Abstract
Background
Patients with sickle cell disease (SCD) visit emergency departments (ED) in rates leading to a significant health system burden. However, limited comprehensive evaluations of utilization patterns have been published using data connecting visits to patients across facilities. This study aims to examine sociodemographic predictors of ED utilization in SCD.
Procedure
This retrospective cohort study employed 2007 data from the California Office of Statewide Health Planning and Development (OSHPD). Data included all ED encounters from California hospitals; identifiers connected each visit to an individual patient, across all facilities in the state. Multivariate regression techniques evaluated sociodemographic predictors of utilization while adjusting for confounding variables.
Results
In 2007, 2,920 California patients with SCD made 16,364 ED visits. Adults ≥ 21 years of age had higher ED visit rates than children and were more likely to both be in the highest tier of users and visit multiple facilities. Patients living further from a self-identified provider of comprehensive SCD care had higher rates of ED visits and a lower likelihood of hospitalization from the ED. Publicly insured patients had higher rates of ED visits and were more likely to be in the highest tier of users than were the privately insured or uninsured.
Conclusions
Adulthood ≥ 21 years of age, distance from comprehensive SCD care, and insurance status are significant predictors of ED utilization in SCD. As a routine source of care decreases ED utilization, these findings prompt concern that these factors act as barriers to accessing comprehensive SCD care.
Keywords: sickle cell disease, emergency department, utilization, distance
Introduction
Sickle cell disease (SCD) affects one in 6,600 California births.[1] With acute and chronic multi-organ complications,[2–3] SCD leads to a considerable burden on the medical system[4–6] including emergency department (ED) utilization;[6–13] in California, the nation’s most populous state, such utilization is particularly burdensome. Effective practices such as newborn screening, penicillin prophylaxis and hydroxyurea have converted SCD from a fatal childhood disease to a chronic disease of middle age.[14–18] Yet in California, an adolescent’s 21st birthday marks the end of federally-mandated Title V eligibility for comprehensive health care coverage for children with special health care needs.[19] A growing adult SCD population, who may not transition effectively from pediatric to adult care, faces [18] considerable medical and psychosocial challenges.
Distance to SCD care has been associated with increased charges[20] and inpatient hospitalizations.[21] Geographic differences in SCD mortality[22] have also been reported. However, SCD geographic utilization studies have approached patient geography by region rather than by individual. Furthermore, most ED utilization descriptions to date in SCD were limited to describing patients connected to care at single institutions[20,23–30] or consortia of institutions.[5,12,31] Broader patient samples have been examined via public data but often lack identifiers to distinguish which visits belong to a particular patient or whether a patient visits a given ED one day and a different ED the next.[13,32–35] Public survey-level data[4,27,32,36] rely on statistical methods to extrapolate to the entire population; this approach may be more appropriately used in common diseases such as asthma.[37–38] Further utilization studies are limited to single payers[6,10–11,21,39–41] or to adult[8–9,35] or pediatric[4,6,11,24,29,36,40–41] populations. While state-based registries may be broadly representative, reported versions represent half the target population.[12] Without population-based data, it is impossible to distinguish how often or where patients use care. To this end, the present study evaluates predictors of ED utilization in California hospitals with links between patients and visits across multiple institutions, including an analysis of patient distance to the nearest source of comprehensive SCD care at the zip code and individual level.
Methods
The original data, obtained from the California Office of Statewide Health Planning and Development (OSHPD), comprises one year of ED visits to California hospitals from January to December 2007 (n=8,786,265) and one year of hospital discharges from January to December 2007 (n=4,012,775). All California hospitals are represented in OSHPD with the exception of Veteran’s Affairs and Department of Defense hospitals; however, in light of the physical limitations of patients with SCD, who are unlikely to participate in the armed services, the exclusion of these hospitals is unlikely to eliminate a significant proportion of the SCD population from the current study. The ED dataset, which includes all ED visits not resulting in admission to the same hospital, was combined with the portion of the inpatient dataset that includes all ED visits resulting in admission to the same hospital. In addition to administrative and demographic data, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) codes are included for a primary and up to 24 secondary diagnoses. Record linkage numbers identify visit-level data, allowing separate visits by one patient to be linked. This research was approved by the Committee for Protection of Human Subjects for the California Health and Human Services Agency. The Children’s Hospital Los Angeles Committee on Clinical Investigations also approved this study.
Data selection
First, patients were selected from original data based on an ICD-9 code reflecting any SCD variant (282.60–282.69 and 282.41–282.42) in the primary or any secondary diagnoses. Visits coded with only sickle-cell trait (282.5) were excluded. Second, record linkage numbers corresponding to visits obtained in the first step were noted and any additional 2007 ED visits identified by them were added to the data set. Finally, inpatient visits were censored to keep only those for which an ED encounter resulted in admission to the same hospital. This three-step procedure yielded 16,364 visits, encompassing all statewide ED encounters in 2007 from patients with SCD. Eight hundred forty visits (roughly 5% of the sample) did not have record linkage numbers; these visits did not have significantly different characteristics compared to the remainder of the cohort. After removing these visits as well as 54 patients without California zip codes and their corresponding visits, a patient-level data set (n=2,920) was created and demographic information and counts of visits, admissions, and facilities used were aggregated to the patient level.
