Abstract
A novel hybrid material is reported as support for a recyclable palladium catalyst via surface immobilization of a ligand onto Co-based magnetic nanoparticles (NPs). A standard “click” reaction is utilized to covalently attach a norbornene tag (Nb-tag) to the surface of the carbon coated cobalt NPs. The hybrid magnetic nanoparticles are produced by initiating polymerization of a mixture containing both Nb-tagged ligand (Nb-tagged PPh 3) and Nb-tagged carbon coated cobalt NPs. In turn, the norbornene units are suitably functionalized to serve as ligands for metal catalysts. A composite material is thus obtained which furnishes a loading that is one order of magnitude higher than the value obtained previously for the synthesis of functionalized Co/C-nanopowders. This allows for its application as a hybrid support with high local catalyst concentrations, as demonstrated for the immobilization of a highly active and recyclable palladium complex for Suzuki-Miyaura cross-coupling reactions. Due to the explicit magnetic moment of the cobalt- NPs, the overall magnetization of this organic/inorganic framework is significantly higher than of polymer coated iron oxide nanoparticles with comparable metal content, hence, its rapid separation from the reaction mixture and recycling via an external magnetic field is not hampered by the functionalized polymer shell.
1. Introduction
Interest in magnetic nanomaterials for applications in electronics [1] as well as in medical [2] and chemical [3] research has steadily increased in recent years. Hence, strategies were developed to coin diverse inorganic and organic frameworks with magnetic properties. Groundbreaking studies in the area of catalysis disclosed materials such as silica, carbon or polymers doped with magnetic nanoparticles. [4] In recent years, many reports focused on defined core/shell assemblies based on super-paramagnetic iron oxide nanoparticles (SPION), such as magnetite. [3, 5] Indeed, the lack of magnetic remanence in this material in the absence of an external magnetic field is especially beneficial for the dispersability of the nanomagnets. However, this advantage is accompanied by a relatively low saturation moment of ferrites (M S,bulk ≤ 92 emu g −1), which is, compared to bulk, usually by far lower in the nano-sized matter, due to partial surface oxidation, and sometimes further diminished through the need for a protective shell. [6] As a consequence, hybrid materials that rely on iron oxide nanoparticles demand a high metal content, e.g., for effective magnetic separation, thus limiting the fraction of other functional material in the composite and their total load capacity. Nanoparticles derived from pure metals exhibit superior magnetic properties to oxides, but their synthesis had been limited to small scale operations. [7, 8] Recently, Grass et al. [9] reported on a continuous process, in which substantial amounts (> 30 g h −1) of carbon coated ferromagnetic nanoparticles are accessible via reducing flame-spray pyrolysis. [10] Despite the comparatively thin graphene layer (1 nm), the intrinsically pyrophoric metal cores exhibit a remarkable thermal and chemical stability [11] and moreover, the carbon layer deposited on the cobalt core has no detrimental effect on the magnetization (158 emu/g). Therefore, Co/C-nanoparticles seem to be a suitable cornerstone for magnetic hybrid materials that ensure high local catalyst loadings. [12]
2. Results and Discussion
Ring-opening metathesis polymerization (ROMP)-derived polymers and oligomers have been of great interest as scaffolds for the immobilization of a plethora of reagents, scavengers and catalysts, [13, 14] since the high loadings (e.g., 7.3 mmol g −1 [13f]) obtained with such resins have clear benefits. [15] ROMP-derived polymers can be tuned to be soluble in a variety of organic solvents, thus, they enable a fully homogeneous process. Alternatively cross-linking can be utilized to yield ROMPgels with favorable properties when compared to traditional resins. Despite the rate-enhancement achieved in solution-phase chemistry, such scaffolds require an additional solvent for selective precipitation out of the reaction mixture. A second solvent can be an environmental drawback in industrial processes and recycling through precipitation/filtration is not necessarily quantitative. In contrast, Co/C-nanomagnets allow for their simple and rapid separation via magnetic decantation but feature only a very limited loading (0.1 mmol/g). [9] Although a low catalyst loading can be advantageous, e.g. when spatial interactions between chiral catalytic moieties shall be suppressed, [16] it is usually outbalanced by the problems associated with the sheer mass of support necessary.
2.1. Synthesis and Characterization of Co/C-ROMPgel
Buchmeiser [17] Grubbs [18] and Whiteside, [19] have demonstrated ring-opening metathesis (ROM) polymerization on silica surfaces, while Buchmeiser and Barrett have also reported ROM polymerization on polystyrene resins. [20] Barrett has demonstrated an increase in load in developing ROMP-spheres, [20] while Roberts has used ROM polymerization on resins to capture Nb-tagged N-hydroxy succinimide in the development of a high-load, resin-immobilized acylation reagent. [21] This complies with the extraordinary stability of the Co/C-NPs and prevents leaching of the nanoparticles from the polymer branches. ROMP-derived oligomers have also been used as surfactants to stabilize maghemite nanoparticles, albeit the magnetization of the resulting composite was not reported. [22] However, the covalent attachment of ROMPgel on magnetic nanoparticles is unprecedented.
In a first step, we utilized a copper(I)-catalyzed alkyne/azide cycloaddition (CuAAC) reaction (“click” reaction) [23] to graft propargylated norbornene derivative 3 onto azide functionalized Co/C-NPs 2 (Scheme 1).
Scheme 1.
