Abstract
The mechanism(s) that increase retinal and visual cortex blood flows in response to visual stimulation are poorly understood. We tested the hypothesis that increased transfer of electrons and protons from glucose to cytosolic free NAD+, reducing it to NADH, evoked by increased energy metabolism, fuels redox-signaling pathways that augment flow. The near-equilibrium between free cytosolic NADH/NAD+ and lactate/pyruvate ratios established by lactate dehydrogenase predicts that transfer of additional electrons and protons from injected lactate to NAD+ will augment the elevated blood flows in stimulated retina and cortex, whereas transfer of electrons and protons from NADH to injected pyruvate will attenuate the elevated flows. These predictions were tested and confirmed in rats. Increased flows evoked by stimulation also were prevented by inhibition of nitric oxide synthase. These findings support an important role for cytosolic free NADH in fueling a signaling cascade that increases •NO production, which augments blood flow in photostimulated retina and visual cortex.
Physiological work, ranging from muscle contraction to neural activity in the brain and phototransduction in the retina, increases energy metabolism and blood flow (1–5). The increase in energy metabolism is explained by increased synthesis of ATP to fuel the work performed. However, the metabolic basis for the increase in blood flow remains enigmatic. Until recently it was widely believed that the increased flow was needed to provide more oxygen and glucose to support increased energy metabolism and to remove the products of energy metabolism that inhibit ATP synthesis. However, increased brain blood flow and glucose utilization evoked by neural activity exceed increased oxygen consumption by as much as 10-fold despite normal or elevated oxygen levels (2, 3). And, enhanced flow is not needed to augment glucose uptake for brief periods of neural activity (6–8).
Observations in rats support the hypothesis that electrons and protons (E&P) carried by free cytosolic NAD (NADc) mediate increased blood flows in contracting skeletal muscle and in stimulated whisker barrel cerebral cortex (1); varying the rates of transfer of E&P to and from free NADc evokes corresponding changes in flow. NAD is the major carrier of E&P from fuels for synthesis of ATP (9). When glucose is the fuel, E&P must be transferred to free 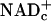 , reducing it to NADHc, before ATP synthesis. ATP is then synthesized in the cytosol by substrate phosphorylation followed by production of pyruvate that is oxidized in mitochondria for ATP synthesis by oxidative phosphorylation. E&P carried by NADHc also are transferred to mitochondrial NAD (
, reducing it to NADHc, before ATP synthesis. ATP is then synthesized in the cytosol by substrate phosphorylation followed by production of pyruvate that is oxidized in mitochondria for ATP synthesis by oxidative phosphorylation. E&P carried by NADHc also are transferred to mitochondrial NAD (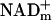 ) via electron shuttles and fuel ATP synthesis by oxidative phosphorylation.
) via electron shuttles and fuel ATP synthesis by oxidative phosphorylation.
The presence of lactate in resting tissues indicates that E&P are transferred to free 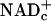 and pyruvate is produced by glycolysis faster than they are used for ATP synthesis by means of oxidative phosphorylation; lactate dehydrogenase (LDH) reoxidizes “excess NADHc” to
and pyruvate is produced by glycolysis faster than they are used for ATP synthesis by means of oxidative phosphorylation; lactate dehydrogenase (LDH) reoxidizes “excess NADHc” to 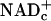 coupled to reduction of “excess pyruvate” to lactate. Elevated lactate levels in working tissues (10–12) attest to increased glycolysis and corresponding increased rates of transfer of E&P to
coupled to reduction of “excess pyruvate” to lactate. Elevated lactate levels in working tissues (10–12) attest to increased glycolysis and corresponding increased rates of transfer of E&P to 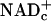 .
.
We propose that the increased rate of transfer of E&P from glucose to 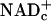 in response to physiological work (and pathological states such as hypoxia and diabetes) exceeds the combined rates of reoxidation of NADHc by electron shuttles and LDH, causing more NADHc to be oxidized by signaling pathways that increase blood flow acutely by vasodilation and chronically by stimulating angiogenesis (1).
in response to physiological work (and pathological states such as hypoxia and diabetes) exceeds the combined rates of reoxidation of NADHc by electron shuttles and LDH, causing more NADHc to be oxidized by signaling pathways that increase blood flow acutely by vasodilation and chronically by stimulating angiogenesis (1).
The aim of these studies was to assess the role of free NADHc in mediating augmented blood flows in retina and visual cortex evoked by visual stimulation.
Methods and Materials
Theoretical Considerations. Free NADHc and 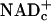 cannot be measured in whole-tissue extracts that also contain enzyme-bound NADHc,
cannot be measured in whole-tissue extracts that also contain enzyme-bound NADHc, 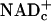 , NADHm, and
, NADHm, and 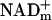 , and free NADHmand
, and free NADHmand 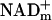 . And, enzyme-bound NAD is 100 times more reduced than free NAD. At the present time, free
. And, enzyme-bound NAD is 100 times more reduced than free NAD. At the present time, free 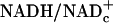 can be estimated only by the redox metabolite indicator method based on the near-equilibria between the ratios of free
can be estimated only by the redox metabolite indicator method based on the near-equilibria between the ratios of free 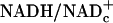 and reduced/oxidized substrates of cytosolic enzymes such as LDH (13).
and reduced/oxidized substrates of cytosolic enzymes such as LDH (13).
The rationale for these experiments is based on the near-equilibrium under steady-state conditions between: (i) free 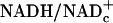 and intracellular lactate/pyruvate (L/P) ratios established by LDH as shown in Eqs. 1 and 2, where KLDH = 1.11 × 10–4 at pH 7.0 (13), and (ii) intracellular and extracellular L/P ratios established by monocarboxylate transporters (14).
and intracellular lactate/pyruvate (L/P) ratios established by LDH as shown in Eqs. 1 and 2, where KLDH = 1.11 × 10–4 at pH 7.0 (13), and (ii) intracellular and extracellular L/P ratios established by monocarboxylate transporters (14).
