Abstract
Perfluorooctanesulfonic acid (PFOS) is an organic contaminant ubiquitous in the environment, wildlife, and humans. Few studies have assessed its chronic toxicity on aquatic organisms. The present study defined the effects of long-term exposure to PFOS on zebrafish development and reproduction. Specifically, zebrafish at 8 h postfertilization (hpf) were exposed to PFOS at 0, 5, 50, and 250 μg/L for five months. Growth suppression was observed in the 250 μg/L PFOS-treated group. The sex ratio was altered, with a significant female dominance in the high-dose PFOS group. Male gonad development was also impaired in a dose-dependent manner by PFOS exposure. Although female fecundity was not impacted, the F1 embryos derived from high-dose exposed females paired with males without PFOS exposure developed severe deformity at early development stages and resulted in 100% larval mortality at 7 d postfertilization (dpf). Perfluorooctanesulfonic acid quantification in embryos indicated that decreased larval survival in F1 offspring was directly correlated to the PFOS body burden, and larval lethality was attributable to maternal transfer of PFOS to the eggs. Lower-dose parental PFOS exposure did not result in decreased F1 survival; however, the offspring displayed hyperactivity of basal swimming speed in a light-to-dark behavior assessment test. These findings demonstrate that chronic exposure to PFOS adversely impacts embryonic growth, reproduction, and subsequent offspring development. Environ.
Keywords: Zebrafish embryo, Chronic exposure, Perfluorooctanesulfonic acid, Sex ratio, Locomotion
INTRODUCTION
Perfluorinated compounds (PFCs) are a group of chemicals widely used as surfactants, lubricants, polymers, and firefighting foams. They are ubiquitous in the environment, wildlife, and humans; and they have recently emerged as a group of persistent organic pollutants [1]. Perfluorooctanesulfonic acid (PFOS) is an end product of the breakdown of many PFCs and also has been one of the most prevalent PFCs detected in wildlife and humans [2,3]. Although PFOS is generally found at low levels over a range of 0.1 to 100 ng/L in surface water, concentrations up to 600 ng/L have been reported in downstream rivers of fluorochemical manufacturing facilities [4]. The total PFCs downstream of spills can also range up to 17,000 μg/L [5]. In addition, this compound is characterized by high bioaccumulation and negligible elimination. For example, PFOS detected in the liver was up to 9,031 μg/kg wet weight for the freshwater eel Anguilla anguilla in Flanders (Belgium), yet such high concentrations of hepatic PFOS were not shown to be linked to fish growth [6].
The toxicity of PFOS has been studied in a number of fish and mammalian model species. Previous studies with aquatic model fish revealed that PFOS exposure causes developmental toxicity in oviparous fish such as medaka Oryzias latipes [7], zebrafish Danio rerio [8–10], and viviparous fish such as the green swordtail Xiphophorus helleri [11]. Analysis of gene expression [12], protein profile [13], and micro-RNA expression [14] showed that PFOS exposure affected multiple pathways involved in development, reproduction, and stress response. In particular, PFOS-induced oxidative stress has been suspected to be one of the main causes for its developmental toxicity in zebrafish embryos [9,15]. Moreover, elevated expression of vitellogenin gene was reported in male fish exposed to PFOS [11,16]. However, most of these observations were derived from acute high PFOS exposures. The impact of long-term continuous exposure to PFOS, which would more accurately resemble the real situation of aquatic animals dwelling in a PFOS-contaminated environment, remains largely unknown. Furthermore, the effects of chronic PFOS exposure on gamete quality have not been defined. Finally, the persistent adverse effects from parental exposure on unexposed offspring have not been explored.
The use of zebrafish as an appropriate vertebrate model for investigating various environmental pollutants has increased in popularity in recent years; however, studies related to PFOS toxicity are limited [8,9]. The present study employed the zebrafish model to define the developmental and reproductive toxicity associated with long-term chronic PFOS exposures. Specifically, we evaluated the effect of long-term PFOS exposure on F0 parental growth and their reproductive potential as assessed by sperm quality in males and fecundity in females, and F1 offspring development, growth, and behavior. A strong correlation between F1 larval mortality and PFOS body burdens was identified. The findings of the present study showed that long-term exposure to PFOS result in altered sex ratio in the F0 generation and maternal-related total mortality in F1 offspring.
MATERIALS AND METHODS
Fish husbandry and embryo collection
Adult zebrafish (Danio rerio) of the wild-type strain (AB) were raised and kept at standard laboratory conditions of 28°C with a 14:10 h light:dark cycle (lights on at 8:00 AM) in a recirculation system according to standard zebrafish culture protocols [17]. Water supplied to the system was filtered by reverse osmosis (pH 7.0–7.5), and Instant Ocean® salt was added to the water to raise the conductivity to 450 to 1,000 μS/cm (system water). The adult fish were fed twice daily with live artemia (Jiahong Feed) and dry flake food (Zeigler). The use of zebrafish for research protocol was approved by the Institutional Animal Care and Use Committee at the Wenzhou Medical College.
Zebrafish embryos were obtained from spawning adults in tanks overnight with a sex ratio of 1:1. Embryos were collected within 1 h after spawning and rinsed in embryo medium [17]. The fertilized embryos were inspected and staged using a stereomicroscope (Nikon, Japan) according to the descriptions of Kimmel et al. [18].
PFOS stock solutions and exposure protocols
Perfluorooctanesulphonic acid (CAS 1763-23-1, purity > 96%) was purchased from Sigma-Aldrich Chemical, and stock solutions were prepared by dissolving in 100% dimethylsulfoxide (DMSO). High-quality 8 h postfertilization (hpf) embryos were divided into four treatment groups: DMSO vehicle control (0.01% v/v), and PFOS at 5, 50, and 250 μg/L. Embryos were first exposed to PFOS in a petri dish (100 embryos/treatment) for 5 d without media change, and all embryos hatched and survived in this stage. After 5 d, the fish were transferred into 2-L tanks for the period of 5 d postfertilization (dpf) to 30 dpf, and after that were raised in 9-L tanks (30 fish/tank) until the end of experiment at 150 dpf. Fish were kept in a static system, and 50% water was renewed with freshly prepared solutions every 5 d. Each tank was checked for morbid fish on a daily basis, and water quality was monitored on a weekly basis. Feeding was initiated at day 5. Between 5 and 14 dpf, fish were fed three times daily with zebrafish larval diet (Aquatic Habitats), and after 14 dpf they were fed twice daily with freshly hatched live Artemia. The experiment was repeated three times with embryos derived from different parental stocks.