Functionalization of azide-tagged Co/C-nanoparticles 2 with alkene 3 via copper(I)-catalyzed CuAAC (“click” chemistry) and subsequent ring-opening metathesis polymerization to covalently grafted ROMPgel on the magnetic nanobeads.
The synthesis of the azide-tagged particles was achieved in a concise two-step reaction based on covalent attachment of diazonium compounds on the graphene layers as described previously. [9a, 24] An azide-loading of 0.1 mmol g −1 was thus obtained as determined by elemental microanalysis. In our strategy, we used the immobilized alkene 3 as a means of loading triphenylphosphine-functionalized norbornenes 6 [25] onto the nanoparticle surface. Briefly, a closed microwave vessel containing alkene modified Co/C-NPs 4 was placed in a tempered (60 °C) ultrasound bath and the nanopowder was dispersed in degassed CH 2 Cl 2 via sonication under an inert atmosphere (argon). A solution of Ru-complex 5 (1.0 equiv. with respect to Co/C-immobilized alkene 4, 2 mol% with respect to PPh3-norbornene derivative 6) was added to generate a ruthenium carbene species on the nanoparticle surface by ring-opening metathesis with the norbornene units in 4. ROM polymerization [26] was then carried out by adding 6 under conditions otherwise suitable for the formation of a 50 mer. During the course of the reaction, a voluminous, black gel was formed, leaving only little residual solvent. Assuming that all Co/C-nanoparticles were coated with the available amount of oligomer, one would expect a Co-content of approximately 33% in the resulting hybrid material, a value which was confirmed by elemental microanalysis. TEM images affirmed that the Co/C-particles were embedded in a polymer matrix, thus “diluting” the otherwise densely packed clusters, a characteristic associated with the high magnetic remanence of the metal cores (Figure 1). Also, no evidence of cross linking was observed in agreement with earlier studies in which metathesis between an immobilized norbornene derivative and dissolved olefins was carried out. [27]
Figure 1.
TEM images of Co/C-nanoparticles 1 (left) and Co/C-ROMPgel 7 (right) at different resolutions; bar size: 100 nm (top) and 20 nm (bottom).
To ensure that the polymer did not only encapsulate the nanomagnets but was covalently attached to the carbon shell, we ran a control experiment utilizing azide-functionalized Co/C-NPs 2 instead of alkene-modified particles 4. In contrast to the previous observation, no gel was formed and the nanoparticles precipitated after the ROM polymerization while the oligomer remained in solution until precipitated from MeOH. The swelling behavior of the Co/C-ROMPgel was found to be in line with the general properties of ROMPgels, showing a pronounced volume increase in THF and CH 2 Cl 2 whereas solvents such as MeOH or Et 2 O did not provoke a significant effect. [28]
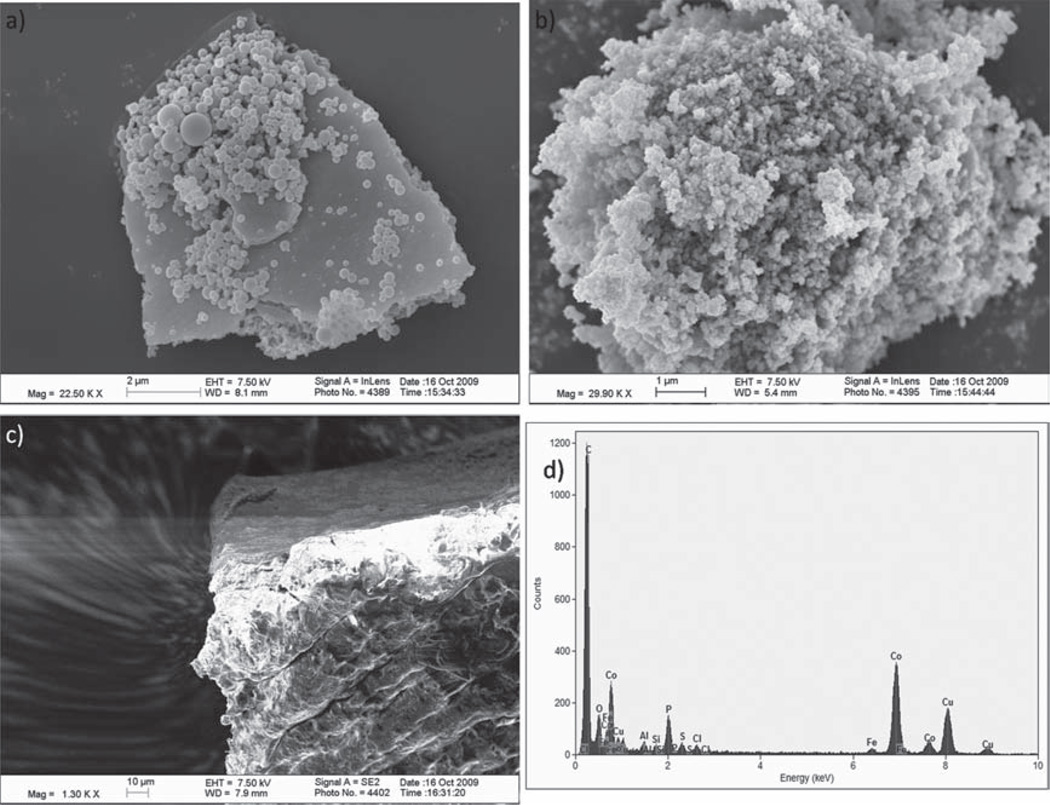
The morphology of the composite was clearly distinct from both parent materials as observed in scanning electron micrographs (SEM, Figure 2). To this end, the aforementioned ROMP oligomer obtained from polymerization in the presence of azide-functionalized Co/C-NPs 2 rather than 4 was analyzed next to unmodified Co/C-nanoparticles 1. In addition, the specimen stub was coated with a silver dispersion prior to deposition of Co/C-ROMPgel 7 to prevent the accumulation of static electric charge on the sample and to display its inherent magnetic field (Figure 2,c). Energy dispersive X-ray (EDX) confirmed the presence of phosphor in the hybrid material (Figure 2,d). The fraction of phosphor was assessed via elemental microanalysis (3.45%), corresponding to 1.1 mmol g−1 P-loading. Hence, approximately 30% of the hybrid material consists of triphenylphosphine (TPP). The immobilized TPP was expected to coin the nanocomposite with the ability to serve as separable reagent/scavenging agent [30] or ligand, i.e. for the formation of a recyclable palladium complex.
