Abstract
The phloem-specific promoter of rice tungro bacilliform virus (RTBV) is regulated in part by sequence-specific DNA-binding proteins that bind to Box II, an essential cis element. Previous studies demonstrated that the bZIP protein RF2a is involved in transcriptional regulation of the RTBV promoter. Here we report the identification and functional characterization of a second bZIP protein, RF2b. RF2b, identified by its interaction with RF2a, binds to Box II in in vitro assays as a homodimer and as RF2a/RF2b heterodimers. Like RF2a, RF2b activates the RTBV promoter in transient assays and in transgenic tobacco plants. Both RF2a and RF2b are predominantly expressed in vascular tissues. However, RF2a and RF2b have different DNA-binding affinities to Box II, show distinctive expression patterns in different rice organs, and exhibit different patterns of subcellular localization. Furthermore, transgenic rice plants with reduced levels of RF2b exhibit a disease-like phenotype. We propose that the regulation of phloem-specific expression of the RTBV promoter and potentially the control of RTBV replication are mainly achieved via interactions of the Box II cis element with multiple host factors, including RF2a and RF2b. We also propose that quenching/titration of these and perhaps other transcription factors by RTBV is involved in the development of the symptoms of rice tungro disease.
Many factors contribute to the regulation of appropriate and accurate gene expression, including interactions between sequence-specific DNA-binding proteins and cognate DNA cis elements (1, 2). A large body of information has been generated to increase understanding of tissue-specific gene expression in plants. However, detailed knowledge of the regulation of gene expression in the vascular system, which conducts water, minerals, and photosynthesis products throughout the plant, is lacking (3).
Many plant DNA viruses, including rice tungro bacilliform virus (RTBV), coconut foliar decay virus, and commelina yellow mottle virus, accumulate specifically in phloem tissues, and the expression of their promoters is restricted to vascular tissues in plants (4–8). Moreover, a number of plant genes that are specifically expressed in vascular tissues have been isolated, including pal2 (9), 4cl (10), PtCesA (11), AtARP2 (12), sh-1 (13), and grp 1.8 (14). Several DNA cis elements that are common among these vascular specific promoters have been identified (15). Prior studies identified four specific DNA cis elements in the RTBV promoter, namely the GATA motif, ASL box, and Box II and I (7, 15, 16). Deletion and point mutation studies showed that Box II is essential for the expression of the RTBV promoter (15, 16).
Greater understanding of the regulation of the RTBV promoter will clarify the mechanisms that control vascular tissue specific development and function and will also reveal the molecular basis of rice tungro diseases (8, 17–19). We previously reported the isolation of rice bZIP transcription factor RF2a through its interaction with the Box II element (19). RF2a activates the promoter of RTBV in in vitro transcription reactions and in vivo assays (18–20). Expression of antisense gene constructs and dominant negative mutants of RF2a in transgenic rice plants caused abnormal developmental phenotypes, indicating that RF2a and potentially other factors are important for development of rice plants (18–20). Furthermore, data from electrophoretic mobility-shift assays (EMSAs) of purified RF2a and rice nuclear extracts suggested that proteins other than RF2a also bind to Box II (19).
We report here the isolation of a second rice bZIP protein, RF2b, which binds to Box II of RTBV and forms heterodimers with RF2a. RF2a, and RF2b shared high amino acid sequence similarity within the bZIP domains, but the biochemical characteristics of their binding to Box II are distinctive. RF2a and RF2b are predominantly localized to vascular tissues; however, the accumulation of RF2a and RF2b in different organs of rice and subcellular localization of RF2a and RF2b in tobacco protoplasts are different. Moreover, antisense gene expression showed that RF2b has a strong impact on rice development during the juvenile stage, whereas RF2a showed its impact on the development of young seedlings.
Materials and Methods
Yeast Two-Hybrid System. Yeast two-hybrid genetic screening was performed by a combination of a modified Matchmaker II system (Clontech) and the pPC86-based rice cDNA expression library with TRP-1 as the selection marker (19). To construct the “bait” pAL-RF2a-3Δ, the coding sequence of the bZIP domain of RF2a (amino acids 108–283) was released from the plasmid pET-RF2a-3Δ (18) and cloned into pAL-2. In pAL-2, TRP-1 of pAS2–1 (Clontech) was replaced with LEU-2 from pACT2 (Clontech) through restriction sites NdeI/BamHI. Yeast strain CG1945 (Clontech) harboring the “bait” construct was used for library screening. Five millimoles of 3-amino-1,2,4-triazole was used in the selection medium to increase screening stringency. β-Galactosidase assays were carried out to eliminate false-positive clones from the His– conditional screening. Yeast mating assays (conducted as recommended for the Matchmaker II system) were performed to further confirm the interactions between the candidates and the bait. The 5′ end DNA sequence of candidates was obtained through RNA ligase-mediated RACE (Invitrogen), and the full-length clone was generated by using fusion PCR.