Visit-level measures
Measures retained from original data included: age in years; sex (male (1) vs. not (0)); ethnicity (Hispanic (1) vs. non-Hispanic (0)); and disposition (admit (1) vs. discharge (0)). Race (American Indian/Alaska Native, Black/African American, White, Asian/Pacific Islander, or “other race”) was combined into a binary measure (Black/African American (1) vs. any other race (0)).
Binary indicators of age (adults ≥ 21 years (1) vs. children < 21 years (0)) and expected source of payment were derived. Insurance categories included public payer (Medicare including Part A, Part B and HMO, Medicaid/Medi-Cal, other government or indigent source, and other source); private payer (HMO, PPO, POS, EPO, automobile insurance, Blue Cross/Blue Shield, worker’s compensation, and commercial insurance); and uninsured. Insurance status was represented in regression analyses by two dummy codes: private payer (1) vs. any other source (0) and uninsured (1) vs. any other source (0).
Patient-level measures
Aggregating demographic information yielded the following measures: Number of ED visits; number of admissions from the ED; number of facilities utilized; mean age; sex; race; ethnicity; geographic residence. All ages with a trailing decimal (i.e., mean age > 20.00) were rounded into the higher age category, as the patient had an ED encounter at the older age during 2007. Finally, the patient’s 5-digit zip code represented the patient’s location of residence in geographic analyses.
Derived measures
To describe individual insurance coverage, the percentage of the year that the patient was covered by any category of insurance (public, private, uninsured) was used as a continuous variable. Thus, if a patient lost eligibility for Title V coverage during the year then this mechanism of describing insurance status would reflect both sources proportionally.
To describe multiple facility utilization, a categorical indicator was created (0 if the patient had only 1 visit; 1 if the patient had multiple visits to a single facility; 2 if the patient had multiple visits to >1 facility). Additionally, a binary indicator was created to designate whether a patient was a “high frequency utilizer” (HFU) in the top 5% of ED users in the cohort (1 if in top user tier; 0 otherwise).
Using the 2000 US Census data, a measure of urbanicity was allocated to each patient based on zip code. Zip codes were assigned a continuous variable representing the percentage of urban dwellings.
To distinguish patients with more serious disease manifestations, a patient-level binary variable “severe disease” was created based on the presence (1) or absence (0) of any of four diagnoses in at least one visit, including cerebrovascular disease, acute chest, and renal failure (acute and chronic). Using the Healthcare Cost and Utilization Project’s Clinical Classification Software categories,[42] we identified 111 unique ICD-9 codes for these diagnoses. The diagnoses were chosen based on their likelihood in both age groups.[2–3] Unable to use the hallmark definition of three or more crises per year[5] in light of the use of visit rate as an outcome, this measure of disease severity was developed based on expert opinion and prior evidence.[2]
A measure of distance to the nearest self-identified provider of comprehensive SCD care (NSC) was derived. Without physician data, it was necessary to develop a de novo list of providers who self identified as providing comprehensive SCD care. First, addresses were compiled of all hematologists who self-identified in the American Society of Hematology Find a Hematologist database as interested in caring for patients with SCD.[43] Second, social networking was used, as in safety net provider studies,[44] to further derive a list of providers who claim to provide comprehensive SCD care. Expert opinion identified fifteen academic centers in the state with large SCD patient rosters treated by academic hematologists active at the regional or national level; stemming from contact with these centers, a list was developed of 45 providers (and what age group is seen) who self-identify as providing comprehensive SCD care. Next, this list was added to the American Society of Hematology list to create the NSC list. Geographic Information Systems software (MapInfo Professional v. 10.0, Pitney Bowes Business Insight, 2009) interpreted street addresses for all 62 locations into geographic coordinates; with street addresses unavailable for the cohort patients, the centroid of the patient’s zip code was used as a proxy for MapInfo to measure the straight-line distance, in miles, from each patient’s residence to the nearest address on the NSC list providing age-appropriate care. This measurement, used as a continuous variable, served as the ultimate distance to care measure.
ED utilization itself was operationalized by evaluating both the ED visit rate[6] and the number of facilities used.[35] Historically, a patient subpopulation is responsible for a substantial proportion of health care utilization;[5,8–9,35,39] thus, presence in the highest (i.e., most frequent) tier of ED users was used to further denote ED utilization.
Analysis
Statistical analyses were performed using SPSS. Primary predictors of interest include age[6,8–9,45], insurance status[9,46–47] and distance to the nearest self-identified provider of comprehensive SCD care.[9,20–21] Additional influential variables include disease severity,[36] urbanicity,[12,31] gender,[6,48] race, and ethnicity*.[1,2] Logistic regression was used to examine disposition from the ED and likelihood of being in the HFU group. Poisson regression was used to evaluate ED visit rate. Finally, multiple facility use was evaluated using multinomial logistic regression with patients making multiple visits to one facility as the reference group.
Results
Demographic and utilization summaries of the patient cohort and visit sample are presented in Table I. Of note, 5% of the sample (n = 154) was responsible for 40% of the visits. The insurance status profile is represented in Table II, and frequency of “severe disease” is presented in Table III. A small proportion of visits (9.28%) and larger proportion of the patient cohort (26.34%) was associated with severe disease. Patients lived an average of 14 miles (range 0.06 to 146.81, SD 19.42) from NSCs, with a modest amount of movement between zip codes. Figure 1 maps patient density per zip code, highlighting patient ‘hot spots.’ Figure 2 compares the densities of patients with NSC locations.
Table I.
