Abstract
Background
This study investigated the effect of changes in inspiratory intrathoracic pressure (ITP) on stroke volume (SV) at rest and during moderate exercise in patients with heart failure and reduced ejection fraction (HFREF) as well as healthy individuals.
Methods and Results
SV was obtained by echocardiography during 2 minutes of spontaneous breathing (S), 2 progressive levels of inspiratory unloading (UL1 and UL2) using a ventilator, and 2 progressive levels of inspiratory loading using resistors in 11 patients with HFREF (61±9 years, EF: 32±4 %, NYHA class I-II) and 11 age-matched healthy individuals at rest and during exercise at 60% of maximal aerobic capacity on a semi-recumbent cycle ergometer. At rest, inspiratory unloading progressively decreased stroke volume index (SVI) (S: 35.2 ± 5.4, UL1: 33.3 ± 5.1, UL2: 32.2 ± 4.4 ml/m2) in healthy individuals while it increased SVI (S: 31.4 ± 4.6, UL1: 32.0 ± 5.9, UL2: 34.0 ± 7.2 ml/m2) in patients with HFREF (p=0.04). During moderate exercise, inspiratory unloading similarly decreased SVI (S: 43.9 ± 7.1, UL1: 40.7 ± 4.7, UL2: 39.9 ± 3.7 ml/m2) in healthy individuals while it increased SVI (S: 40.8 ± 6.5, UL1: 42.8 ± 6.9, UL2: 44.1 ± 4.8 ml/m2) in patients with HFREF (p=0.02). Inspiratory loading did not significantly change SVI at rest or during moderate exercise in both groups.
Conclusions
Inspiratory unloading improved SVI at rest and during moderate exercise in patients with HFREF, possibly due to a reduction in LV afterload.
Keywords: afterload, left ventricular dysfunction, cardiorespiratory
Introduction
Patients with heart failure often demonstrate an excessive ventilatory response to exercise, primarily through an increase in respiratory rate rather than tidal volume (Buller et al. 1990; Johnson et al. 2000). In addition, patients with heart failure often avoid breathing at high lung volumes and maintain a low end-expiratory lung volume (Johnson et al. 2000). A reduced cardiac reserve and increased work of breathing result in augmented heart-lung interdependence in patients with heart failure (Olson et al. 2006b). An enlarged heart also increases competition for space in the thoracic cavity which further accentuates this interdependence between the heart and lungs (Olson et al. 2006a). Exercise influences these cardiopulmonary interactions by augmenting the fluctuations in intrathoracic pressure (ITP) and altering the depth and frequency of breathing while at the same time increasing the demand for cardiac output (Olafsson et al. 1969). Therefore, it has been hypothesized that the abnormal exercising breathing pattern observed in patients with heart failure could be an attempt to minimize the negative influence of breathing-induced changes in ITP on cardiac function (Innes et al. 1993; Lalande et al. 2009).
A more negative ITP during inspiration increases the gradient for systemic venous return which, if maintained over successive respiratory cycles, will result in larger left ventricular (LV) filling and stroke volume (SV) in healthy individuals (Brecher et al. 1955; Kim et al. 1987; Robotham et al. 1989; Innes et al. 1993). Patients with heart failure have a reduced LV contractility and are unresponsive to changes in end-diastolic pressure, therefore increasing the gradient for venous return does not influence SV in this population (Pouleur et al. 1980; Pinsky 1989). The more negative ITP during inspiration also results in an increased LV transmural pressure gradient or LV afterload (Robotham et al. 1978; Karam et al. 1984), and an increase in LV afterload results in a decreased SV (Weber et al. 1982). It is therefore suggested that large decreases in ITP (more negative swing) may reduce SV in patients with heart failure, due to a predominant influence of an increased LV afterload following LV preload insensitivity in this population. The relative contribution of LV preload and LV afterload on SV can be determined by manipulating ITP using a ventilator and/or resistors. While positive pressure ventilation, or inspiratory unloading, reduces SV in healthy men at rest and during maximal exercise (Coast et al. 1988; Naughton et al. 1995; Harms et al. 1998), it increases SV in patients with heart failure at rest (Bradley et al. 1992; Naughton et al. 1995). Similarly, acute volume unloading results in a paradoxical increase in LV end-diastolic volume in patients with heart failure, as a consequence of an accentuated diastolic ventricular interaction due to pulmonary hypertension and volume overload in this population (Atherton et al. 1997). Thus, the normally produced inspiratory ITP seems to be required for an optimal SV in health but could be potentially detrimental to exercising SV in patients with heart failure. Reducing the negative swing in ITP through rapid and shallow breathing at low lung volume may therefore reduce the effects of an increased LV afterload on SV in patients with heart failure. Therefore, this study investigated the effect of changes in ITP on SV at rest and during moderate exercise in patients with heart failure and reduced ejection fraction (HFREF) as well as healthy individuals. It was hypothesized that inspiratory unloading (less negative inspiratory ITP) would improve SV while inspiratory loading (more negative inspiratory ITP) would reduced SV at rest and during moderate exercise in patients with HFREF.
