Abstract
The changes in crude protein, free amino acids, amino acid composition, protein solubility, protein fractionation and protein digestibility after germination of sorghum were investigated. Sorghum varieties (Dorado, Shandaweel-6, Giza-15) were soaked for 20 h followed by germination for 72 h; the results revealed that crude protein and free amino acids in raw sorghum varieties ranged from 10.62 to 12.46% and 0.66 to 1.03 mg/g, respectively. Shandaweel-6 was the highest variety in crude protein and free amino acids content. After germination, crude protein was decreased and free amino acids were increased. There was an increase in content of valine and phenylalanine amino acids after germination. On the other hand, there was a decrease in most of amino acids after germination. After germination protein solubility was significantly increased. Regarding protein fractions, there was an increase in albumin, globulin and kafirin proteins and a decrease in cross linked kafirin and cross linked glutelin after germination.
Introduction
Sorghum (Sorghum bicolor [L.] Moench) one of the most important weaning foods in low-income and high-income countries [1]–[5]. It ranks fifth among the world cereals, following wheat, maize, rice and barley in production area and total production. Sorghum is an extremely important crop in Asia, Africa and other semi-arid regions of the world [6].
The nutrient composition of sorghum indicates that it is a good source of energy, proteins, carbohydrates, vitamins and minerals [7]–[9].
Sorghum components, especially its protein is less digestible than other cereals for human and monogastric animals, because of its anti-nutritional factors such as tannins and phytic acid. Removal of these undesirable components is essential to improve the nutritional quality of sorghum and effectively utilize its potential as human food or animal feed [10], [11].
Interaction between tannins and sorghum proteins reduces both protein and starch digestibility. This is important in both human and animal nutrition. The formation of complexes between sorghum proteins and tannins is thought to render the proteins indigestible as well as inhibit digestive enzymes. Proteins rich in proline bind more sorghum tannins than other proteins. In addition, a protein containing more proline repeats will bind more tannin than one with less such repeats [12].
The low digestibility of sorghum proteins is presumably due to the high protein cross linking. Good quality proteins are those that are readily digestible and contain the essential amino acids in quantities that correspond to human requirements [13]–[18].
The in vitro pepsin digestion assay is mimics the digestive system, and are widely used to study the structural changes, digestibility, and release of food components under simulated gastrointestinal conditions. The most frequently used biological molecules included in the digestion models were digestive enzymes, bile salts, and mucin [19], [20].
Germination is widely used in legumes and cereals to increase their palatability and nutritional value, particularly through the breakdown of certain antinutrients, such as phytate and protease inhibitors [21], [22].
Germination is a common practice in sorghum producing areas. Grains are malted for the production of weaning foods, opaque beers and other traditional dishes. Germination triggers the enzymatic activity of sprouting grains, leading to the breakdown of proteins, carbohydrates and lipids into simpler forms. This processing method activates proteases which are active in degrading proteins, thereby increasing nutrient bioavailability [23].
The objective of this study was to enhance sorghum nutritional value via germination, identify of sorghum protein characteristics, such as protein digestibility, protein solubility and protein fractionation as well as amino acids contents.
Materials and Methods
Materials
Samples and chemicals
Pepsin, pancreatin, α-amylase and L-aspartic acid were obtained from Sigma– Aldrich Chemical Co., St. Louis, USA. All other chemicals used were of analytical reagent grade.
Three white sorghum varieties (Sorghum bicolor L. Moench), Shandaweel-6 was obtained from the Crops Research Institute, Agricultural Research Centre, and Dorado and Giza-15 were obtained from Central Administration for Seed Certification (CASC), Ministry of Agriculture and Land Reclamation, Giza, Egypt. The grains were carefully cleaned and freed from broken grains and extraneous matter.
Germination of grains
Sorghum grains were soaked in distilled water for 20 h with a ratio 1∶5 w/v and the soaked water changed twice. At the end of soaking period, the soaked water was discarded. Soaked grains were germinated for 72 hours at room temperature, and then the grains were dried. The root and shoot portions were manually removed. The grains were milled into fine flour and kept until analysis.
Chemical analysis
Determination of crude protein
Crude protein contents of raw sorghum and treatments were determined according to the methods of A.O.A.C. [24].
Determination of free amino acids
Free amino acids were determined using the method outlined by Rosen [25]. Ninhydrin reagent used for the determination of free amino acids. The free amino acids were calculated as mg/g DW from the standard curve which prepared by using L-aspartic acid as standard.