Patient- and visit-level descriptive statistics (total N = 2,920 identified patients; 16,364 visits.)
| Variable | Range | Patient Median (IQR) | Visit Median (IQR) |
|---|---|---|---|
| Age (years) | 0 to 96 | 28.50 (23) | 30 (19) |
| Number of Visits | 1 to 311 | 2 (4) | ----- |
| Number of Admissions | 0 to 34 | 1 (2) | 5758 (35.2) a |
| Number of Facilities Utilized | 1 to 37 | 1 (1) | ----- |
| Urbanicityb | 0.00 to 1.00 | 1.00 (0.01) | 1.00 (0.004) |
| Distance to Comprehensive | 0.06 to 146.81 | 6.33 (9.45) | 6.00 (10.01) |
| Care (mi.) | |||
|
| |||
| Variable | Categories | Patient N (%) | Visit N (%) |
|
| |||
| Age (years) | Child (0–20) | 833 (29) | 2940 (18) |
| 0–10 | 345 (12) | 1176 (7.2) | |
| 11–20 | 488 (17) | 1764 (10.8) | |
| Adult (21+) | 2087 (72) | 13424 (82) | |
| 21–30 | 721 (25) | 5412 (33.1) | |
| 31–40 | 560 (19) | 3320 (20.3) | |
| 41–50 | 466 (16) | 3133 (19.1) | |
| 51+ | 340 (12) | 1559 (9.5) | |
| Sex | Male | 1213 (42) | 6828 (41.7) |
| Female | 1696 (58) | 9536 (58.3) | |
| Race | Black/African American | 2354 (81) | 14481 (88.5) |
| Other | 566 (19) | 1667 (10.2) | |
| Ethnicity | Hispanic | 230 (8) | 969 (5.9) |
| Non-Hispanic | 2690 (92) | 14917 (91.2) | |
| Geographic Residence | Los Angeles County (LAC) | 1213 (42) | 6351 (38.8) |
| Southern California County (not LAC)c | 1696 (58) | 3345 (20.4) | |
| Northern Californiad | 1075 (37) | 6664 (40.7) | |
| Movement Between Zip Codes | Changed zip code | 613 (21) | ----- |
| Did not change zip code | 2307 (79) | ----- | |
| Distance to Comprehensive Care | Live within 12 miles | 2141 (73) | 12040 (73.6) |
| Live within 25 miles | 2563 (88) | 14206 (86.8) | |
| Multiple Utilization | Utilized > 1 Hospital Facility | 1016 (35) | ----- |
| Utilized 1 Hospital Facility | 1904 (65) | ----- | |
| Only one visit | 886 (30) | ----- | |
| Multiple Visits to Same Facility | 1018 (35) | ----- | |
Visit level number of admissions in total frequency and percent.
Urbanicity is defined as the proportion of housing units per zip code that are urban housing units.
Southern California (not LAC) Counties: San Diego, Orange, Riverside, San Bernardino, Ventura, Kern, Santa Barbara, San Luis Obispo and Imperial
Northern California: Remaining 48 counties
Table II.
Insurance status of SCD patients and visits in 2007
| Insurance Status | Mean (SD)a | N (%) At Least One Visitb | N (%) of Visitsc |
|---|---|---|---|
| Public Insurance | 0.62 (0.46) | 1989 (68) | 11831 (72.3) |
| Private Insurance | 0.25 (0.41) | 876 (30) | 2892 (17.7) |
| Uninsured | 0.13 (0.30) | 560 (19) | 1640 (10) |
For each patient, the percentage of that patient’s ED visits covered by public, private, or no insurance was calculated; means in this column represent the average across patients of the percentage of visits with the corresponding insurance status.
Frequencies (percentages) in this column represent the number of patients who had at least one ED visit in 2007 with the corresponding insurance status.
Frequencies (percentages) in this column represent the number of total visits made to the ED in 2007 by patients with each corresponding insurance status.
Table III.
Frequency of Severe Disease
| Disease | N (%) |
|---|---|
| Visit Level (N = 16364) | |
| Stroke diagnosis | 51(0.31) |
| Acute Chest/Pneumonia diagnosis | 907 (5.54) |
| Acute or Chronic Renal Failure diagnosis | 674 (4.12) |
| Severe illness: Stroke/ACS/Renal Failure | 1518 (9.28) |
| Person Level (N = 2920) | |
| Stroke diagnosis | 42 (1.44) |
| Acute Chest/Pneumonia diagnosis | 618 (21.16) |
| Acute or Chronic Renal Failure diagnosis | 220 (7.53) |
| Severe illness: Stroke/ACS/Renal Failure | 769 (26.34) |
Figure 1.
