Abstract
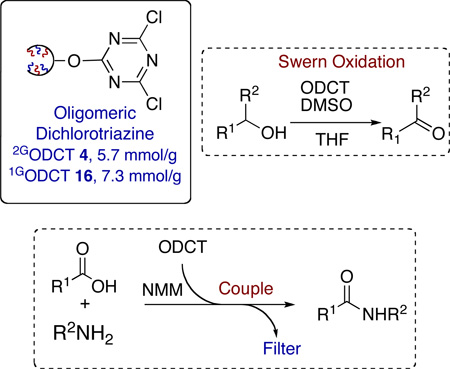
A new high-load, soluble oligomeric dichlorotriazine (ODCT) reagent derived from ring-opening metathesis polymerization (ROMP) is reported as an effective coupling reagent, scavenger of nucleophilic species and activator of DMSO for the classic Swern oxidations. Two variants of this reagent 2GODCT 4 and 1GODCT 16, possessing theoretical loads of 5.3 mmol/g and 7.3 mmol/g, respectively, have been synthesized. Preparation was accomplished via simple synthetic protocols affording free flowing powders, amenable for large-scale production. Removal of the spent oligomeric reagent was achieved via either precipitation of the spent reagent or simple filtration utilizing a silica SPE, followed by solvent removal, to deliver products in excellent yield and purity. In addition, the corresponding norbornenyl monomer 3 was successfully demonstrated in a couple-ROMP-filter protocol utilizing in-situ polymerization, achieving comparable results versus the corresponding oligomeric variant.
Keywords: Ring-Opening Metathesis Polymerization (ROMP), Dichlorotriazine (DCT), Trichlorotriazine (TCT), Oligomer, High-Load, Scavenger, Coupling Reagent, Swern Oxidation
Introduction
Trichlorotriazine (TCT) has been widely used over the years for facilitating an array of transformations, most notably as both a facile scavenger of nucleophilic species and an effective coupling reagent.1 However, in spite of its utility, TCT is a toxic, unstable reagent with a strong stench and hence is undesirable reagent for large scale use and application in the preparation of combinatorial libraries. To overcome these limitations, a number of immobilized TCT derivatives and other polymer-supported acyl group-activating reagents have been reported.2 More recently a cross-linked, polymeric ROMPgel derivative of TCT was reported and demonstrated as a dehydrocondensing reagent for the formation of amides.3 Despite these reports, the design of new and improved variants of TCT that are user-friendly, offering a high degree of versatility are warranted.4
Ring-opening metathesis (ROM) polymerization of functionalized norbornenes has emerged in recent years as a powerful tool for the generation of high-load, supported reagents with tunable properties.5 Originally pioneered by Barrett and co-workers, ROM oligomers/polymers have several salient features which address classical limitations associated with traditional methods of immobilization and therefore positions them as ideal supports for both reagents and scavengers.5,6 Our continued interest in the development of purification protocols based on norbornenyl tagged reagents and ROMP strategies leads us to report the titled oligomeric dichlorotriazine (ODCT) derivatives, designed as readily prepared, scaleable reagents with optimal characteristics for versatile use in synthetic methodology and parallel synthesis.
Results and Discussion
Initial efforts towards the development of a soluble, ROMP-derived DCT-variant focused on the synthesis of an oligomeric DCT derivative (2GODCT50 4) in a three-step protocol.7 Thus, condensation of commercially available carbic anhydride 1 with ethanolamine using a Dean-Stark trap, generated the desired alcohol 2 in high yield (80–89%, Scheme 1).8 Alcohol 2 was subsequently treated with trichlorotriazine (TCT) to afford the desired norbornene monomer 3 in good yield (69–79%). It is worth noting that use of bases other than 2,4,6-collidine yielded additional by-products attributed to multiple additions of alcohol 2 into TCT. With monomer 3 in hand, polymerization was carried out utilizing 2 mol% of the Grubbs second-generation catalyst [(IMesH2)(PCy3)(Cl)2Ru=CHPh; cat-B]9 to afford the corresponding oligomeric reagent 4 (50-mer) as a free flowing powder in high yield (90–98%). Quenching of the ROM polymerization reaction with ethyl vinyl ether, followed by precipitation with diethyl ether, provided the desired reagents as a free-flowing reagent 4 (2GODCT50, theoretical load 5.7 mmol/g)10 in excellent yield and on large scale (15 grams, Scheme 1).11 The high load nature of this soluble oligomeric ROMP reagent/scavenger 4 (5.7 mmol/g) represents a significant advantage over commercially available scavengers and coupling reagents: 1.0–1.6 mmol/g for PS-BnCHO, 3–5 mmol/g for PS-Trisamine, PS-HOBt(HL) 0.9–1.4 mmol/g and PS-carbodiimide 1.0–1.5 mmol/g.12
Scheme 1.
With oligomer 4 in hand, the solubility in a range of organic solvents was examined. It was found that 2GODCT50 4 (50-mer) was soluble in CH2Cl2, DMF, THF, DMSO and insoluble in Et2O, EtOAc, toluene and hexane. This wide solubility profile allows for reactions to be conducted in CH2Cl2 followed by removal of the spent oligomer via precipitation in EtOAc. It is worth noting that ROMP technology enables one to tune the length of the polymer (n = 10, 20, 50 and 100 mer) by control of the amount of catalyst (mol %).13 Thus, the solubility profile and hence the physical properties of the oligomer can be tailored. In general, longer chained polymers (n = 100, 200) in comparison to shorter chained polymers (n = 10, 20, 50) are soluble in a smaller range of organic solvents.