Determination of amino acids composition
Amino acids composition of samples was determined by using amino acid analyzer (Biochrom 30) according to the method outlined in A.O.A.C. [24]. An aliquot sample, were weighed and digested with 25 ml of 6N HCl at 110°C for 24 h. Then HCl was removed by evaporation; the remaining solid fraction was dissolved with 0.2N sodium citrate buffer (pH 2.2). One ml of the solution was filtered though 0.45 µm Millipore membrane filters. The standard amino acids (consist of 17 amino acids) was treated as the same as of the samples. Amino acids were expressed as g/100 g protein on dry weight basis.
Determination of protein solubility
Protein solubility was determined by the method of Sathe and Salunkhe [26]. One gram of samples was dispersed in 25 ml of 1 M NaOH. The obtained suspensions were mixed and stirred in an orbital shaker at 150 rpm for 12 h at room temperature and then centrifuged at 3000 g for 20 min. Soluble proteins in supernatants were determined by Lowry et al. [27]. Bovine Serum Albumin was used as standard protein. Soluble protein was expressed as g/100 g DW sample.
Determination of sorghum protein fractions
Protein fractionation was performed according to the method modified from Landry and Moureaux [28]. Two grams of sorghum were sequentially extracted with the six solvents listed below (20 ml at 25°C and centrifuged at 18,900 g for 10 min at 4°C after each step). Initially, sorghum was extracted with deionized water for 20 min (albumins, fraction 1) and the pellet sequentially extracted with the following solutions: 0.5 M NaCl for 60 min (globulins, fraction 2); 60% 2-propanol (v/v) for 4 h (kafirins, fraction 3); 0.1 M borate buffer, pH 10.8, for 4 h (glutelin-like protein, fraction 4); 60% 2-propanol with 1% dithiothreitol (DTT) for 4 h (cross-linked kafirins, fraction 5); and 0.1 M borate buffer, pH 10.8, containing 1% DTT and 1% sodium dodecyl sulfate (SDS) for 18 h at 4°C (cross-linked glutelins, fraction 6). Soluble protein in supernatants was determined by Lowry et al. [27] for fractions 1 to 4 and by de Wreede and Stegemann [29] from fractions 5 and 6. Protein fractions were expressed as g/100 g protein on dry weight basis.
Determination of in vitro protein digestibility
In vitro protein digestibility was determined according to the method of Akeson and Stahmanna [30]. One gram samples added to HCl (15 ml, 0.1 M), containing 1.5 mg pepsin then the incubated at 37°C for 3 h. The obtained suspension was neutralized with NaOH (7.5 ml, 0.2 M), then treated with 4 mg of pancreatin in 7.5 ml 0.2 M phosphate buffer (pH 8.0). One milliliter of toluene was added to prevent microbial growth and the mixture was gently shaken and incubated for additional 24 h at 37°C. After incubation, the sample was treated with 10 ml of 10% TCA to remove undigested protein and larger peptides and centrifuged at 50000 g for 20 min at room temperature. Protein in the supernatant was estimated using the Kjeldahl method (A.O.A.C.) [24]. The percentage of protein digestibility was calculated by the ratio of protein in supernatant to protein in sample as equation:
Statistic analysis
For the analytical data, mean values and standard deviation are reported. The data obtained were subjected to one-way analysis of variance (ANOVA) and least significant difference (LSD) at P<0.05.
Results and Discussion
Effect of germination of sorghum on crude protein and free amino acids
Table 1 presents crude protein and free amino acids content in sorghum before and after germination. Protein content ranged from 10.62 to 12.46% in raw sorghum varieties. Protein was significantly higher in Shandaweel-6 while Giza-15 was the lowest one. These results are in agreement with Dicko et al. [7] and Johnson et al. [31] who found that crude protein content in whole sorghum grain is ranged from 7 to 15% or 10.30 to 14.90%.
Table 1. Crude protein and free amino acids content of sorghum after germination.
| Treatments | Crude protein (%) | Free amino acids (mg/g DW)* |
| Raw | ||
| Dorado | 10.90±0.14c | 0.66±0.01e |
| Shandaweel-6 | 12.46±0.11a | 1.03±0.03c |
| Giza-15 | 10.62±0.20d | 0.82±0.03d |
| Germination | ||
| Dorado | 10.25±0.20e | 8.78±0.05b |
| Shandaweel-6 | 12.10±0.10b | 9.94±0.04a |
| Giza-15 | 9.77±0.09f | 8.80±0.04b |
| L.S.D | 0.2632 | 0.0561 |
*mg/g DW = mg per gram dry weight.