Methods
The study included 11 patients with HFREF and 11 age-matched healthy individuals. Patients with a history of stable idiopathic or ischemic heart failure of NYHA class I and II, with an ejection fraction ≤ 40%, no history of dangerous arrhythmias (ventricular ectopy or uncontrolled atrial fibrillation) and not pacemaker-dependent were recruited to participate in the study. Of all patients with HFREF, 10 were taking angiotensin-converting enzyme inhibitors, 9 were on non-selective beta blockers/alpha-1 blockers, 2 on beta blockers, 7 were taking aspirin, 8 were on diuretics and 3 were using digoxin. Patients did not stop taking their medication during the study. Age-matched healthy individuals with no history of cardiovascular abnormalities were recruited as control participants. Exclusion criteria for all participants included obesity ≥ 34.9 kg/m2, a smoking history of more than 15 pack/year, an inability to perform exercise, allergies to lidocaine or latex and a deviated nasal septum. All participants were sedentary and performed less than three 20-minutes sessions of moderate intensity exercise per week for two or more years. A blood sample was collected in all participants to ensure that hemoglobin levels were above 11 and 12 mg/dL for females and males, respectively. Recent ejection fraction, LV mass and BNP levels (measures performed within 3 months of the study) were obtained from medical records of patients with HFREF. The study conformed to the standards of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board and all participants provided written informed consent.
Study protocol
Maximal aerobic capacity was first determined in all participants. On a separate day, spirometry measurements were performed before determining the cardiopulmonary response to 2 minutes of 1) spontaneous breathing (S), 2) 2 levels of inspiratory loading and 3) 2 levels of inspiratory unloading at rest and during exercise at a relative intensity of 60% of maximal aerobic capacity. Cardiopulmonary measurements were first performed during spontaneous breathing and were immediately followed by incrementing levels of inspiratory loading and unloading. Inspiratory loading preceded inspiratory unloading by approximately 10 minutes.
Maximal aerobic capacity
Participants performed an incremental workload exercise test on a semi-recumbent ergometer (Ergoselect II 1200, Ergoline, Bitz, Germany) with a 12-lead electrocardiograph (Case®, GE Healthcare) to determine maximal aerobic capacity. Initial workload was set at 25 W and workload increased by 25 W with each 2 minute stage until volitional fatigue. Breath by breath data were collected and analyzed every 5 seconds using a metabolic system (CPX, Medgraphics, St Paul, MN) that was calibrated with room air and standardized gas. Maximal aerobic capacity was considered to be achieved when two of the following criteria were met: an increase in oxygen consumption of less than 100 ml/min with a further increase in workload, respiratory exchange ratio greater than 1.1 or achievement of age-predicted maximal heart rate. The rate of perceived exertion was assessed using the Borg scale (Borg 1970).
Pulmonary function, lung mechanics and manipulation of ITP
Spirometry measurements included measurement of forced vital capacity (FVC) and forced expiratory flow in 1 second (FEV1) (CPFS/DTM USB, Medgraphics, St Paul, MN). During the determination of the cardiopulmonary response to ITP manipulation at rest and during exercise, airflow was assessed by having participants breathe through a mouthpiece attached in series to a pneumotachograph with a switching valve connected to a two-way non-rebreathing valve. Tidal volume was obtained from the digital integration of the linearized flow signal corrected for drift. For assessment of esophageal and gastric pressures, custom-made small latex balloons were simultaneously inserted intranasally into the esophagus and stomach while mouth pressure was measured from a line inserted in the pneumotachograph. Transdiaphragmatic pressure was calculated as the difference between gastric and esophageal pressures. Inspiratory loading was performed by placing fixed diameter resistors on the inhalation arm of the non-rebreathing valve. Diameters were chosen in order to produce a 50% increase in inspiratory ITP (L1) and to double the negative swing in inspiratory ITP (L2). Inspiratory unloading was achieved using a pressure support ventilator producing positive airway pressure (BiPAP Vision®, Respironics, Pittsburgh, PA, USA), which was connected to the inhalation arm of the non-rebreathing valve. Pressures were chosen in order to reduce inspiratory ITP by half (UL1) and to abolish the negative swing in inspiratory ITP (UL2).