Plasmid Construction. To produce RF2b and truncated mutants of RF2b in Escherichia coli, the DNA coding sequences for these proteins were amplified from the original yeast clone TF11 by using Pfu DNA polymerase (Stratagene) and cloned into pET28a (Novagen) through NdeI/BamHI to generate pET-RF2b, pET-RF2b-ΔbZIP, and pET-RF2b-bZIP. In pET-RF2b-ΔbZIP, the bZIP domain (amino acids 131–195) was removed through fusion PCR. In pET-RF2b-bZIP, only DNA coding sequences for the bZIP domain plus certain flanking sequence remain (amino acids 77–254), whereas pET-RF2b contains the full-length coding sequence of RF2b.
Plant expression constructs pCs::RF2b and pCs::RF2b(–) were generated by replacing the uid A gene of pLau-6-GUS, in which the uid A was driven by cassava vein mosaic virus promoter (Cs) (21), with RF2b coding sequence from pET-RF2b through restriction sites BglII (blunt)/BamHI and NdeI (made blunt)/BamHI, respectively. pCs::RF2a was constructed in the same fashion by releasing the RF2a coding sequence from pET-RF2a (18). To construct the binary plasmid pGA-E::GUS/Cs::RF2b, Cs::RF2b was released from pCs::RF2b by using HindIII/ClaI restriction sites and inserted into pGA-E::GUS (18) through ClaI restriction sites. GFP fusion constructs pCs::RF2b:GFP and pCs::RF2a:GFP were generated by replacing the uid A in pLau-6-GUS with fusion PCR products coding for RF2b:GFP or RF2a:GFP.
Southern and Northern Blot Analyses. For Southern blot analysis, 10 μg of rice genomic DNA was digested with restriction enzymes as indicated and separated in 1% agarose gels. For Northern blot analysis, total RNA samples isolated from different tissues as indicated were prepared from 10-day-old rice seedlings, and 10 μg of total RNA was separated in 1.5% agarose denaturing gels. The hybridization procedures for the DNA blots and RNA blots were conducted as described (22) with coding sequences of RF2b or RF2a as probes.
Protein Purification and EMSAs. Proteins produced in E. coli strain BL21(DE3)pLysS were purified as described (20). EMSAs were carried out as described (20) by using 32P-labeled Box IIm1 as probe (15).
Antibody Preparation and Western Blot Assays. The rabbit anti-RF2b polyclonal antibodies were produced by HTI Bio-Products (Ramona, CA) with RF2b-ΔbZIP as antigen. The antibodies were further purified by using protein A Sepharose (Amersham Pharmacia Biosciences). Western blot analysis of different tissue protein samples prepared from 10-day rice seedlings was carried out as described (20) with anti-RF2b-ΔbZIP antibodies as primary antibodies and horseradish peroxidase-conjugated goat anti-rabbit antibodies as secondary antibody (Southern Biotechnology Associates).
BY2 protoplast transfection, Agrobacterium-medicated tobacco transformation, and particle bombardment-mediated transformation of rice were carried out as described (18, 20, 22).
Quantitative and histochemical analyses of β-glucuronidase (GUS) activity were carried out as described (18, 20, 23).
Surface Plasmon Resonance. Purified RF2a and RF2b were studied for binding affinity to the Box II cis element. The top strands of the DNA targets were biotinylated (see ref. 15 for sequence information), and 100 resonance units of each of the double-stranded biotinylated oligos were immobilized to one of the four cells of a streptavidin chip (Biacore, Uppsala); a blank cell was used as control. The Biacore 2000 instrument was used for affinity analysis. Analyses were performed at 25°C in 10 mM Hepes/150 mM NaCl/3.4 mM EDTA/0.005% surfactant P20, pH 7.4. For each cycle, 100 μl of solution containing 500 nM protein was applied. After each cycle, the chips were regenerated by using a solution containing 1 M NaCl and 10 mM NaOH.
In Situ Hybridization. Samples of TP309 rice seedlings were collected at 5 days after germination. Plant materials were fixed, dehydrated, and embedded as described by Sentoku et al. (24). Microtome sections (10–12 μm in thickness) were mounted on poly l-lysine-coated glass slides (Electron Microscopy Sciences, Fort Washington, PA) for in situ hybridization. Digoxigenin-labeled sense and antisense RNAs of RF2b and RF2a were produced by using the T7/SP6 labeling kit (Roche Molecular Biochemicals). Hybridization was performed as described (24), and immunological detection of hybridized probe signals was performed by using the digoxigenin detection kit (Roche Molecular Biochemicals).
