Abstract
Hippocampal inhibitory interneurons are a diverse population of cells widely scattered in hippocampus, where they regulate hippocampal circuit activity. The hippocampus receives cholinergic projections from the basal forebrain, and functional studies have suggested the presence of different subtypes of nicotinic acetylcholine receptors (AChR) on GABAergic interneurons. Single cell PCR-analysis had confirmed that several nAChR subunit mRNAs are coexpressed with glutamate decarboxylase 67 (GAD67), the marker for GABAergic interneurons. In this anatomical study, we systematically investigated the coexpression of GAD67 with different nAChR subunits using double in situ hybridization with a digoxigenin-labeled GAD67 probe and 35S-labeled probes for nAChR subunits (α2, α3, α4, α5, α6, α7, β2, β3, and β4). The results revealed that most GAD67-positive interneurons expressed β2, and 67 % also expressed α7 mRNA. In contrast, mRNA expression of other subunits was limited; only 13 % of GAD67-positive neurons coexpressed α4, and less than 10% expressed transcripts for α2, α3, α5 or β4. Most GAD67/α2 coexpression was located in CA1/CA3 stratum oriens, and GAD67/α5 coexpression was predominantly detected in CA1/CA3 stratum radiatum/lacunosum moleculare and the dentate gyrus. Expression of α6 and β3 mRNAs was rarely detected in the hippocampus, and mRNAs were not coexpressed with GAD67. These findings suggest that the majority of nicotinic responses in GABAergic interneurons should be mediated by a homomeric α7 or heteromeric α7*-containing nAChRs. Other possible combinations such as α2β2*, α4β2*, or α5β2* heteromeric nAChRs could contribute to functional nicotinic response in subsets of GABAergic interneurons but overall would have a minor role.
Keywords: in situ hybridization, hippocampus, nAChR, cholinergic, GAD67
INTRODUCTION
Neurons in the hippocampus are divided into two subpopulations, principal excitatory neurons and local inhibitory interneurons (Freund and Buzsaki, 1996). Although local interneurons are a heterogeneous population, characterized by various chemical markers such as calcium binding proteins or neuropeptides, most of them contain the inhibitory neurotransmitter γ-amino butyric acid (GABA). In the hippocampus, GABAergic interneurons are widely distributed in CA1 and CA3 fields of ammon's horn (CA1, CA3) and in the dentate gyrus (DG), and play an important role in regulating the activity of both excitatory principal neurons, and inhibitory interneurons (Alkondon and Albuquerque, 2001; Ji and Dani, 2000; Jinno et al., 1998, Jones et al., 1999).
Neuronal nicotinic acetylcholine receptors (nAChRs) belong to the superfamily of ligand-gated ion channels, and are found in the central and peripheral nervous system (Sargent, 1993 review). Neuronal nAChR are composed of 5 subunits which can be classified into ligand binding α and structural β subunits. Through molecular cloning at least six mammalian α (α2, α3, α4, α5, α6 and α7) and three β (β2, β3 and β4) subunits have been identified in the brain (Sargent, 1993). Different combinations of α and β subunits form functional heteromeric nAChRs with distinct physiological and pharmacological properties. The exception is the widely distributed α7 subunit, which forms homomeric nAChRs (McGehee and Role, 1995, Chen and Patrick, 1997).
The rat hippocampus receives cholinergic input from the medial septum-diagonal band (MSDB) complex of the basal forebrain, with projections terminating at local inhibitory interneurons and principal excitatory neurons (Dougherty and Milner, 1999, Frotscher and Léránth, 1985, Woolf, 1991). Several studies have shown that rat hippocampal interneurons express functional nAChRs (Alkondon et al., 1997; Frazier et al., 1998; McQuiston and Madison, 1999, and Ji and Dani, 2000), and that nicotinic agonists modulate GABAergic input to other hippocampal interneurons and principal neurons via activation of nAChRs (Alkondon et al., 2001, Jones and Yakel, 1997). Based on their electrophysiological and pharmacological properties, at least three distinct functional nAChR subtypes have been described: α7, α4/β2 and α3/β4 (Alkondon and Albuquerque, 2004), suggesting the expression of several α and β nAchR subunits in GABAergic interneurons. This has been supported by a single cell reverse-transcription polymerase chain reaction (RT-PCR) study that demonstrated the expression of several nAChR subunit mRNAs in the same interneuron, and different expression profiles could be correlated to distinct electrophysiological properties of nAChRs (Yakel and Shao, 2004). In addition, recordings from different types of interneurons in different layers of the hippocampal CA1 region found heterogeneity in the functional responses suggesting different subtypes of nAChR (McQuiston and Madison, 1999). This is supported by results from anatomical in situ hybridization studies and PCR analysis suggesting that interneurons express α5 nAChR subunit mRNA in stratum (s.) radiatum, and α2 mRNA in s. oriens (Winzer-Serhan and Leslie, 2005, Son and Winzer-Serhan, 2006, Sudweeks and Yakel, 2000).
However, a complete overview of the expression of nAChR subunits in GABAergic interneurons is still missing. Therefore, for a comprehensive anatomical analysis we used double-labeling in situ hybridization to identify GABAergic interneurons expressing nAChR subunit mRNAs in the adult rat hippocampus, by using a digoxigenin-labeled riboprobe for glutamic acid decarboxylase 67 (GAD67) as a marker for GABAergic neurons and 35S-labeled cRNA probes for the detection of nAChR subunit mRNAs. In the present study, distinct patterns of expression for the various nAChR subunits in GABAergic interneurons were observed with the majority expressing α7 and β2, and limited and restricted expression of α2, α3, α4, α5 and β4, and no detectable expression of α6 and β3 nAChR subunit mRNAs.
MATERIAL AND METHODS
Tissue preparation
Adult male Sprague-Dawley rats (~ 300 g) (Harlan Inc., Houston, TX) were kept on a 12 h light/dark cycle with free access to food and water according to the protocol approved by the Institutional Animal Care and Use Committee at Texas A&M, and consistent with National Institute of Health guidelines. The animals were anesthetized with isofluorane, decapitated, and brains were immediately removed, frozen in -20°C isopentane, and stored at -80°C until use. From each animal, 20 μm coronal sections were cut on a cryostat for dorsal (Bregma -2.30 to - 4.16 mm) and ventral hippocampus (-4.80 to -5.20 mm) according to the “Rat Brain Atlas” by Paxinos and Watson (1998). For each nAChR subunit probe four dorsal and four ventral sections from three different animals were used. Sections were 200 μm apart from each other for a representative analysis of dorsal and ventral hippocampus. Sections were postfixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) (pH 7.4) for 1 h at room temperature (RT), washed in PB, air dried and stored desiccated at -20°C until use.
cRNA probe preparation
Plasmids containing full-length sequences for nAChR subunits were kindly provided by Dr. J. Boulter (UCLA, CA), and transcripts were subcloned into pBluescript II SK between T3 and T7 promoter sites. 35S-labeled cRNA probes for α2 (1931 bp), α3 (1858 bp), α4 (2110 bp), α5 (1607 bp), α6 (1760 bp), α7 (2100 bp), β2 (2196 bp), β3 (1780 bp), and β4 (2522 bp) were synthesized in antisense and sense orientation in the presence of 35S-UTP (PerkinElmer, Boston, MA). 35S-labeled full-length probes were further subjected to alkaline hydrolysis to yield products with an average size of 600 bp, except for the α2 riboprobe, which was not hydrolyzed. A template for GAD67 (185-650; 466 bp) was generated by RT-PCR (forward primer: 5’-ATGGCATCTTCCACGCCTTCG-3’, reverse primer: 5’- CCAAATTAAAACCTTCCATGCC-3’), subcloned into pPCR-Script Amp SK (+) (Stratagene, La Jolla, CA), and sequenced to verify the sequence for rat GAD67 (M76177). Digoxigenin-labeled GAD67 cRNA probes were synthesized using Dig-UTP (Roche Applied Science, Indianapolis, IN).