Values are mean of three replicates ±SD, number in the same column followed by the same letter are not significantly different at p<0.05 level.
The crude protein was significantly decreased after germination compared with raw sorghum. These results are agreed with Shaker et al. [32] who reported that nutrients loss might be attributed to the leaching of soluble nitrogen, mineral and other nutrients into desired solution.
Free amino acids content ranged from 0.66 to 1.03 mg/g in raw sorghum. Free amino acids amount was significantly higher in Shandaweel-6 than other varieties. After germination free amino acids content were increased and ranged from 8.78 to 9.94 mg/g and this may be due to the activity of proteolytic enzymes. Chavan et al. [33] found that free amino acids content in germinated sorghum (72 h) was 1.20 mg/g.
The nutritive value of food, especially protein mostly would depend not only on its amino acid profile in general but also on the quantities of the essential amino acids content in particular. The nutritive value of dietary protein is determined by the pattern and quantity of essential amino acids present.
Table 2 shows essential amino acids content in sorghum before and after germination. Essential amino acids in raw sorghum ranged from 3.49 to 3.58, 11.74 to 13.56, 2.75 to 3.21, 4.22 to 4.43, 4.40 to 4.98, 2.11 to 2.26, 2.73 to 2.94 and 4.22 to 4.58 g/100 g protein for isoleucine, leucine, threonine, valine, phenylalanin, lysine, methionine and tyrosine, respectively. Shandaweel-6 had the highest amount of isoleucine, leucine, threonine, valine, phenylalanin and tyrosine, respectively. While Dorado had the highest amount of methionine. Finely, Giza-15 had the highest amount of lysine. The results are in the same trend with Mokrane et al. [34] who reported that essential amino acids content in raw sorghum ranged from 3.69 to 4.27, 15.77 to 17.34, 4.47 to 5.07, 4.62 to 5.11, 4.49 to 6.26, 1.58 to 2.05, 1.06 to 1.42 and 2.69 to 3.19 g/100 g protein for isoleucine, leucine, threonine, valine, phenylalanin, lysine, methionine and tyrosine, respectively. Moreover, essential amino acids content in raw sorghum ranged from 3.64 to 3.71, 11.50 to 13.37, 2.99 to 3.45, 4.64 to 5.90, 4.54 to 4.81, 1.66 to 1.88, 1.00 to 1.76 and 3.53 to 3.76 g/100 g protein for isoleucine, leucine, threonine, valine, phenylalanin, lysine, methionine and tyrosine, respectively [35].
Table 2. Essential amino acids content of sorghum after germination (g/100 g protein).
| Treatments | Isoleucine | Leucine | Threonine | Valine | Phenylalanine | Lysine | Methionine | Tyrosine |
| Raw | ||||||||
| Dorado | 3.49±0.01d | 11.74±0.01f | 2.75±0.01d | 4.22±0.02f | 4.40±0.01e | 2.11±0.01d | 2.94±0.02a | 4.22±0.02d |
| Shandaweel-6 | 3.85±0.01a | 13.56±0.02a | 3.21±0.01a | 4.82±0.03d | 4.98±0.01c | 2.17±0.02c | 2.73±0.01c | 4.22±0.02d |
| Giza-15 | 3.58±0.01c | 11.96±0.03c | 2.92±0.01b | 4.43±0.01e | 4.61±0.01d | 2.26±0.02b | 2.82±0.01b | 4.33±0.01b |
| Germination | ||||||||
| Dorado | 3.42±0.02e | 12.00±0.01b | 2.54±0.02e | 5.85±0.05a | 4.00±0.03f | 2.34±0.01a | 2.44±0.01e | 4.29±0.03c |
| Shandaweel-6 | 3.80±0.02b | 11.82±0.01e | 2.89±0.01c | 4.96±0.02c | 5.37±0.02b | 1.98±0.02f | 2.48±0.02d | 4.13±0.01e |
| Giza-15 | 3.38±0.03f | 11.87±0.01d | 2.87±0.02c | 5.32±0.02b | 5.73±0.02a | 2.05±0.03e | 2.15±0.02f | 4.20±0.01d |
| L.S.D | 0.0325 | 0.0299 | 0.0252 | 0.0498 | 0.0325 | 0.0348 | 0.0281 | 0.0325 |
Values are mean of three replicates ±SD, number in the same column followed by the same letter are not significantly different at p<0.05 level.