Figure 2.
SEM images of ROMPgel (a), Co/C-nanoparticles 1 (b), and Co/C-ROMPgel 7 (c); bar size: 2 µm (a), 1 µm (b), and 10 µm (c); EDX-spectrum of 7 (d). [29]
2.1. Catalysis with a Co/C-ROMPgel Immobilized Pd-Complex
Several examples of palladium complexes anchored on different magnetic iron oxide nanoparticles have been reported in the past years. [5, 31] However, highly functionalized architectures that relied on stabilized ferrite cores were naturally less susceptible to magnetic separation.
Doping of such matrices with palladium, an objective that is typically achieved by mixing a metal source together with the ligand-functionalized scaffold (Scheme 2), is prone to further diminish the mass magnetization. Figure 3 highlights the magnetization of composite 8, which was measured via vibrating sample magnetometer (VSM) and found to be 34 emu/g, a value consistent with the mass percentage of ferromagnetic cobalt in the sample. This level is comparable to surfactant stabilized SPIONs, however, polymer or silica coated iron oxide NPs, materials, which resemble 8 in terms of durability, exhibit significantly lower magnetization. [6]
Scheme 2.
Formation of a heterogeneous Palladium-complex on PPh3-functionalized Co/C-ROMPgel 7. The Pd-content of 8 was assessed by AAS (0.48 mmol g−1).
Figure 3.
Magnetization curve of Co/C-nanoparticles 1 (left) and Pd@ Co/C-ROMPgel 8 containing 30% 1 (right) obtained by VSM at room temperature. The saturation magnetization of the carbon coated cobalt NPs was close to bulk cobalt (158 emu g−1), whereas the hybrid material reached 34 emu g−1.
To examine the catalytic efficacy of Pd-catalyst 8, we subjected it to several consecutive Suzuki-Miyaura cross-coupling reactions [32] of aryl halides with phenylboronic acids (Table 1), a reaction that has been used as benchmark for palladium nanocatalysts. [33] As it is common in such reactions, iodides were transformed more rapidly than bromides or chlorides, hence, good to very good yields were achieved for a number of phenylboronic acids when iodobenzene was chosen as substrate (entries 1, 3, 4, and 6). To ensure that the immobilized Pd-complex represents the catalytic active species rather than free metal that is dissociated from the polymer bound ligand under reaction conditions (65 °C) and leaching into the homogeneous phase, we designed the following control experiment: A mixture of catalyst 8, sodium carbonate and THF/water mixture at given ratios (Table 1) was stirred at the reaction temperature for 2 h. Subsequently, catalyst 8 was retracted with the aid of an external magnet and the hot supernatant was transferred into a new vessel before phenylboronic acid 10 and phenyliodide 9 were added to the solution. After further 2 h of stirring at 65 °C, no conversion of reactants into biphenyl 11 was detected by 1H NMR. Thus, it appeared that no significant contribution to the efficacy of the catalytic system originated from dissolved palladium-species. In addition, AAS-analysis of the aqueous layer revealed a rather insignificant Pd-content (1.9 ppm). The performance of this novel hybrid catalyst was comparable to results obtained elsewhere with Pd-phosphine-complexes grafted on heterogenous supports comprising e.g. different polymers or nanoparticles. [5, 33, 34] Importantly, catalyst 8 was quantitatively recovered after each reaction and reused in the next run, proving the suitablitiy of the new Co/C-ROMPgel as a high capacity support that can be readily recovered by magnetic separation.
Table 1.
Iterative Suzuki-Miyaura cross-coupling reactions between phenylhalides 9 and phenylboronic acids 10 catalyzed by recyclable Co/C-ROMPgel immobilized Pd-complex 8.
 | |||||
|---|---|---|---|---|---|
| entry | run | X | R | time(h) | yield(%)[b] |
| 1 | 1 | l | H | 2 | 96 |
| 2 | 2 | Cl | H | 6 | 38 |
| 3 | 3 | l | 2-Br | 2 | 95 |
| 4 | 4 | l | 2-Me | 2 | 90 |
| 5 | 5 | Br | H | 12 | 92 |
| 6 | 6 | l | 4-tBu | 4 | 86 |
| 7 | 7 | l | H | 4 | 90 |
Reagents and conditions: Phenylhalide (0.5 mmol), phenylboronic acid (0.55 mmol), Na2CO3 (1.5 mmol), 1.1 mol% catalyst 8, 65 °C, solvent: 3 mL THF/H2O (1:2,v/v).