In addition, a PCR product for GAD67 (1198- 1474; 277 bp) was used as a template to generate 35S-labeled GAD67 cRNA probe by in vitro transcription after adding the T3 promoter sequence to the reverse primer (forward: 5’-TTATGTCAATGCAACCGCAGGC-3’, reverse: 5’-AATTAACCCTCAAAGGNNNNNNNNNNNNNACACATCTGGTTGCATCCTTGG-3’).
Double in situ hybridization
Sections were pretreated with proteinase K (0.1 μg/ml) for 30 min at RT, acetylated, dehydrated through graded ethanols (50, 70, 95 and 100%) and air-dried. Sections were then incubated for 18 h at 60°C with a 1:1 dilution of Dig-labeled antisense GAD cRNA probe (0.1 μg/ml) and 35S-labeled sense or antisense probes (2 × 107 cpm/ml) in hybridization solution (50% formamide, 10% dextran sulfate, 500 μg/ml tRNA, 10 mM dithiothreitol, 0.3 M NaCl, 10 mM Tris, pH8.0, and 1 mM EDTA, pH 8.0). After hybridization, sections were incubated with RNase A (20 μg/ml) for 30 min at 37°C, followed by two 5 min washes (2X standard saline citrate buffer [SSC]), two 10 min washes with 1X and 0.5X SSC at RT, and a 30 min wash in 0.1X SSC at 65°C. After the hot wash, the slides were incubated in Genius buffer 1 (GB1) (100 mM Tris-HCl, 150 mM NaCl, pH 7.5) for 5 min, followed by a 30 min incubation in 5% nonfat dry milk in GB1 plus 0.25% Triton-X (GB2) at RT. The alkaline-phosphatase conjugated anti-Dig Fab antibody (sheep) (Roche Applied Science, Indianapolis, IN), prepared as 1:1,000 dilution in GB1, was applied to the sections and slides were incubated for 3 h at RT. The slides were washed three times for 1, 5, and 10 min in GB1. Slides were incubated with color reagent [200 μl of NBT/BCIP stock solution (18.75 mg/ml NBT, 9.4 mg/ml BCIP) in 10 ml of GB3 (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2, pH 9.5)] (Roche Applied Science, Indianapolis, IN) at RT overnight. The slides were washed twice in GB4 (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and once in double deionized water, dehydrated with brief dips in graded ethanols (50, 70, 95, and 100%), air-dried and apposed to Kodak Biomax MR film for an appropriate period of time. After film development, slides were coated with 2% parlodion (SPI-Chem, West Chester, PA) in isoamylacetate and dipped in liquid NTB emulsion (VWR, West Chester, PA). After an appropriate period of incubation time, slides were developed in Kodak developer D-19, fixed, cover-slipped, and analyzed.
Analysis
The hippocampus was divided into three subdivisions: CA1, CA3 and DG. The border between the CA1 region and subiculum was defined with a straight line through the point at which the densely packed pyramidal cell layer of the s. pyramidale disappeared. The border between CA1 and CA3 regions was defined with a straight line that linked the tip of the supra-pyramidale blade of the DG with the onset of more loosely packed pyramidal cell of CA3. The border between the CA3 region and the DG was defined with lines drawn from the edges of the two blades of the granule cell layer to the inner tip of the pyramidal cell layer of the CA3 region. The three subdivisions of the hippocampus were further divided into layers: CA1 and CA3 strata oriens, pyramidale, radiatum/lacunosum moleculare, and DG molecular layer, granule cell layer and hilus, identified in relation to the principal cell layers.
The hybridization signal derived from the non-radioactive Dig-labeled GAD67 probe (dark color reaction product) was analyzed by lightfield, the radioactive hybridization signal (silver grains) by darkfield microscopy using an Olympus BX51 microscope. The criterion for the identification of positive GAD67 mRNA expressing interneurons were determined by the area (≥30 and ≤300 μm2) labeled by non-radioactive in situ hybridization, and its integrated density (gray values) using Image J software (www.nih.gov) (≥5000 arbitrary units). To determine positive nAChR subunit mRNA expression the number of silver grains expressed on GAD-positive neurons derived with the sense probes (background) was determined (2.5 ± 1.56/GAD-positive neuron). A positive signal for radioactive in situ hybridization was set as ≥12 silver grains overlying a positive Dig-labeled neuron, which is at more than 4 times higher than background. Thus, the criteria for the identification of double labeled neurons were established by the number of silver grains (≥12) on GAD67 neurons identified as a Dig-labeled area of ≥30 and ≤300 μm2 having an integrated density value of ≥5000.
For counting, images were captured by a DP7-1 digital camera. For each nAChR subunit double-labeled cells were counted in four sections from dorsal and four from ventral hippocampus from three adult males (n = 3) under an objective lens (X40, NA 1.0) and the percentage of double-labeled cells was compared to those of GAD67 and β2 mRNA coexpression. Since most GAD67 positive cells also expressed transcripts for β2, this seemed to provide a reasonable estimate of the relative proportion of cells exhibiting double-labeling. For the statistical analysis results from dorsal and ventral sections were combined and analyzed as a percent of total Dig-GAD/β2 positive cells (defined as 100%) using the student t-test.
Autoradiographic images of sections were obtained using computer-based image analysis system (MCID, Imaging Research Inc. St. Catherine, Canada; now InterFocus Imaging Ltd, UK). Darkfield photomicrographic images were captured by a DP7-1 digital camera (software DP manager, Leeds Instruments, Irving, TX) and adjusted for brightness and contrast using the Adobe Photoshop 9.0 image-editing software package (Adobe System, Mountain View, CA).