Initially, we evaluated the scavenging ability of 2GODCT 4 to sequester nucleophilic species such as amines and alcohols.14 This was undertaken using the corresponding monomer 3, allowing for direct monitoring of the crude monomer using both GC and 1H NMR. With the successful scavenging of excess amines and alcohols utilizing 3, we evaluated the removal of excess amines with 4 in the formation of simple amides (Table 1).15
Table 1.
Application of 2GODCT50 4 as a facile scavenger of amines and alcohols
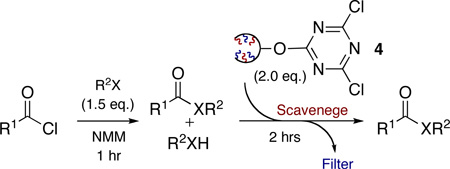 | |||||
|---|---|---|---|---|---|
| entrya | R1C(O)Cl | R2XH | product | yield (%)c | purity (%)b |
| 1 | BnC(O)Cl | BnNH2 | 5 | 92 | >95 |
| 2 | BnC(O)Cl | iPrNH2 | 6 | 92 | >95 |
| 3 | BnC(O)Cl | C5H8NH | 7 | 94 | >95 |
| 4 | p-TolC(O)Cl | iPrNH2 | 8 | 91 | >95 |
| 5 | p-TolC(O)Cl | C5H8NH | 9 | 94 | >95 |
All reactions were carried out on 0.164 mmol scale, 2.0 equiv. of R2XH and scavenged with 1 equiv. of 4 unless stated otherwise.
Purity by GC analysis and confirmed by 1H NMR.
Yields were calculated from pure products after purification via elution through a silica SPE.
With these results in hand, we next evaluated the application of 2GODCT50 4 as a coupling reagent for the formation of amides from commercially available amines and acids (Table 2). Coupling was achieved using 1 equivalent of acid, 1.1 equivalents of amine, 3 equivalents of N-methyl morpholine (NMM) and 2 equivalents of 2GODCT50 4. After stirring for 2 hours at room temperature, silica was added to the crude mixture, the solvent was removed and the crude was loaded onto a silica SPE and eluted with solvent (1:1 EtOAc:hexane) to yield the desired product with no residual oligomer observed in the crude 1H NMR. Additionally, it is worth noting that less than 2 equivalents of 4 can be used, however, slower reaction rates were observed, along with residual amine in certain cases. In the latter case, it is believed that the extra equivalent of 2GODCT50 4 scavenged excess amine in the crude reaction yielding the desired amide in good purity. This latter function displays the dual role inherent to the titled reagents.16
Table 2.
Application of 2GODCT50 4 as an efficient coupling reagent
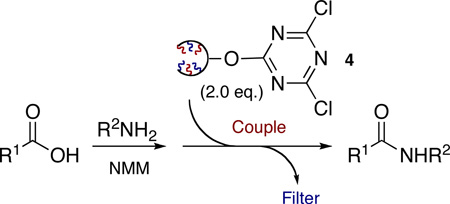 | |||||
|---|---|---|---|---|---|
| entry | R1CO2H | R2NH2 | product | yield (%)c | purity (%)b |
| 1 | BnCO2H | BnNH2 | 5 | 94 | >95 |
| 2 | BnCO2H | iPrNH2 | 6 | 92 | >95 |
| 3 | BnCO2H | C5H8NH | 7 | 93 | >95 |
| 4 | p-TolCO2H | iPrNH2 | 8 | 96 | >95 |
| 5 | p-TolCO2H | C5H8NH | 9 | 97 | >95 |
| 6 | 4-OMe-3-MeBnCO2H | C5H8NH | 10 | 93 | >95 |
| 7 | C7H15CO2H | BnNH2 | 11 | 95 | >95 |
| 9 | C2H5CO2H | BnNH2 | 12 | 93 | >95 |
| 10 | C2H5(CH3)CO2H | iPrNH2 | 13 | 94 | >95 |
All reactions were carried out on 0.164 mmol scale, with addition of 2.0 equivalents of 4 unless stated otherwise.
Purity by GC analysis and confirmed by 1H NMR.
Yields were calculated from pure products after purification via elution through a silica SPE.
To demonstrate the versatility of oligomer 4 and its corresponding monomer 3, a couple-ROMP-filter protocol was examined, whereby 3 was utilized as a coupling reagent for the synthesis of simple amides (Table 3).17 Once the reaction had gone to completion (monitoring via GC/TLC), cat-B was added and the reaction was heated to reflux to initiate in situ polymerization of the spent monomer. Subsequent removal of the spent, oligomerized reagent was achieved by simple precipitation and filtration. Alternatively, the crude reaction mixture can be dry loaded onto silica and eluted through a SiO2 SPE.
Table 3.
Application of DCT monomer 3 in a Couple-ROMP-Filter protocol
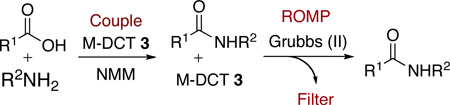 | ||||
|---|---|---|---|---|
| Entry | R1CO2H | R2NH2 | Yield (%)c | Purity (%)b |
| 1 | BnCO2H | BnNH2 | 95 | >95 |
| 2 | C9H19CO2H | BnNH2 | 93 | >95 |
| 3 | p-TolCO2H | iPrNH2 | 92 | >95 |
| 4 | P-TolCO2H | C5H8NH | 93 | >95 |
| 5 | 4-OMe-3-MeBnCO2H | C5H8NH | 96 | >95 |
All reactions were carried out on 0.164 mmol scale, with addition of 2.0 equivalents of 3 unless stated otherwise.