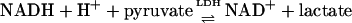 |
[1] |
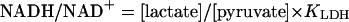 |
[2] |
Because total free NADc (i.e., 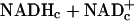 ) is unlikely to change substantially in these brief experiments, and because the ratio of free
) is unlikely to change substantially in these brief experiments, and because the ratio of free 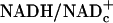 in resting tissues is very low (10–20 × 10–4), changes in free
in resting tissues is very low (10–20 × 10–4), changes in free 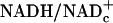 should largely reflect changes in free NADHc. Furthermore, because lactate levels in plasma and in resting tissues are ≈10 times higher than pyruvate and the Km of LDH for pyruvate is lower than for lactate, changes in pyruvate levels have a greater impact on L/P ratio and free
should largely reflect changes in free NADHc. Furthermore, because lactate levels in plasma and in resting tissues are ≈10 times higher than pyruvate and the Km of LDH for pyruvate is lower than for lactate, changes in pyruvate levels have a greater impact on L/P ratio and free 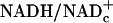 than equimolar changes in lactate.
than equimolar changes in lactate.
Thus, the test hypothesis predicts that (i) injection of l-lactate will increase intracellular lactate, driving oxidation of lactate to pyruvate (Eq. 1) to increase free NADHc and augment blood flow in stimulated retina and visual cortex, and (ii) injection of pyruvate will increase intracellular pyruvate, driving reduction of pyruvate to lactate to decrease free NADHc and attenuate blood flow. (Lactate must be oxidized to pyruvate before it can be further metabolized; pyruvate can be metabolized by multiple enzymes not coupled to oxidation of free NADHc to NAD+.)
Animals. Male Sprague–Dawley rats were used. Housing, care, and all experimental protocols were in conformance with Washington University and National Institutes of Health guidelines.
Experimental Protocols. Preliminary studies were performed to optimize conditions for photostimulation and for injecting lactate and pyruvate to increase or decrease plasma L/P ratios. (Retinal blood flow after 10 min of stimulation was much lower than after 1 min, whereas visual cortex flow remained constant; see Fig. 5, which is published as supporting information on the PNAS web site.) Optimal rates of injection also were determined for NG-nitro-l-arginine methyl ester (l-NAME), a nonselective inhibitor of nitric oxide synthase (NOS) obtained from Sigma–Aldrich, and for SC-52608 [a plasma-soluble Mn2+-caged superoxide dismutase mimic (SODmimic)] provided by Monsanto.
Eyes were stimulated unilaterally or bilaterally at 7.5 Hz for 1 min by checkerboard pattern reversal of 8 × 8-mm black-and-white checks at a spatial period of ≈14° by using a Visual Electrodiagnostic Stimulator model 2510 with a screen height of 25.4 cm and width of 30.5 cm (LKC Diagnostics, Gaithersburg, MD). The light intensity of the pattern (and of white background viewed for 15 min before stimulation) was 1,050 lux. Bilateral stimulation was achieved by positioning a mirror to reflect the pattern on the monitor to the other eye.
Unilateral stimulation was performed for paired comparison of effects of lactate and pyruvate injections on blood flow in stimulated versus contralateral unstimulated retina and visual cortex. Bilateral stimulation was performed in experiments in which one retina was used for measurement of lactate and pyruvate and the other retina was used for assessment of blood flow. Blood flow was quantified by the reference sample microsphere method by using 125I-labeled desmethylimipramine, a plasma-soluble tracer (Mr = 266) (15).
Rats were anesthetized with urethane, and both iliac arteries and femoral veins were cannulated. One arterial cannula was used to monitor blood pressure and obtain blood samples for measurement of metabolites; the other was attached to a constant-flow withdrawal pump to obtain the reference blood sample for measurement of blood flow. One femoral vein was used for injection of 125I-desmethylimipramine and the other was used for injection of test substances.
125I-desmethylimipramine was injected, and the withdrawal pump was started after 1 min of stimulation. One minute later the great vessels were severed, the eyes were enucleated, and retinas were removed for assessment of blood flow and metabolites. The skull was then opened for removal of the left and right visual cortex (≈3 mm in diameter and 1 mm thick) for assessment of blood flow.
Lactate and pyruvate, 1 or 2 mmol/kg of body weight, were given as bolus injections (for 15–20 s) 1 min before stimulation or as a constant infusion beginning 2.5 min before stimulation and continuing until tissues were sampled (≈5 min total duration). Isovolumic injections/infusions of normal saline were given to control rats. l-NAME (Mr = 270) was infused at a rate of 0.5 μmol/kg of body weight per min starting 20 min before tissue sampling. SC-52608 (Mr = 341) was infused at a rate of 0.6 μmol/kg of body weight per min starting 20 min before tissue sampling.
Lactate, pyruvate, and glucose were measured in extracts of arterial blood and retina by standard enzymatic methods (16). Lactate and pyruvate levels are reported as millimoles per liter of plasma and of retinal water. Retinal free 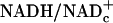 was calculated by using Eq. 2. (Visual cortex lactate and pyruvate levels and ratios are not reported, because we were unable to obtain reproducible values for these metabolites in control rats.)
was calculated by using Eq. 2. (Visual cortex lactate and pyruvate levels and ratios are not reported, because we were unable to obtain reproducible values for these metabolites in control rats.)
Differences in parameters between animals were analyzed with statistical analysis software sas (SAS Institute, Cary, NC), significance set at P < 0.05 (15). Differences in parameters in the same rat were assessed by the two-tailed paired t test. Single- and multiple-factor regression analyses were performed to assess the relative contributions of changes in L/P ratios and lactate and pyruvate levels in regulation of blood flow in retina and visual cortex.