RESULTS
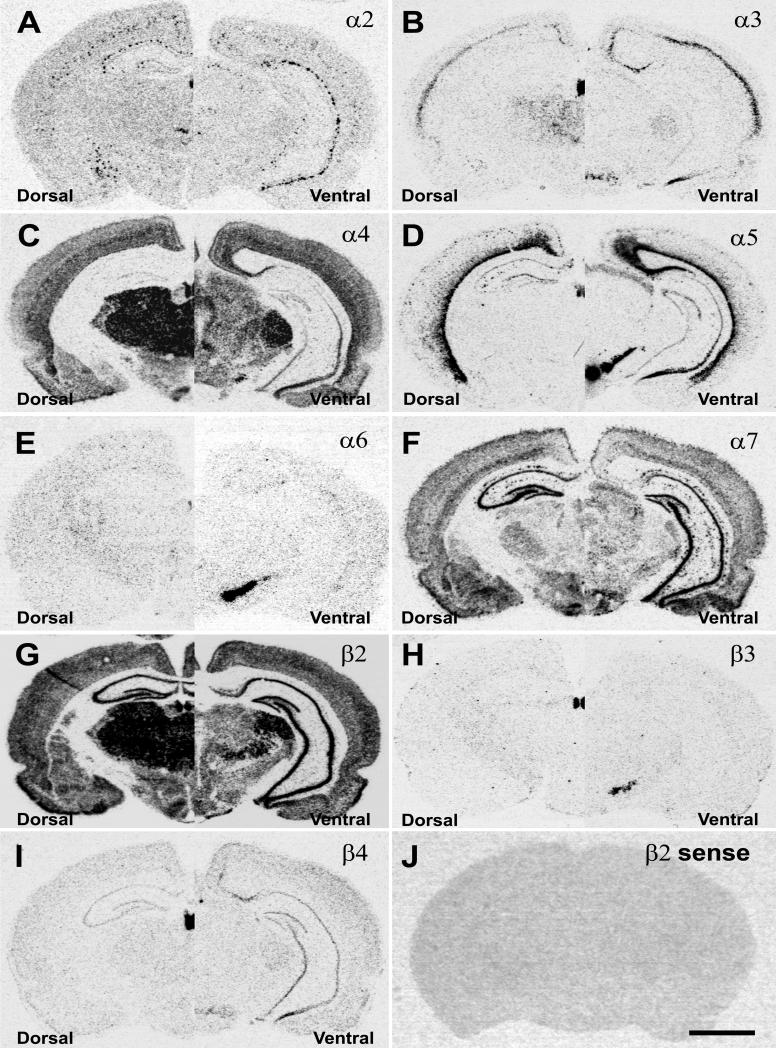
In the adult brain the expression of nAChR subunit mRNAs was studied using radioactive in situ hybridization with subunit specific cRNA probes. The analysis of the autoradiograms showed expression patterns in coronal brain section for the nAChR subunits identical to those previously reported, with α4, α7 and β2 subunit mRNAs being the most widely expressed (Fig. 1). In the hippocampus, strong and widespread mRNA expression was only detected for α7 and β2 (Fig. 1F, G). Other subunits like α4 and β4 exhibited very low (Fig. 1C, I), scattered (α2, α5) (Fig. 1A, D) or barely detectable mRNA expression (α3, α6, β3) (Fig. 1B, E, H) in the hippocampal formation despite intense hybridization signal in other structures such as the medial habenula. The sense probes used to detect non-specific hybridization exhibited only background levels of hybridization signal (Fig. 1J) as shown for the β2 sense probe.
Figure 1.
Autoradiographic images of nAChR subunit mRNA expression pattern in coronal sections of dorsal (left side) and ventral hippocampus (right side). Specific hybridization signals were derived with 35S-labeled antisense probes for A) α2, B) α3, C) α4, D) α5, E) α6, F) α7, G) β2, H) β3, and I) β4; and J) nonspecific hybridization signal detected with a β2 sense probe. Scale bar = 1mm.
Expression of nAChR subunit mRNAs in rat hippocampus
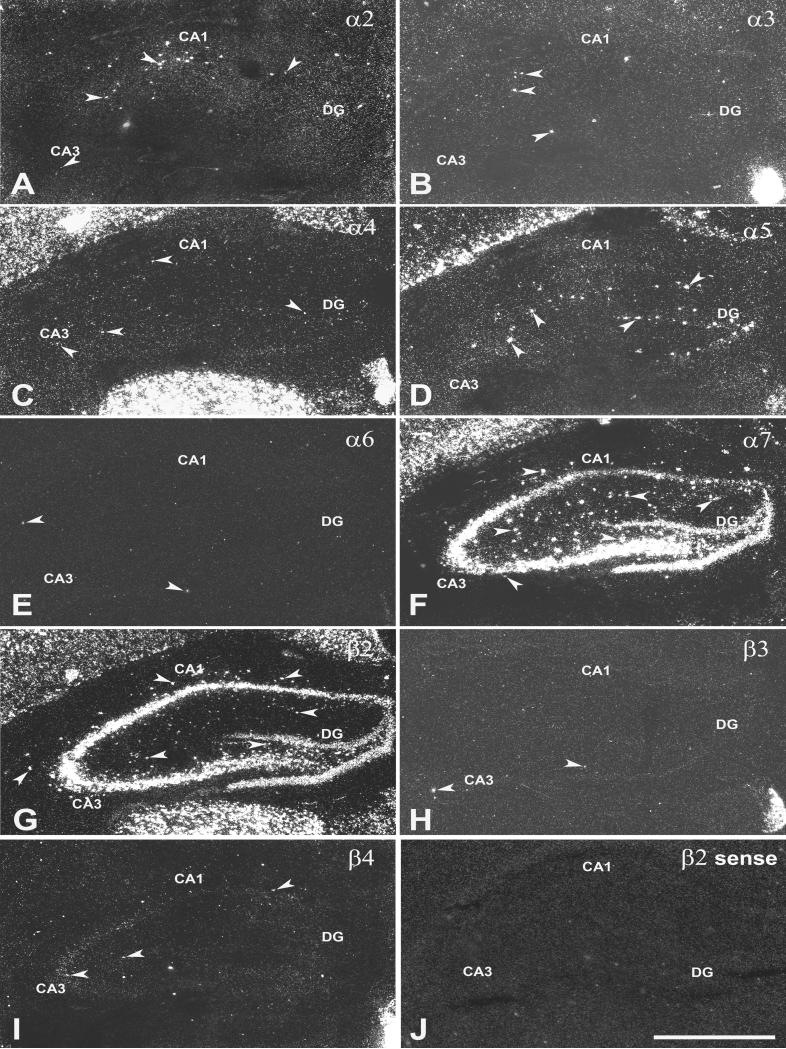
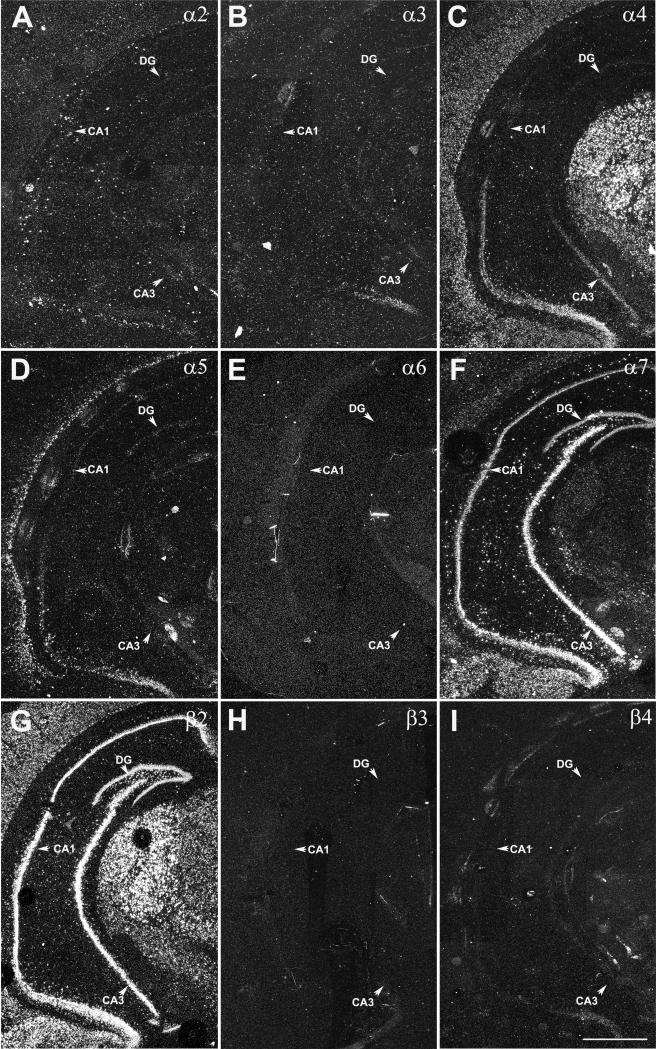
Adult coronal brain sections were analyzed using darkfield microscopy to detect the expression of nAChR subunit mRNAs at a cellular level in dorsal (Fig. 2) and ventral hippocampus (Fig. 3). Spatially restricted expression of α2 mRNA was detected in CA1 s. oriens in dorsal and ventral hippocampus with more cells exhibiting α2 expression in ventral compared to dorsal hippocampus (Fig. 2A and Fig. 3A). In dorsal hippocampus, sporadic expression of α3 subunit mRNA was found on a few scattered cells mostly in CA1 and CA3 s. radiatum. In the ventral hippocampus α3 mRNA expression was restricted to a small area in CA1 s. pyramidale which also exhibited increased expression of α4, α5 and β4 mRNAs (Fig. 2B and Fig. 3B). The expression of α4 mRNA was weak throughout the hippocampus, especially when compared to the robust signal detected in cortex and thalamus (Fig. 2C and Fig. 3C). However, numerous scattered cells exhibiting weak α4 mRNA expression were found in all CA1 and CA3 strata and the DG in dorsal and ventral hippocampus. Strong expression of α5 mRNA was detected in cell in scattered CA1 s. radiatum and DG in dorsal and to a lesser extend in ventral hippocampus (Fig. 2D and Fig. 3D). The expression of α6 mRNA was restricted to a few cells (1 to 3 cells per section) randomly distributed in different regions and layers in dorsal and ventral hippocampus (Fig. 2E and Fig. 3E). Strong expression of α7 was found in all areas of dorsal and ventral hippocampus including strong expression in scattered cells in all strata of CA1 and CA3 and the DG (Fig. 2F and Fig. 3F). Strong expression of β2 mRNAs was found in all