Summary
Dynamic interactions between haematopoietic cells and their specialised bone marrow microenvironments, namely the vascular and osteoblastic ‘niches’ regulate haematopoiesis. The vascular niche is conducive for thrombopoiesis, and megakaryocytes may, in turn, regulate the vascular niche, especially in supporting vascular and haematopoietic regeneration following irradiation or chemotherapy. A role for platelets in tumour growth and metastasis is well established and, more recently, the vascular niche has also been implicated as an area for preferential homing and engraftment of malignant cells. This article aims to provide an overview of the dynamic interactions between cellular and molecular components of the bone marrow vascular niche and the potential role of megakaryocytes in bone marrow malignancy.
Bone marrow haematopoietic niches in normal physiology
Haematopoietic stem cells (HSCs) reside in complex, dynamic microenvironments, or ‘niches’. These are composed of supportive cells, extracellular growth factors, metabolic constituents and matrix factors that actively regulate stem cell function (Table 1)[1–9], and enable a sustainable and responsive HSC pool[1, 10]. Two physiologically distinct HSC niches have been described in the bone marrow; the endosteal (or osteoblastic) niche at the bone-marrow interface, and the vascular niche around the specialized vascular endothelium (Figure 1)[9]. Interactions between stem cells and niches are bidirectional; the niche regulates stem cell self-renewal and cell fate decisions, and stem cells in turn modulate the nurturing microenvironments in which they reside[7].
Table 1.
Cell-extrinsic soluble factors essential for HSC maintenance and their source in bone marrow niches
| Factor | Receptor | Role/Function | Primary Source |
|---|---|---|---|
| SCF (kit ligand) | c-Kit | HSC localization, survival and maintenance. | Bone marrow stromal cells[130] |
| SDF1 | CXCR4 | Principal axis determining HSC/HPC localization [131]. Promotes surface integrin upregulation and HSC survival [45, 132]. |
Mesenchymal stem cells [36] Reticular cells [34] Osteoblasts |
| Ang-1 | Tie2 | Promotes maintenance of quiescent HSCs | Osteoblasts[15], endothelial cells, pericytes[133] |
| TPO | TPO receptor (c-Mpl) | Regulates stem cell quiescence [16, 134] | Liver, kidney, bone marrow stroma, osteoblasts [42] |
| Ca2+ ions | G-protein coupled Ca2+-sensing receptor | Maintains localization of HSCs near the endosteal bone lining[19] | Osteoclast-mediated bone degradation |
Figure 1. Cells, matrix factors and metabolic constituents contributing to osteoblastic and vascular bone marrow niches.
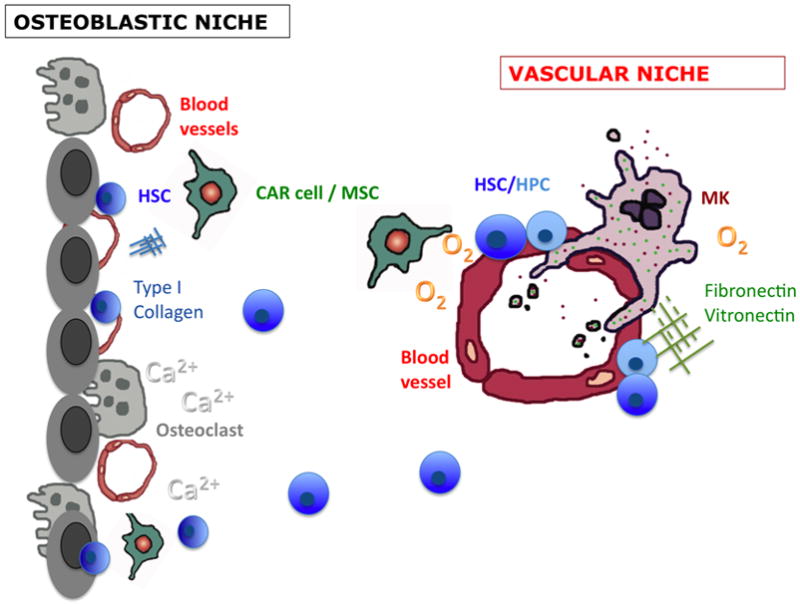
The osteoblastic niche. Osteoblasts and osteoclasts secrete numerous factors that regulate haematopoietic stem cells (HSCs; dark blue). Bone resorption releases calcium ions (Ca2+) influencing HSCs through the Ca2+-sensing receptor. The endosteal surface is also heavily invested with blood vessels. Vascular and perivascular cells, such as CXCL12 (SDF1)-producing reticular cells (CAR) and mesenchymal stem cells (MSCs) are also thought to contribute to HSC niches at the endosteum. The relative hypoxia and abundance of Type I collagen inhibit proplatelet formation by megakaryocytes.
The vascular niche. Perivascular sites maintain HSCs and haematopoietic progenitor cells (HPCs; lighter blue) in the bone marrow and in extramedullary sites including spleen, liver and the placenta. A wide variety of vascular and perivascular cells are likely to contribute to vascular niches, including endothelial cells, CAR cells/MSCs and possibly megakaryocytes. Matrix factors fibronectin, vitronectin and Type III and IV collagen are found around blood vessels, which enhance megakaryocyte development and function.
The endosteal niche: osteoblasts and osteoclasts
The majority of long-term repopulating HSCs are found near the endosteal lining of the bone where they are in close contact with the bone remodelling cells, osteoblasts and osteoclasts[11–14]. Osteoblasts secrete several growth factors required for HSC maintenance, including angiopoietin 1 (Ang-1), thrombopoietin (TPO) and stromal-derived factor-1 (SDF1) (Table 1)[15, 16]. Manipulations that increase the numbers of osteoblasts also increase HSC numbers, suggesting that the availability of osteoblastic niches may limit the HSC pool[13, 14]. Notch and N-cadherin-mediated interactions between HSCs and osteoblasts promote cellular quiescence and self-renewal[14, 17]. However, whether direct cell-cell contact between osteoblasts and HSCs is necessary has yet to be resolved[18].
Osteoclasts also contribute to the HSC niche. Calcium ions (Ca+) released by bone resorption retain HSCs close to the endosteal lining[19] and osteoclast-derived proteolytic enzymes cleave anchorage proteins such as SDF1 and stem cell factor (SCF) enabling mobilization of HSCs from the endosteal region[20].