From the data in Table 2, it could be noticed that after treatments essential amino acids content were changed. There was an increase in content of valine and phenylalanine after germination. On the other hand, there was a decrease in most of amino acids after germination. These findings are in agreement with Elemo et al. [36] who found that germination significantly increased the essential amino acids except for histidine and sulphur amino acids. Germination of cereals and legumes has been shown to be generally advantageous as it also improves the nutritional qualities of cereals and legumes [37].
Table 3 shows non essential amino acids content in sorghum before and after germination. Non essential amino acids content in raw sorghum ranged from 3.58 to 4.01, 6.24 to 7.06, 7.43 to 8.83, 6.66 to 8.99, 18.45 to 20.63, 2.84 to 3.05, 3.49 to 4.17 and 1.93 to 2.17 g/100 g protein for arginine, aspartic acid, alanine, prolin, glutamic acid, glycine, serine, histidine and cysteine. Shandaweel-6 had the highest amount of arginine, aspartic acid, alanine, glutamic acid, glycine, and serine. While Dorado had the highest amount of prolin and cystine. Also, Shandaweel-6 and Giza-15 had the same amounts of histidine. Glutamic acid was found to be the major non-essential amino acids in the tested samples, while cystine was the lowest one.
Table 3. Non essential amino acids content of sorghum after germination (g/100 g protein).
| Treatments | Arginine | Aspartic | Alanine | Proline | Glutamic | Glycine | Serine | Histidine | Cysteine |
| Raw | |||||||||
| Dorado | 3.58±0.02c | 6.24±0.02f | 7.43±0.01e | 8.99±0.01a | 18.45±0.05d | 2.84±0.02d | 3.49±0.01d | 1.93±0.01c | 2.11±0.01a |
| Shandaweel-6 | 4.01±0.01a | 7.06±0.04a | 8.83±0.02a | 6.66±0.02e | 20.63±0.03b | 3.05±0.02a | 4.17±0.03a | 2.17±0.01a | 1.69±0.02d |
| Giza-15 | 3.77±0.02b | 6.87±0.03b | 7.72±0.02d | 7.53±0.02d | 19.77±0.03c | 2.92±0.01b | 3.58±0.02c | 2.17±0.02a | 1.69±0.01d |
| Germination | |||||||||
| Dorado | 3.22±0.02e | 6.44±0.04e | 8.20±0.04c | 8.88±0.02b | 20.78±0.02a | 2.73±0.01e | 3.32±0.01e | 2.05±0.01b | 2.05±0.02b |
| Shandaweel-6 | 3.47±0.03d | 6.78±0.02c | 6.94±0.02f | 6.61±0.01f | 19.75±0.05c | 2.73±0.02e | 3.80±0.02b | 2.07±0.02b | 1.65±0.01e |
| Giza-15 | 3.58±0.02c | 6.65±0.01d | 8.39±0.01b | 7.68±0.02c | 19.75±0.03c | 2.87±0.01c | 3.28±0.01f | 2.05±0.01b | 1.84±0.01c |
| L.S.D | 0.0370 | 0.0513 | 0.0398 | 0.0308 | 0.0643 | 0.0281 | 0.0325 | 0.0252 | 0.0252 |
Values are mean of three replicates ±SD, number in the same column followed by the same letter are not significantly different at p<0.05 level.
The results are in the same trend with other study, which cited that non essential amino acids in raw sorghum ranged from 5.61 to 6.13, 7.75 to 9.39, 7.60 to 8.32, 16.44 to 16.89, 1.92 to 2.95, 3.12 to 4.04 and 1.90 to 2.24 g/100 g protein for aspartic acid, alanine, prolin, glutamic acid, glycine, serine and histidine, respectively [38]. Essential amino acids content in raw sorghum ranged from 7.21 to 7.58, 7.29 to 9.19, 20.03 to 23.16, 2.58 to 2.92, 1.73 to 2.60, 4.44 to 4.73 and 1.89 to 2.96 g/100 g protein for aspartic acid, proline, glutamic acid, glycine, cysteine, serine and histidine, respectively [35].
After germination, most of non essential amino acids content was decreased. Moreover, proline content was decreased except for germinated Giza-15 which seems to be significantly increased than raw Giza-15. The breakdown of protease resistant prolamins and the increase of essential amino acids upon germination have been reported [38].
Effect of germination of sorghum on protein solubility and Protein fractions
Among the functional properties of proteins, solubility is probably the most critical function. Protein solubility characteristics are influenced by factors such as origin, processing conditions, pH, ionic strength and the presence of other ingredients [39], [40].