Evaluation of F0 adult fish
At the end of exposure period (150 dpf), all fish were checked for their sex. A subsample of 10 male and 10 female fish from each batch were also measured for standard body length (from snout to the fork point of caudal fin) and wet weight. Condition factor (K = weight (g) × 100/length (cm)3) was also tabulated to determine their overall fitness.
To evaluate sperm quality in male F0 fish after chronic PFOS exposure, sperm were collected by surgical removal of testis and prepared in Hanks’ balanced salt solution (see Jing et al. [19] for details). Sperm motility was determined by a computer-assisted sperm analysis system (CASA, IVOS ver 12.0, Hamilton Thorne Bioscience) following our previous published method [20]. The total motility indicates the percentage of all motile sperm, and the progressive motility indicates the percentage of motile sperm that expressed vigorous swimming. Sperm membrane was extracted for lipids to characterize the membrane lipid ratio. Lipid extraction was based on the method of Bligh and Dyer [21], and quantification was carried out on an Agilent 1200 liquid chromatography system equipped with an ultraviolet detector. A reversed-phase Zorbas Extend-C18 column (4.6 × 250 mm, 5 μm particle size) was used for cholesterol separation, and a normal-phase Rx-SIL analytical high-performance liquid chromatography column (4.6 × 250 mm, 5 μm particle sizes) was used for detection of phospholipids (phosphatidylethanolamine, phosphatidylcholine, and sphingomyelin). The ratio of cholesterol to phospholipids (C/P) was used as an end readout for membrane lipid composition. A total of 30 male fish with 10 fish per replicate were used for each treatment group.
Evaluation of F1 embryos and larvae
Breeding trials were carried out to produce F1 offspring. Six different crosses were employed between F0 females and males; namely, females were paired with males in the same treatment groups of DMSO control and PFOS-exposed concentrations of 5, 50, and 250 μg/L. Females from the 250-μg/L PFOS treatment group were paired with males from the DMSO controls, and females from the controls were paired with males from the 250-μg/L PFOS treatment group. For each of these crosses, eight randomly selected female fish were paired with four male fish in two separate spawning tanks with four females and two males per tank. Spawning was induced every other day for 5 d, and embryos were used for monitoring their developmental progress. All eggs from each spawn were evaluated for fertilization success. Percent fertilization was expressed as the number of fertilized eggs divided by total number of eggs. Fifty fertilized embryos from each spawn were further monitored for continuous development. Percent hatch was calculated at 72 hpf. Larvae were also assessed for their morphological appearance. Percent survival was monitored until 8 dpf.
Survival larvae at 5 dpf with normal morphology were further subjected to behavior assessment as detailed in our previous study [10]. In brief, larval swimming speeds were recorded when they responded to a 70-min dark to light (10 min for each period) transition stimulation. The test was performed in a ZebraLab behavior monitoring station (ViewPoint Life Sciences).
Quantification of PFOS in fish tissues
The PFOS concentrations in whole-body tissues of larvae or adult zebrafish were determined with waters Acquity UPLC combined Quattro Premier XE Micmass (Waters Corp) modified from So et al. [2]. The method for sample preparation was detailed in our previous study [10]. For adult fish, PFOS content in six females and six males were measured individually after 150 d exposure. For F1 embryos, a total of 40 embryos were pooled as a single sample, and measurements were replicated six times for each of the different crosses.
Immunohistochemistry
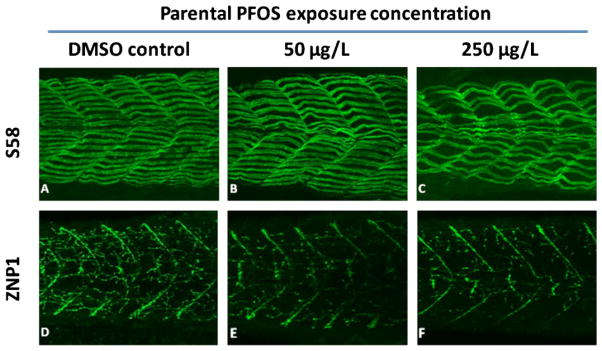
Embryos from each breeding scheme were raised in embryo medium supplemented with 0.003% 1-phenyl-2-thiourea to inhibit pigmentation until 5 dpf. Larvae were fixed in paraformaldehyde, washed in phosphate-buffered saline Tween-20 three times, and blocked in 10% goat serum overnight at 4°C. Samples were then stained with the monoclonal antibodies S58 for slow myofibrils and znp1 for primary motor neurons (Abnova).
Data analysis
One-way analysis of variance and Turkey’s multiple range tests were used to determine significant differences between control and exposure groups. Cholesterol-to-phospholipids ratio was tested with the chi-squared test (χ2 test). All statistical analyses were performed using SPSS 16.0 software, and p <0.05 was considered a statistically significant difference. The data were reported as mean ± standard error.
