 |
| 4 | 3 | Et3N (3) | DMF | 150 | 30 | 47 |
| 5 | 3 | - | DMF | 150 | 30 | 56 |
| 6 | 5 | - | DMF | 150 | 30 | 68 |
| 7 | 5 | - | THF | 150 | 30 | 48 |
| 8 | 5 | - | DMSO | 150 | 30 | 86 |
| 9 | 5 | - | DMSO | 180 | 50 | 90 |
| 10 | 1 | - | DMSO | 180 | 50 | 40 |
| 11 | 5 | DBU (1) | DMSO | 180 | 50 | 94 |
| 12 | 5 | DBU (0.1) | DMSO | 180 | 50 | 95 |
Yields are reported after flash column chromatography on silica gel.
Library Design
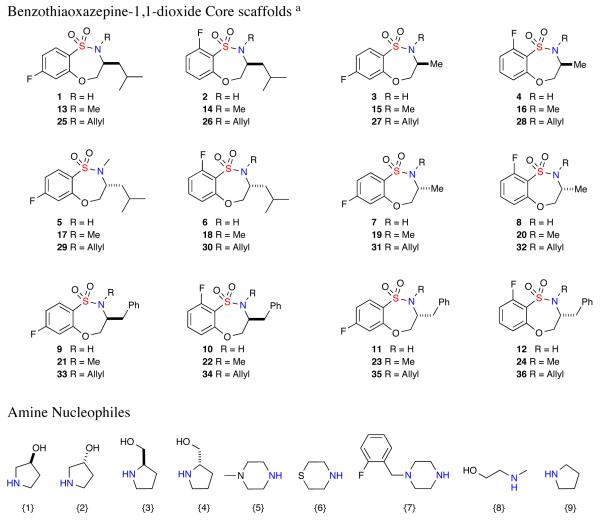
A 144-member, full matrix library was designed using in-silico analysis, literature precedence, and observed synthetic results.16 Twelve benzothiaoxazepine-1,1-dioxide scaffolds 1–12 were designed, composed of two sets of enantiomers at R1 thereby maintaining the ability to generate stereochemical SAR (SSAR) for each building block combination. Each set of enantiomers were varied at R1 = Me, iBu or Ph with fluorine substitution on the benzofused ring at either 4- or 6-position. With the SNAr derived core sultams in hand, a virtual library incorporating all possible building block combinations of 2° amine was constructed for each scaffold (Figure 3). Physico-chemical property filters were applied, guiding the elimination of undesirable building blocks that led to products with undesirable in-silico properties.17 These metric filters included standard Lipinski Rule of 5 parameters (molecular weight <500, ClogP <5.0, number of H-acceptors <10, and number of H-donors <5), in addition to consideration of the number of rotatable bonds (<5) and polar surface area. Absorption, distribution, metabolism and excretion (ADME) properties were calculated along with diversity analysis using standard H-aware 3D BCUT descriptors comparing against the MLSMR screening set (ca. 7/2010; ~330,000 unique chemical structures). Guided by this library design analysis, benzothiaoxazepine-1,1-dioxides (1–36) and amines {1–9} were chosen to generate the aforementioned 144-member library.
Figure 3.
Benzothiaoxazepine-1,1-dioxides (1–36) and Amines {1–9} library building blocks.
aBenzothiaoxazepine-1,1-dioxide scaffolds 13–14 and 24–36
Validation and Library Generation
With these optimized conditions in hand, a 12-member validation library was prepared (General Procedure A) in 1 dram vials using the Anton Parr Synthos 3000® platform. Upon completion, the crude reaction mixtures were diluted, filtered through silica SPE, and purified by automated mass-directed LCMS (Table 2).18 Library validation was essential to assess both substrate and reaction scope, along with evaluating the application of automated mass-directed LCMS as the final analysis and purification method. Key to successful library production was the synthesis of compounds in >90% purity in 40–50 mg quantities, which would be sufficient for HTS screening via the Molecular Library Probe Center Network (MLPCN) (20 mg), external biological outreach screening partners (20 mg), and to retain a sample (10 mg) for follow-up evaluation or to re-supply the NIH MLPCN. Evaluation of this validation library demonstrated that all 12 members where successfully prepared (average purity = 92%, yield = 45%, quantity = 71 mg) in the desired sultams final masses, with 10/12 possessing a final purity >90%.