Cardiovascular function
SV was continuously assessed by echocardiography during the 2 minute period of each condition in order to obtain a maximum of cardiac cycles (Biosound, Esaote, Genoa, Italy). The diameter of the LV outflow tract was determined from the parasternal long axis view at rest and was assumed to remain constant through exercise. The time-velocity integral of the LV outflow tract was obtained in the 5 chamber view of the apical window. SV was calculated with the following equation: (0.785 X (LV outflow tract diameter)2 X time-velocity integral of the LV outflow tract). An average of all SVs measured during each condition was obtained for each individual. Beat-by-beat heart rate, mean arterial pressure (MAP) and systemic vascular resistance (SVR) were obtained by finger arterial pressure waveform analysis (Nexfin, BMEYE, Amsterdam, Netherlands) with the hydrostatic correction system placed at the level of the third intercostal space. Cardiac output was calculated as the product of SV and heart rate. SV and cardiac output were indexed to body surface area as calculated from the Du Bois and Du Bois formula (Du Bois et al. 1989).
Data analysis
Time aligned measurements of tidal volume, intrathoracic and transdiaphragmatic pressures, heart rate, MAP and SVR were continuously acquired at a sampling frequency of 1000 Hz (PowerLab, ADInstruments, Colorado, USA) and analyzed using commercially available software (LabChart 7.1, ADInstruments, Colorado, USA). Analyses of the time-velocity integrals of the LV outflow tract were performed by an observer blinded to both condition and group. All measurements were performed twice, or until 2 measures were within 5% of each other, and an average of both measures is reported. Between group comparisons of participant characteristics were conducted using paired t tests. A mixed factorial analysis of variance was used to test for condition (spontaneous breathing, unloading and loading) and group (healthy individuals vs. patients with heart failure) effects for all measurements. When main effects or a group and condition interaction were significant, post hoc analyses were performed using a Bonferroni correction. Results are expressed as mean ± standard deviations. P values < 0.05 were considered significant.
Results
Age, height, body mass index, FVC, FEV1 and hemoglobin levels were not different between groups (Table 1). Weight was higher and maximal aerobic capacity, maximal workload, MAP and SVR were lower in patients with HFREF (Table 1). Patients with HFREFhad an ejection fraction of 32±4%, a LV mass of 269±67 g, consistent with the observed cardiomegaly in patients with heart failure (Olson et al. 2006a) and BNP levels of 248±193 pg/ml indicating proper treatment. At 60% of maximal aerobic capacity, workload was lower in patients with HFREFthan healthy individuals (69±23 vs. 94±41 W, p=0.03) while the rates of perceived exertion were similar between groups (14±1 vs. 14±3, p=0.20).
Table 1.
Participants’ characteristics
| HFREF | Healthy | |
|---|---|---|
| Female/Male | 1/10 | 2/9 |
| Age (years) | 61 ± 9 | 61 ± 8 |
| Height (cm) | 178 ± 8 | 175 ± 8 |
| Weight (kg) | 96.8 ± 10.5 | 82.9 ± 10.9 * |
| MAP (mmHg) | 78 ± 11 | 100 ± 11 * |
| SVR (dyn·s/cm5) | 930 ± 350 | 1363 ± 359 * |
| BMI (kg/m2) | 30.5 ± 2.4 | 27.3 ± 4.1 |
| VO2max (ml/kg/min) | 19 ± 5.8 | 24.2 ± 4.7 * |
| Maximal workload (W) | 131 ± 42 | 159 ± 39 * |
| FVC (% predicted) | 93.6 ± 16.4 | 95.0 ± 16.1 |
| FEV1 (% predicted) | 91.5 ± 13.3 | 96.7 ± 14.4 |
| Hb (mg/dL) | 14.2 ± 1.0 | 13.4 ± 0.9 |
BMI: body mass index, FEV1: forced expiratory flow in 1 second, FVC: forced vital capacity, Hb: haemoglobin, MAP: mean arterial pressure; SVR: systemic vascular resistance.
p < 0.05 between healthy and HFREF.
Effect of inspiratory unloading on cardiopulmonary function at rest and during moderate exercise
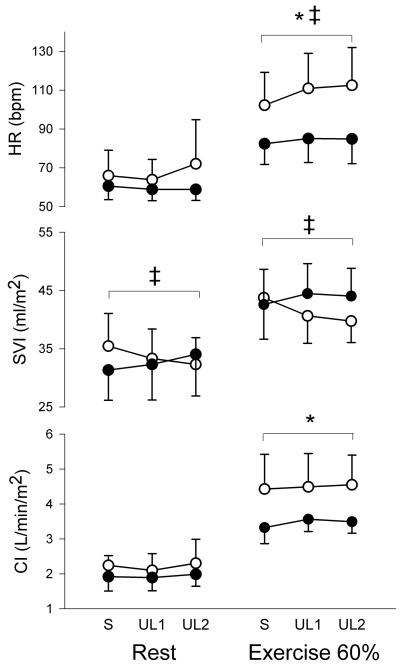
At rest, peak inspiratory ITP was similarly reduced by an average of −86 and −132% of spontaneous breathing values in healthy individuals and by an average of −72 and −162% of spontaneous breathing values in patients with heart failure. Inspiratory unloading resulted in increases in tidal volume, respiratory rate, peak expiratory ITP and a decrease in transdiaphragmatic pressure in both groups (Table 2). Inspiratory unloading did not induce any changes in MAP or in SVR. Inspiratory unloading induced a group and condition interaction on stroke volume index (SVI), with SVI values decreasing in healthy individuals and increasing in patients with HFREF (Figure 1). There were no changes in heart rate or cardiac index with inspiratory unloading in both groups (Figure 1).