The vascular niche
While the osteoblastic niche promotes stem cell quiescence, the perivascular region is thought to be more conducive to HSC expansion[21–24]. Early in vivo studies indicating that the perivascular regions within bone marrow are important for haematopoiesis described restoration of thrombopoiesis following chemokine-induced localization of megakaryocytes to the vascular niche in TPO-deficient mice[25]. Subsequently, immunofluorescence studies using antibodies to the CD150 (SLAM) family of receptors which are differentially expressed among HSCs and haematopoietic progenitor cells (HPCs) suggested that about two-thirds of HSCs in the murine bone marrow and spleen are located adjacent to blood vessels[23]. Studies by Chute et al and others demonstrate that endothelial cells regulate HSC self renewal and cell fate decisions[26]. The relative importance of direct cell-cell contact versus secreted factors in this process remains uncertain.
Cellular components of the vascular niche
Bone marrow vascular endothelium
The bone marrow vasculature is a dense network of smooth muscle-invested afferent arterioles and capillaries, and thin-walled, fenestrated venous sinusoids. It has been suggested that the sinusoidal endothelium is uniquely specialized to support haematopoiesis over other vessel types[27], although this has not been definitively shown and the majority of studies have used pan-endothelial markers such as PECAM (CD31), vWF, or MECA32 (the murine equivalent of CD31). Unlike other vessels, bone marrow sinusoidal endothelium is devoid of pericytes although it is invested with specialized SDF1-secreting reticular cells, as described below. It is presumed that vascular niches in bone marrow and spleen are dedicated for haematopoietic stem/progenitor cells, although non-haematopoietic stem cells such as neural stem cells are thought to reside in analogous vascular niches in other organs[28, 29].
In vitro studies have shown that endothelial cells produce factors that support HSC maintenance, and their unique expression of adhesion factors is important for trafficking and homing of HSCs to the bone marrow[22, 30–33]. Detailed investigation of the bone marrow vasculature has been hampered by the inability to distinguish between arteriole and sinusoidal vessel types. A recent study clarified the immunohistological profile of specific vessel types, reporting that sinusoids can be specifically identified by their expression of VEGFR3, while arterioles uniquely express Sca1[27]. However, the relative contribution of arterioles versus sinusoids to the haematopoietic niche remains to be clarified.
Perivascular stromal cells
Almost all HSCs (97%) are in contact with a small population of perivascular, SDF1-producing reticular cells, termed CXCL12-abundant reticular cells (CAR cells)[34]. SDF1-CXCR4 signalling is essential for HSC colonization of the bone marrow and for HSC maintenance, and while CAR cells are highly enriched for SDF1, comparatively little SDF1 production is observed at the endosteal region[34]. Although endothelial cells produce negligible SDF1, they are often seen surrounded by the cell bodies or cell processes of the CAR cells[34].
A second population of perivascular cells, identified by their expression of nestin, a filament protein typically characteristic of neuroectoderm-derived cells, has also recently been described as key components of the HSC niche[35]. Nestin+ cells were identified as mesenchymal stem cells (MSCs), with colony-forming capacity and osteoblastic, chondrocytic and adipocytic differentiation potential [35]. Previous work revealed that release of HSCs from the bone marrow is orchestrated by circadian rhythms via signals from the sympathetic nervous system (SNS) that control SDF1 expression[36]. Virtually all SNS fibres in the bone marrow were found to be associated with the nestin+ cells, which express high levels of SDF1 and therefore are likely to be the same, or an overlapping, population of cells as the CAR cells. Other groups have described similar subendothelial stromal cells expressing Ang-1 that are able to support HSCs in the bone marrow and that may also establish heterotopic haematopoietic microenvironments following subcutaneous transplantation[37].
Vascular and osteoblastic niches: overlapping or distinct entities?
Classically, dichotomous vascular and osteoblastic haematopoietic microenvironments have been proposed, although whether these niches are indeed anatomically distinct entities is unclear[38]. Advances in confocal and two-photon microscopy have enabled a dynamic exploration of bone marrow niches within their physiological context in vivo[39, 40]. These insights suggest that several cellular and molecular components are shared (Figure 1). The endosteal bone lining is heavily invested with blood vessels[23, 27, 39, 40], with 90% of osteoblasts being within 20 μm of a blood vessel[39]. Furthermore, the nestin+ SDF1-secreting MSCs described by Méndez-Ferrer et al were observed adjacent to HSCs regardless of whether they were in osteoblastic or vascular niches, suggesting that this niche cell may also be common to both osteoblastic and vascular niches[34, 35]. It is therefore possible that the role of osteoblasts may be more indirect, influencing a subset of perivascular niches to promote stem cell quiescence and self-renewal. Although further study is warranted, given the close physical interaction between the cellular elements it seems likely the osteoblastic and vascular niches are not spatially distinct but overlapping entities. Nonetheless, specialized ‘sub-pockets’ of this haematopoietic microenvironment may exist which are more conducive for stem cell renewal vs. differentiation depending on the balance of osteoblastic vs. endothelial derived signals. Further studies are required to delineate the migration patterns of haematopoietic cells between these two areas, and to better characterize the subtypes and maturation state of cells at each site.
Megakaryocyte – niche interactions
The vascular niche supports thrombopoiesis
Mature, polyploid megakaryocytes within the bone marrow are primarily located adjacent to bone marrow vessels, where they extend transendothelial pseudopods or migrate through the endothelium to produce platelets. The processes of megakaryopoiesis and thrombopoiesis have been extensively reviewed elsewhere[41–43]. While the vascular niche supports expansion of all haematopoietic lineages, it appears especially critical for megakaryocyte function and platelet production[22, 24, 25, 44–46]. This was first suggested by in vitro studies demonstrating that, in the absence of exogenous cytokines, bone marrow endothelial monolayers supported differentiation of human CD34+ HSCs to megakaryocyte and myeloid progenitors via their secretion of the thrombopoietic cytokines SCF and interleukin (IL)-6[21, 22]. Notably, endothelial cells derived from bone marrow supported significantly greater megakaryocyte differentiation than did human umbilical vein endothelial cells (HUVECs) or bone marrow stromal cells, suggesting that bone marrow endothelium was uniquely specialized in this regard[22]. One explanation for this is that production of haematopoietic cytokines by the other types of endothelium is not constitutive but occurs only following stimulation with IL-1 or TNF[22]. Furthermore, this study reported that CD34+ cells that were cultured in direct cellular contact with endothelial cells gave rise to a larger number of megakaryocyte progenitors than did CD34+ cells separated from the endothelial cells by transwell coculture chambers[22]. Further evidence that interactions between megakaryocytes and endothelium are important is that administration of SDF1 enhances platelet production only in the presence of bone marrow sinusoids[47, 48].