Purity by GC and confirmed by 1H NMR.
Yields were calculated from pure products after purification via elution through a silica SPE.
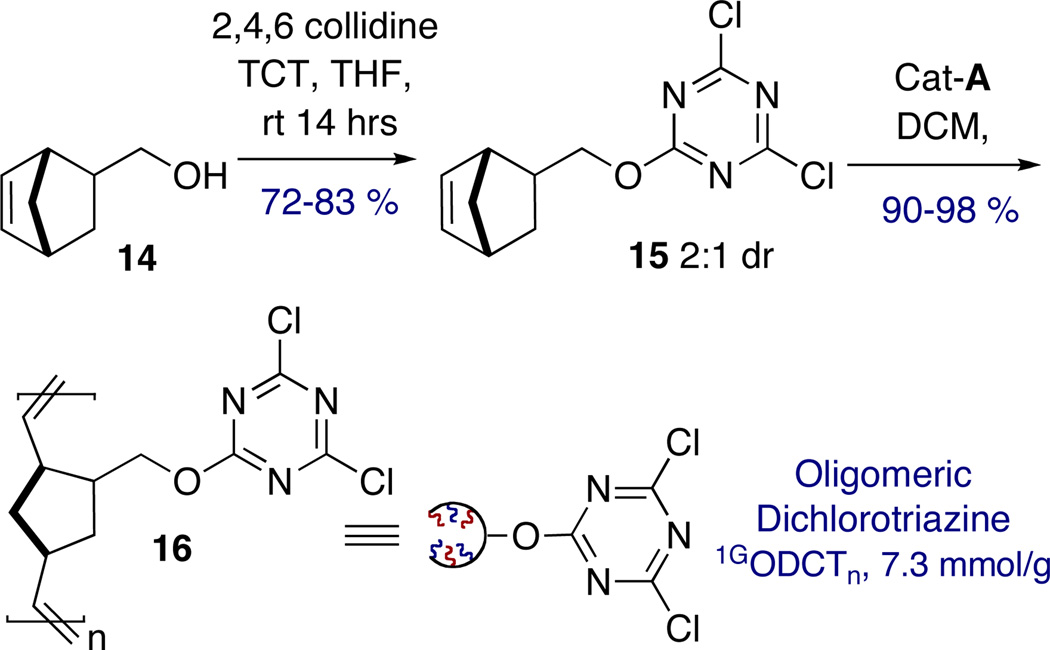
In our continued efforts to develop and generate new high-load oligomeric reagents and scavengers, a second derivative of ODCT possessing a higher load was synthesized (Scheme 2). In this example, commercially available norbornene-2-methanol 14 was condensed with TCT yielding the desired DCT monomer 15 in good yield. Initially, polymerization of the monomer 15 was attempted with cat-B, however, problems occurred with both the polymerization event and precipitation procedure. To circumvent these problems, polymerization and successful precipitation was accomplished with the utilization of the Grubbs first generation catalyst (PCy3)2(Cl)2Ru=CHPh (cat-A),18 followed by precipitation in hexanes to generate 16 in high yield (90–98%).
Scheme 2.
Synthesis of monomer 15 and oligomer 1GODCT 16
Using the aforementioned protocol, a range of oligomeric lengths were generated (20, 50 and 100 mer), which in comparison to ODCT 4, demonstrated a differential solubility profile. In this regard, a greater solubility in a range of organic solvents was observed, including solubility in CH2Cl2, DMF, THF and DMSO, with the notable inclusion of acetone and Et2O. Due to its solubility in Et2O, precipitation of the desired polymer after ROM polymerization was accomplished using hexane.
As demonstrated with 2GODCT 4, the application of 1GODCT50 16 was implemented successfully for the coupling of simple amines and acids giving comparable results as reported with oligomer 4 (Table 4).
Table 4.
Application of 1GODCT50 16 for the formation of simple amides
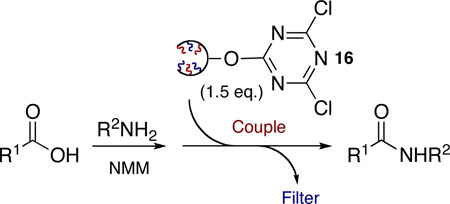 | |||||
|---|---|---|---|---|---|
| entry | R1CO2H | R2NH2 | product | yield (%)c | purity (%)b |
| 1 | BnCO2H | BnNH2 | 5 | 92 | >95 |
| 3 | BnCO2H | C5H8NH | 7 | 94 | >95 |
| 4 | p-TolCO2H | iPrNH2 | 8 | 92 | >95 |
| 5 | p-TolCO2H | C5H8NH | 9 | 95 | >95 |
| 6 | 4-OMe-3-MeBnCO2H | C5H8NH | 10 | 94 | >95 |
| 7 | C7H15CO2H | BnNH2 | 11 | 93 | >95 |
All reactions were carried out on 0.164 mmol scale, with addition of 2.0 equivalents of 16 unless stated otherwise.
Purity by GC analysis and confirmed by 1H NMR of crude isolated products.
Yields were calculated from pure products after purification via elution through a silica SPE.