RESULTS
Altered F0 sex ratio
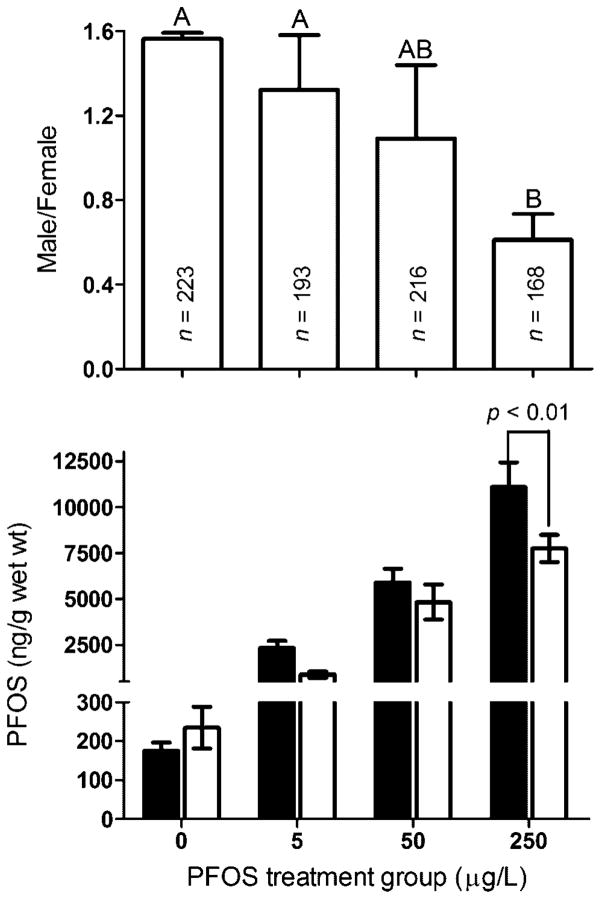
The sex ratio of the F0 population decreased with increasing PFOS expose concentration, and a significant difference was found between the DMSO control (1.56 ± 0.02) and the 250-μg/L treatment group (0.61 ± 0.07) (Fig. 1). Perfluorooctanesulfonic acid accumulation in body tissue increased with exposure concentration, and a significant difference was seen between males (11.1 ± 1.3 μg/g wet wt) and females (7.7 ± 0.7 μg/g) in the 250-μg/L treatment group (Fig. 1).
Fig. 1.
Sex ratio and uptake of perfluorooctanesulfonic acid (PFOS) in male (dark bars) and female (white bars) adult zebrafish after continuous exposure to PFOS at 0 (dimethylsulfoxide control), 5, 50, and 250 μg/L for 5 months starting from embryos at 8 h postfertilization.
F0 growth
Significant reduction in body length and wet weight was found in males and females for the treatment of 250 μg/L, but only in the males for the treatment of 50 μg/L (Table 1). A significant increase in condition factor was only found at 250 μg/L for both males and females (Table 1).
Table 1.
Body length, wet weight, and condition factor of zebrafish after exposure to perfluorooctanesulfonic acid (PFOS) for five months beginning from 8 h post-fertilization
| Sex | PFOS (μg/L) | No. of fish | Length (cm) | Weight (g) | Condition factor |
|---|---|---|---|---|---|
| Female | 0 | 30 | 3.19 ± 0.06 | 0.27 ± 0.01 | 0.84 ± 0.03 |
| 5 | 30 | 3.13 ± 0.05 | 0.29 ± 0.01 | 0.94 ± 0.05 | |
| 50 | 30 | 2.89 ± 0.08 | 0.25 ± 0.01 | 1.07 ± 0.09 | |
| 250 | 30 | 2.68 ± 0.12** | 0.21 ± 0.01** | 1.22 ± 0.15* | |
| Male | 0 | 30 | 3.17 ± 0.06 | 0.22 ± 0.01 | 0.71 ± 0.03 |
| 5 | 30 | 3.04 ± 0.04 | 0.21 ± 0.00 | 0.76 ± 0.02 | |
| 50 | 30 | 2.89 ± 0.05** | 0.19 ± 0.01** | 0.80 ± 0.02 | |
| 250 | 30 | 2.84 ± 0.04** | 0.19 ± 0.01** | 0.83 ± 0.04** |
p <0.05;
p <0.01.
Sperm quantity and quality in F0 males
Chronic exposure of PFOS reduced sperm density in a dose-dependent mode (Table 2). Both total and progressive motility exhibited significant reduction at high PFOS treatment groups of 50 and 250 μg/L. Elevated C/P ratio of sperm plasma membrane was only found at 50 μg/L (Table 2).
Table 2.
Testis weight, sperm number, motility and plasma membrane lipid composition expressed as the ratio of cholesterol to phospholipids (C/P) of male zebrafish (n = 10) after exposure to perfluorooctanesulfonic acid (PFOS) for five months beginning from 8 h postfertilization
| PFOS (μg/L) | Testis weight (mg) | Sperm density (106cell/g testis) | % Motility
|
C/P | |
|---|---|---|---|---|---|
| Total | Progressive | ||||
| 0 | 4.2 ± 1.5 | 2,020 ± 100 | 72 ± 11 | 17 ± 8 | 0.0659 |
| 5 | 4.3 ± 1.9 | 1,671 ± 73** | 67 ± 14 | 15 ± 8 | 0.0681 |
| 50 | 4.0 ± 1.7 | 1,138 ± 95** | 58 ± 16** | 13 ± 7* | 0.1534* |
| 250 | 3.5 ± 1.3 | 1,005 ± 47** | 38 ± 14** | 7 ± 5** | 0.0814 |
p <0.05;
p <0.01.
