Abstract
Background
Toxoplasmosis is an important parasitic zoonosis caused by the protozoan Toxoplasma gondii that is distributed world-wide and infects a variety of hosts. However, the prevalence of T. gondii in the environment (such as soil, water and food) is largely unknown. Due to the technical difficulty in oocyst counting directly, an alternative assay using the serologic status of T. gondii in free-living animals, such as stray or free-living dogs, as an indicator, can be used to evaluate environmental contamination indirectly, as they are exposed to the same risk of infection as humans and other animals.
Results
In the present study, 231 stray or free-living dogs across an urban-rural gradient were examined to assess the frequency of T. gondii in the environment. Specific antibodies to T. gondii were found in 93 dogs (40.3%) by enzyme-linked immunosorbent assay (ELISA), and no statistically significant differences were observed in seroprevalences of T. gondii between urban dogs (38.7%) and rural dogs (41%) (p > 0.05).
Conclusions
A high seroprevalence of T. gondii in stray or free-living dogs in the present study indicates that there would be a wide distribution and a constant infection pressure of T. gondii across an urban-rural gradient, and the oocysts of T. gondii in the environment would be an important source of infection for humans and other animals both in urban and rural areas in China.
Keywords: Prevalence, Toxoplasma gondii, Stray dog, Enzyme-linked immunosorbent assay (ELISA), Environment, Indicator
Background
Toxoplasmosis is an important parasitic zoonosis caused by the protozoan Toxoplasma gondii that is distributed world-wide and infects a variety of hosts including humans, domestic animals and birds [1]. In humans, it was estimated that 30% of humans in the world and 7.88% of population in China were exposed to T. gondii, respectively [2-4]. T. gondii is mainly acquired by ingestion of under-cooked meat containing tissue cysts and water or food contaminated by oocysts in the environment [5,6]. Infection can also occur when T. gondii transmits from an infected mother to the fetus vertically [1,2]. However, it is still unknown which route of transmission is more prevalent for postnatal toxoplasmosis as no methods can differentiate oocyst-induced infection from those cysts formed, although progress is being made [7,8].
Felids, as the only known definitive hosts that shed environmentally-resistant oocysts into the environment, are essential in the life cycle and dispersal of T. gondii in the environment [9]. The shed oocyst is sporulated under favorable climatic conditions, and remains infectious for up to approximately 2 years, leading to a widespread environmental contamination and an important source for exposure of humans, domestic animals and wildlife to T. gondii [5,10-12]. In addition, the marine ecosystem can also be contaminated by environmentally resistant oocysts that are secreted by terrestrial felids and transported into the marine environment via freshwater runoff [13].
As oocyst of T. gondii in the environment is an alternative source for acquired toxoplasmosis and plays a significant role in the transmission dynamics of T. gondii, with constant efforts being made to seek a rapid, accurate and sensitive assay in determining the prevalence of T. gondii in the environment directly. Unfortunately, the progress is not satisfactory [14,15]. Thus, an alternative strategy using seroprevalence of T. gondii infecting free-living animals such as stray dogs which are considered as sentinels has been adopted for measuring the distribution of T. gondii exposure in environment indirectly [16-18].
Stray or free-living dogs are considered as the best indicators of T. gondii in the environment for the following reasons: I) stray dogs without any protection from pathogens roam freely and contact the same contaminated environment which humans are also exposed to; II) they are available both in urban and rural areas in practice, where the sanitary conditions may be very different; III) compared with other free-living animals, dogs have a keen sense of smell and have the behaviour of rolling in cats feces, increasing their exposure [19]; IV) canine toxoplasmosis can be also caused by ingestion of food meats containing cysts of T. gondii, but these food meats are mainly produced by non-carnivorous sources that are most likely to be infected by oocysts in the environment, such as birds, small mammals and under-cooked meat from humans' refuse. Interestingly, an epidemiological study showed a uniform prevalence between human toxoplasmosis and dogs exposed to T. gondii in the same region, indicating that stray dogs might be good sentinels of T. gondii exposure to humans and wildlife [2,17].