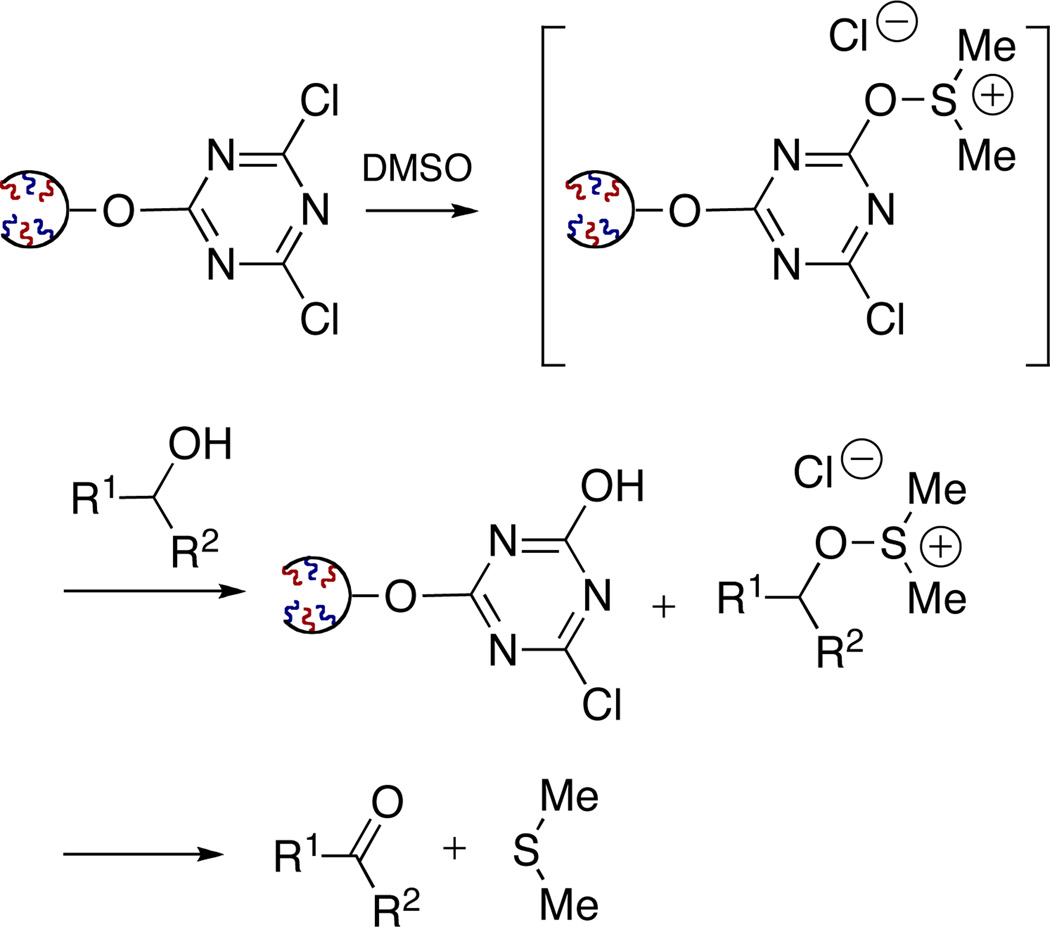
Along with its application as a scavenger and coupling reagent, TCT and the corresponding DCT derivatives have been reported as reagents for a number of other applications, which include chlorination, dehydration of amides to nitriles, deoxygenation of sulfoxides and the Swern oxidation.19 In this regard we examined the application of 1GODCT50 (16) as an activator for the classical Swern oxidation.
TCT has been reported as an effective activator in the Swern oxidation of alcohols.19e Activation of DMSO can be violent and very exothermic, requiring low temperatures as observed with thionyl or oxalyl chloride. Despite previous success with use of TCT, the utilization of TCT as an activator is limited due to its inherent toxicity and moisture sensitivity. With this in mind, ROMP-derived ODCT represents an ideal reagent for the activation of DMSO. To explore this reaction pathway with the application of 1GODCT 16, the Swern oxidation of simple primary alcohols to the corresponding aldehydes was investigated (Table 5).20 In this regard, 1GODCT 16 and DMSO were stirred together at −30 °C, forming the activated DMSO complex in-situ. Subsequent addition of the corresponding primary alcohol, followed by addition of Et3N resulted in a homogeneous solution. The crude mixture was then absorbed onto silica, loaded onto a silica SPE and flushed with an eluent of hexane:EtOAc (2:1) to yield the desired product in high yield and purity.
Table 5.
Application of 1GODCT50 16 in the Swern oxidation of primary alcohols
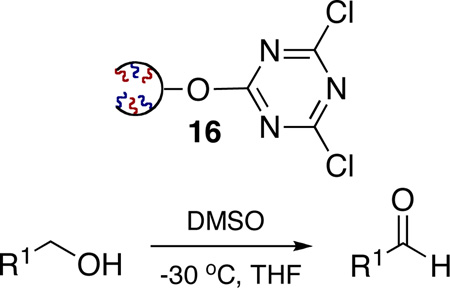 | |||
|---|---|---|---|
| Entry | R1OH | Product | Yield (%)a |
| 1 | C4H9OH | 17 | 90 |
| 2 | (CH3)2CHCH2OH | 18 | 40 |
| 3 | C8H17OH | 19 | 92 |
| 4 | 2,4-OMeBnOH | 20 | 94 |
| 5 | 4-NO2BnOH | 21 | 90 |
| 6 | 4-OMeBnOH | 22 | 95 |
All reactions were carried out on 0.164 mmol scale, with addition of 1.5 eq of 16 unless stated otherwise.
Purity by GC and confirmed by 1H NMR of crude isolated products.