Development and survival of F1 embryos/larvae
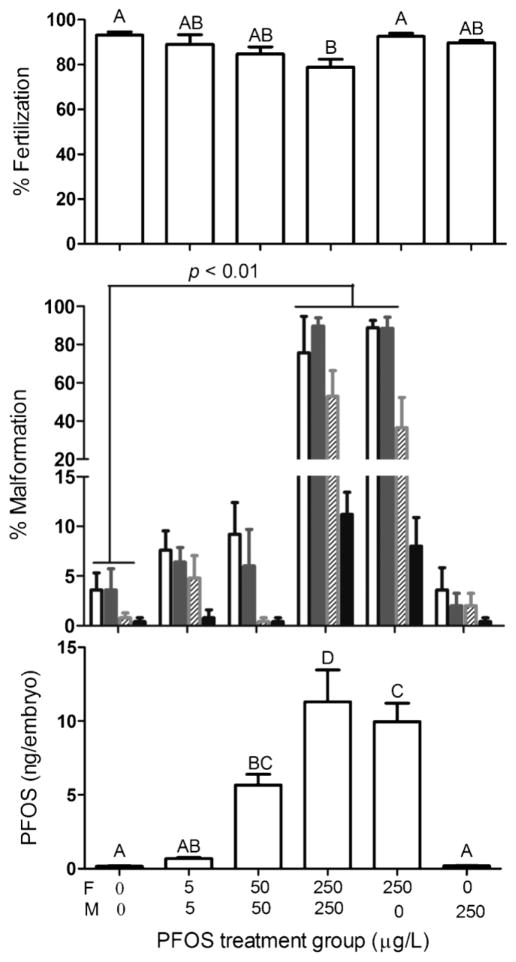
Spawning trials showed no significant difference in egg production of F0 females among all treatment groups (data not shown). Reduction in percent fertilization of F1 embryos was found in the 250-μg/L PFOS treatment group of F0 females and males (Fig. 2). Percent hatch at 72 hpf was above 96% for all groups, and no difference was found among them (data not shown).
Fig. 2.
Percent fertilization, malformation (white bars: total malformed larvae; dark gray bars: larvae with uninflated swim bladders; hatched bars: larvae with bent spine; black bars: other type of malformation), and perfluorooctanesulfonic acid (PFOS) residues in F1 offspring derived from PFOS exposed F0 parental zebrafish (F = female; M = male).
Percent malformation was elevated in F1 larvae at 4 dpf derived from F0 females exposed to 250 μg/L PFOS paired with males from the same PFOS treatment group or the DMSO controls (Fig. 2). F1 larvae derived from parental PFOS exposure to lower concentrations of 5 and 50 μg/L or females of the control group paired with males at 250 μg/L did not exhibit significant increases in malformation when compared with controls. Uninflated swim bladder and bent spine were the predominant malformation observed. Other deformations included pericardial edema, yolk sac edema, and necrosis. Residue PFOS in embryos exhibited similar trends as percent of malformation (Fig. 2).
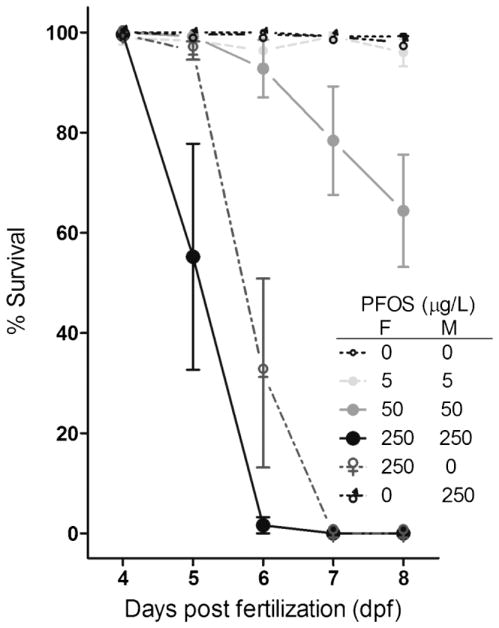
All larvae died at 7 dpf for the treatment groups of F0 females exposed to 250 μg/L PFOS paired with males from the same group or the DMSO controls (Fig. 3). Percent survival in larvae derived from parental PFOS exposure at 50 μg/L decreased 35% from 5 dpf (99.2 ± 0.8%) to 8 dpf (64.4 ± 11.2%). All larvae survived to adulthood for the controls and parental exposure to 5 μg/L PFOS or control females paired with males exposed to 250 μg/L (Fig. 3).
Fig. 3.
Percent survival of F1 larvae derived from perfluorooctanesulfonic acid (PFOS)–exposed F0 parental zebrafish (F = female; M = male).
Larval behavior of F1 offspring
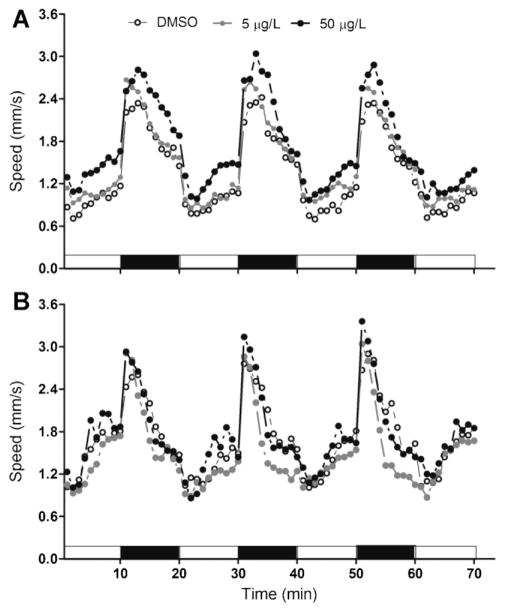
Larvae at 5 dpf with normal morphology derived from F0 DMSO control or 5 or 50 μg/L PFOS exposed groups were subjected to the light stimulation motor behavior test. A rapid transition from light-to-dark resulted in a similar, brief burst of swimming in all groups, and the parental PFOS-exposed groups elicited a higher basal swim rate than the DMSO controls in both light and dark periods (Fig. 4A; Fig. 5A). However, this hyperactive trend was abolished when F1 larvae continued to be exposed to the same PFOS concentration as their parents (Fig. 4B; Fig. 5B).
Fig. 4.
The swimming speed of F1 larvae at 6 d postfertilization when subject to 70 min dark:light photoperiod stimulation. Treatments referred to parental perfluorooctanesulfonic acid (PFOS) exposure concentrations. F1 larvae wereraisedeitherwithout (A) or with continuousPFOS exposure at the same concentrations as their parental group (B). Dots represent the mean speed in 60-s intervals of 30 larvae (three replicates each of 10 larvae).
Fig. 5.
