Abstract
The association of Ca2+-activated K+ channels with voltage-gated Ca2+ channels at the presynaptic active zones of hair cells, photoreceptors, and neurons contributes to rapid repolarization of the membrane after excitation. Ca2+ channels have been shown to bind to a large set of synaptic proteins, but the proteins interacting with Ca2+-activated K+ channels remain unknown. Here, we report that the large-conductance Ca2+-activated K+ channel of the chicken's cochlear hair cell interacts with β-catenin. Yeast two-hybrid assays identified the S10 region of the K+ channel's α-subunit and the ninth armadillo repeat and carboxyl terminus of β-catenin as necessary for the interaction. An antiserum directed against the α-subunit specifically coprecipitated β-catenin from brain synaptic proteins. β-Catenin is known to associate with the synaptic protein Lin7/Velis/MALS, whose interaction partner Lin2/CASK also binds voltage-gated Ca2+ channels. β-Catenin may therefore provide a physical link between the two types of channels at the presynaptic active zone.
The hair cell, the sensory receptor of the internal ear, releases neurotransmitter at presynaptic active zones studding its basolateral membrane surface (for a review, see ref. 1). Examination of hair cells by loose-seal patch recording demonstrated that each active zone bears a cluster of voltage-gated Ca2+ (CaV) channels and Ca2+-activated K+ (KCa) channels (2–4). When CaV channels are activated by a hair cell's depolarization in response to acoustical stimulation, the resultant Ca2+ influx not only triggers synaptic-vesicle release but also opens KCa channels. The efflux of K+ through these channels causes a rapid repolarization that plays a key role in the electrical oscillation that tunes some hair cells (5) and in fast inhibitory synaptic transmission (6). Nerve terminals of the central and peripheral nervous system, including those at the neuromuscular junction, use a similar mechanism to effect repolarization in anticipation of the next action potential (7–11). The presynaptic colocalization of KCa channels with CaV channels is thus indispensable for normal electrical signaling by excitatory cells.
Electrophysiological and molecular-biological analyses have shown that hair cells express L-type CaV channels and large-conductance (BK or Maxi) KCa channels (12–15) that contain, respectively, α1D-subunits (16–18) and slowpoke (slo) α- and slo β-subunits (19–22). In hippocampal neurons, both large- and small-conductance K+ channels occur in presynaptic regions and are associated, respectively, with L- and N-type CaV channels (7, 23). The CaV channels at the presynaptic active zone form a protein complex with several anchoring proteins (24–26), such as Mint-1, CASK, and Rab3-interacting molecule (RIM) binding proteins (RBP). In Drosophila, two cytosolic regulatory proteins, Slob and dSLIP, and two protein kinases, Src and PKAc, are able to assemble with the slo α-subunit (27–29). In vertebrates, however, only kinases, such as Src and Syk, have been reported to interact with this subunit (30–32). To seek other proteins that associate with KCa channels and mediate their presynaptic targeting, we used yeast two-hybrid screening to search a cDNA library made from sensory epithelia of the chicken's cochlea.
Materials and Methods
GST Fusion Proteins. DNA fragments corresponding to amino acid residues 1117–1325 of chicken RBP2 were amplified by PCRs and ligated into the pGEX-4T-1 expression vector (Amersham Biosciences). After the BL21 strain of Escherichia coli had been transformed with this vector, expression of the fusion protein was induced by the addition of 1 mM isopropyl thiogalactopyranoside for 3–4 h at 37°C. Bacteria were lysed by sonication in 1% Triton X-100 and 1 mM EDTA and a mixture of protease inhibitors (Roche Biochemicals) in PBS solution (Invitrogen). After centrifugation, lysates were recovered and the fusion proteins were purified on glutathione-Sepharose.