Megakaryocyte-vessel interactions were subsequently explored in vivo in thrombocytopenic, TPO-deficient mice. Even in the absence of TPO, administration of SDF1 and fibroblast growth factor (FGF)-4 caused a three-fold increase in the platelet count as a result of enhanced interactions between the small number of megakaryocyte progenitors and the bone marrow vascular endothelium[25]. Administration of vascular disrupting agents, which inhibit bone marrow vascular regeneration after chemotherapy-induced myelosuppression, has also been shown to cause prolonged and selective thrombocytopenia which continues after restoration of other haematopoietic lineages[49].
Studies of mice deficient in PECAM-1 provided further evidence that megakaryocyte positioning at the vascular niche is important in thrombopoiesis[45]. Following antibody-induced thrombocytopenia, recovery of the peripheral platelet count was impaired in PECAM-1−/− mice even though megakaryocyte maturation, proplatelet formation and platelet production in vitro was unaffected. This finding was attributed to reduced directional migration in response to SDF1 due to defective polarization of the CXCR4 receptor in the PECAM-1−/− megakaryocytes[45]. Movement of PECAM-1−/−megakaryocytes towards an SDF1 gradient was less than one third of that of wild type megakaryocytes and PECAM-1 deficiency was also associated with an increase in adhesion to the matrix protein fibronectin due to constitutive activation of one of the fibronectin-binding surface integrins, αIIbβ3[45]. These data indicate that PECAM-1 is required for migration of megakaryocytes from osteoblastic to perivascular sites by regulating their directional movement and adhesion to matrix proteins in the bone marrow microenvironment.
Although proplatelet formation (PPF) by megakaryocytes is a spontaneous process in vitro, in vivo it appears to be temporally regulated by interactions with the extracellular matrix, in that while Type I collagen strongly inhibits PPF, collagen types IV and III support PPF[50, 51]. Type I collagen is particularly abundant at the osteoblastic zone while types III and IV are found around the bone marrow vessels. It is possible that this prevents premature release of platelets in the bone marrow prior to megakaryocyte localization at the bone marrow – blood vascular interface. Similarly, the relative hypoxia at the endosteal regions also inhibits PPF[52], while adhesion to perivascular fibronectin and vitronectin enhance megakaryopoiesis and PPF (Figure 1)[53, 54].
Mechanical forces at the vascular niche are also pivotal to platelet release. Blood flow-induced hydrodynamic stress is required for platelet release from proplatelet-forming megakaryocytes, sheering off fragments of megakaryocyte protrusions and enabling release of proplatelets/platelets into the circulation[46, 55].
Reciprocity: Megakaryocytes supporting haematopoietic niches
Evidence suggests that megakaryocytes both support and regulate the bone marrow niches that they inhabit via their abundant production and delivery of growth factors and cytokines (Table 2). Genetic mouse models in which megakaryocyte numbers are increased, such as mice over-expressing TPO or GATA-1 and NF-E2 knockout strains, have an osteosclerotic phenotype[56]. In addition, both mature and immature megakaryocytes stimulate proliferation and differentiation of osteoblasts in vitro and inhibit osteoclast differentiation[57, 58], possibly via α3β1 (VLA4) and α5βmeditated megakaryocyte-osteoblast adhesion and activation[59]. Unlike the majority of haematopoietic cells, megakaryocytes remain functional for around 7 days following irradiation and bone marrow transplantation[60]. It has therefore been postulated that megakaryocytes play a role in the reconstitution of bone marrow niches. An investigation of the bone marrow of mice 48 hours after sub-lethal total body irradiation (TBI) revealed that megakaryocytes had trans-located from the central vascular marrow areas to the diaphysis in response to upregulation of SDF1 in these areas[61]. Expression of platelet-derived growth factor (PDGF)-β and FGF-2, two megakaryocyte-derived factors known to stimulate osteoblast differentiation and proliferation, was significantly increased following TBI[61]. These data suggest that megakaryocytes play an important role in recovery of osteoblastic niches following bone marrow damage.
Table 2.
Megakaryocyte-derived factors that may influence haematopoietic niches
| Osteoblastic niche | Osteoclastogenic promoters | RANKL[135] |
| Osteoclastogenic inhibitors | Osteoprotegerin[57, 135] | |
| Osteoblast promoters/Mesenchymal growth factors | PDGF, FGF2, TGFβ, fibronectin-binding surface integrins[56, 58] | |
| Bone matrix proteins | Osteocalcin, osteonectin, osteopontin, bone sialoprotein, BMP-2, -4 and -6. [56] | |
| Vascular niche | Proangiogenic factors | VEGF, PDGF, TGFβ IGF-2, b-FGF (FGF2), hepatocyte growth factor (HGF), epidermal growth factor (EGF), SDF1 (CXCL12), Lipid mediators e.g. sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) and MMPs[68, 136] |
| Antiangiogenic factors | TSPs, Endostatin, Plasminogen activator inhibitor (PAI) type 1, TGFβ, PF4 (CXCL4)[68, 136] | |
| Chemokines | SDF1[64, 88] (synthesized in low amounts by megakaryocytes-largely taken up from the microenvironment), PF4[137] |
Despite extensive research into the vital contribution of platelets to angiogenesis and maintenance of blood vessel integrity, surprisingly few studies have examined the functional contribution of megakaryocytes to the regulation of bone marrow vasculature. Megakaryocytes are a major source of both proangiogenic and antiangiogenic factors in the bone marrow (Table 2), support the survival of bone marrow-derived sinusoidal endothelial cells in vitro[62] and in vivo evidence from murine studies suggest that angiogenic processes both in the bone marrow and in other tissues are regulated by the absolute number of megakaryocytes in the bone marrow and their content of angiogenic regulatory factors[63, 64]. Immunohistological staining of mouse bone marrow for thrombospondin (TSP)1, the first endogenous angiogenesis inhibitor to be recognized[65], revealed that bone marrow TSP1 expression was limited to megakaryocytes, platelets and the endosteal surface. Other groups have shown that bone marrow vascularity is increased in TSP1−/− mice[64]. Recovery of the bone marrow vasculature following 5-FU treatment occurred significantly faster in TSP1 and TSP2 double knock-out (TSP-DKO) mice than in wild-type mice. Furthermore, vessel sprouting in Matrigel plugs loaded with TSP-DKO megakaryocytes was nearly 2-fold higher than wild-type megakaryocytes, and revascularization of ischaemic hind limbs was also accelerated[64]. Megakaryocyte content of vascular regulatory factors may be a key determinant of bone marrow vascularity and by regulating osteoblast proliferation, megakaryocytes could influence the number and function of both osteoblastic and haematopoietic vascular niches.