Table 2.
Respiratory and hemodynamic responses to loading and unloading at rest
| Healthy | HFREF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | L1 | L2 | UL1 | UL2 | S | L1 | L2 | UL1 | UL2 | |
| Peak inspiratory ITP (cmH2O) | −3.7 ± 1.6 * † | −5.3 ± 1.5 | −9.9 ± 6.4 | −0.5 ± 4.2 | 1.2 ± 3.8 | −3.7 ± 2.0 * † | −4.9 ± 1.9 | −7.5 ± 1.8 | −1.0 ± 1.7 | 2.3 ± 2.3 |
| Peak expiratory ITP (cmH2O) | 2.1 ± 3.7 * | 1.9 ± 3.4 | 2.0 ± 3.4 | 3.6 ± 2.9 | 3.3 ± 2.6 | 3.6 ± 2.0 * | 3.6 ± 2.2 | 3.7 ± 2.1 | 4.1 ± 1.9 | 4.2 ± 2.5 |
| Pdi (cmH2O) | 6.7 ± 5.3 * † | 8.3 ± 5.0 | 12.8 ± 9.9 | 1.9 ± 6.2 | 0.1 ± 6.0 | 5.5 ± 5.1 * † | 6.6 ± 4.71 | 9.2 ± 4.9 | 2.9 ± 6.2 | −0.7 ± 6.0 |
| Tidal volume (L) | 1.33 ± 0.30 * | 1.39 ± 0.30 | 1.44 ± 0.33 | 1.64 ± 0.65 | 1.73 ± 0.77 | 1.29 ± 0.12 * | 1.39 ± 0.19 | 1.40 ± 0.30 | 1.79 ± 0.54 | 1.74 ± 0.74 |
| RR (breath/min) | 14.9 ± 3.3 * † | 14.3 ± 4.1 | 11.6 ± 5.4 | 14.8 ± 5.8 | 17.1 ± 7.0 | 15.1 ± 4.1 * † | 12.9 ± 3.6 | 12.6 ± 4.6 | 15.1 ± 4.8 | 7.5 ± 5.1 |
| MAP (mmHg) | 100 ± 11 † | 101 ± 11 | 103 ± 12 | 99 ± 12 | 100 ± 12 | 78 ± 11 † | 79 ± 12 | 80 ± 12 | 76 ± 8 | 76 ± 7 |
| SVR (dyn·s/cm5) | 1409 ± 342 | 1414 ± 346 | 1395 ± 360 | 1434 ± 301 | 1406 ± 359 | 930 ± 350 | 920 ± 352 | 900 ± 383 | 926 ± 363 | 935 ± 358 |
main effect for unloading,
main effect for loading, HFREF: Heart failure and reduced ejection fraction, ITP: intrathoracic pressure, Pdi: transdiaphragmatic pressure, RR: respiratory rate, MAP: mean arterial pressure, SVR: systemic vascular resistance
Figure 1.
Heart rate (HR), SV index (SVI) and cardiac index (CI) responses to unloading conditions at rest and during moderate exercise in patients with HFREF (black circles) and healthy individuals (white circles). * main effect for group, ‡ group and condition interaction. S: spontaneous breathing, UL1: first level of unloading, UL2: second level of unloading.
During moderate exercise, peak inspiratory ITP was similarly reduced by an average of −31 and −69% of spontaneous breathing values in healthy individuals and by an average of −33 and −72% of spontaneous breathing values in patients with heart failure. Similar to the response at rest, inspiratory unloading during moderate exercise resulted in increases in tidal volume, respiratory rate, peak expiratory ITP and a decrease in transdiaphragmatic pressure in both groups (Table 3). Inspiratory unloading induced a group and condition interaction on MAP and SVR asMAP and SVR decreased with inspiratory unloading in healthy individuals but did not change in patients with heart failure. Inspiratory unloading induced a group and condition interaction on heart rate and SVI (Figure 1). While heart rate significantly increased with both levels of unloading in healthy individuals, it did not change in patients with HFREF (Figure 1). Similar to the response observed at rest, inspiratory unloading during moderate exercise increased SVI in patients with HFREFand reduced SVI in healthy individuals (Figure 1). There were no changes in cardiac index with inspiratory unloading in both groups (Figure 1).
Table 3.