Table 2.
12-Member validation library probing reaction scope
| sultama | purity (%)b |
yield (%)b |
quantity (mg) |
|---|---|---|---|
| 5{2} | 83 | 45 | 69.0 |
| 5{4} | 97 | 51 | 77.6 |
| 5{5} | 99 | 30 | 42.5 |
| 5{6} | 97 | 44 | 66.9 |
| 5{7} | 74 | 36 | 69.0 |
| 5{8} | 91 | 51 | 71.5 |
| 6{4} | 93 | 32 | 49.0 |
| 6{5} | 97 | 47 | 71.9 |
| 6{6} | 98 | 62 | 94.9 |
| 6{8} | 93 | 37 | 54.3 |
| 21{6} | 96 | 51 | 90.3 |
| 33{6} | 94 | 50 | 93.2 |
Reaction conditions: Benzothiaoxazepine-1,1-dioxide 1-12 (1 equiv., 0.434 mmol), dry DMSO (434 μL, 1M), DBU (7 μL, 10 mol%) and amine (5 equiv.).
Purified by automated preparative reverse phase HPLC (detected by mass spectroscopy); purity was assessed by HPLC.
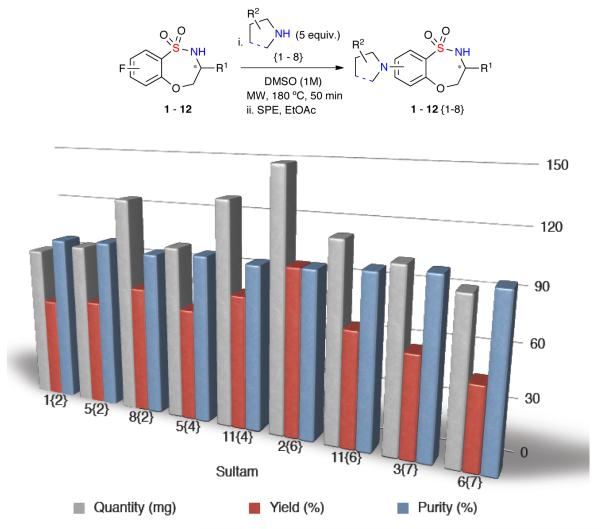
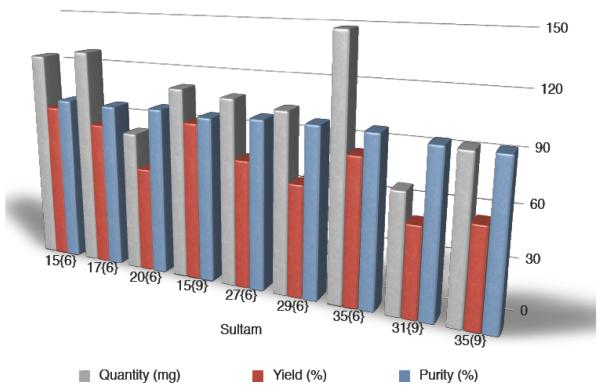
With the validation complete, a 96-membered library (Library I) was proposed for the diversification of core benzothiaoxazepine-1,1-dioxide scaffolds 1–12 with amines {1–9}. Implementing the optimized SNAr reaction conditions, Library I (96-member) was generated and purified by automated mass-directed LCMS. A total of 80 compounds were prepared, with amine enantiomers {1} and {2} excluded due to decomposition under reaction conditions; all products were isolated in good overall yield and quantity, with 78 compounds possessing a final purity >90% after automated purification (Graph 1).19
Graph 1.