Yields were calculated from pure products after purification via elution through a silica SPE.
In conclusion, we have synthesized a high-load, versatile oligomeric variant of TCT derived from ROM polymerization. Initially, a norbornenyl maleimide variant of this reagent was prepared 2GODCTn 4 (5.3 mmol/g) and successfully applied as a facile scavenger of amines and alcohols in addition to its application as a facile coupling reagent for the preparation of simple amides. Expanding the versatility of this reagent, the corresponding monomer 3 was successfully utilized in a couple-ROMP-filter protocol for the formation of amides. A second variant of 1GODCT50 16 was synthesized possessing a significant increase in load. Like 2GODCT50 4, 1GODCT50 16 was successfully applied as both a facile scavenger and coupling reagent. Additional versatility of the reagent was demonstrated with the successful application as an activating reagent of DMSO for the Swern oxidation of a variety of alcohols. Overall, ODCT 4 and 16 offer a high-load, versatile reagent that is readily synthesized from inexpensive starting materials with application to large-scale production.
Experimental Section
Exo-norborenyl Alcohol 2
Into a flame dried flask fitted with a Dean-Stark apparatus under Ar was added exo-carbic anhydride 1 (1g, 6.10 mmol). After addition of dry toluene (8.7 ml) and stirring for 5 mins at RT, ethanolamine (0.75 ml, 12.2 mmol) was added. The reaction was heated at reflux for 14 hrs, which upon completion (monitoring via TLC), was cooled to RT and concentrated under reduced pressure. The resulting crude solid was dissolved in DCM (20 ml), filtered through a silica plug and concentrated to give 1.12 g (89% yield) of 2 as a white solid. Mp 90–92 °C; FTIR (neat) 3442, 2945, 1766, 1696, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.11 (t, J = 1.8 Hz, 2H), 3.63 (t, J = 4.9 Hz, 2H), 3.55 (t, J = 5.5 Hz, 2H), 3.39 (m, 2H), 3.29 (dd, J = 2.9, 1.5 Hz, 2H), 1.74 (t, J = 1.6 Hz, 1H), 1.72 (t, J = 1.6 Hz, 1H), 1.56 (m, 1H), 1.53 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 178.4, 134.4, 60.8, 52.2, 45.8, 44.9, 41.2; HRMS calculated for C11H13NO3Na (M+Na)+ 230.0793; found 230.0786 (TOF MS ES+).
DCT norborenyl monomer 3
Into a flame dried flask under Ar was added TCT (1.78 g, 9.7 mmol). This was dissolved in dry THF (12 mL), cooled to 0 °C and stirred for 15 minutes. After such time, 2,4,6-colidine (2.53 ml, 19.2 mmol) was added followed by addition of 2 (2.0 g, 9.6 mmol). Upon stirring for an additional 15 mins at 0 °C, the reaction was warmed to RT and stirred for 10 hours. The reaction was then concentrated in-situ and purified by flash chromatography (6:4 hexane:EtOAc) to afford 2.59 g (79% yield) of the 3 as a light brown solid. FTIR (neat) 1770, 1699, 1549, 1514, 1255 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.11 (t, J = 1.8 Hz, 2H), 4.52 (t, J = 3.3 Hz, 2H), 3.78 (t, J = 5.5 Hz, 2H), 3.38 (m, 2H), 3.28 (dd, J = 2.9, 1.5 Hz, 2H), 1.73 (t, J = 1.6 Hz, 1H), 1.72 (t, J = 1.6 Hz, 1H), 1.54 (m, 1H), 1.52 (m, 1H); 13C NMR (125 MHz, CDCl3) δ177.4, 172.5, 170.7, 134.3, 65.9, 56.0, 45.9, 45.0, 36.2; HRMS calculated for C14H12Cl2N4O3Na (M+Na)+ 377.0184; found 377.0186 (TOF MS ES+).
General procedure for the polymerization of DCT norborenyl monomer 3 to generate 4
Norbornenyl monomer 3 (0.5g, 1.41 mmol) was dissolved in degassed CH2Cl2 (0.085 M, 16.6 mL), to which cat-B (23.9 mg, 28 µmol) was added and the reaction refluxed for 2 hrs under Ar. After such time, the reaction was cooled to RT, ethyl vinyl ether (0.05 mL) was added and the reaction was stirred for 15 mins. The crude reaction mixture was concentrated and precipitated by dropwise addition to a stirring solution of Et2O (100 mL). The resulting precipitate was filtered, washed with Et2O and dried under vacuum to produce 0.49 g (98% yield) of 4 as a light brown, free flowing solid.
The following represents general experimental procedures for the implementation of the titled oligomer reagents in synthetic transformations. Specific experimental details and characterization data for the aforementioned compounds and other new compounds can be found in the Supporting Information.
General procedure A for the scavenging of amines or alcohols ODCT50 4/16 or monomer 3/15
A vial equiped with a stir bar was charged with amine/alcohol (0.246 mmol, 1.5 equiv.), Et3N and CH2Cl2 (0.075 M). After stiring for 5 minutes, acid chloride (0.164 mmol, 1 equiv.) was added and the reaction was stirred for 1 hr at RT. After the stated time, ODCT50 4/16 or monomer 3/15 (0.328 mmol, 2.0 eq) was added and the crude reaction was stirred for an additional 2 hours. This was monitored by 1H NMR and GC when monomer 3 was applied.