Megakaryocyte and platelet intracellular cargo
Megakaryocytes and platelets store intracellular cargo in α-granules, dense bodies and lysosomes[66]. α-granules, the primary storage vesicle, are formed from vesicular budding of the Golgi apparatus, then mature into multi-vesicular bodies (MVB), which is where organization of specific proteins into distinct subclasses of α-granules is thought to take place prior to their packaging into platelets[67]. Less is known about the origin and packaging of dense bodies, which contain haemostatically active components including serotonin, adenosine 5′diphosphate (ADP), adenosine 5′triphosphate (ATP), catecholamines and calcium.
Megakaryocyte-derived soluble factors may be either synthesized intracellularly or endocytosed and/or pinocytosed. The bone marrow microenvironment may thereby modify the protein content of megakaryocytes and platelets either by altering megakaryocytic gene transcription or by increasing the availability of proteins for cellular uptake.
Regulation of granule release
It was originally assumed that megakaryocyte and platelet granular contents were universally and randomly deployed upon cellular activation. However, it was recently suggested that α-granules are heterogeneous and highly organized, and that selective deployment of granules containing certain protein families may occur[67].
Preliminary evidence for the notion that platelets were able to differentially secrete certain proteins over others was apparent from data showing that the relative release of SDF1 as compared to serotonin was higher by platelets from TSP−/− mice than by platelets from wild-type mice[64]. This observation was supported and extended by subsequent reports indicating that pro- and anti-angiogenic proteins are organized into distinct platelet α-granules[68]. Using double immunofluorescence labelling of human platelets and in vitro cultured mouse megakaryocytes it was shown that angiogenesis stimulators (VEGF, FGF2) and inhibitors (endostatin, TSP1) were segregated into distinct α-granule subpopulations[68]. Similar granule heterogeneity has been reported for the adhesive proteins vWF and fibronectin[69] and for tissue inhibitors of MMPs (TIMPs)[70]. However, a very recent study using quantitative immunofluorescence co-localization confocal microscopy of platelets suggested instead that while α-granule functionally-synergistic cargo may be clustered within the granule, no evidence was found for organized subsets of α-granules with distinct biological activities[71].
Given the implications for therapeutic manipulation, the hypothesis that platelets may selectively release pro-angiogenic mediators over anti-angiogenic factors and enhance tumour growth or healing is an area of intense interest. The selective release of angiogenic simulators versus inhibitors appears to be counter-regulated by specific proteinase-activated receptors (PARs)[68, 72]. Treatment of human platelets with a selective PAR-1 agonist specifically released VEGF granules, while a selective PAR-4 agonist resulted in the release of endostatin but not VEGF[68]. There is some evidence that tumours may modulate the release of angiogenic factors from platelets by the secretion of specific PAR ligands or, alternatively, by proteolytic degradation of opposing PAR receptors[72]. Many tumour cell lines express PAR-1[73] and overexpression of PAR-1 in B16 melanoma cells increases experimental pulmonary metastasis 5-fold[74]. Furthermore, in vitro studies have demonstrated that a breast cancer cell line MCF-7 selectively stimulates platelets to release VEGF and pro-angiogenic releasate, an effect which was inhibited by aspirin treatment[75]. Whether ligation of PAR receptors, or indeed other signalling pathways on megakaryocytes, alters the packaging of proteins into nascent platelets by megakaryocytes is not known.
Platelets in malignancy
Platelets and cancer have been associated since the observation of Trousseau in the 1860s that a migratory thrombophlebitis due to blood clotting and inflammation of the blood vessels was indicative of an occult carcinoma. He later diagnosed the same syndrome in himself and died soon afterwards of gastric carcinoma[76]. It has since been well established that advanced malignancy is associated with platelet abnormalities and hypercoagulability. Higher platelet counts correlate with poor prognosis, and depletion of platelets in animal models reduces metastases in both xenograft and syngeneic tumour models and for a wide range of cancer cell lines[77–79]. Roles for platelets in cancer progression are reviewed elsewhere[80, 81] and outlined briefly below (Table 3).
Table 3.
Roles of platelet/megakaryocyte-derived proteins in carcinogenesis and metastasis
| Protein | Function in carcinogenesis/metastasis |
|---|---|
| TGFβ | |
| SDF1 |
|
| PF4 | |
| TSP1 | Both pro- and anti-metastatic effects have been reported.
|
| P-selectin, vWF, GPIIb/IIIa and Ib-IX | |
| MMP9, MMP2 |
|
| Angiogenic growth factors | See Table 2. |
| Coagulation factors: Thrombin, tissue factor |
|
| Bioactive lipids: S1P and LPA |
|
Primary tumour angiogenesis: The transition of tumours from an avascular state to the active recruitment of blood vessels into the tumour, termed the ‘angiogenic switch’, marks the onset of aggressive and invasive growth[82–85], and a dose-response relationship exists between blood platelet levels and new vessel sprouting in angiogenesis. Platelets may also mediate the recruitment and retention of bone marrow-derived endothelial progenitor cells to sites of vasculogenesis[86–88] and their subsequent maturation[89–92].
Immune evasion: It is thought that circulating tumour cells travel through the circulation coated with platelets that ‘shield’ them from immune destruction. Experimental animal models of haematogenous metastasis suggest that platelets interfere with natural killer (NK) cell anti-tumoural activity[93, 94], possibly via platelet release of TGFβ[94]. Other studies have also shown that the capability of both mouse and human tumour cell lines to activate platelets correlates with their metastatic efficiency[95, 96].