Yields of isolated products.
3. Conclusions
The immanent advantage of Co/C-ROMPgel as a novel hybrid material, e.g. for catalyst immobilization, lies in the combined advantage of high loading ROMP technology and the ease of recycling via magnetic decantation that is provided by the ferromagnetic metal core nanoparticles. The remarkable chemical and thermal stability of the graphene layers surrounding the nanoparticles ranks with the durability of the polymer itself. This composite might be suited as alternative to assemblies that rely on iron oxides as magnetic core material.
4. Experimental Section
General Procedures and Reagents
All air and moisture sensitive reactions were carried out in flame- or oven-dried glassware under argon atmosphere using standard gastight syringes, canellas, and septa. THF, CH2Cl2 and toluene were purified by passage through a Solv-Tek [35] (www.solvtek.com) purification system employing activated Al2O3 and degassed with argon. Flash column chromatography was performed with SiO 2 (Sorbent Technologies 30930M-25, Silica Gel 60Å, 40–63 µm). Thin layer chromatography was performed on silica gel 60F 254 plates. Visualization of TLC spots was effected using KMnO4 stain. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 NMR spectrometer operating at 400 and 100 MHz respectively. The nanoparticles were analyzed by FTIR spectroscopy (1% in KBr using a Tensor 27 Spectrometer, Bruker Optics equipped with a diffuse reflectance accessory, DiffusIR, Pike Technologies), atom absorption spectroscopy (Varian SpectrAA 220FS), elemental microanalysis (LECO CHN-900), transmission electron microscopy (CM30 ST-Philips, LaB6 cathode, operated at 300 kV point resolution ~4 Å), scanning electron microscopy (Hitachi S-2700 equipped with a quartz PCI digital capture) and magnetic hysteresis susceptibility (vibrating sample magnetometer, VSM, Princeton Measurements Corporation, model 3900). The synthesis of carbon coated cobalt nanoparticles 1 and (4-exo-(bicyclo[2.2.1]hept-5-en-2-yl)phenyl)diphenylphosphine 6 has been described elsewhere. [9 , 36] The nanomagnets were azide-functionalized according to literature precedents. [24] All other commercially available compounds were used as received. Second generation Grubbs catalyst 5 was provided by Materia Inc. and used without further purification. Deuterated solvents were purchased from Cambridge Isotope laboratories.
Norbornene-Functionalized Co/C-Nanoparticles 4
The azide-tagged carbon coated cobalt nanobeads 2 (400 mg; 0.1 mmol g−1 azide-loading) were suspended in degassed toluene (3 mL) by the use of an ultrasonic bath (Sonorex RK 255 H-R, Bandelin) before 5-((prop-2-yn-1-yloxy) methyl)bicyclo[2.2.1]hept-2-ene 3 (130 mg, 0.8 mmol), triethylamine (20 µL, 0.12 mmol) and CuI (5 mg, 0.03 mmol) were added. The resulting slurry was sonicated for 48 h at ambient temperature under an argon atmosphere. The nanobeads were recovered from the reaction mixture with the aid of a neodymium based magnet (N48, W-12-N, Webcraft GmbH, side length 12 mm) and washed with toluene (6 × 5 mL). Each washing step consisted of suspending the particles in the solvent, ultrasonication (5 min) and retracting the particles from the solvent by the aid of the magnet. After the last washing step the particles were dried in vacuo to yield 430 mg of 4. IR (ν/cm−1): 2928, 2817, 2097, 1693, 1598, 1505, 1404, 1377, 1253, 1214, 1175, 1096, 1013, 824, 71681; elemental microanalysis: 13.57% C, 0.69% H, 1.18% N.
PPh3-Functionalized Co/C-ROMPgel 7
200 mg of norbornene-functionalized Co/C-nanoparticles 4 were dispersed in CH2Cl2 (2 mL) by sonication in a sealed microwave reaction vessel under argon atmosphere (30 min). A solution of Grubbs II catalyst (17 mg, 0.02 mmol) in CH2Cl2 (1 mL) was injected and the ultrasound bath tempered to 60 °C while sonication of the reaction mixture continued (30 min). (4-exo-(bicyclo[2.2.1]hept-5-en-2-yl)phenyl)diphenylphosphine 6 (353 mg, 1.0 mmol) was added and the dispersion was subjected to sonication at 60 °C for 2 h. Within 50 min the formation of voluminous black gel was observed. After 2 h the reaction was quenched and a single, jelly-like lump was removed from the reaction vessel, crushed and dried in vacuo to yield 490 mg of 7. IR (ν/cm−1): 2929, 2859, 1644, 1584, 1475, 1432, 1400, 1303, 1259, 1177, 1089, 1064, 962, 894, 852, 760, 691, 655; elemental microanalysis: 55.95% C; 4.59% H, 0.47% N, 3.45% P.