Results
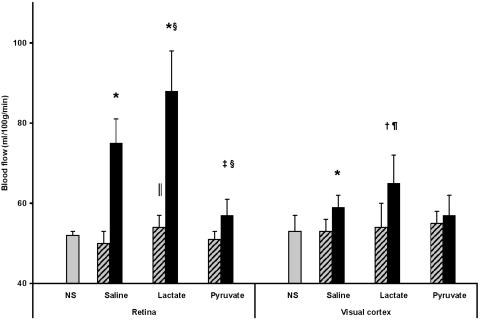
Bolus Lactate and Pyruvate Injection During Unilateral Stimulation. Unilateral stimulation for 1 min increased blood flow 52% in retina and 11% in visual cortex vs. the unstimulated side (Fig. 1). Bolus injection of lactate augmented mean blood flow an additional 18% in retina and 10% in visual cortex. After lactate injection, the difference in flow in the stimulated vs. the unstimulated side was increased 35% in retina and 85% in visual cortex. Lactate injection increased blood flow in unstimulated retina by 8.4% (P = 0.004) but did not affect flow in unstimulated visual cortex.
Fig. 1.
Effects of bolus injections of 1 mmol of pyruvate or lactate per kg of body weight on blood flow in photostimulated (black bars) and contralateral unstimulated (striped bars) retina and visual cortex. NS, control (neither eye stimulated) saline-injected rats; n = 6 –9 rats per group. Values different from unstimulated retina and visual cortex (two-tailed, paired t test): *, P < 0.0001; †, P < 0.004; ‡, P < 0.03. Values different from photostimulated saline-injected rats: §, P < 0.004; and ¶, P = 0.016. Values different from NS saline-injected control rats: ∥, P = 0.004.
Pyruvate injection prevented the increased flow in stimulated visual cortex and attenuated the increased flow in stimulated retina (from 52%) to 12% (P < 0.03 vs. the unstimulated side) (Fig. 1). Pyruvate injection did not affect blood flow in unstimulated retina or visual cortex.
Continuous Lactate and Pyruvate Infusion During Bilateral Stimulation. Bilateral stimulation increased blood flow 44% in retina and 16% in visual cortex in saline-infused controls (Table 1); stimulation did not affect retinal (or plasma) L/P ratios, lactate levels, or pyruvate levels.
Table 1. Effects of infusion of lactate and pyruvate (1 or 2 mmol/kg of body weight) before and during bilateral photostimulation.
| Plasma
|
Retina
|
Visual cortex
|
||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Lactate, mmol/liter | Pyruvate, mmol/liter | L/P ratio | Lactate, mmol/liter | Pyruvate, mmol/liter | L/P ratio | Blood flow, ml·100 g-1·min-1 | Blood flow, ml·100 g-1·min-1 |
| Unstimulated | 1.2 ± 0.4 | 0.14 ± 0.05 | 9.8 ± 2.5 | 7.8 ± 2.2 | 0.55 ± 0.11 | 14.2 ± 1.3 | 52.0 ± 1.0* | 52.6 ± 1.5* |
| PS + saline | 1.2 ± 0.4 | 0.13 ± 0.04 | 10.0 ± 1.9 | 7.0 ± 2.3 | 0.50 ± 0.13 | 13.9 ± 2.7 | 75.4 ± 2.9 | 61.0 ± 1.9 |
| PS + lactate | ||||||||
| 1 mmol/kg | 2.6 ± 0.0‡ | 0.22 ± 0.01‡ | 11.6 ± 0.7 | 8.5 ± 2.9 | 0.59 ± 0.14 | 14.3 ± 2.4 | 77.1 ± 5.6 | 61.7 ± 1.1 |
| 2 mmol/kg | 3.5 ± 0.4* | 0.26 ± 0.05† | 13.5 ± 2.1‡ | 16.4 ± 1.9*¶ | 1.06 ± 0.11‡** | 16.3 ± 0.7 | 87.1 ± 3.3*¶ | 63.3 ± 0.5§ |
| PS + pyruvate | ||||||||
| 1 mmol/kg | 1.4 ± 0.4 | 0.62 ± 0.04* | 2.2 ± 0.7* | 9.2 ± 2.3 | 0.71 ± 0.15 | 12.9 ± 1.2 | 65.2 ± 2.6† | 56.6 ± 2.2§ |
| 2 mmol/kg | 3.9 ± 0.4*¶ | 2.90 ± 1.20*¶ | 1.6 ± 0.8* | 7.5 ± 3.1 | 1.10 ± 0.34†∥ | 6.8 ± 1.8*¶ | 53.2 ± 1.1*¶ | 54.8 ± 1.8† |
PS, photostimulated. Values are means ± SD, n = 4 for each group, except n = 3 for PS + lactate (1 mmol). Different from saline-infused PS group: *, P ≤ 0.0001; †, P ≤ 0.001; ‡, P ≤ 0.005; §, P ≤ 0.05. Different from corresponding 1 mmol of lactate or pyruvate: ¶, P ≤ 0.001; ∥, P ≤ 0.02; **, P ≤ 0.04.
Lactate infusion dose-dependently increased (i) lactate levels 3-fold in plasma and >2-fold in retina, (ii) pyruvate levels 2-fold in plasma and retina, and (iii) L/P ratios 35% in plasma. The 17% higher mean L/P ratio in retina was not statistically significant (P = 0.10). Lactate infusion increased blood flow 16% in the retina and 4% in visual cortex (Table 1).