Tumour cell adhesion/invasion at metastatic sites: Formation of platelet-tumour cell aggregates has been shown to enhance lodgement at metastatic sites via platelet expression of adhesion integrins, in particular GPIIb-IIIa (αIIbβ) and GPIb-IX [97–99]. Once bound to the endothelial cell surface, platelets provide growth factors, coagulation factors and MMPs (Table 3), which support the accumulation of more platelets, leukocytes and tumour invasion. A recent report has suggested that direct cell contacts between platelets and tumour cells during their transit through the bloodstream, and in particular platelet-derived TGFβ and NF-κB signalling, induces cancer cells to undergo an epithelial-mesenchymal-like transition thereby enhancing their metastatic and invasory phenotype[100].
Bone marrow vasculature in haematological malignancy
Tumour vascularisation is well recognized as a fundamental pathological factor in ‘solid’ tumours and there is increasing evidence that the progression of haematological malignancy also requires the induction of new blood vessels within the bone marrow. Increased bone marrow angiogenesis has been reported in acute and chronic leukaemias[101, 102], myelofibrosis and myelodysplastic syndromes[10–105] and multiple myeloma[106]. Bone marrow of acute and chronic leukaemia patients shows enhanced VEGF expression as well as increased microvessel density[101–103, 107]. Leukaemic cells may secrete VEGF and can induce upregulation of VEGF production by BM stromal cells including megakaryocytes[108]. VEGF also has autocrine effects on leukaemic cells, and both VEGF signalling and direct contact with the sinusoidal endothelium, have been shown to promote survival, proliferation, motility of human leukaemia cells and also resistance to chemotherapy[109–111]. The persistence of leukaemic stem cells following chemotherapy is thought to be responsible for disease persistence and relapse, and localization of malignant cells at the vascular niche may confer resistance to chemotherapy and radiotherapy[8, 26, 112, 113].
The bone marrow as a ‘metastatic microenvironment’
The bone marrow is a common site for metastatic disease. Steven Paget’s ‘seed and soil’ hypothesis introduced the concept that a receptive microenvironment was required for malignant cells to engraft distant tissues and form metastases[114]. This microenvironment is now known to comprise supportive (non-malignant) stromal cells, soluble factors, vascular networks, nutrients and metabolic components, and the extracellular matrix[83, 115–119]. A ‘tumour-permissive’ inflammatory microenvironment also contributes to tumour progression by downregulating anti-tumoural immunity[120].
There is evidence that tumour cells preferentially home to, and engraft, the vascular stem cell niche in preference to other areas of bone marrow[121, 122]. Studies in mice using dynamic, intra-vital confocal imaging indicated that fluorescently-labelled human Nalm-6 leukaemic cells injected into immunodeficient mice preferentially engraft the bone marrow vascular niche via SDF1-CXCR4 interactions, suggesting analogous dependence on niche-specific signals to those required by physiological bone marrow-derived cells[121]. Other cell lines, including murine and human leukaemias, multiple myeloma, prostate and melanoma cell lines engrafted perivascular bone marrow regions in a similar fashion[121]. It is well established from both human and animal studies that expression of the CXCR4 receptor on breast, prostate and other carcinomas increases the risk of bone marrow metastases (reviewed in Burger and Kipps, 2006 and Taichman et al., 2002).
Megakaryocytes/platelets in bone marrow metastasis
Despite the accumulating evidence that platelets play important pathological roles in tumourigenesis, metastasis, angiogenesis and inflammation, there have been very few studies examining bone marrow histology and specifically megakaryocytes in metastatic disease. This is perhaps in part due to limited availability of clinical specimens and technical challenges associated with isolation of intact cells from the bone marrow.
An early histological study examined megakaryocyte ploidy in postmortem bone marrow aspirates from 30 patients with metastatic carcinoma (half of whom had paraneoplastic thromboembolic events) and compared these to 3 control groups: patients with localized carcinoma, patients with thromboses but no carcinoma, and healthy controls[123]. Megakaryocyte ploidy was found to be significantly higher in patients with metastatic disease, regardless of whether they had associated thromboembolic disease, than in any of the control groups[123]. The authors speculated that the increased megakaryocyte ploidy resulted in increased platelet production and altered platelet heterogeneity, although this was not specifically examined in this study.
After the bone marrow and the spleen, the next main reservoir for megakaryocytes is the lung, where they may be observed within alveolar capillaries albeit in low numbers. It has been reported that the numbers of pulmonary megakaryocytes associated with lung metastases and pulmonary tumour emboli are higher than in lungs from patients with localized carcinomas and healthy individuals [124–126]. In a study of mammary carcinoma metastasis in rats, highly metastatic cell line clones implanted in the mammary fat pad induced increased numbers of megakaryocytes in the bone marrow which was attributed to secretion of a growth factor with GM-CSF/IL-3-like effect[127]. This was not apparent in animals bearing poorly metastatic tumours.
Megakaryocyte/platelet surface integrin αIIb/β3 may be involved in tumour colonization of the bone marrow[128]. Mice deficient in β3 or mice receiving specific αIIb/β3 inhibitors are protected from spontaneous bone metastases. Notably, following direct intrafemoral tumour cell delivery, although tumour growth occurred in the bone marrow of β3−/− mice, there was no associated bone destruction apparent, suggesting that interactions between platelets/megakaryocytes and tumour cells support tumour-mediated bone destruction[128].
Concluding remarks
Many questions about the role of megakaryocytes in the vascular niche both under physiological conditions and in malignancy remain unanswered. Firstly, the functional contribution of megakaryocytes to the vascular niche and the pathways that determine the net effects of megakaryocyte-derived factors (e.g. the pro- versus anti- angiogenic stimuli) have not been fully clarified. Secondly, the pathways that mediate homing of circulating tumour cells to the bone marrow vascular niche are not fully understood. While a wealth of evidence supports an integral role for platelets in metastasis and the recruitment of bone marrow-derived haematopoietic cells to sites of vasculogenesis, the role of megakaryocytes in recruitment of malignant cells to bone marrow niches has been relatively little studied. It is possible that tumour-derived factors, either acting systemically from distant primary tumours or more directly via tumour cells resident in the bone marrow, may influence the megakaryocyte/platelet ‘phenotype’. Indeed, there are data to suggest that platelet gene expression is altered in metastatic lung cancer as compared to controls[129]. As well as altering megakaryocyte secretion of tumour-regulatory factors within the bone marrow microenvironment, an alteration in the packaging of these factors into nascent platelets would modulate the ‘pro-malignant’ platelet phenotype with widespread, systemic effects.