Respiratory and hemodynamic responses to loading and unloading during moderate exercise
| Healthy | HFREF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | L1 | L2 | UL1 | UL2 | S | L1 | L2 | UL1 | UL2 | |
| Peak inspiratory ITP (cmH2O) | −10.2 ± 4.6 * † | −12.0 ± 4.5 | −13.9 ± 6.1 | −7.0 ± 5.2 | −3.2 ± 5.4 | −7.8 ± 3.4 * † | −10.0 ± 2.9 | −11.3 ± 3.4 | −5.1 ± 3.5 | −2.0 ± 2.7 |
| Peak expiratory ITP (cmH2O) | 3.8 ± 5.0 * | 3.5 ± 5.8 | 4.4 ± 6.6 | 4.8 ± 4.4 | 5.2 ± 4.0 | 4.4 ± 1.9 * | 5.1 ± 1.9 | 5.4 ± 1.7 | 6.4 ± 2.2 | 5.4 ± 1.6 |
| Pdi (cmH2O) | 10.3 ± 4.5 * † | 11.9 ± 4.3 | 13.8 ± 5.9 | 6.9 ± 5.1 | 2.8 ± 5.2 | 9.1 ± 8.2 * † | 11.4 ± 8.0 | 12.8 ± 8.7 | 6.5 ± 7.7 | 3.5 ± 6.6 |
| Tidal volume (L) | 3.07 ± 0.90 * † | 3.33 ± 1.05 | 3.47 ± 1.17 | 3.81 ± 1.13 | 4.03 ± 0.97 | 2.64 ± 0.52 * † | 2.91 ± 0.77 | 2.96 ± 0.76 | 3.49 ± 0.81 | 3.43 ± 0.80 |
| RR (breath/min) | 24.9 ± 6.1 * † | 23.0 ± 7.1 | 22.5 ± 6.8 | 25.8 ± 5.9 | 26.8 ± 3.6 | 25.7 ± 5.8 * † | 22.1 ± 4.3 | 21.1 ± 5.1 | 26.1 ± 7.2 | 29.1 ± 5.9 |
| MAP (mmHg) | 115 ± 12 ‡ | 116 ± 13 | 114 ± 14 | 109 ± 14 | 106 ± 15 | 93 ± 13 ‡ | 97 ± 16 | 94 ± 12 | 98 ± 20 | 97 ± 18 |
| SVR (dyn·s/cm5) | 829 ± 144 ‡ | 795 ± 177 | 791 ± 180 | 729 ± 186 | 715 ± 189 | 728 ± 344 ‡ | 735 ± 341 | 712 ± 307 | 827 ± 436 | 804 ± 407 |
main effect for unloading,
main effect for loading,
group and condition interaction for unloading, HFREF: Heart failure and reduced ejection fraction, ITP: intrathoracic pressure, Pdi: transdiaphragmatic pressure, RR: respiratory rate, MAP: mean arterial pressure, SVR: systemic vascular resistance
Effects of inspiratory loading on cardiopulmonary function at rest and during moderate exercise
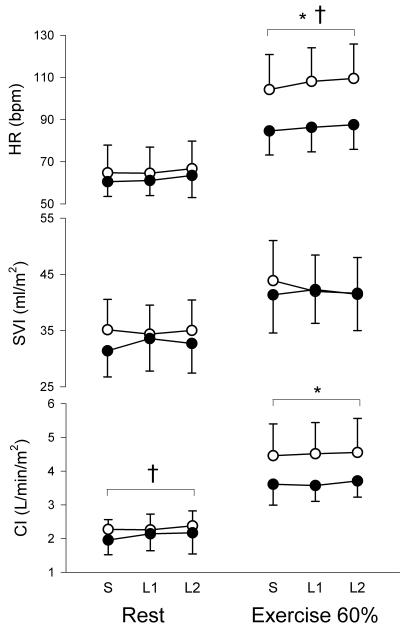
At rest, the addition of inspiratory resistance similarly increased the negative swing in peak inspiratory ITP by an average of 42 and 165% of spontaneous breathing values in healthy individuals and by an average of 38 and 134% of spontaneous breathing values in patients with heart failure. Inducing a more negative peak inspiratory ITP resulted in a slowing of the respiratory rate and increases in transdiaphragmatic pressure while there were no changes in tidal volume or peak expiratory ITP in both healthy individuals and patients with HFREF (Table 2). Inspiratory loading induced an increase in MAP but did not induce any changes in SVR. There was a main effect of inspiratory loading on cardiac index with the first level of loading increasing cardiac index by 9% in patients with heart failure, mainly due to an increased SVI (Figure 2). Inspiratory loading did not induce any changes in heart rate, SVI or cardiac index at rest in healthy individuals (Figure 2).
Figure 2.
Heart rate (HR), SV index (SVI) and cardiac index (CI) responses to loading conditions at rest and during moderate exercise in patients with HFREF (black circles) and healthy individuals (white circles). * main effect for group, † main effect for loading. S: spontaneous breathing, L1: first level of loading, L2: second level of loading.