Pyruvate infusion dose-dependently (i) increased pyruvate levels 22-fold in plasma and 2-fold in retina, (ii) increased lactate levels 3-fold in plasma (but did not affect retinal lactate levels), and (iii) decreased L/P ratios 84% in plasma and 51% in retina. The 51% decrease in retinal L/P ratios corresponds to a decrease in 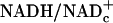 from 15.5 × 10–4 in saline-infused to 7.5 × 10–4 in pyruvate-infused rats. Pyruvate infusion completely prevented the increased blood flows in stimulated retina and visual cortex (P = 0.344 and P = 0.179 vs. nonstimulated controls, respectively).
from 15.5 × 10–4 in saline-infused to 7.5 × 10–4 in pyruvate-infused rats. Pyruvate infusion completely prevented the increased blood flows in stimulated retina and visual cortex (P = 0.344 and P = 0.179 vs. nonstimulated controls, respectively).
Plasma glucose levels (14 ± 2 mM in saline-injected rats) were unaffected by lactate or pyruvate infusion (data not shown).
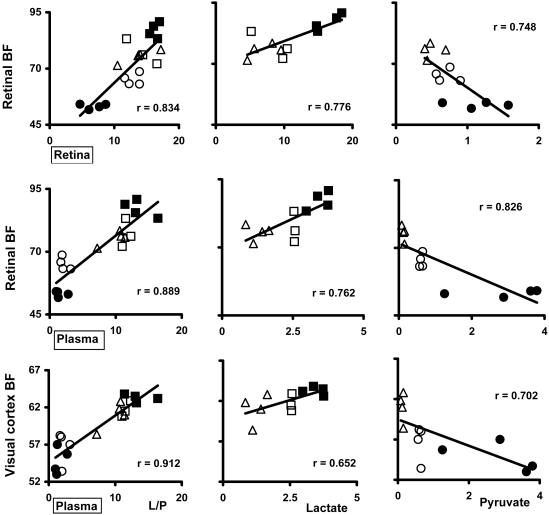
Correlations Between Blood Flows and Plasma and Retinal Lactate, Pyruvate, and L/P Ratios During Bilateral Stimulation. Blood flows in bilaterally stimulated retina and visual cortex correlated positively with plasma L/P ratios (P < 0.0001 for both; see Fig. 2 for r values not shown in text) in all animals infused with saline, lactate, and pyruvate. Retinal blood flows also correlated positively with retinal L/P ratios (P < 0.0001); and retinal and visual cortex blood flows were positively correlated (r = 0.881, P < 0.0001).
Fig. 2.
Correlations (r) between retinal and plasma L/P ratio (and lactate and pyruvate levels) and retinal and visual cortex blood flow. Lactate and pyruvate, 1 and 2 mmol/kg of body weight, were given as a constant infusion during a period of 5 min, beginning 2.5 min before photostimulation (1-min duration) and continuing until tissues were sampled. n = 4 for each condition, except n = 3 for 1 mmol of lactate per kg. (Left) Correlations of retinal blood flow with retinal and plasma L/P ratios and of visual cortex blood flow with plasma L/P ratios include all 19 animals. (Center) Correlations of blood flows with retinal and plasma lactate include only saline- and lactate-infused rats (n = 11). (Right) Correlations of blood flows with retinal and plasma pyruvate include only saline- and pyruvate-infused rats (n = 12). ▵, saline-infused controls; ○, 1 mmol of pyruvate per kg; •, 2 mmol of pyruvate per kg; □, 1 mmol of lactate per kg; ▪, 2 mmol of lactate per kg. P < 0.0001 for correlations of blood flows with L/P ratios; P ≤ 0.01 for correlations of blood flows with lactate and pyruvate levels except visual cortex flow with plasma lactate (P = 0.03).
In saline- and lactate-infused rats retinal and visual cortex flows correlated positively with plasma lactate (P = 0.006 and P = 0.03, respectively); and retinal blood flow correlated positively with retinal lactate (P = 0.005; Fig. 2) and with plasma pyruvate (r = 0.655, P = 0.029, data not shown).
In saline- and pyruvate-infused rats retinal and visual cortex flows correlated negatively with plasma pyruvate levels (P = 0.009 and P = 0.011, respectively; Fig. 2). Retinal and visual cortex blood flows also correlated negatively with plasma lactate levels (r = –0.864, P = 0.0003 and r = –0.612, P = 0.034, respectively, data not shown).
Retinal L/P ratios correlated positively with plasma L/P ratios (r = 0.754, P < 0.0001) in all rats (data not shown). In lactate-infused rats, retinal lactate and pyruvate levels correlated positively with plasma lactate (r = 0.801, P = 0.003 and r = 0.817, P = 0.0021, respectively); however, in pyruvate-infused rats, neither retinal pyruvate nor lactate levels correlated with plasma pyruvate levels (r = 0.509, P = 0.09 and r = –0.316, P = 0.317, respectively).
Because injection of lactate or pyruvate increased plasma levels of both lactate and pyruvate but had opposite effects on plasma L/P ratios and blood flow, the independent effects of changes in plasma and retinal pyruvate, lactate, and L/P ratios on retinal blood flow were assessed by multiple regression analysis. The regression of retinal blood flow vs. plasma and retinal pyruvate, lactate, and L/P ratios was significant only for plasma L/P ratios (P = 0.010). The regression of blood flow in stimulated visual cortex vs. plasma L/P ratios, lactate, and pyruvate also was significant only for plasma L/P ratios (P = 0.001).