Table 4 exhibits the effect of germination of sorghum on protein solubilized under alkaline conditions extracted with NaOH as described in materials and methods. Data showed that solubility of raw sorghum protein ranged from 3.21 to 3.72 g/100 g. Shandaweel-6 recorded the highest variety in protein solubility.
Table 4. Protein solubility of sorghum after germination (g/100 g DW)*.
| Treatments | Protein solubility |
| Raw | |
| Dorado | 3.45±0.08de |
| Shandaweel-6 | 3.72±0.32cd |
| Giza-15 | 3.21±0.13e |
| Germination | |
| Dorado | 4.11±0.08b |
| Shandaweel-6 | 4.90±0.06a |
| Giza-15 | 3.98±0.33bc |
| L.S.D | 0.3597 |
*mg/g DW = mg per gram dry weight.
Values are mean of three replicates ±SD, number in the same column followed by the same letter are not significantly different at p<0.05 level.
There was a significant increase in protein solubility after germination. These findings are in agreement with Elkhalifa and Bernhardt [40], who found that germinated sorghum had a higher protein solubility compared with the un-geminated one. The protein of the germinated sorghum was more soluble than the un-geminated sorghum. This might be due to the high proteolytic activity during germination, which will lead to an increase in the protein solubility resulting from hydrolysis of the storage proteins.
The proteins of the sorghum grain are classically divided, based on solubility in different solvents: water-soluble (albumins), salt-soluble (globulins), aqueous alcohol-soluble (kafirins), aqueous alcohol reducing agent-soluble (cross-linked kafirins) and detergent reducing agent alkaline pH-soluble (cross-linked glutelins) [41].
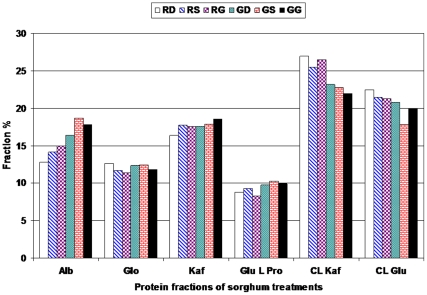
Figure 1 presents the effect of germination treatment on protein fractions based on solubility for each fraction, into albumins, globulins, kafirins, glutelins like protein, cross linked kafirin and cross linked glutelins. From results, it could be noticed that raw sorghum contain 12.77 to 14.95%, 11.37 to 12.67%, 16.37 to 17.75%, 8.30 to 9.35, 25.55 to 27.00% and 21.31 to 22.45% for albumins, globulins, prolamins, glutline like protein, cross linked kafirins and cross linked glutline, respectively. Distribution of protein in fractions extracted with the different solvents suggested that, the three sorghum varieties different in amount of total extractable protein and this is may be due to the differences in total protein. Cross linked kafirins, represented a considerably greater fraction in sorghum varieties. Results are close to Ejeta et al. [42], who found that fractionated protein in raw sorghum ranged from 10.00 to 24.00%, 6 to 16% and 11.00 to 31.00% for albumins plus globulins, prolamins and cross linked kafirins, respectively. Raw corn contain 19.50 to 26.20%, 20.90 to 35.30% and 15.20 to 23.80% for albumins plus globulins, cross linked kafirins and cross linked glutline, respectively [43]. Sorghum prolamins ranged from 42.50 to 81.80% [30]. Since a large percentage of sorghum kafirin storage proteins exist in polymeric forms linked by disulfide bonds in their native state, differences in content of fractions rich in insoluble disulfide proteins, i.e., cross-linked kafirin and cross linked glutelin could contribute to protein digestibility differences. Moreover, kafirins and cross linked kafirin in raw sorghum ranged from 17.30 to 19.90% and 24.50 to 35.10, respectively [44]. Sorghum has a higher proportion of cross linked prolamin than maize [45].
Figure 1. Protein fractions of sorghum after germination (Alb: Albumin; Glo: Globulin; Kaf: Kafirin; Glu L Pro: Glutelins like protein; CL Kaf: Cross linked kafirins; CL Glu: Cross linked glutelins).
Treatments: (RD: Raw Dorado; RS: Raw Shandweel-6; RG: Raw Giza-15; GD: Germinated Dorado; GS: Germinated Shandaweel-6; GG: Germinated Giza-15).
The results revealed an increase in albumin, globulin and kafirin fractions and a decrease in cross linked kafirin and cross linked glutelin after germination. These results are close to previous studies, which found that albumin, globulin and glutelin like protein were increased and prolamins, cross linked kafirin and cross linked glutelin fraction were decreased after germination [46], [47].