General procedure B for the amide coupling of simple acids and amines utilizing ODCT50 4/16
A vial equiped with a stir bar was charged with ODCT50 4/16 (0.328 mmol, 2.0 eq), CH2Cl2 (0.075 M), acid (0.164 mmol, 1 equiv.) and NMM (0.492 mmol, 3 equiv.). After stirring for 10 mins, amine (0.328 mmol, 2 eq.) was added and the reaction was stirred at RT for 2 hours. After such time, the crude mixture was added dropwise to a stirring solution of dry Et2O (10 mL), precipitating the spent oligometric reagent. After successful precipitation, the crude mixture was filtered through a SiO2 SPE, yielding the desired products 5–13.
General procedure C for the amide coupling of simple acids and amines utilizing ODCT50 4/16
A 1 dram vial equipped with a stir bar was charged with ODCT50 4/16 (0.328 mmol, 2.0 eq), CH2Cl2 (0.075 M), acid (0.164 mmol, 1 equiv.) and NMM (0.492 mmol, 3 equiv.). After stirring for 10 mins, amine (0.328 mmol, 2 eq.) was added and the reaction was stirred for 2 hours. Silica was added to the reaction mixture, the solvent was removed under reduced pressure and the resulting powder was loaded onto a silica SPE and eluted with solvent (1:1 EtOAc:hexane) to yeild the desired product 5–13 in high purity.
General procedure D for the amide coupling of simple acids and amines utilizing DCT monomer 3
A vial equiped with a stir bar was charged with DCT monomer 3 (0.328 mmol, 2.0 eq), dry CH2Cl2 (0.075 M), acid (0.164 mmol, 1 eq.) and NMM (0.492 mmol, 3 eq.). After stirring for 10 mins, amine (0.328 mmol, 2 eq.) was added to the crude mixture and the reaction was stirred at RT for 2hrs. After the stated time, cat-B (2 mol%) was added to the crude mixture, which was heated at reflux for 1 hr. After such time the reaction was cooled to RT, quenched via the addition of ethyl vinyl ether (50 µl) and stirred for 15 mins. The spent oligomer was precipitated by the slow addition of the crude mixture to a stirring solution of Et2O (5 mL) and filtered through a silica SPE with solvent (1:1 EtOAc:hexane) to afford the desired product in good purity 5–13.
DCT norborenyl monomer 15
Into a flame dried flask under Ar, a solution of TCT (1.55g, 8.4 mmol in dry THF (10.0 mL) was cooled to 0 °C and stirred for 15 minutes. After such time 2,4,6-collidine (2.12 ml, 16.0 mmol) was added, followed by the slow addition of alcohol 14 (1.0g, 8.0 mmol). After stirring for an additional 15 mins at 0 °C, the reaction was warmed to RT and stirred for 10 hrs. Purification of the crude reaction mixture entailed in-situ concentration followed by flash chromatography (6:4 EtOAc:hexane) to give 1.86 g (83% yield) of 15 (2:1 mixture of diastereoisomers) as a brown viscous oil. FTIR (neat) 1769, 1695, 1550, 1510, 1248 cm −1; [Major isomer] 1H NMR (500 MHz, CDCl3) δ 6.21 (dd, J = 5.7, 3.1 Hz, 1H), 5.97 (dd, J = 5.7, 2.9 Hz, 1H), 4.26 (dd, J = 10.5, 6.6 Hz, 1H), 4.06 (dd, J = 10.4, 9.7 Hz, 1H), 2.99 (s, 1H), 2.87 (s, 1H), 1.92 (ddd, J = 11.9, 9.3, 3.8 Hz, 2H), 1.50 (dd, J = 8.3, 2.1 Hz, 1H), 1.30 (d, J = 8.3 Hz, 1H), 0.64 (ddd, J = 11.8, 4.4, 2.6 Hz, 1H); [Minor] 1H NMR (500 MHz, CDCl3) δ 6.11 (t, J = 1.8 Hz, 2H), 4.56 (dd, J = 10.6, 6.4 Hz, 1H), 4.36 (dd, J = 10.6, 9.4 Hz, 1H), 2.89 (s, 1H), 2.83 (d, J = 1.5 Hz, 1H), 1.92 (ddd, J = 11.9, 9.3, 3.8 Hz, 2H), 1.42 – 1.37 (m, 1H), 1.37 – 1.32 (m, 1H), 1.24 (ddd, J = 11.9, 4.5, 3.5 Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 172.4, 170.9, 138.1, 137.0, 136.0, 131.9, 74.4, 73.8, 49.4, 44.9, 43.7, 43.5, 42.1, 41.6, 37.7, 37.4, 29.3, 28.7; HRMS calculated for C11H11Cl2N3ONa (M+Na)+ 294.0177; found 294.0182 (TOF MS ES+).
General procedure for the polymerization of DCT norborenyl monomer 15 to generate 16
Norbornenyl monomer 15 (1.67g, 6.14 mmol) was dissolved in degassed CH2Cl2 (0.085 M, 72.2 ml), to which cat-A (0.104g, 0.123 mmol) was added. The reaction refluxed for 2 hrs and subsequently cooled to RT, upon which time the reaction was quenched by the addition of ethyl vinyl ether (0.1 mL). After stirring for 15 minutes, the crude mixture was concentrated and precipitated via dropwise addition to a stirring solution of hexane (200 mL). The resulting precipitate was filtered, washed with hexanes and dried under vacuum to produce 1.63 g (98% yield) of 16 as a light brown free flowing solid.