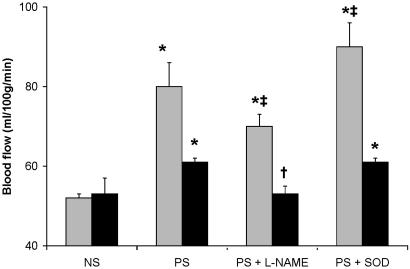
l-NAME. Infusion of l-NAME attenuated the increased blood flow in stimulated retinas by only 36% (Fig. 3). Because l-NAME increased mean arterial blood pressure (from 102 ± 3 to 136 ± 14 mmHg, P < 0.0001), blood flow (milliliters per gram of wet weight per min) was normalized to blood pressure and expressed as conductance (milliliters per gram of wet weight per min per 100 mmHg). The percent increase in conductance was identical with the increase in blood flow; however, it was completely prevented by l-NAME. l-NAME completely prevented increased blood flows in stimulated visual cortex (Fig. 3) and decreased conductance 26% vs. conductance in nonstimulated controls (P < 0.0025).
Fig. 3.
Effects of l-NAME and SODmimic on retinal (shaded bars) and visual cortex (filled bars) blood flow after 1 min of bilateral photostimulation (PS). l-NAME (0.5 μmol/kg of body weight per min) and SODmimic (0.6 μmol/kg of body weight per min) were infused i.v. starting 20 min before and during stimulation. NS, control (neither eye stimulated) saline-injected rats. Values different from NS rats: *, P ≤ 0.001. Values different from PS saline-injected rats: †, P < 0.0001; ‡, P ≤ 0.005.
SODmimic. SODmimic increased retinal blood flow and conductance by 20% but had no effect on visual cortex flow or conductance (Fig. 3). Increasing the dose of SODmimic 2-fold still had no effect on visual cortex blood flow or conductance.
Discussion
Free NADc, the initial acceptor and major carrier of E&P from glucose for ATP synthesis, is well positioned to coordinate blood flow with glucose utilization and energy metabolism in a wide range of physiological and pathological conditions. Rates of transfer of E&P to and from (and/or their accumulation in) free NADc modulate, and are modulated by, rates of energy metabolism and blood flow.
Although glucose is the primary fuel for ATP synthesis in retina and brain, an “astrocyte–neuron lactate shuttle” has been proposed (17, 18) whereby glial cells [primarily astrocytes in brain (18) and Müller cells in retina (19)] metabolize glucose largely by glycolysis to lactate, which diffuses out and is taken up by neurons as the major fuel for mitochondrial ATP synthesis by oxidative phosphorylation. This hypothesis is supported by extensive metabolic studies of isolated glial cells and neurons in vitro, the restricted localization of glycogen to glial cells (19, 20), and the distribution of different LDH and monocarboxylate transporter isoforms in glial cells and neurons in retina and brain.
Other investigators, based on physiological and metabolic considerations including the distribution and kinetics of enzymes involved in energy metabolism and substrate concentrations and gradients, suggest that glucose is more likely to be the major fuel of stimulated neurons in vivo than lactate produced by astrocytes and that ATP synthesis in stimulated astrocytes is not limited to substrate phosphorylation (21–23).
In either case, glucose and lactate must donate E&P to free 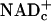 (reducing it to NADHc) before they can fuel ATP synthesis (Fig. 4). Accumulation of lactate in rat cerebral cortex during forepaw stimulation (10) and in visual cortex during photostimulation (11, 12) attests to (i) increased transfer of E&P from glucose to free
(reducing it to NADHc) before they can fuel ATP synthesis (Fig. 4). Accumulation of lactate in rat cerebral cortex during forepaw stimulation (10) and in visual cortex during photostimulation (11, 12) attests to (i) increased transfer of E&P from glucose to free 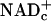 and formation of pyruvate faster than they are used for oxidative phosphorylation by astrocytes and neurons combined, and (ii) increased transfer of E&P from free NADHc to pyruvate by LDH to form lactate.
and formation of pyruvate faster than they are used for oxidative phosphorylation by astrocytes and neurons combined, and (ii) increased transfer of E&P from free NADHc to pyruvate by LDH to form lactate.
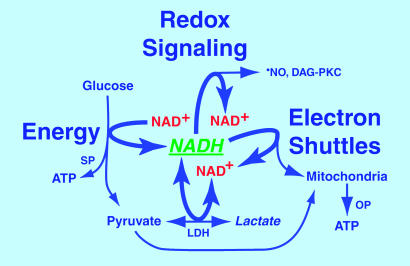
Fig. 4.
The central position of cytosolic free NAD+(H) in energy metabolism and redox signaling. Cytosolic free NAD+(H) is critically positioned to synchronize retinal and visual cortex blood flow with increased glucose utilization and energy metabolism evoked by photostimulation. Increased hydrolysis of ATP to ADP and AMP evoked by stimulation activates phosphofructokinase, which increases metabolism of glucose by means of aerobic glycolysis. Before glucose is used to fuel ATP synthesis, E&P must first be transferred to NAD+, reducing it to NADH. ATP is then synthesized in the cytosol by substrate phosphorylation (SP) followed by production of pyruvate (equimolar to previously formed NADH) for oxidation in mitochondria and synthesis of ATP by oxidative phosphorylation (OP). Even under resting conditions NADH and pyruvate are formed faster than they are used for mitochondrial ATP synthesis by oxidative phosphorylation. LDH reoxidizes “excess NADH” to NAD+ coupled to reduction of “excess pyruvate” to lactate, which diffuses out of the cell. When aerobic glycolysis is increased during work, lactate is formed faster than it can be removed by ambient blood flow. Because rising levels of lactate restrain transfer of E&P to pyruvate by LDH, more E&P carried by NADH are transferred (i.e., “shunted”) to oxidized substrates of enzymes in metabolic “signaling”' pathways that reoxidize NADH and increase production of •NO by cNOS to augment flow. Excess NADH also augments de novo synthesis of diacylglycerol and activation of protein kinase C (DAG-PKC)-mediated signaling pathways. Augmented flows remove lactate and deliver more pyruvate, promoting reoxidation of NADH by LDH. (Rapid reoxidation of NADH to NAD+ is vital to maintain maximal rates of ATP synthesis by substrate phosphorylation. See Supporting Text.) Injection of lactate increases intracellular lactate levels in stimulated tissues, promoting reduction of NAD+ to NADH by LDH and subsequent oxidation of NADH by signaling pathways. In contrast, injection of pyruvate increases intracellular pyruvate levels, promoting reoxidation of NADH by LDH (rather than by signaling pathways) and decreasing flow.