Effect of germination of sorghum on in vitro protein digestibility (IVPD)
The factors that may affect sorghum protein digestibility, divided in two categories: exogenous factors (i.e. interactions of proteins with non-protein components such as polyphenols, starch, non-starch polysaccharides, phytates and lipids), and endogenous factors (factors arising from the sorghum proteins themselves), concluding that the poor digestibility of sorghum proteins appear to be multi-factorial. The main proteins in sorghum were kafirins. These proteins are known to be peptidase resistant because of their S-S bonds. Digestibility may be used as an indicator of protein availability [13], [47].
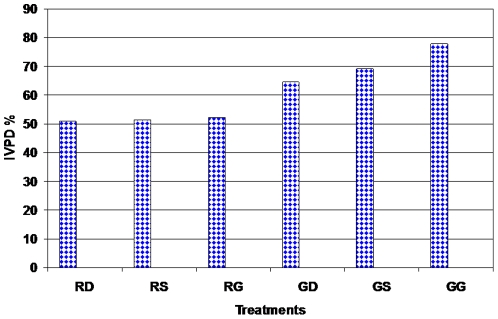
Figure 2 presents the in vitro protein digestibility in sorghum before and after germination. Data showed that in vitro protein digestibility in raw sorghum ranged from 50.94 to 52.09% and Giza-15 was significantly higher in protein digestibility than other varieties. The findings are in agreement with Zhao et al. [13], who showed that the in vitro protein digestibility ranged from 23.00 to 68.20%. The low digestibility of sorghum proteins is presumably due to the high protein cross-linking.
Figure 2. In vitro protein digestibility (IVPD%) of sorghum after germination.
Treatments: (RD: Raw Dorado; RS: Raw Shandweel-6; RG: Raw Giza-15; GD: Germinated Dorado; GS: Germinated Shandaweel-6; GG: Germinated Giza-15).
In addition, in vitro protein digestibility was significantly increased after germination treatments. Germinated Giza-15 was the highest variety in protein digestibility compared with other varieties. These results are similar to Correia et al. [48], who found that germination causes activation of intrinsic amylases, proteases, phytases and fiber-degrading enzymes, thereby increasing nutrient digestibility. The activity of intrinsic proteases in germinated grains leads to an increase in in vitro protein digestibility. Germination is effective in increasing protein digestibility and improving sensory properties Also, processing of sorghum (boiling, germination, fermentation and cooking) greatly improved its nutritive value [49], [50].
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Department of Biochemistry, Faculty of Agriculture, Cairo University, the Department of Crops Technology, the Food Technology Research Institute, and the Agricultural Research Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 2.Shehab GG, Kansowa OA, El-Beltagi HS. Effects of various chemical agents for alleviation of drought stress in Rice plants (Oryza sativa L.). Not Bot Hort Agrobot Cluj. 2010;38:139–148. [Google Scholar]
- 3.Shallan MA, El-Beltagi HS, Mona A, Amera TM, Sohir NA. Effect of amylose content and pre-germinated brown rice on serum blood glucose and lipids in experimental animal. AJBAS. 2010a;4:114–121. [Google Scholar]
- 4.Shallan MA, El-Beltagi HS, Mona A, Amera TM. Chemical Evaluation of Pre-germinated Brown Rice and Whole Grain Rice Bread. EJEAFChe. 2010b;9:958–971. [Google Scholar]
- 5.Abdel-Rahim EA, El-Beltagi HS. Constituents of apple, parsley and lentil edible plants and their therapy treatments for blood picture as well as liver and kidneys functions against lipidemic disease. EJEAFChe. 2010;9:1117–1127. [Google Scholar]
- 6.Dillon SL, Shapter FM, Henry RJ, Cordeiro G, Izouierdo L. Domestication to crop improvement: genetic resources for sorghum and saccharum (Andropogoneae). Ann Bot. 2007;100(5):975–989. doi: 10.1093/aob/mcm192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicko MH, Gruppen H, Traoré AS, Voragen AGJ, van Berkel WJH. Sorghum grain as human food in Africa: relevance of content of starch and amylase activities. Afr J Biotech. 2006;5(5):384–395. [Google Scholar]
- 8.Afify AMR, El-Beltagi HS, Abd El-Salam SM, Omran AA. Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE. 2011a;6(10):1–7. doi: 10.1371/journal.pone.0025512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afify AMR, El-Beltagi HS, Abd El-Salam SM, Omran AA. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. APJTB. 2012;2(3):203–209. doi: 10.1016/S2221-1691(12)60042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soetan KO, Oyewole OE. The need for adequate processing to reduce the anti- nutritional factors in plants used for human food and animal feeds- A Review. Afr J Food Sci. 2009;3(9):123–132. [Google Scholar]