areas of dorsal and ventral hippocampus including strong expression in scattered cells in all strata of CA1 and CA3 and the DG (Fig. 2G and Fig. 3G), however, the hybridization signal in interneurons was less intense than for α7. Hybridization signal for β3 was detected in randomly distributed single cells, but was limited to 1 to 3 cells per section in dorsal and ventral hippocampus (Fig. 2H and Fig. 3H). Weak expression of β4 mRNA was detected in CA3 s. pyramidal in dorsal hippocampus and CA1 s. pyramidal in ventral hippocampus Fig. 2I and Fig. 3I) and in a few scattered cells in CA3 s. radiatum. The sense probes as shown for β2 as an example exhibited only background hybridization signal (Fig. 2J).
Figure 2.
Darkfield images of nAChR subunit mRNA expression in coronal sections of dorsal hippocampus. The expression of A) α2, B) α3, C) α4, D) α5, E) α6, F) α7, G) β2, H) β3, I) β4, and J) β2 sense was detected with 35S-labeled probes. Arrowheads point to specific hybridization in cells in the hippocampus. Abbreviations: CA1 and CA3, CA1 and CA3 hippocampal subfields; DG, dentate gyrus. Scale bar = 1mm.
Figure 3.
Darkfield images of nAChR subunit mRNA expression in coronal sections of ventral hippocampus. The expression of A) α2, B) α3, C) α4, D) α5, E) α6, F) α7, G) β2, H) α3, and I) β4 was detected with 35S-labeled probes. Arrows point to principal cell layers of CA1 and CA3 and DG. Abbreviations: CA1 and CA3, CA1 and CA3 hippocampal field; DG, dentate gyrus. Scale bar = 1 mm.
Co-expression of nAChR subunit mRNAs with GAD67 mRNAs
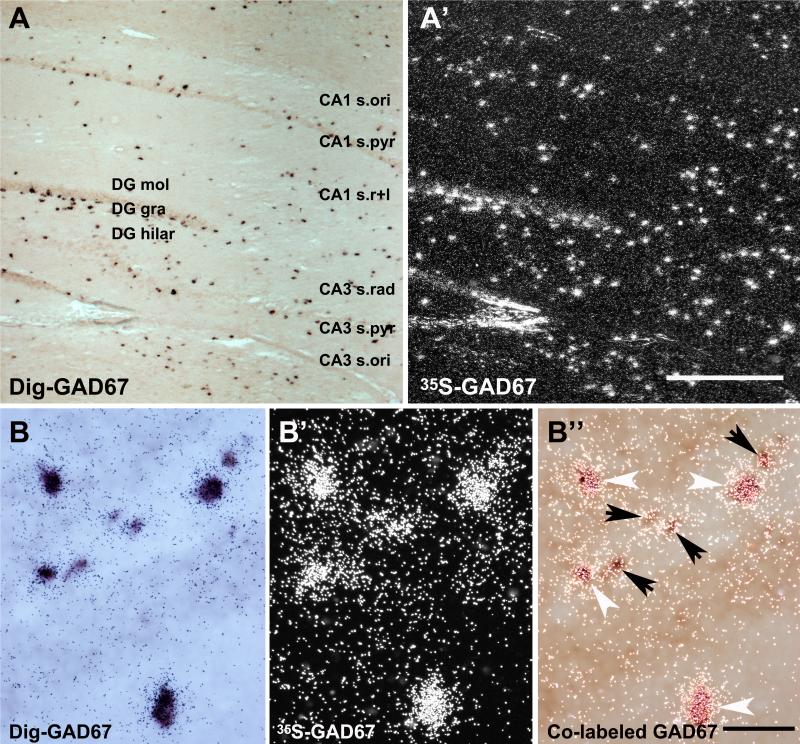
To evaluate the expression of nAChR subunit mRNA in GAD67-positive interneurons, combined radioactive and non-radioactive in situ hybridization was used. The sensitivity of the method was verified by using two probes for GAD67 mRNA, directed against different sequences to avoid competition for the same complementary sequence. The interneurons were labeled with a 277 bp 35S-labeled (1198–1474 bp) and a 466 bp Dig-labeled (185-650 bp) GAD67 cRNA probe (Fig. 4A, A’). Hybridization pattern for GAD67 mRNA derived with the Dig- or the 35S-labeled probes were identical as demonstrated by the pictures taken with lightfield (Dig-signal) (Fig. 4A) or darkfield (35S-signal) (Fig. 4A’) from the same area of the same section. The relative signal intensity for GAD67 mRNA hybridization appeared similar for both probes. Cells strongly labeled by Dig-GAD67 also exhibited high numbers of silver grains, and those exhibiting weak Dig-signal showed lower numbers of silver grains (Fig. 4B, B’ and B’’). This suggests that a strong Dig-signal did not reduce or prevent radiation from the 35S-hybridization signal from reaching the emulsion which is necessary for the formation of the silver grains. Thus, most if not all neurons labeled by one probe were also positive for the other probe. Therefore, the Dig-labeled GAD67 probe used for double in situ hybridization in this study was a highly sensitive and reliable marker for the detection of GABAergic interneurons, and suitable for double in situ hybridization.
Figure 4.