During moderate exercise, the negative swing in peak inspiratory ITP was increased by an average of 18 and 36% of spontaneous breathing values in healthy individuals and by an average of 28 and 45% of spontaneous breathing values in patients with heart failure. Inspiratory loading resulted in slowing of the respiratory rate, increases in tidal volume as well as transdiaphragmatic pressure while there were no changes in peak expiratory ITP in both healthy individuals and patients with HFREF (Table 3). Inspiratory loading did not induce any changes in MAP or in SVR. Inspiratory loading induced an increase in heart rate during both levels of loading in healthy individuals and during the higher loading condition in patients with HFREF (Figure 2). There were no changes in SVI and cardiac index with inspiratory loading during moderate exercise in both groups (Figure 2).
Discussion
Multiple levels of inspiratory unloading and loading were used to determine the SVI response across a wide range of ITP at rest and during moderate exercise in patients with HFREFas well as healthy individuals. In healthy individuals, inspiratory unloading elicited reductions in SVI at rest and during moderate exercise. This detrimental response of SVI to inspiratory unloading suggests that the normally produced inspiratory ITP swing contributes to maintaining LV preload in healthy individuals. In contrast, both levels of inspiratory unloading elicited increases in SVI at rest and during moderate exercise in patients with HFREF. Increases in SVI accompanying inspiratory unloading in patients with HFREF may be due to a decreased LV afterload followinga narrowing of the LV transmural pressure gradient. Inspiratory loading did not induce changes in SVI in patients with HFREFor healthy individuals at rest or during moderate exercise.
Changes in ITP affect both LV preload and LV afterload, which will in turn impact SV. It is generally accepted that the negative ITP generated during inspiration reduces right atrial pressure and increases the pressure surrounding the abdominal vessels, thereby increasing the gradient for systemic venous return and right ventricular filling (Kim et al. 1987; Robotham et al. 1989; Innes et al. 1993). The left ventricle also contracts against a pressure load equivalent to the LV transmural pressure gradient also defined as LV afterload. The LV transmural pressure gradient can be increased through increases in LV pressure or by reducing the pressure surrounding the left ventricle, i.e. ITP. Thus, the more negative ITP accompanying inspiration would increase LV afterload while a less negative ITP would unload the left ventricle (Robotham et al. 1978; Karam et al. 1984). There is therefore an inverse linear relationship between SV and LV afterload (Weber et al. 1982). In the healthy heart, it is typically suggested that changes in LV preload outweigh the influence of changes in LV afterload on SV (Pouleur et al. 1980). However, patients with heart failure have a reduced LV contractility and an unresponsiveness to changes in end-diastolic pressure indicating an inability to augment SV (Pouleur et al. 1980; Pinsky 1989). It is therefore suggested that there is an overriding influence of LV afterload in this population.
Inspiratory unloading
Reductions in SV and cardiac output have previously been observed with inspiratory unloading in resting healthy individuals (Coast et al. 1988; Naughton et al. 1995). These reductions in SV were observed without any changes in LV transmural pressure, therefore it was concluded that the decreased SVs were in response to a reduced LV preload (Naughton et al. 1995). Harms et al. (1998) reported that a 41% reduction in the negative swing in ITP resulted in an 8% decrease in SV during maximal exercise in healthy individuals. We similarly observed a detrimental response of SVI to inspiratory unloading both at rest and during moderate exercise in healthy individuals. The observed decreases of 5 and 9% in SVI following reductions of 31 and 69% in the negative swing in ITP during moderate exercise in healthy individuals are similar to the 8% decrease in SV reported by Harms et al. (1998) during maximal exercise in healthy individuals. It is therefore speculated that the less negative ITP during inspiration reduced venous return thereby resulting in a reduced SVI at rest and during moderate exercise in healthy individuals.
Continuous positive airway pressure has been reported to improve cardiac function in patients with heart failure. Indeed, increases in SV were reported in response to inspiratory unloading at rest in patients with heart failure (Bradley et al. 1992; Naughton et al. 1995). It was further observed that patients with pulmonary capillary wedge pressure above 12 mmHg showed increases in SV and cardiac output with inspiratory unloading while patients with pulmonary capillary wedge pressure below 12 mmHg, which is equivalent to healthy pressures, had a tendency for decreases in SV with inspiratory unloading (Bradley et al. 1992). Further support for the contribution of decreased LV afterload on the increase in SV in patients with heart failure is the finding that inspiratory unloading elicits reductions in end-systolic volume without any change in end-diastolic volume in pigs with heart failure, indicating that the increase in SV is due to a greater LV ejection and not to a greater LV filling (Genovese et al. 1994; Genovese et al. 1995). It is therefore speculated that the improvements in SVI following inspiratory unloading in patients with heart failure are the result of a less negative ITP and the accompanying reduction in LV afterload. However, it is possible that the observed improvements in exercising SVI in patients with heart failure could be the result of modifications in blood pressure, LV contractility or ventricular interdependence triggered by inspiratory unloading (Jardin et al. 1990; Mitchell et al. 2005). Indeed, acute volume unloading increases LV end-diastolic volume in patients with heart failure, due to an accentuated diastolic ventricular interaction influenced by pulmonary hypertension and volume overload (Atherton et al. 1997). There were no changes in exercising MAP with inspiratory unloading in patients with HFREF. Measurements of LV volumes would clarify the respective contribution of LV preload, LV afterload and LV contractility to the improvements in exercising SVI with inspiratory unloading in patients with heart failure.