GST Pull-Down Assays. GST pull-down assays were performed as reported (25). Synaptic plasma membranes obtained from chicken brains by a one-step preparation method were solubilized in PBS containing 1% Triton X-100, 2 mM EDTA, and protease inhibitors and were then centrifuged at 100,000 × g for 15 min at 4°C. Solubilized protein (100 μg) was incubated overnight at 4°C with 30 μl of glutathione-Sepharose beads bound to 5 μg of purified fusion protein. Samples were washed four times at room temperature with 0.05% Triton X-100 in PBS. The material retained on the beads was then eluted with sample buffer solution and analyzed by SDS/PAGE and immunoblotting with anti-chicken slo antiserum.
Yeast Two-Hybrid Screens. Yeast two-hybrid screening was performed with the GAL4 system as described (25, 33). DNA encoding the bait, which consisted of residues 680–1146 from the cytoplasmic carboxyl terminus of the α-subunit from the chicken's slo (cslo) channel (20), was combined by the PCR in frame with the DNA-binding domain of GAL4 and subcloned into the pBDGal4 vector (Stratagene). A sensory epithelial cDNA library of 107 clones was constructed in the pADGal4 prey vector from basilar papillae of late-embryonic chickens and screened with the cslo bait. Positive clones were selected by histidine prototrophy and assayed for β-galactosidase activity. Doubly positive clones were isolated and characterized by sequencing. For the mapping of interaction sites between the slo α-subunit and β-catenin, additional baits of the cytoplasmic domain of the slo α-subunit and additional preys of β-catenin were generated by PCR amplification.
Antisera. A polyclonal rabbit antiserum directed against the α-subunit of the cslo channel was raised against a hexahistidine fusion protein corresponding to the protein's residues 1117–1325 and affinity-purified against the corresponding GST-cslo fusion protein transferred onto nitrocellulose. Antisera directed against murine slo, β-catenin, synaptotagmin/p65, Rab3, and RIM1 were purchased from Transduction Laboratories (Lexington, KY). Antisera against N-methyl-d-aspartate receptor 1, SNAP-25, and synaptophysin were obtained from Affinity Bioreagents (Golden, CO) and Santa Cruz Biotechnology. Antisera against the Ca2+ channel's α1D-subunit and RBP2 were generated as reported (25).
Coimmunoprecipitation. Synaptic membrane proteins were solubilized in PBS containing 1% Triton X-100, 2 mM EDTA, and protease inhibitors and were then centrifuged at 100,000 × g for 15 min at 4°C. Soluble proteins were incubated overnight at 4°C with affinity-purified anti-cslo antiserum covalently bound to protein A-Sepharose beads. The beads were washed four times with 0.05% Triton X-100 in PBS. Purified proteins were resolved by SDS/PAGE and probed with anti-murine slo or anti-β-catenin antiserum.
Subcellular Fractionation and Solubility Analysis. Subcellular fractionation and solubility analysis of chicken brain proteins were performed as described previously (25, 34).
Results
KCa channels are colocalized with CaV channels at the presynaptic active zones of internal-ear hair cells (1), retinal photoreceptors (35), peripheral nerve terminals (7–11), and neurons in the central nervous system (7, 23). We therefore sought a physical link between the two types of channels in native tissues. Because we had observed that RBPs interact with CaV channel α1B (Cav2.2) and α1D (Cav1.3) subunits (25), we constructed a GST-fusion protein containing the second and third Srchomology 3 domains of chicken RBP2 (GST:RBP2) and inquired whether the protein complex that associated with it also included large-conductance KCa channels. Functional KCa channels are composed of slo α- and β-subunits. We prepared a specific antiserum against the α-subunit and analyzed the proteins that bound to the fusion protein by immunoblotting. GST:RBP2 precipitated the chicken's slo α-subunit from brain synaptic proteins (Fig. 1). As reported (25), the precipitants included the α1B- and α1D-subunits of CaV channels (data not shown). Because direct interaction did not occur in vitro between the slo α-subunit and either RBP2 or RIM2, it seemed probable that unknown proteins provided a link between the two channels.
Fig. 1.