- 11.Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010;120:945–959. [Google Scholar]
- 12.Medugu CI, Kwari ID, Igwebuike J, Nkama I, Mohammed ID, et al. Performance and economics of production of broiler chickens fed sorghum or millet as replacement for maize in the semi-arid zone of Nigeria. Agric Biol J North Am. 2010;1(3):321–325. [Google Scholar]
- 13.Zhao R, Bean SR, Ioerger BP, Wang D, Boyle DL. Impact of mashing on sorghum proteins and its relationship to ethanol fermentation. J Agric Food Chem. 2008;56(3):946–953. doi: 10.1021/jf072590r. [DOI] [PubMed] [Google Scholar]
- 14.El-Beltagi HES, Mohamed AA. Variation in fatty acid composition, glucosinolate profile and some phytochemical contents in selected oil seed rape (Brassica napus L.) cultivars. Fats oil, Grasas y Aceites. 2010;61(2):143–150. [Google Scholar]
- 15.Afify AMR, Rashed MM, Ebtesam AM, El-Beltagi HS. Effect of gamma radiation on protein profile, protein fraction and solubility of three oil seeds. Not Bot Hort Agrobot Cluj. 2011b;39(2):90–98. [Google Scholar]
- 16.El-Beltagi HS. Effect of roasting treatments on protein fraction profiles, some enzyme activities of Egyptian peanuts. Inter J Food Sci Nutr. 2011;62(5):453–456. doi: 10.3109/09637486.2010.544642. [DOI] [PubMed] [Google Scholar]
- 17.El-Beltagi HS, Mohamed AA, Mekki BB. Differences in some constituents, enzymes activity and electrophoretic characterization of different rapeseed (Brassica napus L.) cultivars. Ann Univ Oradea –Fascicle Biol Tom. 2011;18(1):39–46. [Google Scholar]
- 18.El-Beltagi HS, Salama ZA, El-Hariri DM. Variations in oil and some phytochemical contents in flaxseed cultivars (Linum usitatissimum L.). EJEAFChe. 2011;10(8):2711–2721. [Google Scholar]
- 19.Coles LT, Moughan PJ, Darragh AJ. In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple stomached animals. Animal Food Sci Tech. 2005;123–124:421–444. [Google Scholar]
- 20.Hur SJ, Lim BO, Decker EA, McClements DJ. In vitro human digestion models for food applications. Food Chem. 2011;125:1–12. [Google Scholar]
- 21.Kayodé APP. Diversity, Users' Perception and Food Processing of Sorghum: Implications for Dietary Iron and Zinc Supply. 2006. pp. 1–151. Ph.D. Thesis, Wageningen Univ., Netherlands, Wageningen.
- 22.Steiner T, Mosenthin R, Zimmermann B, Greiner R, Roth S. Distribution of total phosphorus, phytate phosphorus and phytase activity in legume seeds, cereals and cereal by-products as influenced by harvest year and cultivar. Anim Feed Sci Tech. 2007;133:320–334. [Google Scholar]
- 23.Elkhalifa AO, Bernhardt R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010;121:387–392. [Google Scholar]
- 24.AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed. In: Horwitz W, editor. Washington, D.C.: Association of Official Analytical Chemists; 2000. [Google Scholar]
- 25.Rosen H. A modified ninhydrin calorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- 26.Sathe SK, Salunkhe DK. Solubilization and electrophoretic characterization of the great Northern bean (Phaseolus vulgaris L.) proteins. J Food Sci. 1981;46:82–87. [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Landry J, Moureaux T. Heterogeneity of corn seed glutelin: selective extraction and amino acid composition of the 3 isolated fractions. Bull Soc Chim Biol. 1970;52:1021–1037. [PubMed] [Google Scholar]
- 29.de Wreede I, Stegemann H. Determination of proteins in the presence of large quantities of sodium-dodecyl-sulphate and mercapto-ethanol by a modified biuret-method. Fresenius' J Anal Chem. 1981;308(5):431–433. [Google Scholar]
- 30.Akeson WR, Stahmanna AA. Pepsin pancreatin digest index of protein. J Nutr. 1964;83:257–261. doi: 10.1093/jn/83.3.257. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WB, Ratnayake WS, Jackson DS, Lee K, Herrman TJ, et al. Factors affecting the alkaline cooking performance of selected corn and sorghum hybrids. Cereal Chem. 2010;87(6):524–531. [Google Scholar]