Climates of different geographical locations may also have a great influence on the transmission dynamics of T. gondii in the environment as they cannot become sporulated, survive and remain infectious without favorable climatic conditions [20,21]. However, little is known of the distribution and destiny of T. gondii in the environment of People's Republic of China (PRC) where the environmental hygiene of urban cities and rural areas are very different. Now, PRC is undergoing a rapid urbanization that may also affect the transmission and distribution of T. gondii oocysts in the environment [12]. In view of this background, the objectives of this study were to estimate the distribution and transmission dynamics of T. gondii in the environment by examining the seroprevalence of stray dogs as indicators, as well as identifying the risks of T. gondii in the environment that humans are exposed to in PRC.
Results and Discussion
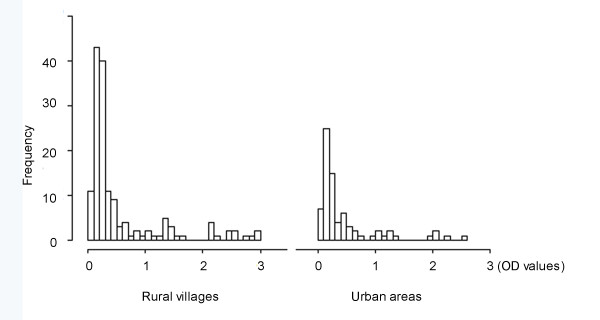
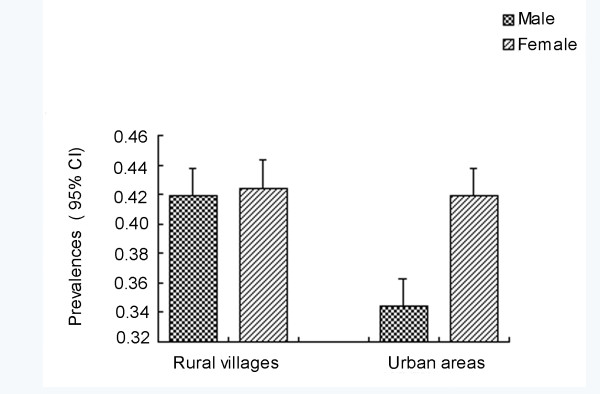
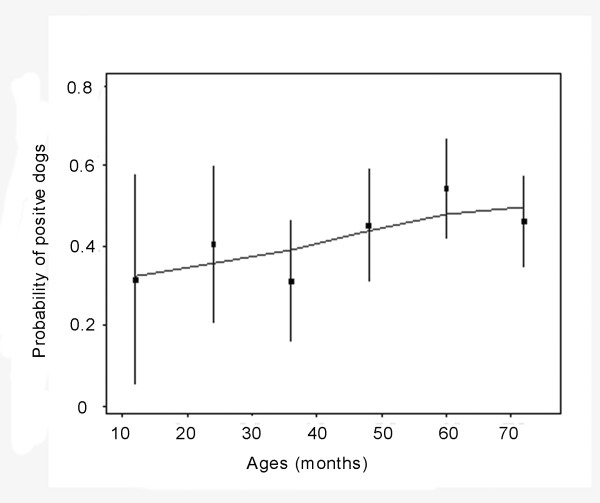
T. gondii antibodies were found in 93 (40.3%) of 231 dogs examined, and four dogs (1.7%) were considered "doubtful". The distribution of individual OD values are presented in Figure 1,and the antibodies levels of positive dogs were high, compared with OD values reported by Meireles et al. [17]. Table 1 shows that seroprevalence of T. gondii in rural village dogs is a little higher than those from urban areas, and the males were slightly lower than female ones, but no associations were found between origins, genders and the seropositivity of T. gondii in dogs (p ≥ 0.05) (Table 1 Figure 2). However, the seropositivity of T. gondii increased with age, showing a relationship between the prevalence of T. gondii and ages of stray or free-living dogs, although this effect is weak (Figure 3).
Figure 1.