E&P carried by free NADHc in stimulated retina and visual cortex (potentially equimolar to pyruvate oxidized in mitochondria) also can be transferred to oxidized substrates of enzymes in metabolic pathways that activate constitutive NOS (cNOS) to increase blood flow (Fig. 4). This conclusion is supported by observations that (i) infusion of pyruvate or l-NAME largely prevented increased flows/conductance in stimulated retina and visual cortex, and (ii) pyruvate infusion decreased L/P ratios (NADHc) in stimulated retina.
Likewise, increased blood flows in numerous resting tissues, including retina, evoked by lactate injection, are prevented by coinfusion of pyruvate and by l-NAME (see Supporting Text, which is published as supporting information on the PNAS web site). Furthermore, elevation of lactate levels in cultured neurons (24) increases free 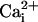 (to activate cNOS); and, increased granulation tissue blood flows evoked by elevated lactate levels are prevented by Ca2+ blockers, pyruvate, and l-NAME (1).
(to activate cNOS); and, increased granulation tissue blood flows evoked by elevated lactate levels are prevented by Ca2+ blockers, pyruvate, and l-NAME (1).
Increased plasma pyruvate levels evoked by lactate infusion and increased plasma lactate levels evoked by pyruvate infusion attest to “whole-body” oxidation of infused lactate to pyruvate and reduction of infused pyruvate to lactate by LDH (Fig. 4) in tissues faster than the pyruvate and lactate formed are used for energy metabolism and/or gluconeogenesis; excess pyruvate and lactate diffuse back into the blood.
Effects of lactate and pyruvate injection on blood flow in stimulated retina and visual cortex and correlations of blood flow with plasma L/P ratios are virtually identical with those in stimulated skeletal muscle and whisker barrel cortex (1). [The minimal impact of pyruvate and lactate injection on flows in unstimulated vs. stimulated retina and visual cortex may be explained by increased transport rate constants for cerebral lactate efflux and influx evoked by neural activity (25).] In contrast to the strong correlation between blood flow and L/P ratios in stimulated retina, L/P ratios in stimulated muscle did not correlate with flow. This difference may be explained largely by the 10- to 20-fold higher L/P ratios in contracting muscle than in stimulated retina, i.e., L/P ratios in retina would likely equilibrate more readily with plasma L/P ratios than muscle L/P ratios would.
The finding that mean L/P ratios and lactate and pyruvate levels did not differ significantly in retinas from stimulated and nonstimulated rats was unexpected in view of the test hypothesis and the strong correlation between retinal L/P ratios and blood flow in stimulated retinas of rats infused with saline, lactate, and pyruvate (Fig. 2). This finding is most likely explained by the 2-fold higher variance of retinal blood flows in photostimulated vs. unstimulated rats (which attests to greater variation in blood flows between rats in the stimulated group) and the small number of animals in each group (n = 4, Table 1) in this correlation experiment; a paired comparison of the difference in L/P ratios in stimulated vs. contralateral unstimulated retina in the same rat could well be significant, as shown for retinal flows after 10 min of photostimulation (Fig. 5). In addition, accumulation of E&P in NADHc in stimulated retinas may be attenuated by the ≈50% higher blood flow in stimulated vs. resting retina, which would increase rates of delivery of plasma pyruvate to reoxidize free NADHc by LDH and removal of lactate.
The finding that l-NAME decreased retinal blood flow by only 36%, but completely prevented the identical increase in conductance, indicates that NOS inhibition prevented the increased flow evoked by stimulation and that the residual elevated flow was caused by the increase in blood pressure evoked by l-NAME. Complete normalization of flows in stimulated visual cortex by l-NAME indicates that inhibition of NOS prevented the increased flow evoked by stimulation without impairing autoregulatory mechanism(s), which prevented an increase in flow due to increased blood pressure. The finding that l-NAME did not impair autoregulation is consistent with observations of Wang et al. (26).
The failure of the SODmimic to reduce blood flow in stimulated retina and visual cortex also was unexpected, because it markedly attenuated increased flows in stimulated whisker barrel cortex and in contracting skeletal muscle (1). This finding indicates that cNOS in stimulated retina and visual cortex was not activated by 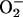 (in contrast to stimulated whisker barrel cortex and skeletal muscle). The likelihood that
(in contrast to stimulated whisker barrel cortex and skeletal muscle). The likelihood that 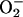 was increased (albeit to a lesser extent than •NO) and reacted with •NO to attenuate increased flows in stimulated retina (see Supporting Text) is supported by the specificity of the SODmimic, which selectively catalyzes dismutation of
was increased (albeit to a lesser extent than •NO) and reacted with •NO to attenuate increased flows in stimulated retina (see Supporting Text) is supported by the specificity of the SODmimic, which selectively catalyzes dismutation of 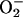 , potentiates •NO-induced vasodilatation without blocking
, potentiates •NO-induced vasodilatation without blocking 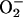 production, and does not interact with •NO, H2O2, or peroxynitrite (refs. 27 and 28; D. P. Riley, personal communication).
production, and does not interact with •NO, H2O2, or peroxynitrite (refs. 27 and 28; D. P. Riley, personal communication).