Library I: Representative library members demonstrating final quantity, purity and overall yield
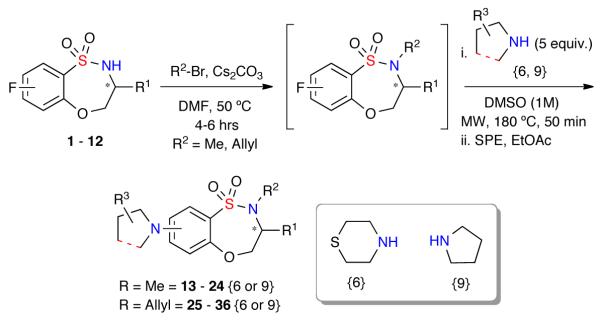
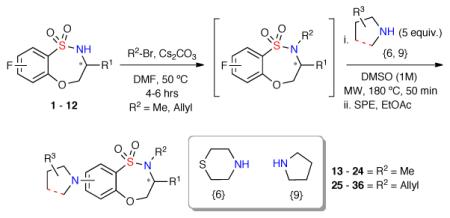
Upon completion of Library I (80-member), a second 48-member compound set (Library II) was investigated implementing a two-step alkylation-SNAr procedure (General procedure B). Hence, benzothiaoxazepine-1,1-dioxides 1–12 were methylated (13–24) or allylated (25–36), concentrated, and submitted to the aforementioned microwave-assisted SNAr diversification with amines {6 and 9} (Scheme 2). A total of 46 compounds from the proposed 48-membered library where prepared with 43 of the 48 possible products having a final purity >90% after automated purification (Graph 2).19
Scheme 2.
Synthesis of Library II utilizing a two-step alkylation-SNAr protocol.
Graph 2.
Library II: Representative library members demonstrating final mass, purity and overall yield.
Final assessment of libraries I and II demonstrated that the primary objectives set out in the library design were achieved; final masses ranged between 8–158 mg and the average final mass was 68 mg (original target being 50 mg).
Conclusion
In conclusion, an efficient microwave-assisted intermolecular-SNAr protocol for the synthesis of a 126-member collection (Libraries I and II) of amino-benzothiaoxazepine-1,1-dioxides has been developed. Employing a variety of commercially available amines, a 126-member library was generated via the microwave assisted-SNAr diversification at the 4-F and 6-F positions. These compounds have been submitted for evaluation of their biological activity in high-throughput screening assays at the NIH MLPCN and the results will be reported in due course.
Experimental Section
General procedure A: Microwave-assisted diversification of benzothiaoxazepine-1,1-dioxides 1- 12 cores. Into a 1-dram vial was added benzothiaoxazepine-1,1-dioxide 1–12 (1 equiv., 0.43 mmol), dry DMSO (0.43 mL, 1M), DBU (7 μL, 10 mol %) and the corresponding amine (5 equiv.). The reaction vessel was capped, placed in Anton Paar Synthos 3000® microwave and heated at 180 °C for 50 min [Power = 1200 W, 8 minute ramp then 50 min hold]. After cooling to RT, the crude reaction mixture was diluted with EtOAc, filtered through a SiO2 SPE and concentrated. The crude product was QC/purified by an automated preparative reverse phase HPLC (detected by mass spectroscopy).
General procedure B: 2-step alkylation-SNAr diversification of benzothiaoxazepine-1,1-dioxides 13–24 and 25–36 cores. Into a round bottom flask, under Ar, was added benzothiaoxazepine-1,1-dioxide 1–12 (1 equiv.), Cs2CO3 (3 equiv.), dry DMF (0.5 M) and alkyl bromide (2 equiv.). The reaction was heated at 50 °C and stirred for 4–6 h (TLC monitoring), after which time the reaction was cooled to RT, filtered through a SiO2 SPE, washed with EtOAc and concentrated to remove solvent and excess alkylating reagent. To the crude reaction mixture was added dry DMSO (0.43 mL, 1M), DBU (7 μL, 10 mol %) and the corresponding amine (5 equiv.). The reaction vessel was capped, placed in Anton Paar Synthos 3000® microwave and heated at 180 °C for 50 mins [Power = 1200 W, 8 minute ramp then 50 min hold]. After cooling to room temperature the reaction was diluted in EtOAc, filtered through a SiO2 SPE and concentrated. The crude reaction mixture was QC/purified by an automated preparative reverse phase HPLC (detected by mass spectroscopy).
Supplementary Material
Figure 2.
Synthesis of benzothiaoxazepine-1,1-dioxides 1 - 12 via MACOS scale-out
Acknowledgment
This work was supported by the National Institute of General Medical Science (Center in Chemical Methodologies and Library Development at the University of Kansas, KU-CMLD, NIH P50 GM069663 and NIH P41-GM076302).