Inspiratory loading
Increasing the negative swing in the inspiratory ITP did not change SVI or cardiac index at rest or during moderate exercise in healthy individuals. This is in accordance with the findings of Harms et al. (1998) that SV and cardiac output were maintained during inspiratory loading at maximal exercise in healthy individuals, indicating that SV and cardiac output are potentially independent of more negative swings in ITP. However, others observed that SV was decreased by 10% during inspiratory loading in resting healthy individuals due to an increase in end-systolic volume without any changes in end-diastolic volume, suggesting that a more negative ITP causes an increased LV afterload (Karam et al. 1984). The discrepancy between these findings could be caused by the greater threshold load used by Karam et al. ((Karam et al. 1984) which could have been sufficient to increase LV afterload to an extent where it reduced SV in healthy individuals. Moreover, the peripheral skeletal muscle pump forcing blood centrally during exercise could have helped maintain venous return and SVI by counteracting the effect of an increased LV afterload on SVI. Therefore, the lack of changes in SVI during inspiratory loading in exercising healthy individuals could be a result of afterload insensitivity or greater preload recruitment. In patients with HFREF, increases in LV afterload caused by a more negative ITP did not reduce SVI at rest or during moderate exercise, which may be explained by an increased LV contractility in order to compensate for the greater LV afterload induced by inspiratory loading.
In conclusion, inspiratory unloading improved SVI at rest and during moderate exercise in patients with heart failure, possibly due to a reduction in LV afterload. Factors causing the transition from preload-dependency to afterload-dependency in patients with HFREFremain unclear, but it can be hypothesized that the SVI response to changes in ITP would be affected by disease severity, pulmonary capillary wedge pressure, volume status and/or heart size. While the influence of altering ITP on SVI was modest, we studied a relatively asymptomatic and stable HFREFcohort, and a larger influence may be observed in patients with greater afterload-dependency and disease severity. Nonetheless, our findings suggest that reducing the negative swing in ITP through inspiratory unloading could potentially improve exercise performance due to an increase in SVI in patients with heart failure. Improving exercise capacity in patients with heart failure is of great importance as peak VO2 is an important predictor of survival in these patients (Mancini et al. 1991). Thus, it remains to be determined whether the observed, although modest, improvements in SVI in response to inspiratory unloading could result in an improved exercise performance in patients with heart failure. Inspiratory unloading has been reported to reduce exertional leg discomfort and increased symptom-limited exercise endurance time in patients with heart failure (O’Donnell et al. 1999). Inspiratory unloading while exercising could therefore serve as a rationale strategy to limit negative cardiorespiratory interactions, dyspnea and fatigue, and facilitate the rehabilitation of patients with heart failure. We did not acquire measurements of right ventricular function which could lead to a better understanding of the role of changes in LV preload on cardiac function. Another study limitation was that LV afterload was solely defined as LV transmural pressure, however wall stress, representing afterload in the myocardial fibers during LV ejection, is also affected by the geometry of the left ventricle (West 1991). We did not perform measurements of LV cavity size or wall thickness during this study. Future studies should include measurements of right ventricular function, LV cavity size, wall thickness as well as simultaneous gas exchange monitoring in order to determine whether exercise performance is improved by inspiratory unloading in patients with HFREF with ranging disease severity.
Acknowledgments
This study was supported by NIH Grant HL71478 and S. Lalande was supported by an American Heart Association Postdoctoral Fellowship (0920054G).
Footnotes
The authors have no conflict of interest.