Pd-PPh3-Functionalized Co/C-ROMPgel 8
200 mg of PPh3-functionalized Co/C-ROMPgel 7 was allowed to swell in CH2Cl2 (2 mL) under sonication at 60 °C under an atmosphere of argon (30 min) before Pd(OAc)2 (40 mg, 0.18 mmol) was added to the reaction vessel. Sonication was maintained for additional 2 h before the magnetic ROMPgel was isolated from the reaction mixture by the aid of an external magnet and dried in vacuo to yield 212 mg of 8. IR (ν/cm−1): 2982, 2360, 2155, 1053, 1033, 1014, 696, 674, 664, 652; elemental microanalysis: 49.23% C; 4.06% H; 0.43% N. AAS analysis: 57.3 mg nanoparticles 8 were extracted with 10 ml HNO 3 (conc), the sample was further diluted with water (1:100) and subjected to AAS to reveal a palladium content of 0.48 mmol palladium per gram nanoparticle 8.
Acknowledgements
This work was supported by the IDK NANOCAT (Elitenetzwerk Bayern) and the Atlantis program (EU/FIPSE). Dr. F. Krumeich (ETH Zurich) and Heather Shinogle (KU) are acknowledged for carrying out SEM and TEM analyses, Dr. H.-P. Hächler (ETH Zurich) for VSM measurements. We thank Materia Inc. for providing Grubbs II catalyst. This work was supported by the International Doktorandenkolleg NANOCAT (Elitenetzwerk Bayern) and the Deutsche Forschungsgemeinschaft (Re 948/8–1, “GLOBUCAT”), the EU-Atlantis program CPTUSA-2006–4560, the National Institute of General Medical Science (Center in Chemical Methodologies and Library Development at the University of Kansas, KU-CMLD, NIH P50 GM069663 and NIH-STTR R41 GM076765) with additional funds from the State of Kansas.
Contributor Information
Alexander Schätz, Institute for Organic Chemistry, University of Regensburg, Universitätsstr. 31, D-93053 Regensburg, Germany.
Toby R. Long, Department of Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS, 66045, USA and The University of Kansas Center for Chemical Methodologies and Library Development (KU-CMLD), 2121 Simons Drive, West Campus, Lawrence, KS, 66047, USA
Robert N. Grass, Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich Wolfang-Pauli-Strasse 10, CH-8093 Zurich, Switzerland
Wendelin J. Stark, Institute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich Wolfang-Pauli-Strasse 10, CH-8093 Zurich, Switzerland
Paul R. Hanson, Department of Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS, 66045, USA and The University of Kansas enter for Chemical Methodologies and Library Development (KU-CMLD), 2121 Simons Drive, West Campus, Lawrence, KS, 66047, USA.
Oliver Reiser, Institute for Organic Chemistry, University of Regensburg, Universitätsstr. 31, D-93053 Regensburg, Germany.
References
- 1.Huber DL. Small. 2005;1:482. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 2.a) Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S. J. Am. Chem. Soc. 2006;128:7383. doi: 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]; b) Xu ZP, Zeng QH, Lu GQ, Yu AB. Chem. Eng. Sci. 2006;61:1027. [Google Scholar]; c) Huh YM, Jun YW, Song HT, Kim S, Choi JS, Lee JH, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J. Am. Chem. Soc. 2005;127:12387. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]; d) Song HT, Choi JS, Huh YM, Kim S, Jun YW, Suh JS, Cheon J. J. Am. Chem. Soc. 2005;127:9992. doi: 10.1021/ja051833y. [DOI] [PubMed] [Google Scholar]; e) Benderbous S, Corot C, Jacobs P, Bonnemain B. Acad. Radiol. 1996;3:292. doi: 10.1016/s1076-6332(96)80560-5. [DOI] [PubMed] [Google Scholar]; f) Pankhurst QA. B T Technol J. 2006;24:33. [Google Scholar]; g) Kohler H, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Small. 2006;2:785. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]; h) Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Angew. Chem. Int. Ed. 2008;47:8438. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]; i) Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. Adv. Funct. Mater. 2009;19:1553. [Google Scholar]; j) Gleeson O, Tekoriute R, Gun’ko YK, Connon SJ. Chem. Eur. J. 2009;15:5669. doi: 10.1002/chem.200900532. [DOI] [PubMed] [Google Scholar]