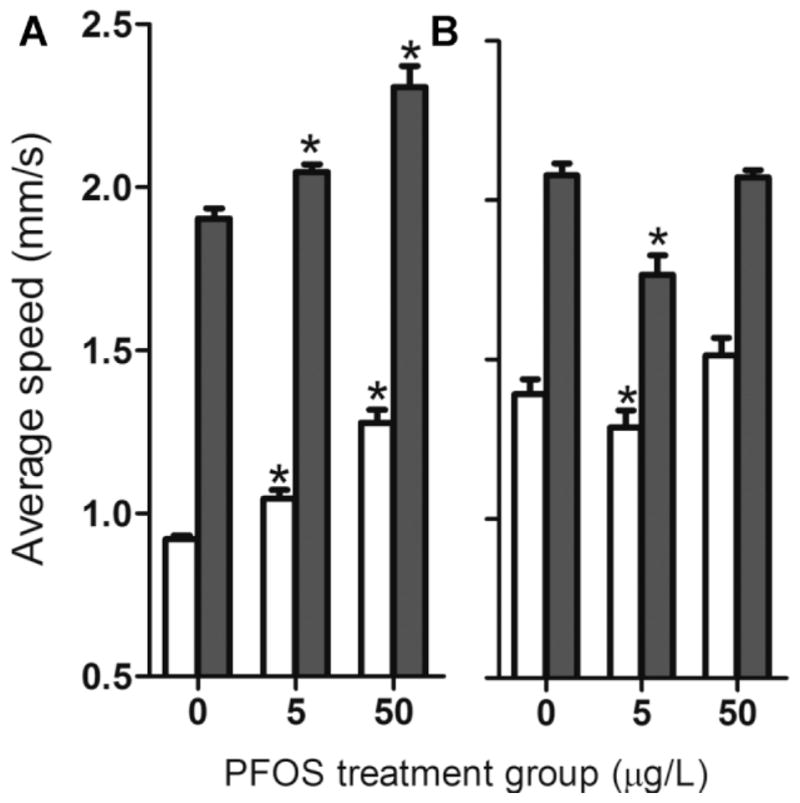
The average swimming speed of F1 larvae at 6 d postfertilization during the light (white bars) and dark photoperiods (dark gray bars). Perfluorooctanesulfonic acid (PFOS) treatment groups refer to parental F0 fish. F1 larvae were raised either without (A) or with continuous PFOS exposure at same concentrations as their parental group (B).
Muscle and neuron development in F1 larvae
Parental PFOS exposure led to disordered and loosened array in slow muscle fiber of 72-hpf F1 larvae (Fig. 6) in comparison with the lathy and compact array observed with normal larvae in controls (Fig. 6A). Primary motor neurons also exhibited slower development in larvae derived from PFOS-exposed parents (Fig. 6D–F).
Fig. 6.
Representative pictures of 72-h postfertilization larva stained for slow myofibrils with S58 and primary motor neuron with znp1. These larvae were derived from parental dimethylsulfoxide (DMSO) control or perfluorooctanesulfonic acid (PFOS)-treated groups. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
DISCUSSION
In this study, we continuously exposed zebrafish to PFOS from embryonic stage to adulthood, and F1 offspring were further evaluated with or without continuous PFOS exposure. Such an exposure scheme could more closely simulate environmental exposures of aquatic animals living in PFOS-contaminated waterways. The present study demonstrated that chronic PFOS exposure altered the sex ratio of the F0 parental population, decreased sperm motility in male fish, and led to maternal-related mortality and behavioral deficits in F1 offspring.
Estrogenic effects of PFOS have been reported in many studies [22,23]. However, no study has yet reported PFOS-induced sex ratio changes. The present study showed a clear trend from a male-dominated population in the control group toward a female-dominated population with increased exposure concentration in PFOS-treated groups. Chronic PFOS exposure of zebrafish at similar concentrations from 14 d larvae to 70 d adulthood; however, did not alter sex ratio, though vitellogenin messenger RNA expression was significantly up-regulated [16]. This suggests that early or persistent long-term exposure is necessary for PFOS to induce sex changes. In zebrafish, no apparent heteromorphy chromosome has been seen [24], and sex determination is thought to be controlled by multiple environmental and genetic factors [25]. Gonad development starts with a juvenile ovary stage in all fish, and in those that develop into males, testis development is initiated at approximately 21 to 30 dpf accompanied by simultaneously immature oocytes loss via apoptosis [24]. How PFOS affected sex determination in this study remains unclear. Recent studies suggest possible regulation of aromatase [26] or involvement of sex genes [27] in zebrafish sex differentiation. Perfluorooctanesulfonic acid could either increase aromatase activities, thus increasing estrogen production, or reduce the expression of male genes such as ar (androgen receptor), sox 9a (SRY-related genes containing a HMG box), and dmrt 1 (the sex-determining gene dmy) and subsequently lead to female-biased sex differentiation. In fathead minnows, PFOS decreased aromatase activities in adult males [28]. However, the expression of aromatase and these male genes during the sex differentiation period after PFOS exposure is unknown. Further investigation is necessary to elucidate the mechanism underlying the PFOS-induced sex changes. In particular, gonad development along with gene expression profiles should be monitored continuously for the sex determination window during development.
Exposure to PFOS affects body weight in rats [29], mice [30], and monkeys [31]. In this study, reduction in body weight and length in females was significant only at the highest exposure concentration of 250 μg/L, but in males it was significant for both 50 and 250 μg/L–treated groups. The higher sensitivity displayed by male fish is most likely related to their higher PFOS body burden than the females. However, the sex difference of PFOS concentration in fish tissue may result mainly from higher excretion rate of females through spawning (see later discussion) rather than higher accumulation rate in males. A similar situation exists in humans: menstrual bleeding, pregnancy, and lactation in women are the routes for PFOS excretion and contribute to their lower PFOS body burden than men [32].