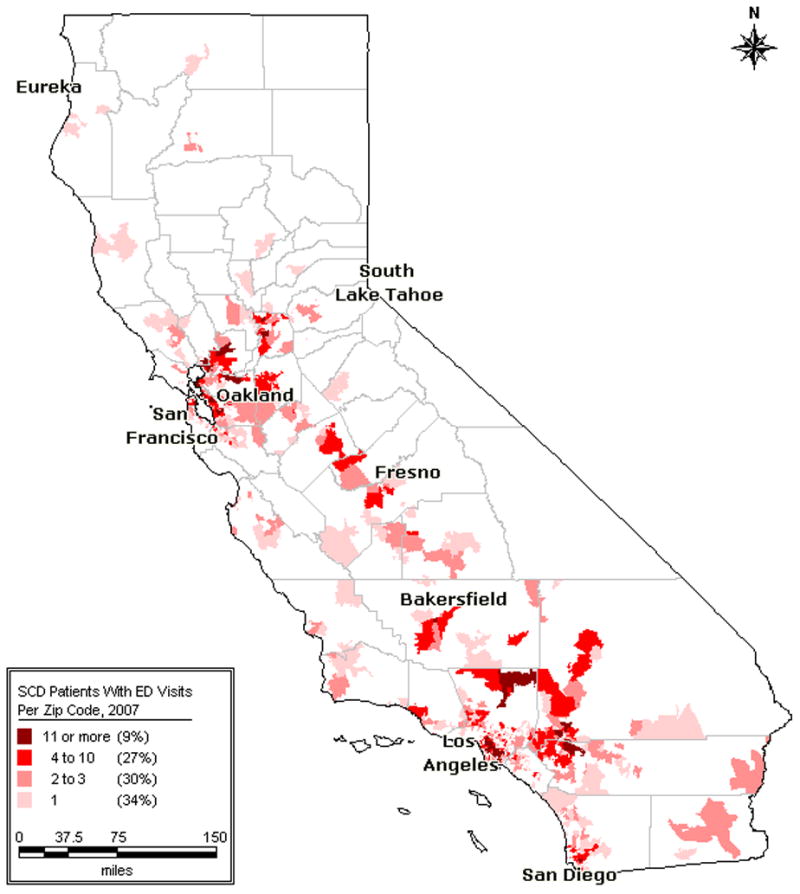
California Patients With SCD: ED Visits Per Zip Code, 2007.
Figure 2.
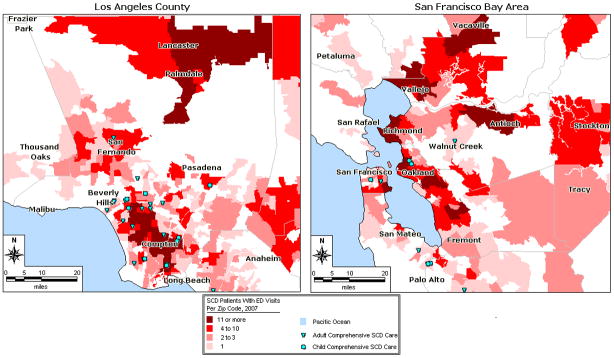
Los Angeles County and San Francisco Bay Area Patients With SCD and Self-Identified Comprehensive Care Sites.
Disposition from the ED
Regression results for disposition, rate of ED visits, and HFU status are presented in Table IV. Patients with more severe disease were more likely to have their ED visit result in hospitalization (OR 13.26, p<0.001, CI[11.38,15.45]). Discharge from the ED was associated with private insurance (OR 0.90, p<0.05, CI[0.82,0.99]) and being uninsured (OR 0.37, p<0.001, CI[0.32,0.43]); being an adult ≥ 21 years (OR 0.68, p<0.001, CI[0.62,0.75]), living further from an NSC (OR 0.91, p<0.001, CI[0.87,0.94]), and living in more urban areas (OR 0.33, p<0.001 CI[0.18,0.58]). Statistically significant race and ethnicity effects will not be described but they are presented in Tables IV and V.
Table IV.
Logistic and Poisson regression results: Disposition, visit rate, and HFU status
| Predictor | Estimate | 95% Confidence Interval | Significance [p-value] |
|---|---|---|---|
| Disposition from the EDa | Odds Ratio | ||
| Adult | 0.68 | [0.62, 0.75] | < 0.01 |
| Private Insurance | 0.90 | [0.82, 0.99] | 0.02 |
| Uninsured | 0.37 | [0.32, 0.43] | < 0.01 |
| Distance to care (mi.) | 0.91 | [0.87, 0.94] | < 0.01 |
| Urbanicity | 0.33 | [0.18, 0.58] | < 0.01 |
| Severe Disease | 13.26 | [11.38, 15.45] | < 0.01 |
| Male | 1.07 | [0.99, 1.15] | 0.06 |
| Black | 1.15 | [0.99, 1.33] | 0.07 |
| Hispanic | 1.43 | [1.19, 1.71] | < 0.01 |
| Rate of ED visitsb | Incidence Rate Ratio | ||
| Adult | 2.32 | [2.22, 2.43] | < 0.01 |
| Private Insurance | 0.59 | [0.56, 0.62] | < 0.01 |
| Uninsured | 0.63 | [0.59, 0.67] | < 0.01 |
| Distance to care (mi.) | 1.04 | [1.02, 1.06] | < 0.01 |
| Urbanicity | 1.34 | [1.01, 1.78] | 0.04 |
| Severe Disease | 1.65 | [1.60, 1.71] | < 0.01 |
| Male | 1.02 | [0.99, 1.06] | 0.16 |
| Black | 0.74 | [0.71, 0.77] | < 0.01 |
| Hispanic | 1.46 | [1.38, 1.54] | < 0.01 |
| High-frequency utilization statusc | Odds Ratio | ||
| Adult | 7.91 | [3.95, 15.85] | < 0.01 |
| Private Insurance | 0.19 | [0.10, 0.37] | < 0.01 |
| Uninsured | 0.44 | [0.23, 0.86] | 0.02 |
| Distance to care (mi.) | 1.17 | [0.98, 1.39] | 0.08 |
| Urbanicity | 1.50 | [0.91, 24.68] | 0.78 |
| Severe Disease | 2.51 | [1.79, 3.53] | < 0.01 |
| Male | 0.99 | [0.71, 1.40] | 0.96 |
| Black | 0.79 | [0.51, 1.24] | < 0.01 |
| Hispanic | 2.26 | [1.33, 3.86] | < 0.01 |
Logistic regression: likelihood of inpatient admission
Poisson regression
Logistic regression: likelihood of being in the top tier of ED users
Table V.