- 32.Shaker AMH, Taha FA, Abdel Fattah SS. Influence of some processing methods on chemical composition of lentil, faba bean and faba bean dishes. Bull Nutr Institute. 1995;15(2):87–93. [Google Scholar]
- 33.Chavan JK, Kadam SS, Salunkhe DK. Changes in tannin, free amino acids, reducing sugars, and starch during seed germination of low and high tannin cultivars of sorghum. J Food Sci. 1981;46:638–639. [Google Scholar]
- 34.Mokrane H, Amoura H, Belhaneche-Bensemra N, Courtin CM, Delcour JA, et al. Assessment of Algerian sorghum protein quality Sorghum bicolor (L.) Moench using amino acid analysis and in vitro pepsin digestibility. Food Chem. 2010;121:719–723. [Google Scholar]
- 35.Ebadi MR, Pourreza J, Jamalian J, Edriss MA, Samie AH, et al. Amino acid content and availability in low, medium and high tannin sorghum grain for poultry. Inter J Poultry Sci. 2005;4(1):27–31. [Google Scholar]
- 36.Elemo GN, Elemo BO, Okafor JNC. Preparation and nutritional compostion of a weaning food formulated from germinated sorghum (Sorghum bicolor) and steamed cooked cowpea (Vigna unguiculata Walp.) Am J Food Tech. 2011;6(5):413–421. [Google Scholar]
- 37.Correia I, Nunes A, Barros AS, Delgadillo I. Protein profile and malt activities during sorghum germination. J Sci Food Agric. 2008;88:2598–2605. [Google Scholar]
- 38.Traoré T, Mouquet C, Icard-Verniere C, Traore AS, Treche S. Changes in nutrient composition, phytate and cyanide contents and α-amylase activity during cereal malting in small production units in Ouagadougou (Burkina Faso) Food Chem. 2004;88:105–114. [Google Scholar]
- 39.Vinay BJ, Sindhu Kanya TC. Effect of detoxification on the functional and nutritional quality of proteins of karanja seed meal. Food Chem. 2008;106:77–84. [Google Scholar]
- 40.Elkhalifa AO, Bernhardt R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010;121:387–392. [Google Scholar]
- 41.Hamaker BR, Bugusu BA. Overview: sorghum proteins and food quality. 2003. Paper presented at: Workshop on the proteins of sorghum and millets: enhancing nutritional and functional properties for Africa. [CD] (Pretoria, South Africa)
- 42.Ejeta G, Hassen MM, Mertz ET. In vitro digestibility and amino acid composition of pearl millet (Pennisetum typhoides) and other cereals. Proc Nat Acad Sci. 1987;84:6016–6019. doi: 10.1073/pnas.84.17.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel Moueium ZS, El Tinay AH, Abdalla AH. Effect of germination on protein fractions of corn cultivars. Food Chem. 1996;51(3):381–384. [Google Scholar]
- 44.Duodu KG, Taylor JRN, Belton PS, Hamaker BR. Factors affecting sorghum protein digestibility. J Cereal Sci. 2003;38:117–131. [Google Scholar]
- 45.McDonougha CM, Floydb CD, Waniskaa RD, Rooney LW. Effect of accelerated aging on maize, sorghum, and sorghum meal. J Cereal Sci. 2004;39:351–361. [Google Scholar]
- 46.Fageer ASM, El Tinay AH. Effect of genotype, malt pretreatment and cooking on in vitro protein digestibility and protein fractions of corn. Food Chem. 2004;84:613–619. [Google Scholar]
- 47.Abu Baker AA, El Tinay AH, Yagoub AA. Protein content and digestibility of sorghum (Sorghum bicolor)-: Effect of supplementation with soybean protein. Elect based fermented gruel. J Environ Agric Food Chem. 2010;9(9):1495–1501. [Google Scholar]
- 48.Correia I, Nunes A, Barros AS, Delgadillo I. Comparison of the effects induced by different processing methods on sorghum proteins. J Cereal Sci. 2010;51:146–151. [Google Scholar]
- 49.Khattak AB, Zeb A, Bibi N, Khalil SA, Khattak MS. Influence of germination techniques on phytic acid and polyphenols contents of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2007;104:1074–1079. [Google Scholar]
- 50.Abdelhaleem WH, El Tinay AH, Mustafa AI, Babiker EE. Effect of fermentation, malt-pretreatment and cooking on antinutritional factors and protein digestibility of sorghum cultivars. Pakistan J Nutr. 2008;7(2):335–341. [Google Scholar]