Frequency distribution of individual optical density (OD) values by ELISA in different environmental conditions.
Table 1.
Prevalence of Toxoplasma gondii in dogs of different genders and geographical origins
| Biometric data | Origins | Genders | ||
|---|---|---|---|---|
| Urban | Rural villages | Male | Female | |
| Sample No. | 75 | 156 | 121 | 110 |
| Doubtful No.* | 4 | 0 | 3 | 1 |
| Positive No. | 29 | 64 | 47 | 46 |
| Prevalence (%) | 38.7 | 41.0 | 38.9 | 41.8 |
| Odds ratio [95% CI] | 0.993 [0.51; 1.756] | 1.051 [0.624; 1.770] | ||
* samples whose OD-value was between 0.9 times and 1.1 times of the mean OD value of critical controls according to manufacturer's recommendations.
Figure 2.
Incidence rates of dogs exposure to T. gondii in different genders and geographical origins by ELISA. Bars represent the 95% confidence interval of the measure.
Figure 3.
Relationship between ages of dogs and the seropositivity of T. gondii exposure. The animals were divided into 6 age groups (< 12, [12-60], ≥ 60 months) and lines represent estimated probability of seropositivity depending on age at 95% confidence intervals.
The prevalence of T. gondii in the environment is largely unknown, and it is definitely associated with different geographical locations, climates, and animal welfare, which have a great influence on transmission of T. gondii [22,23]. In the present study, a high seroprevalence of T. gondii in stray or free-living dogs was obtained, and the seroprevalences in dogs of different origins did not present any statistically significant difference, indicating a wide distribution and a constant infection pressure of T. gondii in different environmental conditions. To illustrate this, a parallel study of 41 stray cats obtained 11 T. gondii isolates by bioassay in this region (unpublished data), therefore, ubiquitous stray cats infected by T. gondii are mainly responsible for the widespread distribution and constant infection pressure in the environment.
Four dogs with OD-value falling in between 0.9 times and 1.1 times of the mean OD-value of critical controls failed to be classified as positive or negative, and they were considered as "doubtful" for T. gondii infection according to manufacture's recommendations, suggesting that those animals were likely in acute phase or long chronic infection with T. gondii as the levels of antibodies to T. gondii were low and close to background [24,25].
In this study, the prevalence of dogs exposed to T. gondii increased with age, suggesting acquisition of infection rather than congenital transmission of T. gondii in the canine population, which is in accordance with reports by others [26-28]. It is interpreted that older dogs have more chance to feed on food or have contact with the surrounding environment that can be contaminated by T. gondii oocysts.
Attempts to evaluate the presence of T. gondii oocysts in the environment directly have been unsuccessful, although PCR assays are considered as the most promising methods for detecting T. gondii DNA in the environment directly [1,29-31]. Therefore, we chose stray or free-living dogs as sentinels of the distribution of T. gondii in the environment indirectly in the present study. Stray dogs of the present study were infected with T. gondii mainly via feeding on contaminated waters, small mammals and birds that are most likely infected by oocysts in the environment since they are non-carnivorous. Besides, ingestion of humans' leftovers including under-cooked animal meat that was produced on animal farms where toxoplasmosis of animals was probably neglected is another probable source for canine toxoplasmosis.
Conclusions
A high seroprevalence of T. gondii in stray or free-living dogs in the present study indicates that there would be a wide distribution and a constant infection pressure of T. gondii across the urban-rural gradient, and the oocysts of T. gondii in the environment would be an important source of infection for humans and other animals both in urban and rural areas in China. Therefore, the knowledge of the distribution and transmission dynamics of T. gondii in the environment should be further recognized and explored as humans are likewise potentially at risk of exposure to T. gondii. Furthermore, measures that eliminate the sources of T. gondii in the environment, for example, the campaign of capturing stray dogs and cats and hygienic education programs for preventing T. gondii exposure to residents should be executed.
Methods
Ethics Statement
All dogs were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People's Republic of China, and the study was approved by the Animal Ethics Committee of Xuzhou Medical College (No. SCXK<SU>2010-0003).