Microscopy. Specimens for in situ hybridization, GFP localization, and GUS accumulation in leaf tissues were visualized by using either the Olympus (Melville, NY) SEX12 microscope or Nikon Eclipse E800 microscope, and images were collected by using a Nikon DXM1200 charge-coupled device camera.
Results
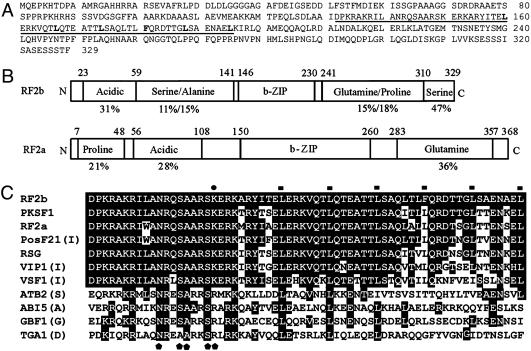
Isolation of RF2b. A yeast two-hybrid system was used for genetic screening of candidate proteins that interact with RF2a. The bZIP domain (comprising amino acids 108–283; constructed in plasmid pAL-RF2a-3Δ) was an ideal target, because bZIP proteins form dimers through the leucine-zipper region (25). After screening of ≈1.7 × 106 clones on His– conditional medium, false-positive clones were eliminated through the LacZ second reporter gene and by yeast mating assays. Nucleotide sequence analysis revealed that, among the positive clones, only two different bZIP proteins were isolated in addition to RF2a. Each of these two bZIP proteins was cloned at least twice. One of these, RF2b, was isolated from two independent clones, TF11 and TC15; TC15 is shorter at the 5′ end than TF11 but is otherwise identical in sequence. The 5′ end of TF11 was obtained through RNA ligase-mediated RACE (Invitrogen). The full-length clone of RF2b is 1,497 bp in length with an ORF encoding a protein of 329 aa (Fig. 1A).
Fig. 1.
Predicted amino acid sequence and putative domain structure of RF2b. (A) Deduced amino acid sequence of RF2b. The bZIP domain region is indicated by underlining. These sequence data have been submitted to GenBank under accession no. AY466471. (B) Schematic domain structure of RF2b. Domain structure of RF2a is presented for comparative purposes. (C) Multiple amino acid sequence alignment of the bZIP domains of RF2b and RF2a (19), PKSF1 (26), PosF21 (27), RSG (28), VIP1 (29), VSF1 (30), and representatives from each major subgroup of bZIP proteins, ATB2 (42), ABI5 (43), GBF1 (44), and TGA1 (45), of Arabidopsis thaliana (31). Residues identical to RF2b are highlighted. Solid rectangles indicate positions of conserved leucine residues in the bZIP proteins. Solid circle shows the –10 position in bZIP domain related to the first conserved leucine residue. Amino acids that are important for DNA-binding specificity are indicated by a solid pentagon (36).
Structure of RF2b. Southern blot analysis demonstrated that the rice genome contains a single copy of the RF2b gene (Fig. 4A). RF2b was predicted to encode a bZIP protein of 329 aa (Fig. 1 A and B) with the bZIP domain located near the midpoint of the protein (amino acids 131–195) (Fig. 1B). An acidic domain (amino acids 23–59; with 31% acidic amino acids) is located at the N terminus. Between the acidic domain and the bZIP domain is a 69-aa-long region in rich of serine (16%) and alanine (23%). A glutamine-(15%) and proline-rich domain (18%) stretched from amino acids 242–310, and a short serine-rich domain (47% within a 19-aa region) was identified at the C terminus of RF2b (Fig. 1B).
Fig. 4.
Gene copy number and expression of RF2b. (A) Southern blot analysis of RF2b. Ten micrograms of TP309 genomic DNA was digested with the enzymes indicated, and the blot was hybridized with RF2b probe. (B) Accumulation of mRNA of RF2a and RF2b in different tissues of rice. RNA gel blot analysis was performed by using total RNA from different tissues of 10-day-old seedlings of rice cultivar TP309. Ten micrograms of RNA was loaded per lane and stained with ethidium bromide (rRNA). The blots were hybridized with either RF2b- or RF2a-specific probes. (C) Accumulation of RF2b in different tissues of 10-day-old rice seedlings. Forty micrograms of protein samples from each tissue type was separated by SDS/PAGE. Equal loading was monitored by staining the blot with Ponceau S (Sigma) before the antibody reaction. Antibodies were prepared against RF2b mutant that lacked the bZIP domain. Arrow indicates the position of RF2b.