- 3.a) Shokouhimehr M, Piao Y, Kim J, Jang Y, Hyeon T. Angew. Chem. Int. Ed. 2007;119:7169. doi: 10.1002/anie.200702386. [DOI] [PubMed] [Google Scholar]; b) Liu J, Peng X, Sun W, Zhao Y, Xia W. Organometallics. 2008;10:3933. doi: 10.1021/ol801478y. [DOI] [PubMed] [Google Scholar]; c) Sobal NS, Hilgendorff M, Möhwald H, Giersig M. Nano Lett. 2002;2:621. [Google Scholar]; d) Ceylan S, Friese C, Lammel C, Mazac K, Kirschning A. Angew. Chem. Int. Ed. 2008;47:8950. doi: 10.1002/anie.200801474. [DOI] [PubMed] [Google Scholar]; e) Hu A, Yee GT, Lin W. J. Am. Chem. Soc. 2005;127:12486. doi: 10.1021/ja053881o. [DOI] [PubMed] [Google Scholar]; f) Gleeson O, Tekoriute R, Gun’ko YK, Connon SJ. Chem. Eur. J. 2009;15:5669. doi: 10.1002/chem.200900532. [DOI] [PubMed] [Google Scholar]; g) Chouhan G, Wang D, Alper H. Chem. Commun. 2007:4809. doi: 10.1039/b711298j. [DOI] [PubMed] [Google Scholar]; h) Lu AH, Salabas EL, Schüth F. Angew. Chem. 2007;119:1242. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:1222. [Google Scholar]
- 4.a) Lu A-H, Li WC, Kiefer A, Schmidt W, Bill E, Fink G, Schüth F. J. Am. Chem. Soc. 2004;126:8616. doi: 10.1021/ja0486521. [DOI] [PubMed] [Google Scholar]; b) Lu A-H, Schmidt W, Matoussevitch N, Bönnemann H, Spliethoff B, Tesche B, Bill E, Kiefer W, Schüth F. Angew. Chem. Int. Ed. 2004;43:4303. doi: 10.1002/anie.200454222. [DOI] [PubMed] [Google Scholar]; c) Lu A-H, Li W, Matoussevitch N, Spliethoff B, Pennemann HB, Schüth F. Chem. Commun. 2005:98. doi: 10.1039/b414146f. [DOI] [PubMed] [Google Scholar]; d) Sen T, Sebastianelli A, Bruce IJ. J. Am. Chem. Soc. 2006;128:7130. doi: 10.1021/ja061393q. [DOI] [PubMed] [Google Scholar]; e) Ge J, Huynh T, Hu Y, Yin Y. Nano Lett. 2008;8:931. doi: 10.1021/nl080020f. [DOI] [PubMed] [Google Scholar]; e) Michalek F, Lagunas A, Jimeno C, Pericàs MA. J Mater Chem. 2008;18:4692. [Google Scholar]
- 5.a) Stevens PD, Fan J, Gardimalla HMR, Yen M, Gao Y. Org. Lett. 2005;7:2085. doi: 10.1021/ol050218w. [DOI] [PubMed] [Google Scholar]; b) Duanmu C, Saha I, Zheng Y, Goodson BM, Gao Y. Chem. Mater. 2006;18:5973. [Google Scholar]; c) Abu-Reziq R, Alper H, Wang D, Post ML. J. Am. Chem. Soc. 2006;128:5279. doi: 10.1021/ja060140u. [DOI] [PubMed] [Google Scholar]; d) Gill CS, Long W, Jones CW. Catal. Lett. 2009;131:425. [Google Scholar]; e) Rosario-Amorin D, Wang X, Gaboyard M, Clerac R, Nlate S, Heuze K. Chem. Eur. J. 2009;15:12636. doi: 10.1002/chem.200901866. [DOI] [PubMed] [Google Scholar]; f) Hua D, Tang J, Dai L, Pu Y, Cao X, Zhu X. J Nanosci Nanotechnol. 2009;9:6681. doi: 10.1166/jnn.2009.1338. [DOI] [PubMed] [Google Scholar]; g) Arai T, Sato T, Kanoh H, Kaneko K, Oguma K, Yanagisawa A. Chem. Eur. J. 2008;14:882. doi: 10.1002/chem.200701371. [DOI] [PubMed] [Google Scholar]; h) Jin M-J, Lee D-H. Angew. Chem. 2010;122:1137. doi: 10.1002/anie.200905626. [DOI] [PubMed] [Google Scholar]
- 6.a) Butterworth MD, Illum L, Davis SS. Colloids Surf, A. 2001;179:93. [Google Scholar]; b) Sun Y, Duan L, Guo Z, Mu YD, Ma M, Xu L, Zhang Y, Gu N. J. Magn. Magn. Mater. 2005;285:65. [Google Scholar]; c) Deng Y-H, Wang C-C, Hu J-H, Yang W-L, Fu S-K. Colloids Surf, A. 2005;262:87. [Google Scholar]; d) Lee J, Lee Y, Youn JK, Na HB, Yu T, Kim H, Lee S-M, Koo Y-M, Kwak JH, Park HG, Chang HN, Hwang M, Park J-G, Kim J, Hyeon T. Small. 2008;4:143. doi: 10.1002/smll.200700456. [DOI] [PubMed] [Google Scholar]
- 7.a) Saito Y. Carbon. 1995;33:979. [Google Scholar]; b) Scott JHJ, Majetich SA. Phys. Rev B. 1995;52:12564. doi: 10.1103/physrevb.52.12564. [DOI] [PubMed] [Google Scholar]; c) Jiao J, Seraphin S, Wang X, Withers JC. J. Appl. Phys. 1996;80:103. [Google Scholar]
- 8.a) Wang ZH, Choi CJ, Kim BK, Kim JC, Zhang ZD. Carbon. 2003;41:1751. [Google Scholar]; b) Flahaut E, Agnoli F, Sloan J, O’Connor C, Green MLH. Chem. Mater. 2002;14:2553. [Google Scholar]