Multiple logistic regression results: Multiple utilization statusa
| Predictor | Odds Ratio | 95% Confidence Interval | Significance [p-value] |
|---|---|---|---|
| Likelihood of making one visit to one facility | |||
| Adult | 0.77 | [0.63, 0.94] | 0.01 |
| Private Insurance | 1.05 | [0.84, 1.31] | 0.67 |
| Uninsured | 2.74 | [1.95, 3.85] | < 0.01 |
| Distance to care (mi.) | 0.91 | [0.82, 1.01] | 0.07 |
| Urbanicity | 0.89 | [0.20, 3.94] | 0.88 |
| Severe Disease | 0.46 | [0.36, 0.58] | < 0.01 |
| Male | 1.11 | [0.92, 1.34] | 0.28 |
| Black | 0.96 | [0.74, 1.26] | 0.77 |
| Hispanic | 0.70 | [0.47, 1.05] | 0.09 |
| Likelihood of making multiple visits to multiple facilities | |||
| Adult | 2.33 | [1.86, 2.92] | < 0.01 |
| Private Insurance | 0.49 | [0.39, 0.63] | < 0.01 |
| Uninsured | 1.43 | [1.01, 2.03] | 0.04 |
| Distance to care (mi.) | 1.04 | [0.94, 1.15] | 0.45 |
| Urbanicity | 2.10 | [0.43, 10.33] | 0.36 |
| Severe Disease | 1.18 | [0.96, 1.44] | 0.11 |
| Male | 0.90 | [0.74, 1.08] | 0.25 |
| Black | 0.67 | [0.52, 0.86] | < 0.01 |
| Hispanic | 0.89 | [0.63, 1.28] | 0.54 |
Multinomial logistic regression: Reference group is patients making multiple visits to one facility
Number of ED Visits
Higher rates of ED visits were seen in adults (IRR 2.32, p<0.001, CI[2.22,2.43]) and patients with severe disease (IRR 1.65, p<0.001, CI[1.60,1.71]). Patients living further from NSCs (IRR 1.04, p<0.001, CI[1.02,1.06]) and in more urban areas (IRR 1.34, p<0.05, CI[1.01,1.78]) also had higher rates of ED visits. Private insurance (IRR 0.59, p<0.001, CI[0.56,0.62]) and being uninsured (IRR 0.63, p<0.001, CI[0.59,0.67]) were associated with lower rates of ED visits.
High-frequency utilizer status
Adulthood (OR 7.91, p<0.001, CI[3.95,15.85]) and disease severity (OR 2.51, p<0.001, CI[1.79,3.53]) were positively associated with likelihood of being in the highest 5% of ED utilizers, i.e., HFU. Having private insurance (OR 0.19, p<0.001, CI[0.10,0.37]) or no insurance (OR 0.44, p<0.05, CI[0.23,0.86]) was associated with a lower likelihood of HFU status. Distance and urbanicity were not statistically significant in predicting HFU.
Multiple utilization status
The likelihood of making only one visit to a single facility rather than multiple visits to that facility was increased among the uninsured (OR 2.74, p<0.001, CI[1.95,3.85]); a greater likelihood of making multiple visits to the same facility was seen among adults (OR 0.77, p<0.05, CI[0.63,0.94]) and those with severe disease (OR 0.46, p<0.01, CI[0.36,0.58]). Furthermore, the likelihood of making multiple visits to multiple different facilities rather than to only one facility was higher among adults (OR 2.33, p<.001, CI[1.86,2.92]) and the uninsured (OR 1.43, p<0.05, CI[1.01,2.03]; the likelihood of multiple facility use was decreased among the privately insured (OR 0.49, p<0.01, CI[0.39,0.63]).
Discussion
Using unique California data with linkages between patient and visit across institutions statewide, predictors of ED utilization in SCD were evaluated. Statistically significant predictors of interest included distance to NSC, age, insurance status, disease severity and urbanicity.
Although 88% of patients lived within 25 miles of NSCs, the distance metric is deceptive: it may take over two hours to travel that distance in Los Angeles, or three buses and a subway to travel from a rural home to an urban provider. Patients further from NSCs had higher rates of ED visits with a lower likelihood of inpatient admission from the ED, adjusting for a rural/urban bias.[12,31] Furthermore, near-significant relationships emerged between living further from NSC and being a HFU visiting multiple facilities. Tennessee claims data have previously shown more hospitalizations and fewer outpatient visits in the region closest to the SCD center with no difference in ED utilization by region or age.[21] Representation of multiple payers may explain these differences; additionally, the distance measure differed greatly in the current study as a continuous variable was used, and care was defined not as the region with one federally-funded sickle cell center, but rather the nearest self-identified provider. No studies using distance as a continuous measurement have been published in SCD utilization; in the present study, distance represents a likely barrier to care.