The amino acid sequences of the bZIP domain of RF2a and RF2b share 85% identity. Although similarity within the remaining sequences of RF2b and RF2a is low, the proteins are similar in that each contains multiple functional domains (Fig. 1B). The amino acid sequence of the bZIP domains of RF2a and RF2b shows strong similarity with the bZIP region of PKSF1 (Paulownia kawakamii) (26), PosF21 (Arabidopsis) (27), RSG (tobacco) (28), VIP1 (Arabidopsis) (29), and VSF1 (tomato) (30), and less similarity with other bZIP proteins in Arabidopsis (Fig. 1C). We conclude that RF2a and RF2b, with PKSF1, PosF21, RSG, VIP1, and VSF-1, form a distinctive subgroup of bZIP proteins related to the group “I” bZIP proteins in Arabidopsis as classified by Jakoby et al. (31).
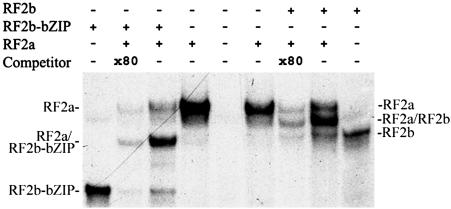
DNA-Binding and Dimer Formation of RF2b. To determine whether RF2b can bind to the Box II cis element and form heterodimers with RF2a, EMSAs were conducted. Because RF2a bound to Box IIm1, a mutant of Box II with a higher affinity than wild-type Box II (19), the mutant was chosen as probe. Like RF2a, RF2b and the bZIP domain (RF2b-bZIP) bound Box IIm1 as homodimers (Fig. 2). When RF2a was added to the reactions with RF2b or RF2b-bZIP, heterodimers were formed, as shown by reactions that contain RF2a and RF2b or RF2a and RF2b-bZIP, respectively (Fig. 2). The data in Fig. 2 also showed that the heterodimers of RF2b and RF2a or RF2b-bZIP and RF2a had stronger binding to the Box IIm1 probe than did the homodimers of RF2b, RF2b-bZIP, or RF2a. All protein–DNA complexes in this study can be effectively competed by addition of 80× molar excess of unlabeled probe.
Fig. 2.
DNA-binding and dimer formation of RF2b. EMSAs of RF2b and the bZIP domain of RF2b and their cobinding with RF2a by using 32P-labeled Box IIm1 probe are shown. Proteins used in each reaction are labeled on top of each lane. The unlabeled probe was used as competitor in reactions as indicated. DNA–protein complexes are labeled at both sides of the image to show the formation of homodimers and heterodimers.
DNA-Binding Constants of RF2b and RF2a. To compare the DNA-binding characteristics of RF2b and RF2a, surface plasmon resonance measurements were conducted by using a Biacore 2000 instrument (Biacore). The binding affinities of RF2a and RF2b to Box II and its mutants were measured in real time with the same set of chips. Association and dissociation constants were determined by using biaevaluation 3.1 software (Biacore), and results are shown in Table 1. RF2a exhibits relatively higher binding affinities to Box II than does RF2b, and the binding behaviors of RF2a and RF2b are different from each other. RF2a binds to the DNA target relatively rapidly and dissociates slowly from the DNA target, whereas RF2b binds slowly and dissociates from the target DNA relatively rapidly.
Table 1. Box II DNA-binding constants of RF2a and RF2b.
| Ka, 1/MS | Kd, M | |
|---|---|---|
| RF2a | 8.67 × 106 | 1.15 × 10-7 |
| RF2b | 2.19 × 106 | 4.57 × 10-7 |
Ka, association constant; Kd, dissociation constant.