- 9.a) Grass RN, Athanassiou EK, Stark WJ. Angew. Chem. Int. Ed. 2007;46:4909. doi: 10.1002/anie.200700613. [DOI] [PubMed] [Google Scholar]; b) Herrmann IK, Grass RN, Mazunin D, Stark WJ. Chem. Mater. 2009;21:3275. [Google Scholar]; c) Grass RN, Stark WJ. J. Mater. Chem. 2006;16:1825. [Google Scholar]; c) Wittmann S, Schätz A, Grass RN, Stark WJ, Reiser O. Angew. Chem. Int. Ed. 2010;49:1867. doi: 10.1002/anie.200906166. [DOI] [PubMed] [Google Scholar]
- 10.Stark WJ, Madler L, Maciejewski M, Pratsinis SE, Baiker A. Chem. Commun. 2003:588. doi: 10.1039/b211831a. [DOI] [PubMed] [Google Scholar]
- 11.a) Koehler FM, Rossier M, Waelle M, Athanassiou EK, Limbach LK, Grass RN, Günther D, Stark WJ. Chem. Commun. 2009:4862. doi: 10.1039/b909447d. [DOI] [PubMed] [Google Scholar]; b) Rossier M, Koehler FM, Athanassiou EK, Grass RN, Aeschlimann B, Günther D, Stark WJ. J. Mater. Chem. 2009;19:8239. [Google Scholar]
- 12.Fuhrer R, Athanassiou EK, Luechinger NA, Stark WJ. Small. 2009;5:383. doi: 10.1002/smll.200801091. [DOI] [PubMed] [Google Scholar]
- 13.a) Preishuber-Pflügl P, Buchacher P, Eder E, Schitter RM, Stelzer F. J. Mol. Catal A. 1998;133:151. [Google Scholar]; b) Moore JD, Herpel RH, Lichtsinn JR, Flynn DL, Hanson PR. Org. Lett. 2003;5:105. doi: 10.1021/ol0270273. [DOI] [PubMed] [Google Scholar]; c) Zhang M, Moore JD, Flynn DL, Hanson PR. Org. Lett. 2004;6:2657. doi: 10.1021/ol049209y. [DOI] [PubMed] [Google Scholar]; d) Harned AM, Sherrill WM, Flynn DL, Hanson RR. Tetrahedron. 2005;61:12093. [Google Scholar]; e) Zhang M, Flynn DL, Hanson PR. J. Org. Chem. 2007;72:3194. doi: 10.1021/jo0620260. [DOI] [PubMed] [Google Scholar]; f) Rolfe A, Probst D, Volp K, Omar I, Flynn D, Hanson PR. J. Org. Chem. 2008;73:8785. doi: 10.1021/jo801578f. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Harned AM, Zhang M, Vedantham P, Mukherjee S, Herpel RH, Flynn DL, Hanson PR. Aldrichimica Acta. 2005;38:3. [Google Scholar]
- 14.Reviews: Barrett AGM, Hopkins BT, Köberling J. Chem. Rev. 2002;102:3301. doi: 10.1021/cr0103423. Flynn DL, Hanson PR, Berk SC, Makara GM. Curr. Opin. Drug Discovery Dev. 2002;5:571.
- 15.Ley SV, Schucht O, Thomas AW, Murray PJ. J. Chem. Soc., Perkin Trans. 1999:1251. [Google Scholar]
- 16.Schätz A, Hager M, Reiser O. Adv. Funct. Mater. 2009;19:2109. [Google Scholar]
- 17.a) Buchmeiser MR, Seeber G, Mupa M, Bonn GK. Chem. Mater. 1999;11:1533. [Google Scholar]; b) Buchmeiser MR, Sinner F, Mupa M, Wurst K. Macromolecules. 2000;33:32. [Google Scholar]; c) Gatschelhofer C, Magnes C, Pieber TR, Buchmeiser MR, Sinner FM. J. Chromatogr. A. 2005;1090:81. doi: 10.1016/j.chroma.2005.06.098. [DOI] [PubMed] [Google Scholar]
- 18.Rutenberg IM, Scherman OA, Grubbs RH, Jiang W, Garfunkel E, Zhenan B. J. Am. Chem. Soc. 2004;126:4062. doi: 10.1021/ja035773c. [DOI] [PubMed] [Google Scholar]
- 19.Kim NY, Jeon NL, Hoi IS, Takami S, Harada Y, Finnie KR, Girolami GS, Nuzzo RG, Whitesides GM, Laibinis PE. Macromolecules. 2000;33:2793. [Google Scholar]
- 20.Barrett AGM, Cramp SM, Roberts RS. Org. Lett. 1999;7:1083. [Google Scholar]
- 21.Roberts RS. J. Comb. Chem. 2005;7:21. doi: 10.1021/cc049915q. [DOI] [PubMed] [Google Scholar]
- 22.Belfield KD, Zhang L. Chem. Mater. 2006;18:5929. [Google Scholar]
- 23.a) Tornøe CW, Meldal M. In: LebI M, Houghten RA, editors. American Peptide Symposium; American Peptide Society, Kluwer Academic Publishers; San Diego, CA. 2001. p. 263. [Google Scholar]; b) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; c) Tornøe CW, Christensen C, Meldal M. J. Org.Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 24.a) Schätz A, Grass RN, Stark WJ, Reiser O. Chem. Eur. J. 2008;14:8262. doi: 10.1002/chem.200801001. [DOI] [PubMed] [Google Scholar]; b) Schätz A, Grass RN, Kainz Q, Stark WJ, Reiser O. Chem. Mater. 2010;22:305. [Google Scholar]
- 25.a) ROMPgel-supported PPh3: Årstad E, Barrett AGM, Hopkins BT, Köbberling J. Org. Lett. 2002;4:1975. doi: 10.1021/ol026008q. Harned AM, Song He H, Toy PH, Flynn PHDL, Hanson PR. J. Am. Chem. Soc. 2005;127:52. doi: 10.1021/ja045188r. Yang Y-C, Luh T-YJ. Org. Chem. 2003;68:9870. doi: 10.1021/jo035318z.