The investigated regions
This study was performed in Xuzhou City which is located in Eastern China, and shares borders with Shandong Province, Anhui Province and Henan Province between 33°43'~34°58' North latitudes and 116°22'~118°40' East longitudes. The city covers an area of approximately 11,000 square kilometers and has a 9.4 million population. The climate of this region is a seasonal temperate semi-humid monsoon with an average annual temperature of 14°C and an average annual rainfall ranging from 800 mm to 930 mm. This information was available at http://www.xz.gov.cn/xzgl/zrdl/.
Sampling of naturally infected dogs
The animals in the present study consisted of 75 stray dogs from a stray animal shelter in Xuzhou city, and 156 stray or free-living dogs slaughtered for animal meat, which originated from surrounding rural villages. Blood samples were collected randomly and the information such as sex, age and source were also obtained and matched. Blood was allowed to clot at room temperature, then centrifuged at 1800 g for 10 min. The sera were separated and stored at -20°C for further analysis.
Serological assay
The presence of T. gondii antibodies was determined using a commercial ELISA kit according to the manufacturer's recommendations (Zhuhai S.E.Z. Haitai Pharmaceutlcals Co., Ltd, Zhuhai, China). This kit has been confirmed by specific PCR assay for detecting tissue cysts in experimentally infected pigs in our laboratory [32]. Briefly, sera diluted 1:100 were incubated in a T. gondii antigen-coated 96-well plate at 37°C for 30 min, and the plate was washed 3 times, then a drop of HRP-labelled conjugate was added into each well. After a final washing, "A" and "B" solution were then added and incubated at 37°C for 10 min. The reaction was blocked by adding a drop of "stop solution". The optical density (OD) at 450 nm was read using a photometer (BioTek Instrument Inc, Vermont, USA).
Positive control, negative control, three cut-off controls and blank control provided by the manufacturer were included in each test. All OD-values for the test sera were corrected according to blank controls, and OD-values of samples were authentic if the OD-value of positive control was > 0.6 and negative control was ≤ 0.2 in each test. The threshold value was determined by the mean of 3 critical controls in each test. Samples were considered positive if OD-values were greater than 1.1 times of threshold values and negative if OD-values were less than 0.9 times of threshold values, respectively. Those sera with the OD-values falling in between 0.9 times and 1.1 times of the threshold values were considered dubitable and should be re-tested.
Statistical analysis
Data were analyzed by the chi-square test using Chi Square Test in SPSS for Windows (Release 16.0 standard version, SPSS Inc., Chicago, America) and excel 2003 (Microsoft®). Statistically significant difference was observed if the p value was < 0.05.
Conflict of interest statement
The authors declare that they have no competing interests.
Authors' contributions
Conceived and designed the experiments: XQZ, KYZ, CY and FFL. Performed the experiments: CY and FFL; Analyzed the data: PZ and CY; Contributed reagents/materials/analysis tools: CLY, RXT, YSL, LL, NS, PZe, PZh, DHW, DHZ. Wrote the paper: CY, QXZ and KYZ. All authors read approved the final version of the manuscript.
Contributor Information
Chao Yan, Email: yanchao6957@163.com.
Lin-Lin Fu, Email: fulinlin_327@163.com.
Cai-Ling Yue, Email: Yuecailing506@163.com.
Ren-Xian Tang, Email: tangrenxian-t@163.com.
Yi-Sheng Liu, Email: liuyisheng52@163.com.
Liang Lv, Email: ll494223984@126.com.
Na Shi, Email: 992188949@qq.com.
Ping Zeng, Email: zpstat@xzmc.edu.cn.
Peng Zhang, Email: qldzhp@gmail.com.
Dong-Hui Wang, Email: 1550694452@qq.com.
Dong-Hui Zhou, Email: 281970075@qq.com.
Xing-Quan Zhu, Email: Xingquanzhu1@hotmail.com.
Kui-Yang Zheng, Email: ZKY02@163.com.
Acknowledgements
Project support was provided, in part, by the Science Foundation of Xuzhou Medical College (Grant Nos. 2011KJ09), the National Natural Science Foundation of China (Grant Nos. 81171590, 31172316 and 31101812), the Undergraduate Innovation Program in Science and Technology of Colleges and Universities in Jiangsu Province, 2010 (Grant Nos. 800), the Open Funds of the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Grant Nos. SKLVEB2011KFKT010, SKLVEB2010KFKT009, SKLVEB2009KFKT014, SKLVEB2010KFKT010 and SKLVEB2011KFKT004) and the Yunnan Provincial Program for Introducing High-level Scientists (Grant No. 2009CI125).
References
- Dubey JP. CRC Press Inc. 2. Boca Raton, New York; 2010. Toxoplasmosis of animals and humans. [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 2008;24:190–196. doi: 10.1016/j.pt.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Zhou P, Chen Z, Li HL, Zheng H, He S, Lin RQ, Zhu XQ. Toxoplasma gondii infection in humans in China. Parasit Vectors. 2011;4:165. doi: 10.1186/1756-3305-4-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel JK, Ruiz A, Chinchilla M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am J Trop Med Hyg. 1975;24:439–443. doi: 10.4269/ajtmh.1975.24.439. [DOI] [PubMed] [Google Scholar]
- Pereira KS, Franco RM, Leal DA. Transmission of toxoplasmosis (Toxoplasma gondii) by Foods. Adv Food Nutr Res. 2010;60:1–19. doi: 10.1016/S1043-4526(10)60001-0. [DOI] [PubMed] [Google Scholar]
- Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–442. doi: 10.1016/S1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- Munoz-Zanzi CA, Fry P, Lesina B, Hill D. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerg Infect Dis. 2010;16:1591–1593. doi: 10.3201/eid1610.091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Lappin MR, Thulliez P. Diagnosis of induced toxoplasmosis in neonatal cats. J Am Vet Med Assoc. 1995;207:179–185. [PubMed] [Google Scholar]
- Yilmaz SM, Hopkins SH. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. J Parasitol. 1972;58:938–939. doi: 10.2307/3286589. [DOI] [PubMed] [Google Scholar]
- Sroka J, Wojcik-Fatla A, Szymanska J, Dutkiewicz J, Zajac V, Zwolinski J. The occurrence of Toxoplasma gondii infection in people and animals from rural environment of Lublin region-estimate of potential role of water as a source of infection. Ann Agric Environ Med. 2010;17:125–132. [PubMed] [Google Scholar]
- Lehrer EW, Fredebaugh SL, Schooley RL, Mateus-Pinilla NE. Prevalence of antibodies to Toxoplasma gondii in woodchucks across an urban-rural gradient. J Wildl Dis. 2010;46:977–980. doi: 10.7589/0090-3558-46.3.977. [DOI] [PubMed] [Google Scholar]
- Miller MA, Gardner IA, Kreuder C, Paradies DM, Worcester KR, Jessup DA, Dodd E, Harris MD, Ames JA, Packham AE, Conrad PA. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int J Parasitol. 2002;32:997–1006. doi: 10.1016/S0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Dumètre A, Dardé ML. Purification of Toxoplasma gondii oocysts by cesium chloride gradient. J Microbiol Methods. 2004;56:427–430. doi: 10.1016/j.mimet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Jones JL, Dubey JP. Waterborne toxoplasmosis-recent developments. Exp Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Frenkel JK. Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology. Vet Parasitol. 1998;77:1–32. doi: 10.1016/S0304-4017(97)00227-6. [DOI] [PubMed] [Google Scholar]
- Meireles LR, Galisteo AJ Jr, Pompeu E, Andrade HF Jr. Toxoplasma gondii spreading in an urban area evaluated by seroprevalence in free-living cats and dogs. Trop Med Int Health. 2004;9:876–881. doi: 10.1111/j.1365-3156.2004.01280.x. [DOI] [PubMed] [Google Scholar]