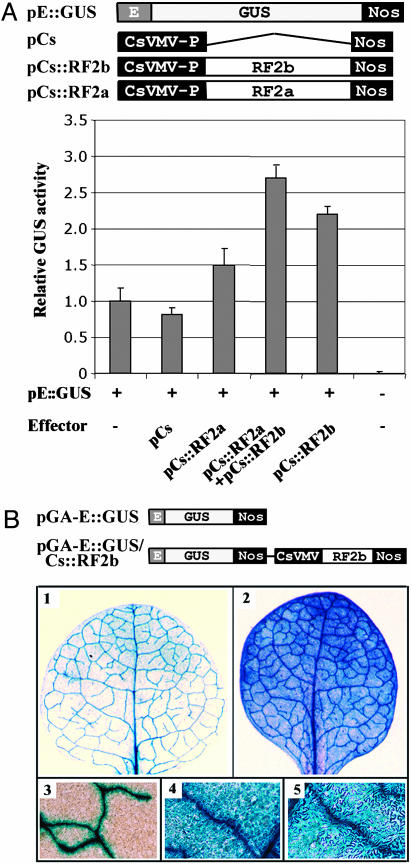
Transcriptional Activity of RF2b. As described above, RF2b has as many as four putative functional domains and binds to the RTBV promoter. To determine whether RF2b can regulate expression of the RTBV promoter, the RF2b gene was placed under the control of Cs. The Cs promoter is a strong constitutive promoter in transgenic tobacco and rice plants and in tobacco protoplasts (32, 33). The effector plasmids were transfected to tobacco BY-2 protoplasts with pE::GUS as reporter. The E promoter in the reporter contains five defined DNA cis elements, including Box II, and retains the properties of full RTBV promoter (7, 15). For comparison, pCs::RF2a was constructed and included in the experiments. In BY-2 protoplasts, the E::GUS reporter was activated 2-fold by expression of RF2b and to a lesser degree by RF2a (Fig. 3A). In samples that included both RF2a and RF2b, transcriptional activation is stronger than with either protein alone. Considering the relatively high background expression level of the E::GUS gene in BY-2 protoplasts (20), both RF2a (18) and RF2b are considered to be strong activators of expression of the reporter gene.
Fig. 3.
Activity of RF2b in regulation of RTBV promoter. (A Upper) Diagram of plasmids used in the BY-2 cell cotransfection assays. The E promoter in reporter construct of pE::GUS that contains five defined DNA cis elements (15), including Box II, with which RF2a and RF2b interact. In the effector constructs pCs::RF2a and pCs::RF2b, RF2a and RF2b coding sequences were driven by Cs (32, 33). (Lower) GUS activity of transfected BY-2 protoplasts samples. The E::GUS reporter gene was cotransfected into BY-2 protoplasts with Cs::RF2b, Cs::RF2a, Cs::RF2b/Cs::RF2b, and controls, as labeled. The results are the average with SD of three independent experiments, three samples per experiment, after normalization with 35S::GFP that was cointroduced and served as internal control. (B Upper) diagram of T-DNA regions of plasmids used in Agrobacterium-mediated tobacco transformation. (Lower) Histochemical localization of GUS in leaf tissues from transgenic tobacco plants. GUS activity is indicated in transgenic tissue by an indigo dye precipitate after staining with 5-bromo-4-chloro-3-inodyl-β-d-glucuronic acid. (1 and 3) Leaf with pGA-E::GUS construct; (2, 4, and 5) leaf with pGA-E::GUS/Cs::RF2b; (4) highlighting the expression of GUS in mesophyll cells; (5) highlighting the expression of GUS in epidermal cells.
To further confirm the role of RF2b in enhancing expression of the pE:GUS gene, the Cs::RF2b fusion gene was cloned into a Ti binary plasmid, pGA-E::GUS (18), and transgenic tobacco plants with pGA-E::GUS/Cs::RF2b were developed through Agrobacterium-mediated transformation. Eight parental lines and/or T1 seedlings were used in GUS histochemical analyses with similar expression patterns in all plants tested. When transgenic plants were transformed with E::GUS alone, GUS accumulation was restricted to the vascular system (Fig. 3B1) (18). The constitutive expression of RF2b altered the pattern of expression of the E::GUS gene from phloem specific to constitutive (Fig. 3B2–5). Similar results were reported with RF2a (18).
Tissue and Subcellular Localization of RF2b. The similarity of DNA-binding and gene activation functions of RF2a and RF2b suggests that they may have redundant functions in rice. RNA blot analysis was performed to compare expression of RF2a and RF2b in different rice tissues. As shown in Fig. 4B, mRNA of RF2b accumulated predominantly in leaf sheath, with lower accumulation in roots and very low accumulation in leaf blade tissues. In contrast, RF2a mRNA accumulated predominantly in leaf sheath and less in leaf blade and root tissues (19). Western immunoblot assays showed that RF2b protein accumulated to high levels in roots and low levels in leaf sheath but was difficult to detect in leaf blade tissues (Fig. 4C). RF2a was not detected in roots (19). The mRNA levels of RF2a and RF2b did not show change significantly from 9-day-old seedlings to 60-day-old plants as monitored by using RT-PCR (data not shown).
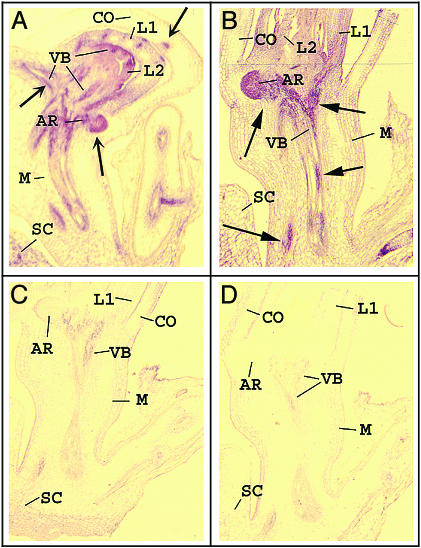
RNA in situ hybridization was used to localize the expression patterns of RF2a and RF2b in seedling tissues where primary phloem tissues are abundant. Fig. 5 shows that both RF2b and RF2a are predominantly localized in vascular tissues and in root tips of rice seedlings 5 days after germination.