General procedure E for the swern oxidation of simple primary alcohols utilizing 1GODCT50 16
To a stirring solution of 1GODCT50 16 (0.246, 1.5 eq.) in dry THF (1 mL) at −30 °C, was added DMSO (0.984 mmol, 6 eq.) slowly under Ar. After stirring for 30 mins, a solution of alcohol (0.164 mmol, 1 eq.) in dry THF (0.5 mL) was slowly added maintaining the reaction temperature at −30 °C. After stirring for 30 minutes, Et3N (0.825 mmol, 5 eq.) was added and the reaction mixture was stirred for an additional for 30 min. After warming to RT, silica was added to the reaction mixture, solvent was removed and the resulting powder was loaded onto an SiO2 SPE. The desired products 17–22 were isolated via elution with a solvent mixture (2:1 hexane:EtOAc).
Supplementary Material
Scheme 3.
Activation of DMSO utilizing 1GODCT50 16.
Acknowledgement
This work was generously supported by funds provided by an NIH STTR Grant (R41 GM076765 STTR-Phase-I), the Center for Chemical Methodologies and Library Development at the University of Kansas (KU-CMLD) [P50 GM069663] and NIH COBRE award P20 RR015563 with additional funds from the State of Kansas. Undergraduate funding was provided by the NIH K-INBRE award (K.V) and the KU Center of Research (K.V.). The authors also thank the Umaer Basha Research fellowship for student support (I.O.) and Materia, Inc. for supplying catalyst and helpful discussion.
Footnotes
Supporting Information Available. Copies of NMR spectra are provided along with supporting information for products 5–13 and 17–22. This material is available free of charge via the World Wide Web at http://pubs.acs.org.
References
- 1.(a) Albright JD. J. Org. Chem. 1974;39:1977–1979. [Google Scholar]; (b) Olah GA, Narang SC, Fung AP, Gupta BGB. Synthesis. 1980:657–658. [Google Scholar]; (c) Senier A. Ber. 1886;18:311. [Google Scholar]; (d) De Luca L, Giacomelli G, Taddei M. J. Org. Chem. 2001;66:2534–2537. doi: 10.1021/jo015524b. [DOI] [PubMed] [Google Scholar]; (e) Lu Y, Zhang W. QSAR Comb. Sci. 2006;25:728–731. doi: 10.1002/qsar.200640042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Luo G, Xu L, Poindexter GS. Tetrahedron. Lett. 2002;43:8909–8912. [Google Scholar]; (b) Falchi A, Taddei M. Tetrahedron. Lett. 2000;22:3429–3431. doi: 10.1021/ol0002222. [DOI] [PubMed] [Google Scholar]; (c) Zhang W, Lu Y. J. Comb. Chem. 2007;9:836–843. doi: 10.1021/cc070055y. [DOI] [PubMed] [Google Scholar]; (d) Dilly SJ, Carlisle SJ, Clark AJ, Shepherd AR, Smith SC, Taylor PC, Marsh A. J. Pol. Sci, Part A: Pol. Chem. 2006;44:2248–2259. [Google Scholar]; (e) Marsh A, Carlisle SJ, Smith SC. Tetrahedron Lett. 2001;42:493–496. [Google Scholar]
- 3.Hioki K, Kameyama S, Tani S, Kunishima M. Chem. Pharm. Bull. 2007;55:825–828. doi: 10.1248/cpb.55.825. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cohen BJ, Karoly-Hafeli H, Patchornik A. J. Org. Chem. 1984;49:922–924. [Google Scholar]; (b) Kim K, Le K. Synlett. 1999:1957–1959. [Google Scholar]; (c) Adamczyk M, Fishpaugh JR, Mattingly PG. Tetrahedron Lett. 1999;40:463–466. [Google Scholar]; (d) Chinchilla R, Dodsworth DJ, Najera C, Soriano JM. Tetrahedron Lett. 2001;42:4487–4489. [Google Scholar]; (e) Barrett AGM, Cramp SM, Roberts RS, Zecri F. J. Org. Lett. 2000;2:261–264. doi: 10.1021/ol991208w. [DOI] [PubMed] [Google Scholar]; (f) Arnauld T, Barrett AGM, Hopkins BT, Zecri FJ. Tetrahedron Lett. 2001;42:8215–8217. [Google Scholar]
- 5.For reviews concerning ROMP Reagents, see: Barrett AGM, Hopkins BT, Köbberling J. Chem. Rev. 2002;102:3301–3324. doi: 10.1021/cr0103423. Flynn DL, Hanson PR, Berk SC, Makara GM. Curr. Opin. Drug Discov. Devel. 2002;5:571–579. Harned AM, Probst DA, Hanson PR. The Use of Olefin Metathesis in Combinatorial Chemistry: Supported and Chromatography-Free Syntheses. In: Grubbs RH, editor. Handbook of Metathesis. Wiley-VCH: Weinheim; 2003. pp. 361–402. Harned AM, Zhang M, Vedantham P, Mukherjee S, Herpel RH, Flynn DL, Hanson PR. Aldrichimica Acta. 2005;38:3–16.
- 6.(a) Zhang M, Flynn DL, Hanson PR. J. Org. Chem. 2007;72:3194–3198. doi: 10.1021/jo0620260. [DOI] [PubMed] [Google Scholar]; (b) Harned AM, Sherrill WM, Flynn DL, Hanson PR. Tetrahedron. 2005;61:12093–12099. [Google Scholar]; (c) Zhang, Moore JD, Flynn DL, Hanson PH. Org. Let. 2004;6:2657–2660. doi: 10.1021/ol049209y. [DOI] [PubMed] [Google Scholar]
- 7.XODCTy (x = generation of Grubbs catalyst, y = polymer length/number of monomer units in the oligomeric chain).