Footnotes
Supporting Information Available: Experimental procedures, tabulated results for all libraries, and full characterization data for representative compounds. This material is available free of charge via the Internet at http://acs.pubs.org/
Reference and Notes
- (1) (a).Dolle RE, Bourdonnec BL, Worm K, Morales GA, Thomas CJ, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2009. J. Comb. Chem. 2010;12:765–806. doi: 10.1021/cc100128w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dolle RE, Bourdonnec BL, Goodman AJ, Morales GA, Thomas CJ, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2008. J. Comb. Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]; (c) Dolle RE, Bourdonnec BL, Goodman AJ, Morales GA, Thomas CJ, Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2007. J. Comb. Chem. 2008;10:753–802. doi: 10.1021/cc800119z. [DOI] [PubMed] [Google Scholar]
- (2) (a).Drews J. Drug Discovery: A Historical Perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]; (b) Navia MA. A Chicken in Every Pot, Thanks to Sulfonamide Drugs. Science. 2000;288:2132–2133. doi: 10.1126/science.288.5474.2132. [DOI] [PubMed] [Google Scholar]
- (3).For an extensive list of biologically-active sultams see Rolfe A, Young K, Hanson PR. Domino Heck-Aza-Michael Reactions: A One-pot, Multi-Component Approach to 1,2-Benzisothiazoline-3-acetic acid 1,1-dioxides. Eur. J. Org. Chem. 2008:5254–5262. doi: 10.1002/ejoc.200800651.
- (4) (a).Palani A, Qin J, Zhu X, Aslanian RG, McBriar MD. 954468P US Patent. 2007; (b) Paige MI. β-Sultams Mechanism of Reactions and Use as Inhibitors of Serine Proteases. Acc. Chem. Res. 2004;37:297–303. doi: 10.1021/ar0200899. [DOI] [PubMed] [Google Scholar]; (c) Hanessian S, Sailes H, Therrien E. Synthesis of functionally-diverse bicyclic sulfonamides as constrained proline analogues and application to the design of potential thrombin inhibitors. Tetrahedron. 2003;59:7047–7056. [Google Scholar]; (d) Cherney RJ, Mo R, Meyer DT, Hardman K, Liu R-Q, Covington MB, Qian M, Wasserman ZR, Christ DD, Trzaskos JM, Newton RC, Decicco CP. Sultam Hydroxamates as Novel Matrix Metalloproteinase Inhibitors. J. Med. Chem. 2004;47:2981–2983. doi: 10.1021/jm049833g. [DOI] [PubMed] [Google Scholar]; (e) Nhien ANV, Tomassi C, Len C, Marco-Contelles JL, Balzarini J, Pannecouque C, Clerq ED, Postel D. First Synthesis and Evaluation of the Inhibitory Effects of Aza Analogues of TSAO on HIV-1 Replication. J. Med. Chem. 2005;48:4276–4284. doi: 10.1021/jm050091g. [DOI] [PubMed] [Google Scholar]; (f) Cordi A, Lacoste J–M, Audinot V, Millan M. Design, synthesis and structure-activity relationships of novel strychnine-insensitive glycine receptor ligands. Bioorg. Med. Chem. Lett. 1999;9:1409–1414. doi: 10.1016/s0960-894x(99)00194-8. [DOI] [PubMed] [Google Scholar]; (g) Chen Z, Demuth TP, Jr., Wireko FC. Stereoselective synthesis and antibacterial evaluation of 4-amido-isothiazolidinone oxides. Bioorg. Med. Chem. Lett. 2001;11:2111–2115. doi: 10.1016/s0960-894x(01)00409-7. [DOI] [PubMed] [Google Scholar]
- (5).Rocher J-P. Preparation of diarylsultam derivatives as antipsychotic agents. PCT Int. Appl. 1997 WO 9730038 A1 19970821. [Google Scholar]
- (6).Santora VJ, Covel JA, Ibarra JB, Semple G, Smith B, Smith J, Weinhouse MI, Schultz JA. Biphenylsulfonamides as modulators of the histamine H3-receptor useful for the treatment of disorders related thereto and their preparation. PCT Int. Appl. 2008 WO 2008005338 A1 20080110. [Google Scholar]
- (7) (a).McKerrecher D, Pike KG, Waring MJ. Preparation of heteroaryl benzamide derivatives for use as glucokinase activators in the treatment of type 2 diabetes. PCT Int. Appl. 2006 WO 2006125972 A1 20061130. [Google Scholar]; (b) Campbell L, Pike KG, Suleman A, Waring MJ. Preparation of benzoyl amino heterocyclyl compounds as glucokinase activators for treating type 2 diabetes and other diseases mediated by GLK. PCT Int. Appl. 2008 WO 2008050101 A2 20080502. [Google Scholar]