Fig. 5.
Localization of RF2a and RF2b transcripts in rice seedlings. Longitudinal sections of the 5-day-old seedlings were hybridized with antisense and sense probes of RF2a and RF2b labeled with digoxigenin-UTP. Hybridization signal is visualized by red color; arrows indicate strong signals. (A) Antisense probe of RF2b; (B) antisense probe of RF2a; (C) sense RF2b probe; (D) sense probe of RF2a. AR, adventitious root; CO, coleoptile; L1, first leaf; L2, second leaf; M, mesocotyl; SC, scutellum; VB, vascular bundle.
To visualize RF2a and RF2b in transient assays, RF2a:GFP and RF2b:GFP fusion proteins were produced in BY-2 protoplasts after transfection with plasmid DNAs. As shown in Fig. 6, RF2b:GFP clearly localized to nuclei, whereas RF2a:GFP did not localize to nuclei in this assay.
Fig. 6.
Accumulation of RF2a and RF2b in tobacco BY-2 protoplasts. BY-2 protoplasts were transfected with plasmids encoding RF2b:GFP (a–c) and RF2a:GFP (d–f) fusion proteins. (a and d) Images of GFP fusion proteins visualized with blue light excitation. (c and f) Images visualized in white field. (b and e) Overlay of a and c and d and f, respectively.
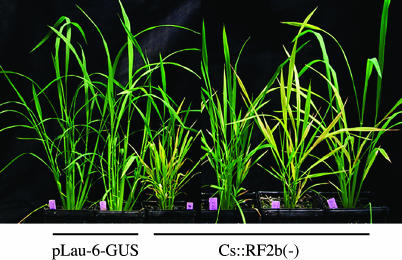
Role of RF2b in Rice Development. Transgenic rice plants were developed via particle cobombardment with a hygromycinresistant selection marker and genes that encode either sense or antisense sequences of RF2b. For these studies, gene sequences were under the control of the constitutive Cs. Eleven of 14 transgenic rice plants with the antisense gene construct, pCs:RF2b(–) were strongly stunted, and their leaves were yellow in color after the transgenic plants was transplanted into soil (Fig. 7); stunted plants had reduced amounts of RF2b transcripts compared to control plants (data not shown). After ≈2 mo of growth in the greenhouse, transgenic plants were increased in growth, and leaves gradually turned green. Similar observations were made on T1 generation plants. In contrast, plants in which RF2b was overexpressed (pCs::RF2b) did not exhibit abnormal phenotype in general. Although 4 of 27 lines showed phenotypes similar to that shown in Fig. 7, the effect is likely due to gene silencing, because a Northern blot analysis of these lines revealed lower levels of RF2b transcripts in stunted plant lines (not shown).
Fig. 7.
Impact of RF2b on rice development. Photograph was taken 1.5 mo after transgenic rice plants were transplanted to soil. The two transgenic plants on the left carry control plasmid pLau-6-GUS, in which a GUS (uid A) gene is driven by CsVMV promoter. The four transgenic plants on the right carry the Cs:RF2b(–) antisense gene.
Discussion
Box II, a cis sequence element in the phloem specific promoter of RTBV, is essential for the expression of the promoter (15, 16) and binds the bZIP transcription factor RF2a (18–20, 34). These studies indicated that other proteins bind to Box II and led to the present study of RF2b.
RF2a and RF2b are members of a subgroup of plant bZIP proteins with DNA-binding sites common to several vascular specific promoters (15, 19, 28, 35). The DNA-binding domains of RF2a, RF2b, and other bZIP proteins in this group are very highly conserved (Fig. 1) and possess a signature sequence for DNA binding characterized as NxxxSAxxSK (20, 36). Furthermore, these proteins have a lysine rather than an arginine residue at the –10 position (relative to the first leucine residue of the leucine zipper region), apparently at variance with known interactions between the guanidinium group of the arginine residues at this position and the N7 atom of the G residue in the core ACGT (37) that exists in many plant bZIP protein-binding sites (38). The features of the DNA-binding domain and the prediction of nonpalindromic DNA-binding sites that lack the ACGT core sequence make this group of proteins unique.