The difference of condition factor among all treatments was also examined, because this parameter is often used to evaluate changes in food intake, lipid deposition, and protein budgets [33]. Significant changes were only observed at 250 μg/L, with an increase found in both females and males. This was partially in agreement with findings in an earlier study in which condition factor was increased in male fish and unchanged in females [16]. Previous study with fathead minnow also showed an elevated condition factor in males after PFOS exposure at 300 μg/L for 28 d [34]. The consistent increase of condition factor in males across different studies suggests that PFOS body burden could be the direct cause, because PFOS accumulation in males is generally high. The discrepancy in females between the present study and that of Du et al. [16] could be attributable to different exposure schemes between these two studies; in the present study we exposed embryos at a much earlier stage (8 hpf vs 14 dpf) and for a much longer duration period (150 d vs 70 d). Higher body burden of PFOS associated with longer exposure period could contribute to the increased condition factor in females of the present study.
Despite the fact that PFOS body burden was often found to be higher in males than in females, few studies explore the potential effect of PFOS on male reproduction traits. Sperm density reflects gamete production, and motility represents sperm quality [35]. This study showed that both parameters were significantly reduced in a dose-dependent manner by PFOS exposure, suggesting that PFOS affects male gonadal development. However, the underlying mechanism for this effect is unknown. Reduced plasma testosterone and 11-ketotestosterone levels were found in both male and female fathead minnows after exposure to PFOS [28]. Decreased male hormones along with increased vitellogenin expression [16] after continuous PFOS exposure may halt male gonad development and thus lead to low sperm production. The decreased sperm motility could result from impaired mitochondrial membrane potential induced deficiency in adenosine triphosphate production, because PFOS can induce production of reactive oxygen species and dissipation of mitochondria membrane potential [36,37]. In humans, serum PFC concentrations have a negative correlation with sperm quality [38], and earlier studies indicated that PFCs are associated with increased serum estradiol and reduced testosterone [39].
Because PFOS partitions into lipid bilayer [40] and its exposure increases free cholesterol or lipid drops in the liver [16,30], it also might affect lipid composition of the sperm membrane. The C/P ratio affects sperm function through regulation of membrane fluidity [41]; we thus measured the sperm membrane lipids. A significant increase of C/P ratio was seen only in the 50-μg/L PFOS-treated groups. Why the C/P ratio did not show a dose-dependent response to PFOS exposure and how PFOS altered the lipid composition of the sperm membrane is unclear. Future studies are necessary to reveal the underlying mechanism. Though sperm quantity and quality were both severely impaired by PFOS exposure, the male contribution to parental PFOS-dependent offspring responses was negligible. This is somewhat expected, because during natural fertilization sperm were often produced in excessive numbers, and only those with vigorous swimming were able to successfully fertilize eggs; that is, impaired or damaged sperm that resulted from PFOS exposure were excluded for fertilization by natural selection.
Female fecundity measured by egg production was not affected by PFOS exposure (data not shown). However, F1 offspring development and survival were severely affected by parental PFOS treatment, in particular maternal PFOS exposure. Predominant malformation and 100% total mortality at 7 dpf were only observed in offspring derived from 250 μg/L PFOS-exposed females paired with males with or without PFOS exposure. The PFOS concentration measurements in embryos revealed a direct correlation between PFOS body burden and embryonic deformity and larval mortality. For example, the fact that parental exposure of 5 μg/L PFOS did not cause any significant reduction in F1 offspring survival can be well explained by the low PFOS body burden in this group of embryos. Our measurements also showed negligible contribution of sperm to PFOS burden in embryos. All together, these findings provide direct evidence of maternal transfer of PFOS from adult females to their eggs. This was also verified in a recent study on PFOS isomer accumulation in zebrafish, in which an estimate of 10% (wt) adult PFOS body burden was transferred to the developing embryos, and this maternal transfer of branched and linear PFOS is nonisomer specific [42]. Oviparous transfer not only explained this maternal related developmental deformity and larval mortality but also contributed to the lower PFOS body burden in adult females than males.
Among all of the deformed phenotypes, uninflated swim bladder was the major abnormality found in the present study, suggesting its crucial role in PFOS-induced malformation in zebrafish. Previous studies with acute exposure also showed that the swim bladder is an important target site for PFOS [9,10]. The swim bladder in fish is homologous to the tetrapod lung in morphology as well as on the molecular basis [43,44]. In rodents, PFOS-induced neonatal mortality is related to respiratory dysfunction, either through direct interference with the function of pulmonary surfactant (see Lau et al. [45] for review) or by alternating the epithelial walls in the lungs [46] or indirectly through the intracranial blood vessel dilatation [47]. In zebrafish, swim bladder gas gland cells can also produce surfactant [48], but how PFOS affects its function and whether larval lethality at high-dose PFOS exposure is attributable to inflated swim bladder is unknown. Inflated swim bladder was found to be deflated at 7 dpf for F1 offspring derived from parental PFOS treatment at 50 μg/L (our unpublished data). To elucidate the underlying mechanism for PFOS-induced uninflated swim bladder and possibly the F1 larval total mortality, future studies should look into gene expression profile for larvae derived from chronic PFOS exposure.
Behavioral analysis often serves as a more sensitive tool for detecting sublethal chemical effects [49]. In the present study, surviving larvae showing normal morphological appearance were subjected to a light:dark stimulation test [10]. An elevated basal swimming rate was observed in F1 larvae derived from low-dose parental PFOS exposure. However, continuous exposure of these larvae to PFOS abolished these hyperactivity effects. Given that PFOS body burden was relatively low in these morphologically normal larvae (in the 5 and 50 μg/L groups), the altered larval swimming speed indicated that even a low amount of PFOS could play a significant role in larval behavior response. Further immunohistoanalysis indicated disorganization in muscle fiber and motor neuron development, both of which have been shown to play significant roles in locomotor behavior [50–52] and thus could contribute to their behavioral response. Despite this, the hyperactivity observed in the present study could represent a more general stress response associated with chemical exposure that are not specific to PFOS, because similar observation was found in larval zebrafish with parental exposure to other chemicals such as polybrominated diphenyl ethers or trimethyltin chloride (our unpublished results). Future studies should explore further the stress-related behavioral responses in larval zebrafish, in particular their relationship with whole-body cortisol level, because it is the main mediator of physiological response to stress in fish [53].