- 26.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 27.a) Cuny GD, Cao J, Hauske JR. Tetrahedron Lett. 1997;38:5237. [Google Scholar]; b) Cao J, Cuny GD, Hauske JR. Mol. Diversity. 1998;3:173. doi: 10.1023/a:1009663720800. [DOI] [PubMed] [Google Scholar]; c) Week M, Jackiw JJ, Rossi RR, Weiss PS, Grubbs RH. J. Am. Chem. Soc. 1999;121:4088. [Google Scholar]; d) Barrett AGM, Cramp SM, Roberts RS. Org. Lett. 1999;1:1083. [Google Scholar]; e) Aarstad E, Barrett AGM, Hopkins BT, Koebberling J. Org. Lett. 2002;4:1975. doi: 10.1021/ol026008q. [DOI] [PubMed] [Google Scholar]; Roberts RSJ. Comb. Chem. 2005;7:21. doi: 10.1021/cc049915q. [DOI] [PubMed] [Google Scholar]
- 28.Santini R, Griffith MC, Qi M. Tetrahedron Lett. 1998;39:8951. [Google Scholar]
- 29.The Cu-signal in the EDX-spectrum is rather originated by the copper grid, on which the sample was supported during measurement, than residual copper from the “click”-reaction. No traces of the ruthenium based ROMP-catalyst 5 appeared in any spectrum.
- 30.a) McKinley SV, Rakshys JW. J. Chem. Soc., Chem. Commun. 1972:134. [Google Scholar]; b) Bernard M, Ford WT. J. Org. Chem. 1983;48:326. [Google Scholar]; c) Tunoori AR, Dutta D, Georg GI. Tetrahedron Lett. 1998;39:8751. [Google Scholar]; d) Regen SL, Lee DP. J. Org. Chem. 1975;40:1669. [Google Scholar]; e) Charette AB, Boezio AA, Janes MK. Org. Lett. 2000;2:3777. doi: 10.1021/ol006432w. [DOI] [PubMed] [Google Scholar]; f) Westman J. Org. Lett. 2001;3:3745. doi: 10.1021/ol0167053. [DOI] [PubMed] [Google Scholar]
- 31.a) Stevens PD, Li G, Fan J, Yenb M, Gao Y. Chem. Commun. 2005:4435. doi: 10.1039/b505424a. [DOI] [PubMed] [Google Scholar]; b) Zheng Y, Stevens PD, Gao Y. J. Org. Chem. 2006;71:537. doi: 10.1021/jo051861z. [DOI] [PubMed] [Google Scholar]; c) Baruwati B, Reddy KM, Manorama SV, Singh RK, Parkash O. Appl. Phys. Lett. 2004;85:2833. [Google Scholar]; d) Guin D, Baruwati B, Manorama SV. J. Mol. Catal. A Chem. 2005;242:26. [Google Scholar]; e) Guin D, Baruwati B, Manorama SV. Org. Lett. 2007;9:1419. doi: 10.1021/ol070290p. [DOI] [PubMed] [Google Scholar]; f) Baruwati B, Guin D, Manorama SV. Org. Lett. 2007;9:5377. doi: 10.1021/ol702064x. [DOI] [PubMed] [Google Scholar]; g) Ceylan S, Friese C, Lammel C, Mazac K, Kirschning A. Angew. Chem. Int. Ed. 2008;47:8950. doi: 10.1002/anie.200801474. [DOI] [PubMed] [Google Scholar]; h) Liu J, Peng X, Sun W, Zhao Y, Xia C. Org. Lett. 2008;10:3933. doi: 10.1021/ol801478y. [DOI] [PubMed] [Google Scholar]; i) Lv G, Mai W, Jin R, Gao L. Synlett. 2008;9:1418. [Google Scholar]
- 32.a) Miyaura N, Yamada K, Suzuki A. Tetrahedron Lett. 1979;20:3437. [Google Scholar]; c) Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]; d) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 33.a) Stevens PD, Li GF, Fan JD, Yen M, Gao Y. Chem. Commun. 2005:4435. doi: 10.1039/b505424a. [DOI] [PubMed] [Google Scholar]; b) Narayanan R, El-Sayed MA. J. Am. Chem. Soc. 2003;125:8340. doi: 10.1021/ja035044x. [DOI] [PubMed] [Google Scholar]; c) Gopidas KR, Whitesell JK, Fox MA. Nano Lett. 2003;3:1757. [Google Scholar]; d) Kim SW, Kim M, Lee WY, Hyeon T. J. Am. Chem. Soc. 2002;124:7642. doi: 10.1021/ja026032z. [DOI] [PubMed] [Google Scholar]
- 34. Uozumi Y, Nakai Y. Org. Lett. 2002;4:2997. doi: 10.1021/ol0264298. Bergbreiter DE, Liu Y-S. Tetrahedron Lett. 1997;38:7843. Uozumi Y, Danjo H, Hayashi T. Tetrahedron Lett. 1998;39:8303. Uozumi Y, Danjo H, Hayashi T. J. Org. Chem. 1999;64:3384. doi: 10.1021/jo982438b. Uozumi Y, Watanabe T. J. Org. Chem. 1999;64:6921. doi: 10.1021/jo990631f. Kim J-W, Kim J-H, Lee D-H, Lee Y-S. Tetrahedron Lett. 2006;27:4745. Review: Uozumi Y. Bull. Chem. Soc. Jpn. 2008;81:1183.
- 35.Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518. [Google Scholar]
- 36.Andrew M, He H, Song T, Patrick H, Flynn DL, Hanson PR. J. Am. Chem. Soc. 2005;127:52. [Google Scholar]