Acknowledgments
B.P. received funding from a Kay Kendall Leukaemia Fund Travelling Fellowship and a Fulbright Scholarship in Cancer Research. D.L. receives support from the NCI; Children’s Cancer and Blood foundation; Stavros S. Niarchos Foundation; Champalimaud Foundation; Nancy C. and Daniel P. Paduano Foundation; Hartwell Foundation; Witmer Family Foundation; Malcolm Hewitt Wiener Foundation; Mary Kay Ash Charitable Foundation; the AHEPA 5th District; National Foundation for Cancer Research and Susan G. Komen for the Cure. I.R. is funded by Leukaemia & Lymphoma Research Specialist Programme Award 08030 and the Imperial College London Comprehensive Biomedical Research Centre.
References
- 1.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–7. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 2.Balduino A, Hurtado S, Frazao P, Takiya C, Alves L, Nasciutti L, El-Cheikh M, Borojevic R. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319:255–66. doi: 10.1007/s00441-004-1006-3. [DOI] [PubMed] [Google Scholar]
- 3.Haylock DN, Nilsson SK. Stem cell regulation by the hematopoietic stem cell niche. Cell Cycle. 2005;4:1353–5. doi: 10.4161/cc.4.10.2056. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RN, Psaila B, Lyden D. Niche-to-Niche migration of bone marrow-derived cells. Trends in Molecular Medicine. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–9. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 7.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 8.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, Laurenti E, Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–8. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord BI, Testa NG, Hendry JH. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- 13.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 15.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–97. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–63. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiel MJ, Acar M, Radice GL, Morrison SJ. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell. 2009;4:170–9. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 20.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 21.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–86. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 22.Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, Asch AS. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–63. [PubMed] [Google Scholar]
- 23.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 25.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 26.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2011 doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 27.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colmone A, Sipkins DA. Beyond angiogenesis: the role of the endothelium in teh bone marrow vascular niche. Transl Res. 2008;151:1–9. doi: 10.1016/j.trsl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004;32:1226–37. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–9. [PubMed] [Google Scholar]
- 32.Naiyer AJ, Jo DY, Ahn J, Mohle R, Peichev M, Lam G, Silverstein RL, Moore MA, Rafii S. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94:4011–9. [PubMed] [Google Scholar]
- 33.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25:862–4. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom A, MacArthur B, Lira S, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Coordinated Regulation of Hematopoietic and Mesenchymal Stem Cells in a Bone Marrow Niche. Blood (ASH annual meeting abstracts) 2009;114:2. [Google Scholar]
- 36.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 37.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, Bertoncello I, Nilsson SK. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118:1516–24. doi: 10.1182/blood-2010-08-303800. [DOI] [PubMed] [Google Scholar]
- 39.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, Park J, Haug JS, Wunderlich JP, Li H, Zhang S, Johnson T, Feldman RA, Li L. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 41.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–6. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geddis AE. Megakaryopoiesis. Semin Hematol. 2011;47:212–9. doi: 10.1053/j.seminhematol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–86. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 45.Dhanjal TS, Pendaries C, Ross EA, Larson MK, Protty MB, Buckley CD, Watson SP. A novel role for PECAM-1 in megakaryocytokinesis and recovery of platelet counts in thrombocytopenic mice. Blood. 2007;109:4237–44. doi: 10.1182/blood-2006-10-050740. [DOI] [PubMed] [Google Scholar]
- 46.Junt T, Schulz H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JEJ, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 47.Wang JF, Liu ZY, Groopman JE. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–64. [PubMed] [Google Scholar]
- 48.Hamada T, Mohle R, Hesselgesser J, Hoxie J, Nachman RL, Moore MA, Rafii S. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor-1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188:539–48. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopp HG, Rafii S. Thrombopoietic cells and the bone marrow vascular niche. Ann NY Acad Sci. 2007;1106:175–9. doi: 10.1196/annals.1392.004. [DOI] [PubMed] [Google Scholar]
- 50.Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, Balduini CL, Torti M. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–7. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson SK, Debatis ME, Dooner MS, Madri JA, Quesenberry PJ, Becker PS. Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem. 1998;46:371–7. doi: 10.1177/002215549804600311. [DOI] [PubMed] [Google Scholar]
- 52.Pallotta I, Lovett M, Rice W, Kaplan DL, Balduini A. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS One. 2009;4:e8359. doi: 10.1371/journal.pone.0008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leven RM, Tablin F. Extracellular matrix stimulation of guinea pig megakaryocyte proplatelet formation in vitro is mediated through the vitronectin receptor. Exp Hematol. 1992;20:1316–22. [PubMed] [Google Scholar]
- 54.Fox NE, Kaushansky K. Engagement of integrin alpha4beta1 enhances thrombopoietin-induced megakaryopoiesis. Exp Hematol. 2005;33:94–9. doi: 10.1016/j.exphem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Dunois-Larde C, Capron C, Fichelson S, Bauer T, Cramer-Borde E, Baruch D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009;114:1875–83. doi: 10.1182/blood-2009-03-209205. [DOI] [PubMed] [Google Scholar]
- 56.Kacena MA, Gundberg CM, Horowitz MC. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone. 2006;39:978–84. doi: 10.1016/j.bone.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–9. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Ciovacco WA, Cheng YH, Horowitz MC, Kacena MA. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem. 2010;109:774–81. doi: 10.1002/jcb.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemieux JM, Horowitz MC, Kacena MA. Involvement of integrins alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem. 2010;109:927–32. doi: 10.1002/jcb.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanum G. The megakaryocyte DNA content and platelet formation after the sublethal whole body irradiation of rats. Blood. 1984;63:917–20. [PubMed] [Google Scholar]