GAD67 mRNA expression detected with double in situ hybridization in the hippocampus. Hybridization signals for GAD67 viewed in bright-field detecting non-radioactive signal generated with the Dig-labeled probe (A) and in darkfield detecting radioactive hybridization signal generated with the 35S-labeled probe (A’). Higher magnification lightfield (B), darkfield (B’) and dual exposure (B’’) to light- and darkfield simultaneously detecting GAD67 expression in interneurons of the CA3. White arrowheads point to strong and black arrows to moderate expression of GAD67. Abbreviations: CA1 s.ori, CA1 stratum oriens; CA3 s.pyr, CA3 stratum pyramidale; CA1 s.r+l, CA1 stratum radiatum/lacunosum moleculare; DG mol, dentate gyrus molecular layer; DG gra, dentate gyrus granule cell layer; DG hilar, dentate gyrus hilar region. Scale bar = 200 μm (A’) and 50 μm (B’’).
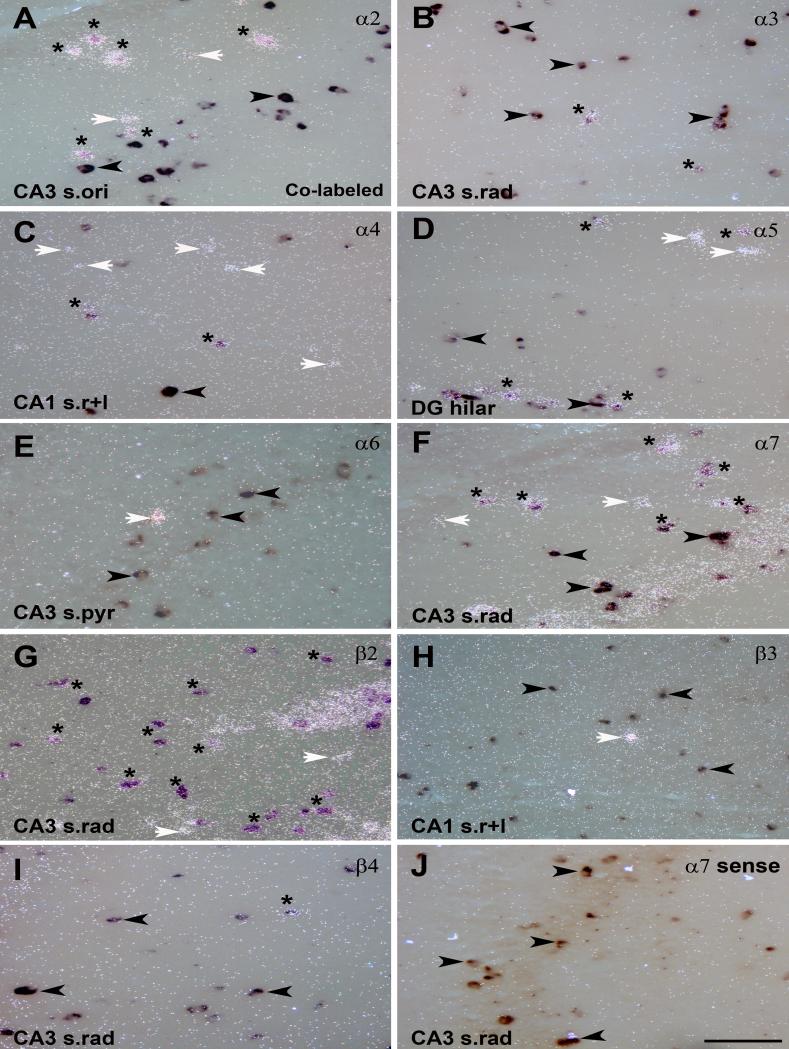
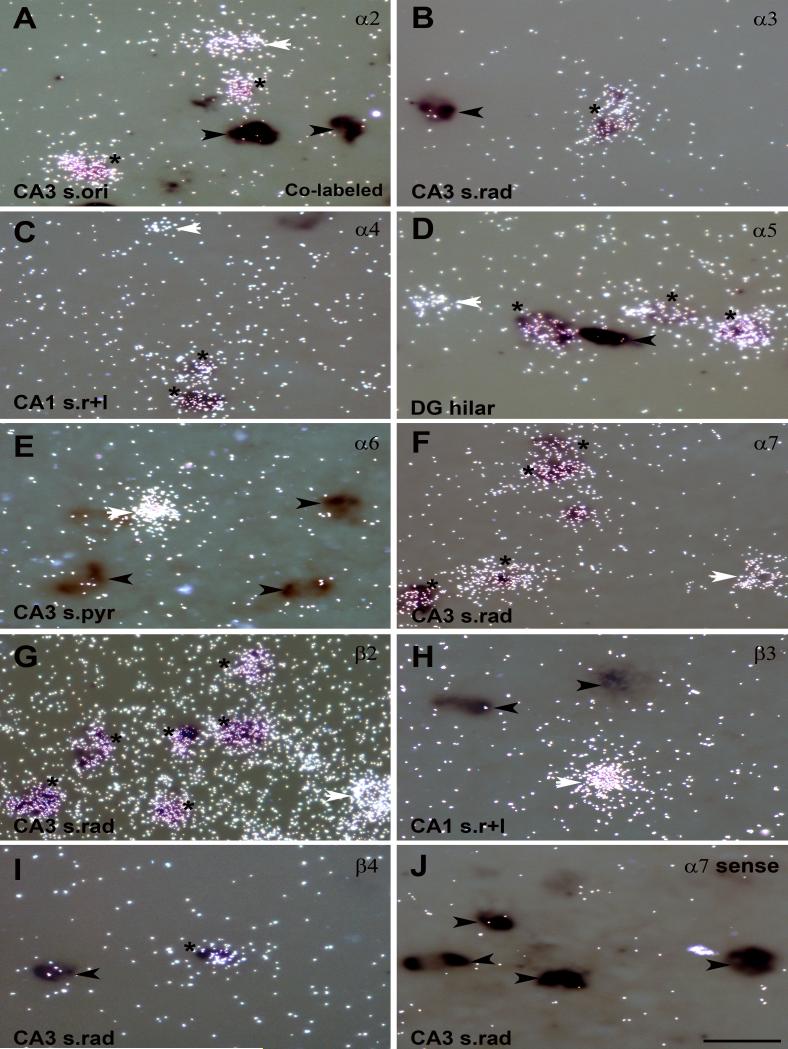
The double in situ hybridization showed co-expression of GAD67 mRNA with α2, α3, α4, α5, α7, β2 and β4 nAChR subunit mRNAs in adult rat hippocampus, but not with α6 and β3 (Fig. 5, Fig. 6). Quantitative analysis revealed that most GAD67 positive interneurons also expressed transcripts for β2, and the majority exhibited co-expression with α7 mRNA, but less than 20% exhibited co-expression with the other subunits (Fig. 7A). Sense probes were used as negative controls for double in situ hybridization and exhibited only background signal on Dig-labeled neurons as shown for the α7 sense probe (Fig. 5J and Fig. 6J).
Figure 5.
Co-expression of GAD67 and nAChR subunit mRNAs in hippocampal interneurons detected with double in situ hybridization. Expression of GAD67 and nAChR subunit mRNA for A) α2, B) α3, C) α4, D) α5, E) α6, F) α7, G) β2, H) β3, and I) β4 detected by dual exposure to light- and darkfield. J) Double in situ hybridization with the Dig-GAD67 probe and a 35S-nAChR sense probe for α7 used as a negative control. Asterisks indicate neurons with co-expression of mRNAs for GAD67 and a nAChR subunit; white arrows point to single-labeled neurons expressing nAChR subunit mRNA only; black arrows point to single-labeled GAD67 expressing neurons. Abbreviations: CA1 s.ori, CA1 stratum oriens; CA3 s.pyr, CA3 stratum pyramidale; CA1 s.rad/LM, CA1 stratum radiatum/lacunosum moleculare; DG mol, dentate gyrus molecular layer. Scale bar = 200 μm.