- Salb AL, Barkema HW, Elkin BT, Thompson R, Whiteside DP, Black SR, Dubey JP, Kutz SJ. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canada. Emerg Infect Dis. 2008;14:60–63. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel JK, Lindsay DS, Parker BB, Dobesh M. Dogs as possible mechanical carriers of Toxoplama, and their fur as a source of infection of young children. Int J Infect Dis. 2003;7:292–293. doi: 10.1016/S1201-9712(03)90112-3. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasma gondii oocyst survival under defined temperatures. J Parasitol. 1998;84:862–865. doi: 10.2307/3284606. [DOI] [PubMed] [Google Scholar]
- Afonso E, Thulliez P, Gilot-Fromont E. Local meteorological conditions, dynamics of seroconversion to Toxoplasma gondii in cats (Felis catus) and oocyst burden in a rural environment. Epidemiol Infect. 2010;138:1105–1113. doi: 10.1017/S0950268809991270. [DOI] [PubMed] [Google Scholar]
- Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Afonso E, Thulliez P, Gilot-Fromont E. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus) Int J Parasitol. 2006;36:1373–1382. doi: 10.1016/j.ijpara.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP, Butler JM, Blagburn BL. Experimental tissue cyst induced Toxoplasma gondii infections in dogs. J Eukaryot Microbiol. 1996;43:113S. doi: 10.1111/j.1550-7408.1996.tb05032.x. [DOI] [PubMed] [Google Scholar]
- Piergili-Fioretti D. Problems and limitations of conventional and innovative methods for the diagnosis of toxoplasmosis in humans and animals. Parassitologia. 2004;46:177–181. (In Italian) [PubMed] [Google Scholar]
- Zhang H, Zhou DH, Chen YZ, Lin RQ, Yuan ZG, Song HQ, Li SJ, Zhu XQ. Antibodies to Toxoplasma gondii in stray and household dogs in Guangzhou, China. J Parasitol. 2010;96:671–672. doi: 10.1645/GE-2352.1. [DOI] [PubMed] [Google Scholar]
- Lopes AP, Santos H, Neto F, Rodrigues M, Kwok OC, Dubey JP, Cardoso L. Prevalence of antibodies to Toxoplasma gondii in dogs from northeastern Portugal. J Parasitol. 2011;97:418–420. doi: 10.1645/GE-2691.1. [DOI] [PubMed] [Google Scholar]
- Wu SM, Huang SY, Fu BQ, Liu GY, Chen JX, Chen MX, Yuan ZG, Zhou DH, Weng YB, Zhu XQ, Ye DH. Seroprevalence of Toxoplasma gondii infection in pet dogs in Lanzhou, Northwest China. Parasit Vectors. 2011;4:64. doi: 10.1186/1756-3305-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schares G, Herrmann DC, Beckert A, Schares S, Hosseininejad M, Pantchev N, Globokar Vrhovec M, Conraths FJ. Characterization of a repetitive DNA fragment in Hammondia hammondi and its utility for the specific differentiation of H. hammondi from Toxoplasma gondii by PCR. Mol Cell Probes. 2008;22:244–251. doi: 10.1016/j.mcp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Aubert D, Villena I. Detection of Toxoplasma gondii oocysts in water: proposition of a strategy and evaluation in Champagne-Ardenne Region, France. Mem Inst Oswaldo Cruz. 2009;104:290–295. doi: 10.1590/S0074-02762009000200023. [DOI] [PubMed] [Google Scholar]
- Du F, Feng HL, Nie H, Tu P, Zhang QL, Zhou YQ, Zhao JL. Survey on the contamination of Toxoplasma gondii oocysts in the soil of public parks of Wuhan, China. Vet Parasitol. in press . [DOI] [PubMed]
- He Y, Zhu YP, Yuan ZG, Yin CC, Zhou DH, Lin RQ, Zhu XQ, Weng YB. Comparison of five ELISA kits for detection of anti-Toxoplasma gondii antibodies in experimentally infected swine. Chin Anim Husb Vet Med. 2011;38:218–221. (in Chinese) [Google Scholar]