Large-conductance KCa channels associate with Src-homology 3 domains of RBP2. The detergent-extracted synaptic fraction of chicken brain proteins was incubated with either a GST fusion protein alone (second lane) or protein containing the Src-homology 3 domains of presynaptic RBP2 (third lane). Proteins bound to glutathione-Sepharose were analyzed by Western blotting with an antiserum directed against the cslo α-subunit. Solubilized protein (30 μg) was loaded to the first lane as a positive control for the immunoreactivity of the α-subunit. Because GST:RBP2 interacts with CaV channels, the result indicates that the CaV and KCa channels coexist in a protein complex.
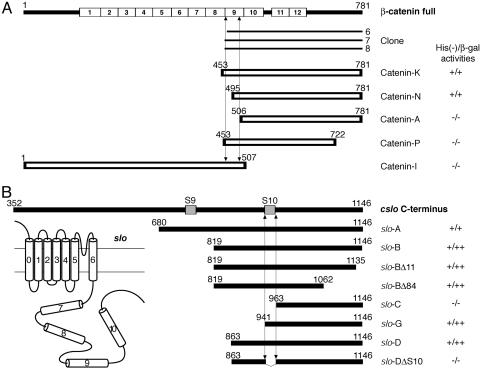
To seek molecules that bind the cslo α-subunit, we screened a cDNA library of chicken sensory epithelia by the yeast two-hybrid technique (25, 33) with a bait comprising 467 amino acid residues from the channel's carboxyl terminus. We obtained three independent positive colonies, each of which encoded a carboxyl-terminal fragment of chicken β-catenin (Fig. 2A).
Fig. 2.
Characterization of the interaction sites of β-catenin and the cslo α-subunit by two-hybrid experiments. (A) Using the carboxyl-terminal region of cslo α-subunit as bait, we obtained from a sensory epithelial library three positive clones, each of which represented a part of chicken β-catenin. Five β-catenin fragments were additionally tested with bait constructs to clarify the domains necessary for the interaction with the α-subunit. (B) To map the interaction site in the cslo α-subunit, we examined eight fragments in bait vectors with prey clone 7 from A. Interaction requires the S10 region of the cslo α-subunit and both the carboxyl-terminal half of the ninth armadillo repeat and the carboxyl terminus of β-catenin. (Inset) Schematic diagram of the slo channel.
To map the interaction sites between β-catenin and the cslo α-subunit, we conducted additional two-hybrid experiments with deletion mutants (Fig. 2 A). The constructs catenin K and catenin N, each of which encodes a region extending from the second half of the ninth armadillo repeat to the carboxyl terminus, bound to the carboxyl terminus of the cslo α-subunit. Catenin A, which lacked all the ninth armadillo repeat, no longer associated with the α-subunit. Moreover, catenin P, which retained the repeat but lacked the 59 amino acid residues of the carboxyl terminus, did not to bind the α-subunit (Fig. 1 A). We therefore concluded that the carboxyl-terminal half of the ninth armadillo repeat and the carboxyl terminus of β-catenin are required for the interaction.
We next attempted to map the interacting domain in the cslo α-subunit. The potential PDZ domain-binding motif localized at the carboxyl terminus of the α-subunit was not needed for the binding to β-catenin, because slo-BΔ11 and slo-BΔ84 constructs that lacked, respectively, the final 11 and 84 amino acid residues still interacted with β-catenin. The mutants slo-B, slo-G, and slo-D, which lacked the hydrophobic S9 domain, interacted with β-catenin; the constructs slo-C and slo-DΔ84, which lacked the S10 domain, were unable to associate. These results imply that the S10 region of the α-subunit's cytoplasmic carboxyl terminus is responsible for the interaction with β-catenin.
We next performed GST pull-down and coimmunoprecipitation experiments on transfected mammalian cells to determine whether the interaction of β-catenin with the slo α-subunit is direct. Expressed alone or together with auxiliary β1, β2, β3, or β4-subunits in fibroblastic COS or epithelial Madin–Darby canine kidney cells (36), the slo α-subunit did not associate with endogenous β-catenin or a GST-β-catenin fusion protein (data not shown). These results suggested that the interaction of the slo α-subunit and β-catenin is indirect or that the association depends on the conformational state of one or both proteins.