In summary, the present study demonstrates that chronic PFOS exposure decreased body length and body weight, altered sex ratio, and impaired male gamete function in parental F0 generation. For F1 offspring, parental PFOS exposure had an adverse effect on embryonic development and larval survival. Specifically, the high-dose parental PFOS exposure induced total larval mortality in F1, which was mainly attributable to the high PFOS body burden derived from maternal transfer. The low-dose parental PFOS exposure caused hyperactivity in morphological normal F1 larvae despite their lower PFOS body burden, suggesting that low levels of PFOS impact embryonic development and function.
Acknowledgments
We thank S. Rodenburg for critical comments and English editing. This work was supported in part by funding from the major project of Science and Technology Department of Zhejiang Province (2008C03001-2), the National Environmental Protection Public Welfare Science and Technology Research Program of China (200909089), the International Collaboration Project from Wenzhou City Government (H20070037), the key project of Science and Technology Department of Wenzhou (S20060023), and the National Institute of Environmental Health Sciences grant P30 00210.
References
- 1.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 2.So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PK. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan. China Environ Sci Technol. 2006;40:2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 3.Naile JE, Khim JS, Wang T, Chen C, Luo W, Kwon BO, Par J, Koh CH, Jones PD, Lu Y, Giesy JP. Perfluorinated compounds in water, sediment, soil and biota from estuarine and coastal areas of Korea. Environ Pollut. 2010;158:1237–1244. doi: 10.1016/j.envpol.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Hansen KJ, Johnson HO, Eldrige JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol. 2002;36:1681–1685. doi: 10.1021/es010780r. [DOI] [PubMed] [Google Scholar]
- 5.Moody CA, Martin JW, Kwan WC, Muir DC, Mabury SA. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek. Environ Sci Technol. 2002;36:545–551. doi: 10.1021/es011001+. [DOI] [PubMed] [Google Scholar]
- 6.Hoff PT, Van Campenhout K, Van de Vijver K, Covaci A, Bervoets L, Moens L, Huyskens G, Goemans G, Belpaire C, Blust R, De Coen W. Perfluorooctane sulfonic acid and organohalogen pollutants in liver of three freshwater fish species in Flanders (Belgium) relationships with biochemical and organismal effects. Environ Pollut. 2005;137:324–333. doi: 10.1016/j.envpol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Ji K, Kim Y, Oh S, Ahn B, Jo H, Choi K. Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Moina macrocopa) and fish (Oryzias latipes) Environ Toxicol Chem. 2008;27:2159–2168. doi: 10.1897/07-523.1. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, Du Y, Lam PK, Wu RS, Zhou B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol. 2008;230:23–32. doi: 10.1016/j.taap.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Shi X, Liu C, Wu G, Zhou B. Waterborne exposure to PFOS causes disruptionof thehypothalamus-pituitary-thyroid axisin zebrafishlarvae. Chemosphere. 2009;77:1010–1018. doi: 10.1016/j.chemosphere.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, Tanguay RL, Dong Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS) Aquat Toxicol. 2010;98:139–147. doi: 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Fang Z. Estrogenic effects, reproductive impairment and developmental toxicity in ovoviparous swordtail fish (Xiphophorus helleri) exposed to perfluorooctane sulfonate (PFOS) Aquat Toxicol. 2010;99:281–290. doi: 10.1016/j.aquatox.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Hagenaars A, Knapen D, Meyer IJ, van der Ven K, Hoff P, De Coen W. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio) Aquat Toxicol. 2008;88:155–163. doi: 10.1016/j.aquatox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Yeung LW, Lam PK, Wu RS, Zhou B. Protein profiles in zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate. Toxicol Sci. 2009;110:334–340. doi: 10.1093/toxsci/kfp111. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Li YY, Zeng HC, Wei J, Wan YJ, Chen J, Xu SQ. MicroRNA expression changes during zebrafish development induced by perfluorooctane sulfonate. J Appl Toxicol. 2011;31:210–222. doi: 10.1002/jat.1583. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci. 2010;115:391–400. doi: 10.1093/toxsci/kfq066. [DOI] [PubMed] [Google Scholar]
- 16.Du Y, Shi X, Liu C, Yu K, Zhou B. Chronic effects of water-borne PFOS exposure on growth, survival and hepatotoxicity in zebrafish: A partial life-cycle test. Chemosphere. 2009;74:723–729. doi: 10.1016/j.chemosphere.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 17.Westerfield M. The Zebrafish Book:A Guide for the Laboratory Use of Zebrafish (Danio rerio) 3. University of Oregon; Eugene, OR, USA: 1995. pp. 267–272. [Google Scholar]
- 18.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Jing R, Huang C, Bai C, Tanguay R, Dong Q. Optimization of activation, collection, dilution, and storage methods for zebrafish sperm. Aquaculture. 2009;290:165–171. [Google Scholar]
- 20.Wang X, Wang F, Wu X, Zhao X, Liu J, Huang C, Dong Q. The use of cryomicroscopy in guppy sperm freezing. Cryobiology. 2010;61:182–188. doi: 10.1016/j.cryobiol.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Du Y, Zhou B. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat Toxicol. 2007;85:267–277. doi: 10.1016/j.aquatox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim WK, Lee SK, Jung J. Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. J Hazard Mater. 2010;180:395–400. doi: 10.1016/j.jhazmat.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- 25.Baroiller JF, Guiguen Y. Endocrine and environmental aspects of sex differentiation in gonochoristic fish. EXS (Basel) 2001;91:177–201. doi: 10.1007/978-3-0348-7781-7_9. [DOI] [PubMed] [Google Scholar]