Increased NADHc in stimulated retina and visual cortex may activate cNOS by pathways independent of 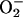 . (i) NADH stimulates Ca2+ release from purified inositol 1,4,5-trisphosphate receptors reconstituted in phospholipid vesicles and also mobilizes Ca2+ from inositol 1,4,5-trisphosphate-sensitive stores in cerebellar Purkinje cells in response to chemical hypoxia (NAD, NADP, and NADPH had little or no effect); and inhibitors of glycolysis (and associated formation of free NADHc) prevent the increase in free
. (i) NADH stimulates Ca2+ release from purified inositol 1,4,5-trisphosphate receptors reconstituted in phospholipid vesicles and also mobilizes Ca2+ from inositol 1,4,5-trisphosphate-sensitive stores in cerebellar Purkinje cells in response to chemical hypoxia (NAD, NADP, and NADPH had little or no effect); and inhibitors of glycolysis (and associated formation of free NADHc) prevent the increase in free 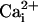 evoked by hypoxia (29). (ii) E&P carried by NADH reduce quinonoid-H2biopterin (formed by NOS) to H4biopterin coupled to oxidation of NADH to NAD+ by dihydropteridine reductase (9, 30). H4biopterin promotes activation of NOS by stabilizing the active dimer of NOS in addition to its cofactor function (31). (iii) Reoxidation of NADHc by cytosolic glycerol 3-phosphate dehydrogenase coupled to reduction of dihydroxyacetone phosphate to glycerol 3-phosphate is the first step in one pathway for de novo synthesis of diacylglycerol and activation of PKC, which phosphorylates NOS, increasing NOS activity (32). Further studies are needed to elucidate which if any of these potential mechanisms may activate cNOS in stimulated retina and visual cortex.
evoked by hypoxia (29). (ii) E&P carried by NADH reduce quinonoid-H2biopterin (formed by NOS) to H4biopterin coupled to oxidation of NADH to NAD+ by dihydropteridine reductase (9, 30). H4biopterin promotes activation of NOS by stabilizing the active dimer of NOS in addition to its cofactor function (31). (iii) Reoxidation of NADHc by cytosolic glycerol 3-phosphate dehydrogenase coupled to reduction of dihydroxyacetone phosphate to glycerol 3-phosphate is the first step in one pathway for de novo synthesis of diacylglycerol and activation of PKC, which phosphorylates NOS, increasing NOS activity (32). Further studies are needed to elucidate which if any of these potential mechanisms may activate cNOS in stimulated retina and visual cortex.
The optimal positioning of free NADc for fueling signaling pathways that coordinate blood flow with energy metabolism is underscored by the fact that glucose and lactate must donate E&P to 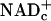 before fueling ATP synthesis. This ensures availability of E&P carried by NADHc to fuel signaling pathways that augment blood flow as needed for synthesis of ATP. Likewise, oxidation of ketones and fatty acids requires transfer of E&P to
before fueling ATP synthesis. This ensures availability of E&P carried by NADHc to fuel signaling pathways that augment blood flow as needed for synthesis of ATP. Likewise, oxidation of ketones and fatty acids requires transfer of E&P to 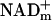 (and FADm) to fuel ATP synthesis. The redox state of free NAD(H) [and NADP(H)] also markedly influences the activity of transcription factors (33, 34); Rutter et al. (33) suggest that changes in NADH/NAD+ may regulate entrainment of the circadian clock by fluctuation in retinal neuronal activity associated with the light–dark cycle.
(and FADm) to fuel ATP synthesis. The redox state of free NAD(H) [and NADP(H)] also markedly influences the activity of transcription factors (33, 34); Rutter et al. (33) suggest that changes in NADH/NAD+ may regulate entrainment of the circadian clock by fluctuation in retinal neuronal activity associated with the light–dark cycle.
The effects of lactate and pyruvate injection on blood flow are consistent both with (i) the need for higher flows in stimulated tissues to remove lactate, accumulation of which inhibits reoxidation of free NADHc by LDH, and (ii) the evidence that increased flows appear to exceed the need for oxygen and glucose to support increased energy metabolism (2, 3, 6–8, 35).
In conclusion, these observations provide additional support for the hypothesis that E&P carried by free NADHc fuel signaling pathways that mediate increased blood flows evoked by physiological stimulation (1). The hypothesis gains additional support from observations of Mintun et al. (36) that blood flow in photostimulated human visual cortex is augmented by lactate injection, and the increased flows correlate with plasma L/P ratios.
Supplementary Material
Acknowledgments
We thank Michael L. Wolf for the use of the Visual Electrodiagnostic Stimulator for these studies; David Grosoff for advice on conditions for visual stimulation; Andrei Vlassenko, Mark Mintun, and Marc Raichle for their comments and advice; and Eva Ostrow, Sam Smith, and Judi Burgan for expert technical assistance. This work was supported by National Institutes of Health Grants HL-39934 and EY-06600 and the Kilo Research Foundation (St. Louis).
Abbreviations: E&P, electrons and protons; LDH, lactate dehydrogenase; L/P ratio, lactate/pyruvate ratio; NOS, NO synthase; cNOS, constitutive NOS; SODmimic, superoxide dismutase mimic SC-52608; l-NAME, NG-nitro-l-arginine methyl ester.