- 61.Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, Paolucci P, Conte P, Horwitz EM. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–43. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–8. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varner JA. The sticky truth about angiogenesis and thrombospondins. J Clin Invest. 2006;116:3111–3. doi: 10.1172/JCI30685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M, Milde T, Ramos CA, Zhang F, Kopp T, Bornstein P, Jin DK, Marcus AJ, Rafii S. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–91. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Italiano JE, Jr, Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7 (Suppl 1):173–6. doi: 10.1111/j.1538-7836.2009.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro-and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5:2009–16. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
- 70.Villeneuve J, Block A, Le Bousse-Kerdiles MC, Lepreux S, Nurden P, Ripoche J, Nurden AT. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: a novel organization for these secreted proteins. Exp Hematol. 2009;37:849–56. doi: 10.1016/j.exphem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Kamykowski J, Carlton P, Sehgal S, Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet {alpha}-granules. Blood. 2011;118:1370–3. doi: 10.1182/blood-2011-01-330910. [DOI] [PubMed] [Google Scholar]
- 72.Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, Wallace JL. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci U S A. 2005;102:216–20. doi: 10.1073/pnas.0406682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nierodzik ML, Chen K, Takeshita K, Li JJ, Huang YQ, Feng XS, D’Andrea MR, Andrade-Gordon P, Karpatkin S. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood. 1998;92:3694–700. [PubMed] [Google Scholar]
- 74.Nierodzik ML, Bain RM, Liu LX, Shivji M, Takeshita K, Karpatkin S. Presence of the seven transmembrane thrombin receptor on human tumour cells: effect of activation on tumour adhesion to platelets and tumor tyrosine phosphorylation. Br J Haematol. 1996;92:452–7. doi: 10.1046/j.1365-2141.1996.d01-1494.x. [DOI] [PubMed] [Google Scholar]
- 75.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegelman ES, Needleman L. Venous thrombosis and cancer. N Engl J Med. 1993;328:885. author reply 6–7. [PubMed] [Google Scholar]
- 77.Gupta GP, Massague J. Platelets and metastasis: a novel fatty link. Journal of Clinical Investigation. 2004;114:1696–3. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karpatkin S, Pearlstein E. Role of Platelets in tumor cell metastases. Ann Intern Med. 1981;95:646–1. doi: 10.7326/0003-4819-95-5-636. [DOI] [PubMed] [Google Scholar]
- 79.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–51. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 81.Sierko E, Wojtukiewicz MD. Platelets and angiogenesis in malignancy. Seminars in thrombosis and hemostasis. 2004;30:95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- 82.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 83.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 84.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 85.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 86.Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–22. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 87.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1a and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. The Journal of Experimental Medicine. 2006;203:1211–33. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4(+) hemangiocytes. Nat Med. 2006;12:557–66. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, Bigalke B, Schonberger T, Geisler T, Siegel-Axel D, Oostendorp RA, Lindemann S, Gawaz M. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. Faseb J. 2006;20:2559–61. doi: 10.1096/fj.06-6265fje. [DOI] [PubMed] [Google Scholar]
- 90.de Boer HC, Verseyden C, Ulfman LH, Zwaginga JJ, Bot I, Biessen EA, Rabelink TJ, van Zonneveld AJ. Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol. 2006;26:1653–9. doi: 10.1161/01.ATV.0000222982.55731.f1. [DOI] [PubMed] [Google Scholar]
- 91.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 92.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–70. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 93.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300. [PubMed] [Google Scholar]
- 94.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69:7775–83. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 95.Heinmoller E, Weinel RJ, Heidtmann HH, Salge U, Seitz R, Schmitz I, Muller KM, Zirngibl H. Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol. 1996;122:735–44. doi: 10.1007/BF01209121. [DOI] [PubMed] [Google Scholar]
- 96.Heinmoller E, Schropp T, Kisker O, Simon B, Seitz R, Weinel RJ. Tumor cell-induced platelet aggregation in vitro by human pancreatic cancer cell lines. Scand J Gastroenterol. 1995;30:1008–16. doi: 10.3109/00365529509096346. [DOI] [PubMed] [Google Scholar]
- 97.Burdick MM, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol. 2004;287:C539–47. doi: 10.1152/ajpcell.00450.2003. [DOI] [PubMed] [Google Scholar]
- 98.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104:9024–8. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Labelle M, Begum S, Hynes RO. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-Like Transition and Promotes Metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–13. [PubMed] [Google Scholar]
- 102.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–21. [PMC free article] [PubMed] [Google Scholar]
- 103.Lundberg LG, Lerner R, Sundelin P, Rogers R, Folkman J, Palmblad J. Bone marrow in polycythemia vera, chronic myelocytic leukemia, and myelofibrosis has an increased vascularity. Am J Pathol. 2000;157:15–9. doi: 10.1016/S0002-9440(10)64511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mesa RA, Hanson CA, Rajkumar SV, Schroeder G, Tefferi A. Evaluation and clinical correlations of bone marrow angiogenesis in myelofibrosis with myeloid metaplasia. Blood. 2000;96:3374–80. [PubMed] [Google Scholar]
- 105.Pruneri G, Bertolini F, Soligo D, Carboni N, Cortelezzi A, Ferrucci PF, Buffa R, Lambertenghi-Deliliers G, Pezzella F. Angiogenesis in myelodysplastic syndromes. Br J Cancer. 1999;81:1398–401. doi: 10.1038/sj.bjc.6693515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vacca A, Ribatti D, Roncali L, Dammacco F. Angiogenesis in B cell lymphoproliferative diseases. Biological and clinical studies. Leuk Lymphoma. 1995;20 :27–38. doi: 10.3109/10428199509054750. [DOI] [PubMed] [Google Scholar]
- 107.Shanafelt TD, Kay NE. The clinical and biologic importance of neovascularization and angiogenic signaling pathways in chronic lymphocytic leukemia. Semin Oncol. 2006;33:174–85. doi: 10.1053/j.seminoncol.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 108.Ghannadan M, Wimazal F, Simonitsch I, Sperr WR, Mayerhofer M, Sillaber C, Hauswirth AW, Gadner H, Chott A, Horny HP, Lechner K, Valent P. Immunohistochemical detection of VEGF in the bone marrow of patients with acute myeloid leukemia. Correlation between VEGF expression and the FAB category. Am J Clin Pathol. 2003;119:663–71. doi: 10.1309/331Q-X7AX-KWFJ-FKXM. [DOI] [PubMed] [Google Scholar]
- 109.Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, Hicklin DJ, Witte L, Moore MA, Rafii S. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106:511–21. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood. 2006;107:1608–16. doi: 10.1182/blood-2005-06-2530. [DOI] [PubMed] [Google Scholar]