The S10 domain of the slo α-subunit belongs to the so-called “calcium bowl,” a region formerly believed to bind Ca2+ and to undergo conformational changes during channel activation (ref. 37; see also ref. 38). To test the effect of depolarization or elevated intracellular Ca2+ concentration on the interaction, we depolarized transfected cells with a high external K+ concentration or exposed them to a Ca2+ ionophore. Neither condition promoted a stable interaction between the slo α-subunit and β-catenin (data not shown). At the same time, overexpression of β-catenin in COS cells transfected with cslo α-subunit and the β1-subunit did not change the current density, activation threshold, or Ca2+ sensitivity of large-conductance K+ channels (data not shown). We also examined the phosphorylation status of β-catenin. Recently, it was shown that β-catenin accumulates at synaptic sites after depolarization of hippocampal slices, a redistribution mimicked by a tyrosine kinase (39). Moreover, the Tyr-654 point mutation of β-catenin alters its distribution; Y654E-β-catenin is concentrated in dendritic shafts, whereas Y654F-β-catenin accumulates in spines. We coexpressed these two mutant forms of β-catenin with the slo α-subunit but did not observe any direct interaction (data not shown).
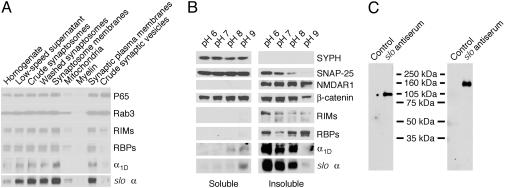
Because our repeated attempts to discriminate between indirect or conformation-dependent interactions were unsuccessful, we focused on the colocalization and association of β-catenin and the slo α-subunit in the brain. To characterize the α-subunit's localization, we first prepared subcellular fractions of chicken brain proteins and probed them with antisera directed against a variety of synaptic markers (Fig. 3A). We found that the cslo α-subunit, together with RIMs, RBPs, and the α1D-subunit of CaV channels, was enriched in a fraction corresponding to synaptic plasma membranes but not in that containing synaptic vesicles (Fig. 3A). To separate presynaptic particles and postsynaptic densities, we next extracted brain synaptosomes with 1% Triton X-100 at different pH values (34). As expected for a protein present in the presynaptic specialization, SNAP-25 was present in the pellet at the lowest pH levels but was extracted at pH values >7. Consistent with its linkage to postsynaptic densities, N-methyl-d-aspartate receptor 1 was found in the insoluble fraction regardless of the pH. The vesicle protein synaptophysin occurred exclusively in the soluble fraction. β-Catenin, which is present at both pre- and postsynaptic membranes (39–42), was found in both the insoluble and the soluble fractions. As reported (25, 34), RIMs and RBPs are presynaptic proteins that remain associated with postsynaptic densities under conditions in which the presynaptic particles are extracted. In these experimental conditions, we observed that the extraction pattern of the cslo α-subunit was almost identical with that of the Cav channel's α1D-subunit (Fig. 3B). Both channel subunits were extracted at pH levels >8, a result compatible with their presynaptic localization. Altogether, these results support the colocalization of β-catenin (41), RIMs, RBPS, and the two types of channels at presynaptic active zones in the brain.
Fig. 3.