References
- 1.Ido, Y., Chang, K., Woolsey, T. A. & Williamson, J. R. (2001) FASEB J. 15, 1419–1421. [DOI] [PubMed] [Google Scholar]
- 2.Fox, P. T. & Raichle, M. E. (1986) Proc. Natl. Acad. Sci. USA 83, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. (1988) Science 241, 462–464. [DOI] [PubMed] [Google Scholar]
- 4.Ames, A., III, & Nesbett, F. B. (1981) J. Neurochem. 37, 867–877. [DOI] [PubMed] [Google Scholar]
- 5.Michelson, G., Patzelt, A. & Harazny, J. (2002) Retina 22, 336–343. [DOI] [PubMed] [Google Scholar]
- 6.Lindauer, U., Megow, D., Schultze, J., Weber, J. R. & Dirnagl, U. (1996) Neurosci. Lett. 216, 207–210. [DOI] [PubMed] [Google Scholar]
- 7.Powers, W. J., Hirsch, I. B. & Cryer, P. E. (1996) Am. J. Physiol. 270, H554–H559. [DOI] [PubMed] [Google Scholar]
- 8.Cholet, N., Seylaz, J., Lacombe, P. & Bonvento, G. (1997) J. Cereb. Blood Flow Metab. 17, 1191–1201. [DOI] [PubMed] [Google Scholar]
- 9.Stryer, L. (1995) Biochemistry (Freeman, New York).
- 10.Ueki, M., Linn, F. & Hossmann, K. A. (1988) J. Cereb. Blood Flow Metab. 8, 486–494. [DOI] [PubMed] [Google Scholar]
- 11.Prichard, J., Rothman, D., Novotny, E., Petroff, O., Kuwabara, T., Avison, M., Howseman, A., Hanstock, C. & Shulman, R. (1991) Proc. Natl. Acad. Sci. USA 88, 5829–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sappey-Marinier, D., Calabrese, G., Fein, G., Hugg, J. W., Biggins, C. & Weiner, M. W. (1992) J. Cereb. Blood Flow Metab. 12, 584–592. [DOI] [PubMed] [Google Scholar]
- 13.Williamson, D. H., Lund, P. & Krebs, H. A. (1967) Biochem. J. 103, 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole, R. C. & Halestrap, A. P. (1993) Am. J. Physiol 264, C761–C782. [DOI] [PubMed] [Google Scholar]
- 15.Chang, K., Ido, Y., LeJeune, W., Williamson, J. R. & Tilton, R. G. (1997) Am. J. Physiol 273, E164–E173. [DOI] [PubMed] [Google Scholar]
- 16.Van den Enden, M. K., Nyengaard, J. R., Ostrow, E., Burgan, J. H. & Williamson, J. R. (1995) Invest. Ophthalmol. Visual Sci. 36, 1675–1685. [PubMed] [Google Scholar]
- 17.Poitry-Yamate, C. L., Poitry, S. & Tsacopoulos, M. (1995) J. Neurosci. 15, 5179–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magistretti, P. J. & Pellerin, L. (1999) Philos. Trans. R. Soc. London B 354, 1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman, E. & Reichenbach, A. (1996) Trends Neurosci. 19, 307–312. [DOI] [PubMed] [Google Scholar]
- 20.Swanson, R. A. (1992) Can. J. Physiol. Pharmacol. 70, Suppl., S138–S144. [DOI] [PubMed] [Google Scholar]
- 21.Chih, C. P., Lipton, P. & Roberts, E. L., Jr. (2001) Trends Neurosci. 24, 573–578. [DOI] [PubMed] [Google Scholar]
- 22.Dienel, G. A. & Hertz, L. (2001) J. Neurosci. Res. 66, 824–838. [DOI] [PubMed] [Google Scholar]
- 23.Gruetter, R. (2002) Neurochem. Int. 41, 143–154. [DOI] [PubMed] [Google Scholar]
- 24.Nedergaard, M. (1995) Am. J. Physiol 268, R506–R513. [DOI] [PubMed] [Google Scholar]
- 25.Lear, J. L. & Kasliwal, R. K. (1991) J. Cereb. Blood Flow Metab. 11, 576–580. [DOI] [PubMed] [Google Scholar]
- 26.Wang, Q., Paulson, O. B. & Lassen, N. A. (1992) Acta Physiol. Scand. 145, 297–298. [DOI] [PubMed] [Google Scholar]
- 27.Hardy, M. M., Flickinger, A. G., Riley, D. P., Weiss, R. H. & Ryan, U. S. (1994) J. Biol. Chem. 269, 18535–18540. [PubMed] [Google Scholar]
- 28.Kilgore, K. S., Friedrichs, G. S., Johnson, C. R., Schasteen, C. S., Riley, D. P., Weiss, R. H., Ryan, U. & Lucchesi, B. R. (1994) J. Mol. Cell. Cardiol. 26, 995–1006. [DOI] [PubMed] [Google Scholar]
- 29.Kaplin, A. I., Snyder, S. H. & Linden, D. J. (1996) J. Neurosci. 16, 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon, N. S., Nathan, C. F. & Stuehr, D. J. (1989) J. Biol. Chem. 264, 20496–20501. [PubMed] [Google Scholar]
- 31.Panda, K., Rosenfeld, R. J., Ghosh, S., Meade, A. L., Getzoff, E. D. & Stuehr, D. J. (2002) J. Biol. Chem. 277, 31020–31030. [DOI] [PubMed] [Google Scholar]
- 32.Nakane, M., Mitchell, J., Forstermann, U. & Murad, F. (1991) Biochem. Biophys. Res. Commun. 180, 1396–1402. [DOI] [PubMed] [Google Scholar]
- 33.Rutter, J., Reick, M., Wu, L. C. & McKnight, S. L. (2001) Science 293, 510–514. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Q., Piston, D. W. & Goodman, R. H. (2002) Science 295, 1895–1897. [DOI] [PubMed] [Google Scholar]
- 35.Mintun, M. A., Lundstrom, B. N., Snyder, A. Z., Vlassenko, A. G., Shulman, G. L. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA. 98, 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintun, M. A., Vlassenko, A. G., Rundle, M. M. & Raichle, M. E. (2004) Proc. Natl. Acad. Sci. USA 101, 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.