- 111.Veiga JP, Costa LF, Sallan SE, Nadler LM, Cardoso AA. Leukemia-stimulated bone marrow endothelium promotes leukemia cell survival. Exp Hematol. 2006;34:610–21. doi: 10.1016/j.exphem.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Gallay N, Anani L, Lopez A, Colombat P, Binet C, Domenech J, Weksler BB, Malavasi F, Herault O. The role of platelet/endothelial cell adhesion molecule 1 (CD31) and CD38 antigens in marrow microenvironmental retention of acute myelogenous leukemia cells. Cancer Res. 2007;67:8624–32. doi: 10.1158/0008-5472.CAN-07-0402. [DOI] [PubMed] [Google Scholar]
- 113.Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 2006;5:3113–21. doi: 10.1158/1535-7163.MCT-06-0228. [DOI] [PubMed] [Google Scholar]
- 114.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 115.Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3:1516–619. doi: 10.4161/cc.3.12.1289. [DOI] [PubMed] [Google Scholar]
- 116.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–21. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 118.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Psaila B, Lyden D. The metastatic niche: adapting the foreignsoil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 121.Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means JK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–5. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 123.Winkelmann M, Stockler J, Grassmuck J, Pfitzer P, Schneider W. Ploidy pattern of megakaryocytes in patients with metastatic tumors with and without paraneoplastic thrombosis and in controls. Haemostasis. 1984;14:501–7. doi: 10.1159/000215112. [DOI] [PubMed] [Google Scholar]
- 124.Sharnoff JG, Kim ES. Evaluation of pulmonary megakaryocytes. AMA Arch Pathol. 1958;66:176–82. [PubMed] [Google Scholar]
- 125.Aabo K, Hansen KB. Megakaryocytes in pulmonary blood vessels. I. Incidence at autopsy, clinicopathological relations especially to disseminated intravascular coagulation. Acta Pathol Microbiol Scand A. 1978;86:285–91. [PubMed] [Google Scholar]
- 126.Soares FA. Increased numbers of pulmonary megakaryocytes in patients with arterial pulmonary tumour embolism and with lung metastases seen at necropsy. J Clin Pathol. 1992;45:140–2. doi: 10.1136/jcp.45.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McGary CT, Miele ME, Welch DR. Highly metastatic 13762NF rat mammary adenocarcinoma cell clones stimulate bone marrow by secretion of granulocyte-macrophage colony-stimulating factor/interleukin-3 activity. Am J Pathol. 1995;147:1668–81. [PMC free article] [PubMed] [Google Scholar]
- 128.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher KN. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calverley DC, Phang TL, Choudhury QG, Gao B, Oton AB, Weyant MJ, Geraci MW. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci. 2010;3:227–32. doi: 10.1111/j.1752-8062.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, Martin FH, Williams DA. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992;89:7350–4. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 133.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 134.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–84. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 135.Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE. Synthesis of osteoprotegerin and RANKL by megakaryocytes is modulated by oestrogen. Br J Haematol. 2004;126:244–51. doi: 10.1111/j.1365-2141.2004.05024.x. [DOI] [PubMed] [Google Scholar]
- 136.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–70. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110:1153–60. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--adouble-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 139.Kolber DL, Knisely TL, Maione TE. Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. J Natl Cancer Inst. 1995;87:304–9. doi: 10.1093/jnci/87.4.304. [DOI] [PubMed] [Google Scholar]
- 140.Sharpe RJ, Byers HR, Scott CF, Bauer SI, Maione TE. Growth inhibition of murine melanoma and human colon carcinoma by recombinant human platelet factor 4. J Natl Cancer Inst. 1990;82:848–53. doi: 10.1093/jnci/82.10.848. [DOI] [PubMed] [Google Scholar]
- 141.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–42. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 142.Gupta SK, Singh JP. Inhibition of endothelial cell proliferation by platelet factor-4 involves a unique action on S phase progression. J Cell Biol. 1994;127:1121–7. doi: 10.1083/jcb.127.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gentilini G, Kirschbaum NE, Augustine JA, Aster RH, Visentin GP. Inhibition of human umbilical vein endothelial cell proliferation by the CXC chemokine, platelet factor 4 (PF4), is associated with impaired downregulation of p21(Cip1/WAF1) Blood. 1999;93:25–33. [PubMed] [Google Scholar]
- 144.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lawler J, Miao WM, Duquette M, Bouck N, Bronson RT, Hynes RO. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am J Pathol. 2001;159:1949–56. doi: 10.1016/S0002-9440(10)63042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–56. [PubMed] [Google Scholar]
- 147.Bertin N, Clezardin P, Kubiak R, Frappart L. Thrombospondin-1 and -2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res. 1997;57:396–9. [PubMed] [Google Scholar]
- 148.Tuszynski GP, Gasic TB, Rothman VL, Knudsen KA, Gasic GJ. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987;47:4130–3. [PubMed] [Google Scholar]
- 149.Yee KO, Connolly CM, Duquette M, Kazerounian S, Washington R, Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat. 2009;114:85–96. doi: 10.1007/s10549-008-9992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taraboletti G, Roberts DD, Liotta LA. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987;105:2409–15. doi: 10.1083/jcb.105.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ko HM, Kang JH, Jung B, Kim HA, Park SJ, Kim KJ, Kang YR, Lee HK, Im SY. Critical role for matrix metalloproteinase-9 in platelet-activating factor-induced experimental tumor metastasis. Int J Cancer. 2007;120:1277–83. doi: 10.1002/ijc.22450. [DOI] [PubMed] [Google Scholar]
- 152.Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, Karpatkin S. Growth-regulated oncogene is pivotalin thrombin-induced angiogenesis. Cancer Res. 2006;66:4125–32. doi: 10.1158/0008-5472.CAN-05-2570. [DOI] [PubMed] [Google Scholar]
- 153.Hu L, Roth JM, Brooks P, Luty J, Karpatkin S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008;68 :4666–73. doi: 10.1158/0008-5472.CAN-07-6276. [DOI] [PubMed] [Google Scholar]
- 154.Hu L, Roth JM, Brooks P, Ibrahim S, Karpatkin S. Twist is required for thrombin-induced tumor angiogenesis and growth. Cancer Res. 2008;68:4296–302. doi: 10.1158/0008-5472.CAN-08-0067. [DOI] [PubMed] [Google Scholar]
- 155.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992;89:11832–6. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fischer EG, Ruf W, Mueller BM. Tissue factor-initiated thrombin generation activates the signaling thrombin receptor on malignant melanoma cells. Cancer Res. 1995;55:1629–32. [PubMed] [Google Scholar]
- 157.Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–8. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 159.Boucharaba A, Guillet B, Menaa F, Hneino M, van Wijnen AJ, Philippe C, Oliver P. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol Res. 2009;18 :173–84. doi: 10.3727/096504009790217399. [DOI] [PMC free article] [PubMed] [Google Scholar]