Large-conductance KCa channels assemble with β-catenin in the presynaptic active zones of the chicken's brain. (A) Subcellular fractions were prepared as described in Materials and Methods and analyzed by Western blotting. In conjunction with other synaptic proteins, the cslo α-subunit is enriched in the fraction containing synaptic plasma membranes (second lane from the right). Note that the crude fraction of synaptic vesicles (right lane) contains little of the cslo α-subunit, whose distribution pattern resembles those of other constituents of the presynaptic active zone, such as RIMs, RBPs, and the Cav channel α1D-subunit. (B) In a solubility analysis of synaptic proteins, synaptosomes were extracted with 1% Triton X-100 at the indicated pH levels; equal volumes of soluble and insoluble materials were then analyzed by immunoblotting. Both the cslo α-subunit and the α1D (Cav1.3) Ca2+-channel subunit were extracted at pH levels >8, a result compatible with their presynaptic localization. β-Catenin is present in both pre- and postsynaptic locations. (C) Brain synaptic proteins were purified on an anti-cslo α-subunit affinity column. The precipitants were then separated by SDS/PAGE, transferred to blots, and detected with antiserum directed against either anti-β-catenin (Left) or anti-slo α-subunit (Right). The result indicates that β-catenin and the slo α-subunit associate in a protein complex.
We confirmed the close association between β-catenin and the cslo α-subunit in vivo by copurification (Fig. 3C). An affinity column was used to purify the slo α-subunit from solubilized synaptic membranes. The purification was verified by detecting this subunit with a cross-reacting antiserum against the murine slo α-subunit. Reprobing of the blot with an antiserum directed against β-catenin demonstrated that β-catenin occurred in the same protein complex.
Discussion
In this study, we have demonstrated that the α-subunit of the cslo K+ channel binds β-catenin in two-hybrid assays and that the two proteins belong to the same protein complex at presynaptic active zones in the brain. β-Catenin has recently been reported to be necessary for relocation of a PDZ-domain anchoring protein, Lin7/Velis/MALS, to an epithelial junction (43). Because β-catenin and Lin7 are both localized in presynaptic terminals (41, 43), β-catenin may play an essential role in recruitment of a variety of molecules to a specific region. For example, the kainate-receptor subunit glutamate receptor 6 is recruited to the synaptic membrane by cadherin–catenin complexes through its interaction with β-catenin (44). Expressed at the basolateral membrane of hair cells (45), β-catenin may thus direct cslo KCa channels to the presynaptic active zones of those cells and of neurons.
Other presynaptic proteins have been found to form a complex including β-catenin. CASK, Mint1, and Lin7/Velis/MALS associate tightly in the brain (46). In turn, the Cav channel's α1B-subunit that predominates in the presynaptic terminals of brain neurons interacts with CASK and Mint1 (24). Because β-catenin directly binds a PDZ domain of Lin7/Velis/MALS through its carboxyl terminus (43), it is possible that slo KCa channels indirectly associate with Cav channels through a physical bridge including β-catenin. Cav channels directly bind SNARE proteins, so the large protein complex including the slo α-subunit and β-catenin might additionally associate with the exocytotic machinery. Tight colocalization of these different elements would ensure the fast regulation of synaptic transmission. Although large-conductance KCa channels are colocalized with L-type Cav channels in the hair cell, this colocalization does not occur in certain neurons. In hippocampal neurons, for example, large- and small-conductance K+ channels couple with N- and L-type Cav channels, respectively (23). Therefore, a divergence of the adaptor proteins that associate with large- or small-conductance KCa channels may occur in vivo and determine the selective targeting and coupling of the channels in a microdomain of the synaptic terminal.
The subcellular localization of β-catenin is dynamically regulated. β-Catenin plays a central role in the developmentally important Wnt signaling pathway (for review, see ref. 47). In this case, the localization of β-catenin is regulated by ligand–receptor interactions at the cell surface. In the brain, the distribution of β-catenin is influenced by neural activity. The resulting change in adhesion molecules with activity may coordinate presynaptic and postsynaptic plasticity by affecting synaptic size and strength (39). As previously reported for the glutamate receptor subunit 6, the association between β-catenin and the slo α-subunit may be indirect. A conformation-dependent interaction between the slo α-subunit and β-catenin may constitute another functional link between electrical activity and synaptic plasticity. Further studies are needed to clarify the mechanism of association of the slo α-subunit and β-catenin.