- (8).Gerard B, Duvall JR, Lowe JT, Murillo T, Wei J, Akella LB, Marcaurelle LA. Synthesis of a Stereochemically Diverse Library of Medium-Sized Lactams and Sultams via SNAr Cycloetherification. ACS Combi Sci. 2011 doi: 10.1021/co2000218. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9) (a).Rolfe A, Hanson PR. Microwave-assisted Sequential One-pot Protocol to Benzothiadiazin-3-one-1,1-dioxides via a Copper-catalyzed N-Arylation Strategy. Tetrahedron Lett. 2009;50:6935–6937. doi: 10.1016/j.tetlet.2009.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rolfe A, Young K, Volp K, Schoenen F, Neuenswander B, Lushington GH, Hanson PR. A One-Pot, 3-Component, Domino Heck-aza-Michael Approach to Libraries of Functionalized 1,1-Dioxido-1,2-benzisothiazoline-3-acetic acids. J. Comb. Chem. 2009;11:732–738. doi: 10.1021/cc900025e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jeon KO, Rayabarapu D, Rolfe A, Volp K, Omar I, Hanson PR. Metathesis Cascade Strategies (ROM-RCM-CM): A DOS Approach to Skeletally Diverse Sultams. Tetrahedron. 2009;65:4992–5000. doi: 10.1016/j.tet.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Rayabarapu D, Zhou A, Jeon KO, Samarakoon T, Rolfe A, Siddiqui H, Hanson PR. α -Haloarylsulfonamides: Multiple Cyclization Pathways to Skeletally Diverse Benzofused Sultams. Tetrahedron. 2009;65:3180–3188. doi: 10.1016/j.tet.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rolfe A, Young K, Hanson PR. Domino Heck-Aza-Michael Reactions: A One-pot, Multi-Component Approach to 1,2-Benzisothiazoline-3-acetic acid 1,1-dioxides. Eur. J. Org. Chem. 2008:5254–5262. doi: 10.1002/ejoc.200800651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10) (a).Zhou A, Rayabarapu D, Hanson PR. “Click, Click, Cyclize”: A DOS Approach to Sultams Utilizing Vinyl Sulfonamide Linchpins. Org. Lett. 2009;11:531–534. doi: 10.1021/ol802467f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou A, Hanson PR. Synthesis of Sultam Scaffolds via Intramolecular Oxa-Michael and Diastereoselective Baylis–Hillman Reactions. Org. Lett. 2008;10:2951–2954. doi: 10.1021/ol8009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).Samarakoon TB, Hur MY, Kurtz RD, Hanson PR. A Formal [4+4] Complementary Ambiphile Pairing (CAP) Reaction: A New Cyclization Pathway for ortho-Quinone Methides. Org. Lett. 2010;12:2182–2185. doi: 10.1021/ol100495w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rolfe A, Samarakoon TB, Hanson PR. Formal [4+3] Epoxide Cascade Reaction via a Complementary Ambiphilic Pairing. Org. Lett. 2010;12:1216–1219. doi: 10.1021/ol100035e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rolfe A, Lushington GH, Hanson PR. Reagent Based DOS: A Click, Click, Cyclize Strategy to Probe Chemical Space. Org. Biomol. Chem. 2010;8:2198–2203. doi: 10.1039/b927161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13) (a).Chen W, Li Z, Ou L, Giulianott MA, Houghten RA, Yu Y. Solid-phase synthesis of skeletally diverse benzofused sultams via palladium-catalyzed cyclization. Tetrahedron Lett. 2011;52:1456–1458. [Google Scholar]; (b) Rolfe A, Smarakoon TB, Klimberg SV, Brzozowski M, Neuenswander B, Lushington GH, Hanson PR. SNAr-Based, Facile Synthesis of a Library of Benzothiaoxazepine-1,1′-dioxides. J. Comb. Chem. 2010;12:850–854. doi: 10.1021/cc1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rolfe A, Probst D, Volp KA, Omar I, Flynn D, Hanson PR. High-load, Oligomeric dichlorotriazine (ODCT): A Versatile ROMP-derived Reagent and Scavenger. J. Org. Chem. 2008;73:8785–8790. doi: 10.1021/jo801578f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14) (a).Achanta S, Liautardm V, Paugh R, Organ MG. The Development of a General Strategy for the Synthesis of Tyramine-Based Natural Products by Using Continuous Flow Techniques. Chem. Eur. J. 2010;16:12797–12800. doi: 10.1002/chem.201002102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shore G, Yoo WJ, Li CJ, Organ MG. Propargyl Amine Synthesis Catalysed by Gold and Copper Thin Films by Using Microwave-Assisted Continuous-Flow Organic Synthesis (MACOS) Chem. Eur. J. 2010;16:126–133. doi: 10.1002/chem.200902396. [DOI] [PubMed] [Google Scholar]; (c) Shore G, Organ MG. Gold-Film-Catalysed Hydrosilylation of Alkynes by Microwave-Assisted, Continuous-Flow Organic Synthesis (MACOS) Chem. Eur. J. 2008;14:9641–9646. doi: 10.1002/chem.200801610. [DOI] [PubMed] [Google Scholar]; (d) Shore G, Organ MG. Diels-Alder Cycloadditions by Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS): The Role of Metal Films in the Flow Tube. Chem. Commun. 2008:838–840. doi: 10.1039/b715709f. [DOI] [PubMed] [Google Scholar]; (e) Shore G, Morin S, Mallik D, Organ MG. Pd PEPPSI-IPr-Mediated Reactions in Metal-Coated Capillaries Under Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS): The Synthesis of Indoles by Sequential Aryl Amination/Heck Coupling. Chem. Eur. J. 2008;14:1351–1356. doi: 10.1002/chem.200701588. [DOI] [PubMed] [Google Scholar]; (f) Bremner S, Organ MG. Multi-Component Reactions (MCR) to Form Heterocycles by Microwave-Assisted Continuous Flow Organic Synthesis (MACOS) J. Comb. Chem. 2007;9:14–16. doi: 10.1021/cc060130p. [DOI] [PubMed] [Google Scholar]; (g) Shore G, Morin S, Organ MG. Catalysis in Capillaries by Pd Thin Films Using Microwave-Assisted Continuous Flow Organic Synthesis (MACOS) Angew. Chem. 2006;118:2827–2832. doi: 10.1002/anie.200503600. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2006;45:2761–2766. doi: 10.1002/anie.200503600. [DOI] [PubMed] [Google Scholar]; (h) Organ MG, Comer E. A Microcapillary System for Microwave Assisted, High Throughput Synthesis of Molecular Libraries. Chem. Eur. J. 2005;11:7223–7227. doi: 10.1002/chem.200500820. [DOI] [PubMed] [Google Scholar]; (i) Comer E, Organ MG. A Microreactor for Microwave-Assisted Capillary (Continuous Flow) Organic Synthesis (MACOS) J. Am. Chem. Soc. 2005;127:8160–8167. doi: 10.1021/ja0512069. [DOI] [PubMed] [Google Scholar]
- (15) (a).Ullah F, Samarakoon TB, Rolfe A, Kurtz RD, Hanson PR, Organ MG. Scaling Out by Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS): Multi-Gram Synthesis of Bromo- and Fluoro-benzofused Sultams Benzthiaoxazepine-1,1-dioxides. Chem. Eur J. 2010;16:10959–10962. doi: 10.1002/chem.201001651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Organ MG, Hanson PR, Rolfe A, Samarakoon TB, Ullah FJ. Accessing Stereochemically Rich Sultams via Microwave-Assisted, Continuous Flow Organic Synthesis (MACOS) Scale-out. J. Flow Chem. (Special Edition) 2011 doi: 10.1556/jfchem.2011.00008. Manuscript in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zang Q, Javed S, Ullah F, Zhou A, Knudton CA, Bi D, Basha FZ, Organ MG, Hanson PR. Application of a Double aza-Michael Reaction in a “Click, Click, Cy-Click” Strategy: From Bench to Flow. Synthesis. 2011 doi: 10.1055/s-0030-1260112. Thematic Issue on Click Chemistry: Invited. Manuscript in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Akella LB, Marcaurelle LA. Application of a Sparse Matrix Design Strategy to the Synthesis of DOS Libraries. ACS Comb. Sci. 2011 doi: 10.1021/co200020j. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Full in-silico data and detailed calculation information is provided in the supplementary information
- (18). [accessed 11-10-10]; http://www.anton-paar.com/
- (19).Representative compounds with full numeric data for each compound provided in supplementary information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.