- 8.(a) Davies GR, Gibson VC, Hursthouse MB, Light ME, Marshall EL, North M, Robson DA, Thompson I, White AJP, Williams DJ, Williams PJ. J. Chem. Soc., Perkin Trans. 1. 2001:3365–3381. [Google Scholar]; (b) Berlin JM, Goldberg SD, Grubbs RH. Angew. Chem. Int. Ed. 2006;45:7591–7595. doi: 10.1002/anie.200602469. [DOI] [PubMed] [Google Scholar]
- 9.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 10.Loading of ODCT is calculated as mmol Cl/g.
- 11.We have previously found that there is a good correlation between the mol % of Grubbs catalyst added and the Gaussian distribution of oligomers formed, which is the case with several other oligomers formed [See Ref 5d.] We have made these reagents several times with good reproducibility and consistency. MALDITOF and/or GPC data is normally attained on all oligomers; however, both methods have failed to give good results for our previously published reactive oligomeric bis-acid chloride (OBAC) and sulfonyl chloride resin (OSC).
- 12. www.biotage.com (5-11-08).
- 13.We have previously found that there is good correlation between the mol % of Grubbs catalyst added and the Gaussian distribution of oligomers formed, which we believe is the case with the ODCT shown in Scheme 1. We have made this reagent several times (i.e. the preparation and reactivity are repeatable and consistently reliable). We normally obtain MALDI-TOF and/or GPC data on all oligomers formed; however, both methods have failed to give good results for this reactive oligomer.
- 14.For additional ROMP-derived scavengers, see: Moore JD, Herpel RH, Lichtsinn JR, Flynn DL, Hanson PR. Org. Lett. 2003;5:105–107. doi: 10.1021/ol0270273. Moore JD, Byrne RJ, Vedantham P, Flynn DL, Hanson PR. Org. Lett. 2003;5:4241–4244. doi: 10.1021/ol0352759. Herpel RH, Vedantham P, Flynn DL, Hanson PR. Tetrahdron Lett. 2006;47:6429–6432. Stoianova DS, Yao L, Rolfe A, Samarakoon T, Hanson PR. Tetrahedron Lett. 2008;49:4553–4555. doi: 10.1016/j.tetlet.2008.05.011.
- 15.(a) Wan Y, Alterman M, Larhed M, Hallberg A. J. Org. Chem. 2002;67:6232–6235. doi: 10.1021/jo025965a. [DOI] [PubMed] [Google Scholar]; (b) Furuya Y, Ishihara K, Yamamoto H. J. Am. Chem. Soc. 2005;127:11240–11241. doi: 10.1021/ja053441x. [DOI] [PubMed] [Google Scholar]; (c) Lawrence RM, Biller SA, Fryszman OM, Poss MA. Synthesis. 1987;5:553–558. [Google Scholar]; (d) Cooke MP, Jr, Pollock CM. J. Org. Chem. 1993;58:7474–7481. [Google Scholar]; (e) Kimpe ND, Verhe R, Buyck LD, Chys J, Schamp N. J. Org. Chem. 1978;43:2670–2672. [Google Scholar]
- 16.As observed in the crude 1H NMR spectra of reactions where spent-oligomer was removed by precipitation in Et2O, followed by filtration and removal of solvent under reduced pressure.
- 17.Of notable importance is the seminal advances made by Barrett and coworkers demonstrating the concept of reagent annihilation (norbornenyl-tagged DEAD) see: Barrett AGM, Roberts RS, Schröder J. Org. Lett. 2000;2:2999–3002. doi: 10.1021/ol006313g. For additional examples of in situ scavenging, see: Moore JD, Harned AM, Henle J, Flynn DL, Hanson PR. Org. Lett. 2002;4:1847–1849. doi: 10.1021/ol0257880.
- 18.(a) Schwab P, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1996;118:100–110. [Google Scholar]; (b) Schwab P, France MB, Ziller JW, Grubbs RH. Angew. Chem., Int. Ed. Engl. 1995;34:2039–2041. [Google Scholar]
- 19.(a) Venkataraman K, Wagle DR. Tetrahedron Lett. 1979;20:3037–3040. [Google Scholar]; (b) Matetz P, Rodriguez M. Tetrahedron Lett. 1997;38:4221–4222. [Google Scholar]; (c) Olath GA, Fung AP, Gupta BGB, Narang SC. Synthesis. 1980:221. [Google Scholar]; (d) Albright JD. J. Org. Chem. 1974;39:1977–1979. [Google Scholar]; (e) Luca LD, Giacomelli G, Porcheddu A. J. Org. Chem. 2001;66:7907–7909. doi: 10.1021/jo015935s. [DOI] [PubMed] [Google Scholar]; (f) Luca LD, Giacomelli G, Porcheddu A. Org. Lett. 2002;4:553–555. doi: 10.1021/ol017168p. [DOI] [PubMed] [Google Scholar]
- 20.(a) Rezaeifard A, Jafarpour M, Moghaddam GK, Amini F. Bioorg. Med. Chem. 2007;15:3097–3101. doi: 10.1016/j.bmc.2007.01.032. [DOI] [PubMed] [Google Scholar]; (b) Joesph JK, Jain SL, Sain B. Eur. J. Org. Chem. 2006:590–594. [Google Scholar]; (c) Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J. Bioorg. Med. Chem. 2005;13:433–441. doi: 10.1016/j.bmc.2004.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.