Acknowledgments
We thank J. D. Lippiat (University of Leicester, Leicester, U.K.) for the gift of pIRES-hsloα+β constructs, S. Murase and E. M. Schuman (California Institute of Technology, Pasadena) for β-catenin plasmids, and B. Attali and Y. Mori for helpful comments on the manuscript. During the initial portion of this study, H.H. was supported by Human Frontier Science Program Grant LT0318/1999-B. This research was supported by National Institutes of Health Grant DC00241. A.J.H. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: CaV, voltage-gated Ca2+; slo, slowpoke; cslo, chicken slo; KCa, Ca2+-activated K+; RIM, Rab3-interacting molecule; RBP, RIM-binding protein.
References
- 1.Hudspeth, A. J. (1997) Neuron 19, 947–950. [DOI] [PubMed] [Google Scholar]
- 2.Roberts, W. M., Jacobs, R. A. & Hudspeth, A. J. (1990) J. Neurosci. 10, 3664–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Issa, N. P. & Hudspeth, A. J. (1996) Proc. Natl. Acad. Sci. USA 93, 9527–9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Issa, N. P. & Hudspeth, A. J. (1996) J. Neurocytol. 25, 257–266. [DOI] [PubMed] [Google Scholar]
- 5.Hudspeth, A. J. & Lewis, R. S. (1988) J. Physiol. (London) 400, 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver, D., Klocker, N., Schuck, J., Baukrowitz, T., Ruppersberg, J. P. & Fakler, B. (2000) Neuron 26, 595–601. [DOI] [PubMed] [Google Scholar]
- 7.Hu, H., Shao, L. R., Chavoshy, S., Gu, N., Trieb, M., Behrens, R., Laake, P., Pongs, O., Knaus, H. G., Ottersen, O. P. & Storm, J. F. (2001) J. Neurosci. 21, 9585–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robitaille, R. & Charlton, M. P. (1992) J. Neurosci. 12, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robitaille, R., Garcia, M. L., Kaczorowski, G. J. & Charlton, M. P. (1993) Neuron 11, 645–655. [DOI] [PubMed] [Google Scholar]
- 10.Blundon, J. A., Wright, S. N., Brodwick, M. S. & Bittner, G. D. (1995) J. Neurophysiol. 73, 178–189. [DOI] [PubMed] [Google Scholar]
- 11.Wang, Z. W., Saifee, O., Nonet, M. L. & Salkoff, L. (2001) Neuron 32, 867–881. [DOI] [PubMed] [Google Scholar]
- 12.Lewis, R. S. & Hudspeth, A. J. (1983) Nature 304, 538–541. [DOI] [PubMed] [Google Scholar]
- 13.Hudspeth, A. J. & Lewis, R. S. (1988) J. Physiol. (London) 400, 237–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Art, J. J., Wu, Y. C. & Fettiplace, R. (1995) J. Gen. Physiol. 105, 49–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zidanic, M. & Fuchs, P. A. (1995) Biophys. J. 68, 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollmar, R., Fak, J., Montgomery, L. G. & Hudspeth, A. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14889–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollmar, R., Montgomery, L. G., Fak, J., Henry, L. J. & Hudspeth, A. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14883–14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platzer, J., Engel, J., Schrott-Fischer, A., Stephan, K., Bova, S., Chen, H., Zheng, H. & Striessnig, J. (2000) Cell 102, 89–97. [DOI] [PubMed] [Google Scholar]
- 19.Navaratnam, D. S., Bell, T. J., Tu, T. D., Cohen, E. L. & Oberholtzer, J. C. (1997) Neuron 19, 1077–1085. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblatt, K. P., Sun, Z.-P., Heller, S. & Hudspeth, A. J. (1997) Neuron 19, 1061–1075. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan, K., Michael, T. H., Jiang, G. J., Hiel, H. & Fuchs, P. A. (1999) Science 283, 215–217. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan, K., Michael, T. H. & Fuchs, P. A. (2000) J. Neurosci. 20, 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrion, N. V. & Tavalin, S. J. (1998) Nature 395, 900–905. [DOI] [PubMed] [Google Scholar]