References
- Atherton JJ, Moore TD, Lele SS, Thomson HL, Galbraith AJ, Belenkie I, Tyberg JV, Frenneaux MP. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Bradley TD, Holloway RM, McLaughlin PR, Ross BL, Walters J, Liu PP. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis. 1992;145:377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- Brecher GA, Hubay CA. Pulmonary blood flow and venous return during spontaneous respiration. Circ Res. 1955;3:210–214. doi: 10.1161/01.res.3.2.210. [DOI] [PubMed] [Google Scholar]
- Buller NP, Poole-Wilson PA. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J. 1990;63:281–283. doi: 10.1136/hrt.63.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast JR, Jensen RA, Cassidy SS, Ramanathan M, Johnson RL., Jr. Cardiac output and O2 consumption during inspiratory threshold loaded breathing. J Appl Physiol. 1988;64:1624–1628. doi: 10.1152/jappl.1988.64.4.1624. [DOI] [PubMed] [Google Scholar]
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311. discussion 312-303. [PubMed] [Google Scholar]
- Genovese J, Huberfeld S, Tarasiuk A, Moskowitz M, Scharf SM. Effects of CPAP on cardiac output in pigs with pacing-induced congestive heart failure. Am J Respir Crit Care Med. 1995;152:1847–1853. doi: 10.1164/ajrccm.152.6.8520745. [DOI] [PubMed] [Google Scholar]
- Genovese J, Moskowitz M, Tarasiuk A, Graver LM, Scharf SM. Effects of continuous positive airway pressure on cardiac output in normal and hypervolemic unanesthetized pigs. Am J Respir Crit Care Med. 1994;150:752–758. doi: 10.1164/ajrccm.150.3.8087348. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Innes JA, De Cort SC, Kox W, Guz A. Within-breath modulation of left ventricular function during normal breathing and positive-pressure ventilation in man. J Physiol. 1993;460:487–502. doi: 10.1113/jphysiol.1993.sp019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology. 1990;72:966–970. doi: 10.1097/00000542-199006000-00003. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117:321–332. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- Karam M, Wise RA, Natarajan TK, Permutt S, Wagner HN. Mechanism of decreased left ventricular stroke volume during inspiration in man. Circulation. 1984;69:866–873. doi: 10.1161/01.cir.69.5.866. [DOI] [PubMed] [Google Scholar]
- Kim BH, Ishida Y, Tsuneoka Y, Matsubara N, Hiraoka T, Takeda H, Inoue M, Kamada T, Kimura K, Kozuka T. Effects of spontaneous respiration on right and left ventricular function: evaluation by respiratory and ECG gated radionuclide ventriculography. J Nucl Med. 1987;28:173–177. [PubMed] [Google Scholar]
- Lalande S, Johnson BD. Breathing strategy to preserve exercising cardiac function in patients with heart failure. Med Hypotheses. 2009;74:416–421. doi: 10.1016/j.mehy.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr., Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Whitelaw WA, Sas R, Smith ER, Tyberg JV, Belenkie I. RV filling modulates LV function by direct ventricular interaction during mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H549–557. doi: 10.1152/ajpheart.01180.2004. [DOI] [PubMed] [Google Scholar]
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91:1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160:1804–1811. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in normal subjects. J Clin Invest. 1969;48:564–573. doi: 10.1172/JCI106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TP, Beck KC, Johnson JB, Johnson BD. Competition for intrathoracic space reduces lung capacity in patients with chronic heart failure: a radiographic study. Chest. 2006a;130:164–171. doi: 10.1378/chest.130.1.164. [DOI] [PubMed] [Google Scholar]
- Olson TP, Snyder EM, Johnson BD. Exercise-disordered breathing in chronic heart failure. Exerc Sport Sci Rev. 2006b;34:194–201. doi: 10.1249/01.jes.0000240022.30373.a2. [DOI] [PubMed] [Google Scholar]
- Pinsky MR. Effects of changing intrathoracic pressure on the normal and failing heart. In: L C, Scharf SM, Cassidy SS, editors. Lung Biology in Health and Disease, Heart-Lung Interactions in Health and Disease. Marcel Dekker; New York: 1989. pp. 839–878. [Google Scholar]
- Pouleur H, Covell JW, Ross J., Jr. Effects of nitroprusside on venous return and central blood volume in the absence and presence of acute heart failure. Circulation. 1980;61:328–337. doi: 10.1161/01.cir.61.2.328. [DOI] [PubMed] [Google Scholar]
- Robotham J, Peter J. Mechanical effects of intrathoracic pressure on ventricular performance. In: Scharf S, Cassidy S, editors. Heart-Lung Interactions in Health and Disease. Marcel Dekker; New York: 1989. pp. 251–284. [Google Scholar]
- Robotham JL, Lixfeld W, Holland L, MacGregor D, Bryan AC, Rabson J. Effects of respiration on cardiac performance. J Appl Physiol. 1978;44:703–709. doi: 10.1152/jappl.1978.44.5.703. [DOI] [PubMed] [Google Scholar]
- Weber KT, Janicki JS, Hunter WC, Shroff S, Pearlman ES, Fishman AP. The contractile behavior of the heart and its functional coupling to the circulation. Prog Cardiovasc Dis. 1982;24:375–400. doi: 10.1016/0033-0620(82)90020-2. [DOI] [PubMed] [Google Scholar]
- West JB. Best & Taylor’s Physiological Basis of Medical Practice. Williams & Wilkins; Baltimore, MD: 1991. [Google Scholar]