Disease severity was associated with higher rates of ED visits as well as higher likelihood of being HFUs, making multiple visits and being admitted from the ED, and a lower likelihood of visiting multiple facilities. The infrequent identification of stroke as compared to renal failure or acute chest highlights the limitations of this method, as acute events in prior years are missed with only one year of data with which to develop the model. However, the pattern identified is consistent with both SCD and general ED population patterns,[27,47] affirming the ability of this novel method to adjust for some bias conferred by disease severity in the model.
Adulthood ≥ 21 years of age and reliance on public insurance was predictive of higher ED visit rates and HFU status. When making multiple visits in a year, adults were also more likely to visit multiple different facilities. This utilization pattern that emerges according to both age and reliance on public insurance is concerning for the insufficiency of public coverage for adults. In contrast to adults, the uninsured, and the publicly insured who are ‘shopping around’ at multiple facilities, children and privately insured patients tended to utilize one facility. This is also consistent with the premise that uninsured and publicly insured patients, especially adults, lack a consistent provider and access to comprehensive SCD care. The cohesiveness and continuity of both public pediatric comprehensive coverage and private disease-specific coverage may explain this; public coverage for adults may be complicated by discontinuity.[10]
One explanation for the age- and coverage-biased utilization pattern presented is default use of the ED in the wake of insufficient coverage for comprehensive SCD care. Though California is one of only two states to cover adults with genetic diseases including SCD (twelve cover cystic fibrosis; eight cover hemophilia[49]), very few patients with SCD in the state are enrolled (personal communication, GHPP). Alternate explanations for this utilization pattern include adults engaging in less self-care, more high-risk behavior, and being more seriously ill. However, the access to care premise is supported by adjustment of disease severity in the model and by adults’ lower likelihood of inpatient admission from the ED; the latter is consistent with the notion that EDs are used for indications that could be triaged to a clinic or day hospital.[50–51] To this end, several provisions of the 2010 Patient Protection and Affordable Care Act (PPACA) aim to improve access and affordability for this population by enacting prohibitions of lifetime limits on coverage, rescinding coverage, and excluding coverage for pre-existing conditions; limitations on the ways in which premiums can vary; and expansion of Medicaid eligibility criteria along with the federal funding to support it. Furthermore, the PPACA’s promotion of medical homes will be key to this population. Despite the applicability of PPACA preventive care measures to patients with SCD, a comprehensive SCD evaluation should also include many additional procedures such as echocardiograms and retinal examinations. Although the optimal solution would be for plans to include adult SCD comprehensive care in their networks, currently there is a dearth of specialized providers providing care to adults with SCD, such that it is unlikely that every patient will be able to receive care within network at a center. If cost-sharing is imposed when these services are delivered out-of-network, there is a risk that the appropriate care will not be delivered.
Although the present study represents a broader patient population than single-institution, single-payer, or multiple-institution studies of patients connected to care, administrative data have several significant limitations. This study is limited to patients presenting for ED care, although 34% of children and 12% of 18- to 30-year-olds with SCD may not visit an ED in any given year.[45] Despite common use as the best available source of clinical information from administrative data,[52] concerns about the validity of ICD-9 codes in ED data have been raised.[52–54] Data such as OSHPD necessitate numerous sites and modes of collection, leading to inevitable reporting inconsistencies. Furthermore, limitations in race and ethnicity accuracy include single historically-defined categories and inconsistencies in self-identification (identification that may be performed by administrative hospital employees). Finally, twelve months of data may omit patients admitted at either end of the year. Nevertheless, these data present a unique SCD cohort, with identifiers connecting each visit to an individual patient across all facilities in the state. This population-level access to a heterogeneous cohort with little travel across state borders for health care facilitates novel analyses of geography, utilization and insurance status.
In summary, predictors associated with higher ED utilization in California patients with SCD include age ≥ 21 years, distance from NSC, insurance status, urbanicity and disease severity. As a routine source of care both in SCD[28,55] and non-SCD populations[56] decreases ED utilization, such utilization patterns are consistent with the premise that these factors act as barriers to accessing comprehensive SCD care. Guidelines for preventive comprehensive SCD care were endorsed by the National Institutes of Health[57]; these include annual hematologist visits and screening procedures to stem the tide of severe disease that leads to premature death. Findings here prompt concern that such quality care is not being uniformly accessed. Further population-level investigation into outpatient utilization and delivery of care in SCD is needed to explore the access to care provided according to evidence-based guidelines.
Acknowledgments
This project was supported by the Los Angeles Basin Clinical and Translational Science Institute. The California Office of Statewide Health Planning and Development provided the data to Children’s Hospital Los Angeles. The authors would like to thank Joyce A. Sutjeda for her contributions to the data preparation. The authors would like to thank David Zingmond, MD, PhD and Ninez Ponce, PhD for their contributions to the study design.
Footnotes
Economic status, operationalized as median household income by zip code, was not a significant predictor of any outcome.
Conflict of Interest Statement
The authors have no relevant conflicts of interest to disclose.