Figure 6.
Higher power images of co-expression of GAD67 and nAChR subunit mRNAs in hippocampal interneurons detected with double in situ hybridization. Expression of GAD67 and nAChR subunit mRNA for A) α2, B) α3, C) α4, D) α5, E) α6, F) α7, G) β2, H) β3, and I) β4 detected by dual exposure to light- and darkfield. J) Double in situ hybridization with the Dig-GAD67 probe and a 35S-nAChR sense probe for α7 used as a negative control. Asterisks indicate neurons with co-expression of mRNA s for GAD67 and a nAChR subunit; white arrows point to single-labeled neurons expressing nAChR subunit mRNA only; black arrows point to single-labeled GAD67 expressing neurons. Abbreviations: CA1 s.ori, CA1 stratum oriens; CA3 s.pyr, CA3 stratum pyramidale; CA1 s.rad/LM, CA1 stratum radiatum/lacunosum moleculare; DG mol, dentate gyrus molecular layer. Scale bar = 50 μm.
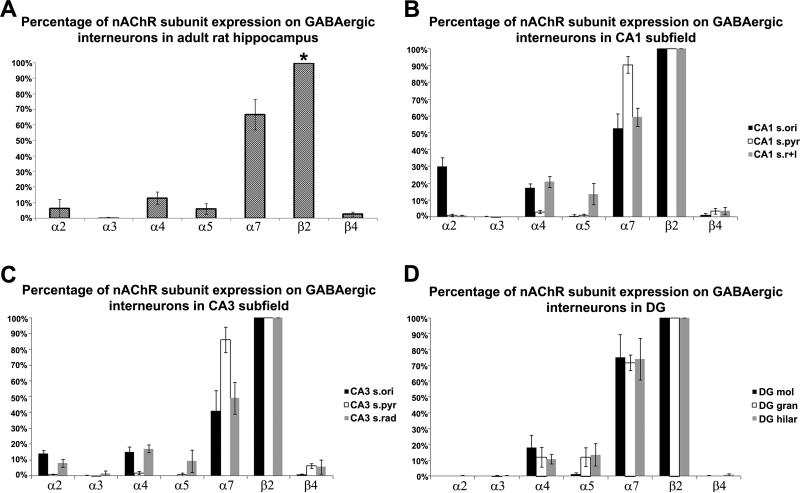
Figure 7.
Percent of GAD67 interneurons coexpressing nAChR subunit mRNAs for α2, α3, α4, α5, α7, and β4 in (A) entire hippocampus, (B) CA1 subfield, (C) CA3 subfield, and (D) in the dentate gyrus. The percent coexpression for each nAChR subunit was compared to that of β2 (representing 100%). * indicates that the percentage of GAD67/β2 neurons was significantly different from those of all other subunits. (*p < 0.001, Student t-test analysis, n=3).
Co-expression of GAD67 with α2 mRNA was mostly detected in CA1 s. oriens (30.1 ± 5.3 %) (Fig. 5A, Fig 6A and Fig. 7B), and a few double-labeled neurons were found in CA3 s. oriens (14.1 ± 1.9 %) and radiatum (8.1 ± 2.4 %) (Fig. 7C) but not in the DG. A comparison between dorsal and ventral CA1 s. oriens showed significantly higher coexpression (p≤0.05) in ventral (37.4 ± 3.5%) than dorsal hippocampus (22.8 ± 2.0%). Coexpression of GAD67 and α3 nAChR mRNAs was restricted to a few neurons located in the CA3 s. radiatum where 1.8 ± 1.5% of GAD67 positive neurons also expressed α3 mRNA (Fig. 5B, Fig. 6B and Fig. 7C).
Expression intensity for α4 mRNA was very low throughout the hippocampus including expression of α4 in interneurons (Fig. 5C and Fig. 6C). However, 12.9 ± 3.9% of GAD67 positive neurons also expressed α4 mRNA (Fig. 7A). Coexpression was found in CA1 (17.4 ± 2.4%) and CA3 s. oriens (15.2 ± 3.3%) and radiatum (17.1 ± 2.5%) and in the molecular layer (18.0 ± 8.0%), granule cell layer (12.1 ± 6.3%) and hilar region (10.8 ± 2.9%) of the DG (Fig. 7B, 7C and 7D). Coexpression of GAD67 and α5 mRNA was detected in 5.9 ± 3.6% of GABAergic neurons in the hippocampus (Fig. 5D, Fig. 6D and Fig. 7A), but was restricted to CA1 s. radiatum/ lacunosum moleculare (13.7 ± 6.3%), CA3 s. radiatum (9.4 ± 7.1%), and the granule cell layer (12.1 ± 5.9%) and hilar region (13.6 ± 7.1%) of the DG (Fig. 7B, 7C and 7D). The coexpression of GAD67 and α5 mRNA was mostly restricted to dorsal hippocampus.
Expression of α6 was detected in very few cells which exhibited very strong mRNA expression. However, there was no coexpression with GAD67 mRNA detected in any of the α6 expressing cells (Fig. 5E and Fig. 6E). Strong coexpression of GAD67 and α7 mRNAs was also found throughout the hippocampus in 66.7% (± 9.8%) of GABAergic interneurons (Fig. 5F, Fig. 6F and Fig. 7A). Double-labeling was high in CA1 (90.5 ± 4.99%) and CA3 s. pyramidal layers (86.0 ± 8.02%) and DG granule cell layer (71.6 ± 4.97%) and hilar region (74.1 ± 13.14%) (Fig. 7B, C and D). However, due to the high expression of α7 mRNA in pyramidal and granule cells it was not possible to distinguish expression of α7 mRNA in principal cells from those in interneurons. In other regions such as CA1 s. oriens (52.7 ± 8.5%) and radiatum/LM (59.4 ± 5.5%), CA3 s. oriens (41.2 ± 12.9%) and radiatum (49.2 ± 10.02%), coexpression was significantly lower.
Of the beta subunits expressed in the hippocampus, only β2 and β4 were coexpressed with GAD67 mRNA (Fig. 5G, I and Fig. 6G, I); β3 mRNA, although expressed in very few cells, was never detected in GAD67 positive interneurons (Fig. 5H and Fig. 6H). β2 exhibited strong and widespread mRNA expression, whereas hybridization signal intensity for β4 mRNA was low and restricted to a few cells. The microscopic analysis revealed that most GABAergic interneurons also expressed transcripts for β2 mRNA in all areas of the hippocampus (Fig. 5G, Fig. 6G and Fig. 7A). In contrast, β4 mRNAs was restricted to less than 2.5 ± 1.36% of interneurons (Fig. 5I, Fig. 6I and Fig. 7A), mostly located in CA1 and CA3 s. radiatum/moleculare.
DISCUSSION
This study is the first to systematically analyze the expression of nAChR subunit mRNAs in GABAergic interneurons in the rat hippocampus using double in situ hybridization. The results show that most GAD67 positive GABAergic interneurons expressed β2 subunit mRNA and about 66% also expressed α7 mRNA. In contrast, the expression of other subunits was very limited; only 13 % of GABAergic neurons expressed α4 mRNA followed by 6% for α5 and α2 and less than 2% for α3 and β4. In addition, the relative expression intensities for α4, α3 and β4 in GABAergic interneurons were low compared to their expression in other brain areas. No expression of α6 and β3 mRNAs was detected in GABAergic interneurons, although, a limited number of randomly scattered cells which exhibited very intense mRNA expression were found throughout the hippocampus but their identity remains to be determined.