- 26.von Hofsten J, Olsson PE. Zebrafish sex determination and differentiation: Involvement of FTZ-F1 genes. Reprod Biol Endocrinol. 2005;3:63. doi: 10.1186/1477-7827-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen A, Morthorst JE, Andersen O, Rasmussen LJ, Bjerregaard P. Expression profiles for six zebrafish genes during gonadal sex differentiation. Reprod Biol Endocrinol. 2008;6:25. doi: 10.1186/1477-7827-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ankley GT, Kuehl DW, Kahl MD, Jensen KM, Linnum A, Leino RL, Villeneuvet DA. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2005;24:2316–2324. doi: 10.1897/04-634r.1. [DOI] [PubMed] [Google Scholar]
- 29.Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, Butenhoff JL. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology. 2003;183:117–131. doi: 10.1016/s0300-483x(02)00511-5. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, Dong GH, Jin YH, He QC. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch Toxicol. 2009;83:679–689. doi: 10.1007/s00204-008-0361-3. [DOI] [PubMed] [Google Scholar]
- 31.Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol Sci. 2002;68:249–264. doi: 10.1093/toxsci/68.1.249. [DOI] [PubMed] [Google Scholar]
- 32.Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Bervoets L, Blust R. Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: Relationship with fish condition factor. Environ Pollut. 2003;126:9–19. doi: 10.1016/s0269-7491(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 34.Oakes KD, Sibley PK, Martin JW, MacLean DD, Solomon KR, Mabury SA, Van Der Kraak GJ. Short-term exposures of fish to perfluorooctane sulfonate: acute effects on fatty acyl-CoA oxidase activity, oxidative stress, and circulating sex steroids. Environ Toxicol Chem. 2005;24:1172–1181. doi: 10.1897/04-419.1. [DOI] [PubMed] [Google Scholar]
- 35.Farrell PB, Presicce GA, Brockett CC, Foote RH. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology. 1998;49:871–879. doi: 10.1016/S0093-691X(98)00036-3. [DOI] [PubMed] [Google Scholar]
- 36.Kawamoto K, Nishikawa Y, Oami K, Jin Y, Sato I, Saito N, Tsuda S. Effects of perfluorooctane sulfonate (PFOS) on swimming behavior and membrane potential of paramecium caudatum. J Toxicol Sci. 2008;33:155–161. doi: 10.2131/jts.33.155. [DOI] [PubMed] [Google Scholar]
- 37.Hu XZ, Hu DC. Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2cells. Arch Toxicol. 2009;83:851–861. doi: 10.1007/s00204-009-0441-z. [DOI] [PubMed] [Google Scholar]
- 38.Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jorgensen N. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect. 2009;117:923–927. doi: 10.1289/ehp.0800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook JC, Murray SM, Frame SR, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol. 1992;113:209–217. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- 40.Lehmler HJ, Xie W, Bothun GD, Bummer PM, Knutson BL. Mixing of perfluorooctanesulfonic acid (PFOS) potassium salt with dipalmitoyl phosphatidylcholine (DPPC) Colloids Surfaces B: Biointerfaces. 2006;51:25–29. doi: 10.1016/j.colsurfb.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinsko SP, Love CC, Bauer JE, Macpherson ML, Varner DD. Cholesterol-to-phospholipid ratio in whole sperm and seminal plasma from fertile stallions and stallions with unexplained subfertility. Anim Reprod Sci. 2007;99:65–71. doi: 10.1016/j.anireprosci.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Sharpe RL, Benskin JP, Laarman AH, Macleod SL, Martin JW, Wong CS, Goss GG. Perfluorooctane sulfonate toxicity, isomer-specific accumulation, and maternal transfer in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss) Environ Toxicol Chem. 2010;29:1957–1966. doi: 10.1002/etc.257. [DOI] [PubMed] [Google Scholar]
- 43.Finney JL, Robertson GN, McGee CA, Smith FM, Croll RP. Structure and autonomic innervation of the swim bladder in the zebrafish (Danio rerio) J Comp Neurol. 2006;495:587–606. doi: 10.1002/cne.20948. [DOI] [PubMed] [Google Scholar]
- 44.Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, Gong Z. Development of zebrafish swimbladder: The requirement of Hedgehog signaling in specification and organization of the three tissue layers. Dev Biol. 2009;331:222–236. doi: 10.1016/j.ydbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 46.Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol. 2009;56:338–349. doi: 10.1007/s00244-008-9194-6. [DOI] [PubMed] [Google Scholar]
- 47.Yahia D, Tsukuba C, Yoshida M, Sato I, Tsuda S. Neonatal death of mice treated with perfluorooctane sulfonate. J Toxicol Sci. 2008;33:219–226. doi: 10.2131/jts.33.219. [DOI] [PubMed] [Google Scholar]
- 48.Daniels CB, Orgeig S, Sullivan LC, Ling N, Bennett MB, Schurch S, Val AL, Brauner CJ. The origin and evolution of the surfactant system in fish: Insights into the evolution of lungs and swim bladders. Physiol Biochem Zool. 2004;77:732–749. doi: 10.1086/422058. [DOI] [PubMed] [Google Scholar]
- 49.Kane AS, Salierno JD, Gipson GT, Molteno TC, Hunter C. A video-based movement analysis system to quantify behavioral stress responses of fish. Water Res. 2004;38:3993–4001. doi: 10.1016/j.watres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 50.Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- 51.Drapeau P, Ali DW, Buss RR, Saint-Amant L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J Neurosci Meth. 1999;88:1–13. doi: 10.1016/s0165-0270(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 52.Levin E, Aschner M, Heberlein U, Ruden D, Welsh-Bohmer K, Bartlett S, Berger K, Chen L, Corl A, Eddins D, French R, Hayden K, Helmcke K, Hirsch H, Linney E, Lnenicka G, Page G, Possidente D, Possidente B, Kirshner A. Genetic aspects of behavioral neurotoxicology. NeuroToxicology. 2009;30:741–753. doi: 10.1016/j.neuro.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]