RF2a and RF2b share a high degree of sequence similarity in DNA-binding domains, and both proteins function as transcription activators. However, they are apparently not redundant in function based on several types of data. First, the expression patterns of RF2a and RF2b differ from each other. RF2b accumulates predominantly in roots and leaf sheath (Fig. 4 B and C), whereas RF2a is more abundant in leaf blade and leaf sheath, and accumulation is very low in roots (19).
Second, subcellular localization of RF2a and RF2b is different in tobacco protoplasts. Although both RF2a and RF2b have bipartite nuclear localization signals, only RF2b showed clear nuclear localization in BY-2 protoplasts, in contrast with our previous report, which demonstrated that RF2a was localized to nuclei in vascular tissues in rice (19). The difference between current and previous data may be a result of differences in cell types and may indicate additional levels of regulation of the functions of these proteins. It was reported that different members of G box binding and the common plant regulatory factor family of bZIP proteins showed different subcellular localization characteristics (39, 40). Tobacco bZIP protein RSG, which shares high similarity with RF2b and RF2a in bZIP domains, did not show clear nuclear localization in tobacco leaf protoplasts (41).
Third, RF2a and RF2b bind to Box II with different biochemical characteristics (Table 1). The heterodimers between RF2a and RF2b showed stronger binding to Box IIm1 DNA than did the homodimers of either RF2a or RF2b (Fig. 2), which may indicate that RF2a and RF2b have different roles in regulating common target genes. Alternatively, this may suggest that a diversity of endogenous targets are regulated by these proteins. RF2a, RF2b, and perhaps other members of this group of bZIP proteins regulate a wide spectrum of endogenous genes through formation of homo- or heterodimers, temporal and spatial patterns of expression, and perhaps through interactions with other regulatory proteins.
Last, RF2a and RF2b appear to have different roles in rice development. Reducing the expression of RF2a in transgenic rice plants with an antisense gene construct caused stunting and abnormal development of vascular tissues in early but not in later stages of development (19). In contrast, transgenic rice plants expressing antisense sequences of the RF2b gene caused clear stunting and yellowish leaves in 3- to 4-wk-old transgenic rice plants. Furthermore, the phenotypes associated with expression of the antisense gene of RF2b are similar to the disease symptoms caused by infection of RTBV (8).
As reported earlier, the Box II cis element is crucial for function of the RTBV promoter (15, 16). RF2a and RF2b regulate the promoter through interaction with Box II (Figs. 2, 3, 4) (18–20), and each protein interacts with the rice TATA-binding protein (20, 34) (S.D. and R.N.B., unpublished work). We propose that these proteins contribute to viral gene expression by recruiting one or more factors to the promoter to activate transcription.
In summary, we conclude that RF2a and RF2b play important roles in regulating tissue-specific expression of the RTBV promoter and thereby regulate virus replication. The similarity between the abnormal phenotype caused by an antisense gene of RF2b and the rice symptoms of rice tungro disease (8) may indicate that infection by RTBV reduces the availability of RF2a, RF2b, and perhaps related factors for expression of endogenous genes that are important for plant development through quenching and/or titration. Altering the expression of host genes may result in the development of disease symptoms.
We anticipate that knowledge of the biological functions of RF2a, RF2b, and the transcriptional regulation networks in which they participate will lead to more complete understanding of the basis and control of rice tungro disease.
Acknowledgments
We thank Drs. Y. Yin, Silvana Petruccelli, W. Kim, C. Reichel, M. Fujiki, and H. Berg for their help. We thank Drs. Yi Liu and Maria Soto-Aguilar for critical reading of and suggestions for this manuscript. This study was supported by grants from the U.S. Department of Energy (DE-FG02–99ER20355), the Rohm and Haas Company, and the Donald Danforth Plant Science Center.
Abbreviations: RTBV, rice tungro bacilliform virus; EMSA, electrophoretic mobility-shift assay; GUS, β-glucuronidase; Cs, cassava vein mosaic virus promoter.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY466471).
References
- 1.Lemon, B. & Tjian, R. (2000) Genes Dev. 14, 2551–2569. [DOI] [PubMed] [Google Scholar]
- 2.Levine, M. & Tjian, R. (2003) Nature 424, 147–151. [DOI] [PubMed] [Google Scholar]
- 3.Smith, H., B. (1999) Plant Cell 11, 2061–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya-Pakrasi, M., Peng, J., Elmer, J. S., Laco, G., Shen, P., Kaniewska, M. B., Kononowicz, H., Wen, F., Hodges, T. K. & Beachy, R. N. (1993) Plant J. 4, 71–79. [DOI] [PubMed] [Google Scholar]
- 5.Hehn, A. & Rohde, W. (1998) J. Gen. Virol. 79, 1495–1499. [DOI] [PubMed] [Google Scholar]
- 6.Medberry, S. L. & Olszewski, N. E. (1993) Plant J. 3, 619–626. [DOI] [PubMed] [Google Scholar]
- 7.Yin, Y. & Beachy, R. N. (1995) Plant J. 7, 969–980. [DOI] [PubMed] [Google Scholar]
- 8.Saito, Y., Hibino, H., Omura, T. & Inoue, H. (1986) in Virus Diseases of Rice and Legumes in the Tropics, eds. Kajiwara, T. & Konno, K. (Tropic Agriculture Research Center, Minister of Agriculture, Forestry and Fisheries, Tsukuba, Japan), pp. 3–13.