- 24.Maximov, A., Südhof, T. C. & Bezprozvanny, I. (1999) J. Biol. Chem. 274, 24453–24456. [DOI] [PubMed] [Google Scholar]
- 25.Hibino, H., Pironkova, R., Onwumere, O., Vologodskaia, M., Hudspeth, A. J. & Lesage, F. (2002) Neuron 34, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maximov, A. & Bezprozvanny, I. (2002) J. Neurosci. 22, 6939–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schopperle, W. M., Holmqvist, M. H., Zhou, Y., Wang, J., Wang, Z., Griffith, L. C., Keselman, I., Kusinitz, F., Dagan, D. & Levitan, I. B. (1998) Neuron 20, 565–573. [DOI] [PubMed] [Google Scholar]
- 28.Xia, X., Hirschberg, B., Smolik, S., Forte, M. & Adelman, J. P. (1998) J. Neurosci. 18, 2360–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, J., Zhou, Y., Wen, H. & Levitan, I. B. (1999) J. Neurosci. 19, RC4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling, S., Woronuk, G., Sy, L., Lev, S. & Braun, A. P. (2000) J. Biol. Chem. 275, 30683–30689. [DOI] [PubMed] [Google Scholar]
- 31.Alioua, A., Mahajan, A., Nishimaru, K., Zarei, M. M., Stefani, E. & Toro, L. (2002) Proc. Natl. Acad. Sci. USA 99, 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezzonico, R., Schmid-Alliana, A., Romey, G., Bourget-Ponzio, I., Breuil, V., Breittmayer, V., Tartare-Deckert, S., Rossi, B. & Schmid-Antomarchi, H. (2002) J. Bone Miner. Res. 17, 869–878. [DOI] [PubMed] [Google Scholar]
- 33.Hibino, H., Pironkova, R., Onwumere, O., Rousset, M., Charnet, P., Hudspeth, A. J. & Lesage, F. (2003) Proc. Natl. Acad. Sci. USA 100, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips, G. R., Huang, J. K., Wang, Y., Tanaka, H., Shapiro, L., Zhang, W., Shan, W. S., Arndt, K., Frank, M., Gordon, R. E., et al. (2001) Neuron 32, 63–77. [DOI] [PubMed] [Google Scholar]
- 35.Stockbridge, N. & Ross, W. N. (1984) Nature 309, 266–268. [DOI] [PubMed] [Google Scholar]
- 36.Lippiat, J. D., Standen, N. B., Harrow, I. D., Phillips, S. C. & Davies, N. W. (2003) J. Membr. Biol. 192, 141–148. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber, M. & Salkoff, L. (1997) Biophys. J. 73, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piskorowski, R. & Aldrich, R. W. (2002) Nature 420, 499–502. [DOI] [PubMed] [Google Scholar]
- 39.Murase, S., Mosser, E. & Schuman, E. M. (2002) Neuron 35, 91–105. [DOI] [PubMed] [Google Scholar]
- 40.Uchida, N., Honjo, Y., Johnson, K. R., Wheelock, M. J. & Takeichi, M. (1996) J. Cell Biol. 135, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benson, D. L. & Tanaka, H. (1998) J. Neurosci. 18, 6892–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg, M. S. & McNutt, P. M. (1999) Curr. Opin. Cell Biol. 11, 554–560. [DOI] [PubMed] [Google Scholar]
- 43.Perego, C., Vanoni, C., Massari, S., Longhi, R. & Pietrini, G. (2000) EMBO J. 19, 3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coussen, F., Normand, E., Marchal, C., Costet, P., Choquet, D., Lambert, M., Mege, R. M. & Mulle, C. (2002) J. Neurosci. 22, 6426–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warchol, M. E. (2002) J. Neurosci. 22, 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butz, S., Okamoto, M. & Südhof, T. C. (1998) Cell 94, 773–782. [DOI] [PubMed] [Google Scholar]
- 47.Moon, R. T., Bowerman, B., Boutros, M. & Perrimon, N. (2002) Science 296, 1644–1646. [DOI] [PubMed] [Google Scholar]