References
- 1.Michlitsch J, Azimi M, Hoppe C, et al. Newborn screening for hemoglobinopathies in California. Pediatr Blood Cancer. 2009;52(4):486–490. doi: 10.1002/pbc.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. American Journal of Hematology. 2010;85(1):6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orkin SH, Nathan DG, Ginsburg D, et al., editors. Nathan and Oski’s Hematology of Infancy and Childhood. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 4.Panepinto JA, Brousseau DC, Hillery CA, et al. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44(2):182–186. doi: 10.1002/pbc.20180. [DOI] [PubMed] [Google Scholar]
- 5.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 6.Shankar SM, Arbogast PG, Mitchel E, et al. Medical care utilization and mortality in sickle cell disease: a population-based study. Am J Hematol. 2005;80(4):262–270. doi: 10.1002/ajh.20485. [DOI] [PubMed] [Google Scholar]
- 7.Bilenker JH, Weller WE, Shaffer TJ, et al. The costs of children with sickle cell anemia: preparing for managed care. J Pediatr Hematol Oncol. 1998;20(6):528–533. doi: 10.1097/00043426-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Epstein K, Yuen E, Riggio JM, et al. Utilization of the office, hospital and emergency department for adult sickle cell patients: a five-year study. J Natl Med Assoc. 2006;98(7):1109–1113. [PMC free article] [PubMed] [Google Scholar]
- 9.Hand R, Koshy M, Dorn L, et al. Health insurance status and the use of emergency and other outpatient services by adults with sickle cell disease. Ann Emerg Med. 1995;25(2):224–229. doi: 10.1016/s0196-0644(95)70328-4. [DOI] [PubMed] [Google Scholar]
- 10.Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323–327. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 11.Raphael JL, Dietrich CL, Whitmire D, et al. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatr Blood Cancer. 2009;52(2):263–267. doi: 10.1002/pbc.21781. [DOI] [PubMed] [Google Scholar]
- 12.Telfair J, Haque A, Etienne M, et al. Rural/urban differences in access to and utilization of services among people in Alabama with sickle cell disease. Public Health Rep. 2003;118(1):27–36. doi: 10.1093/phr/118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38(4 Suppl):S536–541. doi: 10.1016/j.amepre.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagar RW, Vichinsky EP. Major changes in sickle cell disease. Adv Pediatr. 2000;47:249–272. [PubMed] [Google Scholar]
- 15.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 17.Vichinsky E, Hurst D, Earles A, et al. Newborn screening for sickle cell disease: effect on mortality. Pediatrics. 1988;81(6):749–755. [PubMed] [Google Scholar]
- 18.Quinn CT, Rogers ZR, McCavit TL, et al. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medi-Cal Policy Institute: A Project of the California HealthCare Foundation. 2000. Medi-Cal Facts: California Children’s Services & Medi-Cal. Report. [Google Scholar]
- 20.Nietert PJ, Abboud MR, Zoller JS, et al. Costs, charges, and reimbursements for persons with sickle cell disease. J Pediatr Hematol Oncol. 1999;21(5):389–396. doi: 10.1097/00043426-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Shankar SM, Arbogast PG, Mitchel E, et al. Impact of proximity to comprehensive sickle cell center on utilization of healthcare services among children with sickle cell disease. Pediatr Blood Cancer. 2008;50(1):66–71. doi: 10.1002/pbc.21066. [DOI] [PubMed] [Google Scholar]
- 22.Davis H, Gergen PJ, Moore RM., Jr Geographic differences in mortality of young children with sickle cell disease in the United States. Public Health Rep. 1997;112(1):52–58. [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein AM, Ayanian JZ. Racial disparities in medical care. N Engl J Med. 2001;344(19):1471–1473. doi: 10.1056/NEJM200105103441911. [DOI] [PubMed] [Google Scholar]
- 24.Frei-Jones MJ, Baxter AL, Rogers ZR, et al. Vaso-occlusive episodes in older children with sickle cell disease: emergency department management and pain assessment. J Pediatr. 2008;152(2):281–285. doi: 10.1016/j.jpeds.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshy M, Leikin J, Dorn L, et al. Evaluation and Management of Sickle Cell Disease in the Emergency Department (An 18-year Experience): 1974–1992. Am J Ther. 1994;1(4):309–320. doi: 10.1097/00045391-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Loureiro MM, Rozenfeld S, Sa Carvalho M, et al. Factors associated with hospital readmission in sickle cell disease. BMC Blood Disord. 2009;9:2. doi: 10.1186/1471-2326-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer ML, Konrad TR, Dvorak CC. Hospital resource utilization among patients with sickle cell disease. J Health Care Poor Underserved. 2003;14(1):122–135. [PubMed] [Google Scholar]
- 28.Okpala I, Thomas V, Westerdale N, et al. The comprehensiveness care of sickle cell disease. Eur J Haematol. 2002;68(3):157–162. doi: 10.1034/j.1600-0609.2002.01523.x. [DOI] [PubMed] [Google Scholar]
- 29.Teach SJ, Lillis KA, Grossi M. Compliance with penicillin prophylaxis in patients with sickle cell disease. Arch Pediatr Adolesc Med. 1998;152(3):274–278. doi: 10.1001/archpedi.152.3.274. [DOI] [PubMed] [Google Scholar]
- 30.Yang YM, Shah AK, Watson M, et al. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Health Rep. 1995;110(1):80–86. [PMC free article] [PubMed] [Google Scholar]