Technical considerations
Radioactive-labeled cRNA probes for the detection of nAChR subunit mRNAs together with a dig-labeled probe to visualize GAD67 mRNA were used in this double in situ hybridization study. In general double in situ hybridization is a powerful tool for the localization of two mRNA transcripts in a single neuron. This method is a well-established technique to measure colocalization of nAChR subunit mRNAs with marker genes to identify specific neuronal populations (Winzer-Serhan and Leslie, 1997, Azam et al., 2002, 2003). In fact, the results for the expression of nAChR subunit mRNAs in tyrosine hydroxylase positive dopaminergic neurons in the substantia nigra and ventral tegmental area were similar to those obtained with single-cell PCR (Azam et al., 2002, Klink et al.,2001). However, there are limitations with this method. In general non-radioactive in situ hybridization is considered to be less sensitive, and quenching of the radioactive signal by the nonradioactive one could lower the number of detectable double-labeled cells. We compared the hybridization signal for GAD67 double in situ hybridization using two GAD67 probes. The results showed that although the S35-labled GAD67 probe was much shorter than the Dig-labeled GAD67 probe (277 vs. 466 bp), the hybridization pattern for both probes were identical. Furthermore, there was no sign of quenching by the color precipitate, because neurons which were strongly labeled by the non-radioactive probe were also strongly labeled by the radioactive probe. For the double in situ hybridization with nAChR subunits, S35-labeled probes were transcribed from full-length transcripts and had probe length between 1607 and 2522 bp to increase the sensitivity of the radioactive signal, and to assure that most if not all cells coexpressing transcripts for nAChR subunits were accounted for. Although, it does not seem that digoxigenin labeling reduced detection of radioactive labeling, we cannot rule out that interneurons, expressing low levels of a nAChR subunit (such as α3, α4, or β4), did not reach the criteria of 12 silver grains which was set as 4 times above background to avoid false positive results. This could result in an underestimation of coexpressing interneurons, especially. To control for the limitations of the method, the data are calculated as the percentage of GAD67/β2 double labeled cells in the hippocampus, which offers a reasonable estimate of the relative proportion of cells expressing both markers.
Anatomical evaluation
Our data are in complete agreement with previous anatomic studies. In here, the hybridization patterns derived with the different cRNA probes for the nAChR subunits are identical to those reported previously showing robust and widespread expression for α7 and β2 mRNA, but limited expression for other subunits (Patrick et al., 1989, Duvoisin et al., 1989, Wada et al., 1990, Wada et al., 1989, Séquéla et al., 1993). These findings suggest the presence of mainly homomeric nAChRs but very limited expression of the major heteromeric nAChR subtypes in the hippocampus. This is supported by receptor autoradiographic studies demonstrating that in rodent hippocampus, the major nAChR subtype is the α7 homomeric receptor, exhibiting strong binding throughout the hippocampal formation including interneurons (Clarke et al., 1985, Fuchs and Schwark, 1993, Freedman et al., 1993, Tribollet et al., 2004). In contrast, the number of binding sites for heteromeric receptors, detected with radiolabeled ligands such as nicotine, epibatidine, or cytosine are very low (Clarke et al., 1985, Pauly et al., 1989, Perry and Kellar, 1995, Happe et al., 1995, Tribollet et al., 2004). Although, β2 mRNA is strongly expressed in the hippocampal formation, heteromeric binding sites are not present because of the lack of a corresponding α subunit required for β2 to form heteromeric receptors.
A similar expression profile of nAChR subunit mRNAs in GABAergic neurons has been described before (Azam et al., 2003). The majority of GABAergic neurons in the basal forebrain express mRNAs for α7 and β2, with only a small subpopulation expressing other subunit mRNAs, mainly for α2, α4, and β4. In addition, GABAergic neurons located in the substantia nigra/ventral tegmental area also show mRNA expression of only a limited number of subunits: α4, α7 and β2 (Azam et al., 2002). This is in stark contrast to dopaminergic and noradrenergic neurons which express a wide array of different nAChR subunit mRNAs (Azam et al., 2002, Klink et al., 2001, Gallardo et al., 1997).
However, a single-cell RT-PCR study suggests that in addition to α7 and β2 other nAChR subunits such as α2, α3, α4, α5, β3 and β4 are expressed within GAD-positive neurons (Sudweeks and Yakel, 2001). Given the higher sensitivity of RT-PCR over in situ hybridization it is possible that very low levels of mRNA expression did not result in a positive hybridization signal and remained undetected in this study.
Correlation with functional studies
Our anatomical data for α7 mRNA expression are widely supported by functional studies reporting that the majority of nicotinic responses are mediated by an α7-type nAChR (Alkondon and Albuquerque, 1993). However, despite the strikingly low number of heteromeric nAChR binding sites, and low and restricted expression of α subunits in the hippocampus, functional studies have repeatedly suggested that heteromeric nAChRs located on GABAergic interneurons play a major role in the regulation of hippocampal activity (for review see Alkondon and Albuquerque 2004, Yakel and Shao, 2004). At least three distinct physiological responses to nicotinic agonists have been described in GABAergic interneurons and attributed to different nAChRs subtypes such as the homomeric α7 (type IA), and the heteromeric α4β2 (type II) and α3β4 (type III) nAChRs (Alkondon and Albuquerque, 1993, Alkondon et al., 1997, Jones and Yakel, 1997, Frazier et al., 1998, McQuiston and Madison, 1999, Ji and Dani, 2000). One explanation for this discrepancy is that the characterization of functional nAChRs has been done mostly in hippocampal slices from postnatal rat pups. Several anatomical studies have documented that the expression of nAChR subunit mRNAs is developmentally regulated with increased expression of α2, α3, α4, α5, α7 and β4 in postnatal hippocampus (Winzer-Serhan and Leslie, 1997, 2005, Adams et al., 2002, Son and Winzer-Serhan, 2006, Huang and Winzer-Serhan, 2006). However, a recent study by Alkondon and co-worker (2007) using hippocampal slices from adult and postnatal animals came to the conclusion that, although distinct age-dependent differences in the functional response to nicotinic activation exist, the type III response (presumably an α3β4-type nAChR) is still present in slices from adult animals.
An alternative explanation is that even very low numbers of mRNA transcripts, not detected by in situ hybridization, could be translated into subunit proteins, and subsequently be incorporated into functional nAChRs. It remains to be seen however, if sufficient amounts of proteins are synthesized to significantly contribute to nicotinic hippocampal activity.