- 31.Haque A, Telfair J. Socioeconomic distress and health status: the urban-rural dichotomy of services utilization for people with sickle cell disorder in North Carolina. J Rural Health. 2000;16(1):43–55. doi: 10.1111/j.1748-0361.2000.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 32.Davis H, Moore RM, Jr, Gergen PJ. Cost of hospitalizations associated with sickle cell disease in the United States. Public Health Rep. 1997;112(1):40–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Lanzkron S, Haywood C, Jr, Segal JB, et al. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81(12):927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 34.Strouse JJ, Jordan LC, Lanzkron S, et al. The excess burden of stroke in hospitalized adults with sickle cell disease. Am J Hematol. 2009;84(9):548–552. doi: 10.1002/ajh.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods K, Karrison T, Koshy M, et al. Hospital utilization patterns and costs for adult sickle cell patients in Illinois. Public Health Rep. 1997;112(1):44–51. [PMC free article] [PubMed] [Google Scholar]
- 36.Ellison AM, Bauchner H. Socioeconomic status and length of hospital stay in children with vaso-occlusive crises of sickle cell disease. J Natl Med Assoc. 2007;99(3):192–196. [PMC free article] [PubMed] [Google Scholar]
- 37.Machlin S, Cohen J, Elixhauser A, et al. Sensitivity of household reported medical conditions in the medical expenditure panel survey. Med Care. 2009;47(6):618–625. doi: 10.1097/MLR.0b013e318195fa79. [DOI] [PubMed] [Google Scholar]
- 38.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5(3):143–151. [PubMed] [Google Scholar]
- 39.Carroll CP, Haywood C, Jr, Fagan P, et al. The course and correlates of high hospital utilization in sickle cell disease: Evidence from a large, urban Medicaid managed care organization. Am J Hematol. 2009;84(10):666–670. doi: 10.1002/ajh.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhlthau K, Ferris TG, Beal AC, et al. Who cares for medicaid-enrolled children with chronic conditions? Pediatrics. 2001;108(4):906–912. doi: 10.1542/peds.108.4.906. [DOI] [PubMed] [Google Scholar]
- 41.Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057–1061. doi: 10.1001/jama.290.8.1057. [DOI] [PubMed] [Google Scholar]
- 42.HCUP CCS. Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. Rockville, MD: Agency for Healthcare Research and Quality; [Accessed December, 2009]. < www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. >. [Google Scholar]
- 43.American Society of Hematology. [Accessed 2009 September 15.];American Society of Hematology: Find a Hematologist. 2008 Sep 15; < http://www.bloodthevitalconnection.org/find-a-hematologist.aspx>.
- 44.Ponce N. Personal communication. 2009 nponce@ucla.edu. [Google Scholar]
- 45.Brousseau DC, Owens PL, Mosso AL, et al. Acute Care Utilization and Rehospitalizations for Sickle Cell Disease. JAMA. 2010;303(13):1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 46.Brousseau D, Panepinto JA, Owens P, et al. Acute Care Visits in Sickle Cell Disease: a Population-Based Multi- State Study. ASH Annual Meeting Abstracts. 2008;112(11):165. [Google Scholar]
- 47.Zuckerman S, Shen YC. Characteristics of occasional and frequent emergency department users: do insurance coverage and access to care matter? Med Care. 2004;42(2):176–182. doi: 10.1097/01.mlr.0000108747.51198.41. [DOI] [PubMed] [Google Scholar]
- 48.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 49.Grosse SD, Schechter MS, Kulkarni R, et al. Models of Comprehensive Multidisciplinary Care for Individuals in the United States With Genetic Disorders. Pediatrics. 2009;123(1):407–412. doi: 10.1542/peds.2007-2875. [DOI] [PubMed] [Google Scholar]
- 50.Benjamin LJ, Swinson GI, Nagel RL. Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood. 2000;95(4):1130–1136. [PubMed] [Google Scholar]
- 51.Raphael JL, Kamdar A, Beavers MB, et al. Treatment of uncomplicated vaso-occlusive crises in children with sickle cell disease in a day hospital. Pediatric Blood & Cancer. 2008;51(1):82–85. doi: 10.1002/pbc.21483. [DOI] [PubMed] [Google Scholar]
- 52.Iezzoni LI. Assessing Quality Using Administrative Data. Annals of Internal Medicine. 1997;127(8 Part 2):666–674. doi: 10.7326/0003-4819-127-8_part_2-199710151-00048. [DOI] [PubMed] [Google Scholar]
- 53.Gorelick MH, Knight S, Alessandrini EA, et al. Lack of agreement in pediatric emergency department discharge diagnoses from clinical and administrative data sources. Acad Emerg Med. 2007;14(7):646–652. doi: 10.1197/j.aem.2007.03.1357. [DOI] [PubMed] [Google Scholar]
- 54.Kahn LH, Blustein J, Arons RR, et al. The validity of hospital administrative data in monitoring variations in breast cancer surgery. Am J Public Health. 1996;86(2):243–245. doi: 10.2105/ajph.86.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahimy MC, Fanou L, Somasse YE, et al. When to start supplementary iron to prevent iron deficiency in early childhood in sub-Saharan Africa setting. Pediatr Blood Cancer. 2007;48(5):544–549. doi: 10.1002/pbc.21103. [DOI] [PubMed] [Google Scholar]
- 56.Ryan S, Riley A, Kang M, et al. The Effects of Regular Source of Care and Health Need on Medical Care Use Among Rural Adolescents. Arch Pediatr Adolesc Med. 2001;155(2):184–190. doi: 10.1001/archpedi.155.2.184. [DOI] [PubMed] [Google Scholar]
- 57.NIH. NIH Publication No. 02-2117. Bethesda, MD: National Heart Lung and Blood Institute; 2002. The management of sickle cell disease. Report. [Google Scholar]