On the other hand, the pharmacological tools used to characterize nicotinic responses in hippocampal slices are not selective enough to clearly distinguish between nAChR subtypes. Although, 90% of nicotinic responses are believed to be mediated via activation of α7 (type IA) nAChRs, discrepancies between the properties of hippocampal type IA and expected properties of homomeric α7 nAChRs have lead to speculations that not all α7 nAChRs are homomeric (Yakel and Shao, 2004, Khirough et al., 2002). If in fact α7 subunits also form functional heteromeric nAChRs in hippocampal interneurons, then those could contribute to the functional diversity seen in the nicotinic responses despite an apparent lack of α4 and in particular α3 mRNA expression in most interneurons. Support for this idea comes from a recent study presented by Liu et al. (2007) describing a novel α7β2 heteromeric nAChR which is highly sensitive to inhibition by β-amyloid peptides (Aβ), and is expressed in forebrain cholinergic neurons. Basal forebrain cholinergic neurons exhibit a similarly restricted expression pattern of mostly α7 and β2 nAChR subunits (Azam et al., 2003). Interestingly, Aβ also inhibits whole-cell and single-channel nicotinic currents from rat hippocampal interneurons at low concentrations (Pettit et al., 2001). Therefore, in addition to forming homomeric receptors, α7 may also form heteromeric nAChRs and thus, could contribute to the diverse nicotinic responses typically seen in hippocampal interneurons.
In this study we found that α2 mRNA was clearly expressed in 30% of CA1 s. oriens/alveus GABAergic interneurons, presumably in oriens-lacunosum moleculare (OLM) cells (Son and Winzer-Serhan, 2006, Wada et al., 1989). This finding is supported by the RT-PCR study also describing co-expression of α2 transcripts with GAD67 in s. oriens (Sudweeks and Yakel, 2001). OLM cells potently inhibit excitatory input to pyramidal cells (Yanovsky et al., 1997), and exhibit two different nicotinic responses, a fast α7, and a slow non-α7 mediated response (McQuiston and Madison, 1999). The slow response is insensitive to the α3β2 nAChR antagonist α-CTX MII, and relatively insensitive to the α4β2 nAChR antagonist DHβE, therefore, suggesting other types of nAChRs, perhaps with participation of α2. A functional study has recently underscored the importance of α2-containing nAChRs in regulating long-term potentiation in the hippocampal CA1 region (Nakauchi et al., 2007). Thus, α2-containing nAChRs appear to be a major functional contributor to the slow nicotinic response in these neurons and could either be a new type of nicotinic response mediated by α2β2 nAChRs or represent one of the previously described (type II or III) once.
The α5 nAChR subunit mRNA exhibited spatially restricted but robust expression in GABAergic neurons in CA1 and CA3 s. radiatum and in the DG. The α5 stands out among α nAChR subunits because α5 does not form functional nAChRs when expressed alone or in combination with another β subunit (Ramirez-Latorre et al., 1996). However, when combined with another α and β subunit, α5 does contribute to the formation of heteromeric channels and significantly alters functional properties such as increasing single channel conductance. In a previous anatomical study co-expression of α5 and α7 mRNA in a subpopulation of hippocampal interneurons was detected (Winzer-Serhan and Leslie, 2005), and since most GABAergic interneurons express β2 mRNA (this study), a heteromeric α5α7β2 nAChRs may exist. However, since α4 mRNA is also found in the same areas as α5 mRNA, an α4α5β2 heteromeric receptor type seems also a possibility.
Hippocampal inhibitory interneurons are very diverse, and differ in their projection and innervation patterns, physiology, expression of neurotransmitter receptors and calcium binding proteins. This diversity is thought to reflect functional and structural specializations which evolved to control distinct network operations such as synchronization of neuronal firing, pyramidal cell generated theta rhythm, or basket cell generated gamma oscillations to name a few (Freund and Buzsaki, 1996). The complex expression pattern of nAChR subunits, most likely tailored to the specific functional needs of different interneuron populations contributes to the interneuronal diversity. Depending on the physiological properties of distinct nAChR subtypes nicotine or nicotinic activation could differentially influence subpopulations of interneurons and contribute to the differential control of the hippocampal network. Thus, nAChRs are in a position where they can play a critical regulatory role in hippocampal circuit activity in line with their role in enhancing cognitive functions.
CONCLUSION
In summary, based on the anatomical analysis presented in this study, the majority of nicotinic responses should be mediated by an α7-containing nAChR and some by non-α7 heteromeric receptors such as an α2β2* subtype in the s. oriens and an α5 containing nAChRs in the s. radiatum and DG. Heteromeric α4β2* nAChRs should represent only a small fraction of the nicotinic response since less than 20% of GAD67 positive neurons were identified as co-expressing α4 mRNA. Nicotinic responses mediated by heteromeric α3β4* nAChRs should be at best very rare, because the number of GAD67 positive neurons co-expressing α3 was almost negligible and very limited for β4.
Acknowledgments
Grant sponsor: NIH grants DA 106487.
REFERENCES
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. Age-dependent changes in the functional expression of two nicotinic receptor subtypes in CA1 stratum radiatum interneurons in the rat hippocampus. Biochem Pharmacol. 2007;74:1134–1144. doi: 10.1016/j.bcp.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Patrick JW. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain:autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KD, Milner TA. Cholinergic septal afferent terminals preferentially contact neuropeptide Y-containing interneurons compared to parvalbumin-containing interneurons in the rat dentate gyrus. J Neurosci. 1999;19:10140–10152. doi: 10.1523/JNEUROSCI.19-22-10140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta4. Neuron. 1989;4:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Wetmore C, Strömberg I, Leonard S, Olson L. α-Bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Léránth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Schwark HD. Distribution of [3H]QNB and [125I]alpha-bungarotoxin binding and acetylcholinesterase activity in visual system and hippocampal structures of eleven mammalian species. J Comp Neurol. 1993;329:427–437. doi: 10.1002/cne.903290402. [DOI] [PubMed] [Google Scholar]
- Gallardo KA, Winzer-Serhan UH, Chen YC, Leslie FM. Ontogenetic expression of neuronal nicotinic acetylcholine receptor subunits in rat locus coeruleus. Soc Neurosci Abstr. 1997;23:383. [Google Scholar]
- Happe HK, Peters JL, Bergman DA, Murrin LC. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H] cytisine. Neuroscience. 1994;62:929–944. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Jinno S, Aika Y, Fukuda T, Kosaka T. Quantitative analysis of GABAergic neurons in the mouse hippocampus, with optical dissector using confocal laser scanning microscope. Brain Res. 1998;814:55–70. doi: 10.1016/s0006-8993(98)01075-0. [DOI] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurons in the rat hippocampus. J Physiol. 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chang Y, Zhang J, Xue F, Dechon J, Lukas RJ, WU J. A novel α7β2-nicotinic acetylcholine receptor in forebrain cholinergic neurons is highly sensitive to amyloid beta peptides. Neuroscience Abstract. 2007;2007:39.1. [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via activation of α2* nicotinic ACh receptors. Eur J Neurosci. 2007;25:2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- Patrick J, Boulter J, Deneris E, Wada K, Wada E, Connolly J, Swanson L, Heinemann S. Structure and function of neuronal nicotinic acetylcholine receptors deduced from cDNA clones. Prog Brain Res. 1989;79:27–33. doi: 10.1016/s0079-6123(08)62463-2. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–459. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edition Academic Press; San Diego, CA: 1998. [Google Scholar]
- Perry DC, Kellar KJ. [3H] epibatidine labels nicotinic receptors in rat brain: an autoradiographic study. J Pharmacol Exp Ther. 1995;275:1030–1034. [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. β-Amyloid (1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21(RC120(1-5)) doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning functional properties, and distribution of rat brain alpha7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. J Chem Neuroanat. 2006;32:179–190. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic acetylcholine receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol. 1997;386:540–554. doi: 10.1002/(sici)1096-9861(19971006)386:4<540::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Shao Z. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat hippocampal interneurons. Prog Brain Res. 2004;145:95–107. doi: 10.1016/S0079-6123(03)45006-1. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]