- 9.Seguin, A., Laible, G., Leyva, A., Dixon, R. A. & Lamb, C. J. (1997) Plant Mol. Biol. 35, 281–291. [DOI] [PubMed] [Google Scholar]
- 10.Becker-Andre, M., Schulze-Lefert, P. & Hahlbrock, K. (1991) J. Biol. Chem. 266, 8551–8559. [PubMed] [Google Scholar]
- 11.Wu, L., Joshi, C. P. & Chiang, V. L. (2000) Plant J. 22, 495–502. [DOI] [PubMed] [Google Scholar]
- 12.Klahre, U. & Chua, N. H. (1999) Plant Mol. Biol. 41, 65–73. [DOI] [PubMed] [Google Scholar]
- 13.Graham, M. W., Craig, S. & Waterhouse, P. M. (1997) Plant Mol. Biol. 33, 729–735. [DOI] [PubMed] [Google Scholar]
- 14.Keller, B. & Baumgartner, C. (1991) Plant Cell 3, 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin, Y., Chen, L. & Beachy, R. (1997) Plant J. 12, 1179–1188. [DOI] [PubMed] [Google Scholar]
- 16.He, X., Hohn, T. & Futterer, J. (2000) J. Biol. Chem. 275, 11799–808. [DOI] [PubMed] [Google Scholar]
- 17.Saito, Y., Iwaki, M. & Usugi, T. (1976) Ann. Phytopathol. Soc. Japan 43, 375. [Google Scholar]
- 18.Petruccelli, S., Dai, S., Carcamo, R., Yin, Y., Chen, S. & Beachy, R. N. (2001) Proc. Natl. Acad. Sci. USA 98, 7635–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin, Y., Zhu, Q., Dai, S., Lamb, C. & Beachy, R. N. (1997) EMBO J. 16, 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai, S., Petruccelli, S., Ordiz, M. I., Zhang, Z., Chen, S. & Beachy, R. N. (2003) J. Biol. Chem. 278, 36396–36402. [DOI] [PubMed] [Google Scholar]
- 21.Verdaguer, B., de Kochko, A., Fux, C. I., Beachy, R. N. & Fauquet, C. (1998) Plant Mol. Biol. 37, 1055–1067. [DOI] [PubMed] [Google Scholar]
- 22.Dai, S., Zheng, P., Marmey, P., Zhang, S., Tian, W., Chen, S., Beachy, R. N. & Fauquet, C. (2001) Mol. Breeding 7, 25–33. [Google Scholar]
- 23.Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. (1987) EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sentoku, N., Sato, Y., Kurata, N., Ito, Y., Kitano, H. & Matsuoka, M. (1999) Plant Cell 11, 1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevray, P. M. & Nathans, D. (1992) Proc. Natl. Acad. Sci. USA 89, 5789–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low, R., Prakash, A., Swarup, S., Goh, C.-J. & Kumar, P. (2001) Plant Cell Rep. 20, 696–700. [Google Scholar]
- 27.Aeschbacher, R. A., Schrott, M., Potrykus, I. & Saul, M. W. (1991) Plant J. 1, 303–316. [PubMed] [Google Scholar]
- 28.Fukazawa, J., Sakai, T., Ishida, S., Yamaguchi, I., Kamiya, Y. & Takahashi, Y. (2000) Plant Cell 12, 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzfira, T., Vaidya, M. & Citovsky, V. (2001) EMBO J. 20, 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ringli, C. & Keller, B. (1998) Plant Mol. Biol. 37, 977–988. [DOI] [PubMed] [Google Scholar]
- 31.Jakoby, M., Weisshaar, B., Droge-Laser, W., Vicente-Carbajosa, J., Tiedemann, J., Kroj, T. & Parcy, F. (2002) Trends Plant Sci. 7, 106–111. [DOI] [PubMed] [Google Scholar]
- 32.Padidam, M., Gore, M., Lu, D. L. & Smirnova, O. (2003) Transgenic Res. 12, 101–109. [DOI] [PubMed] [Google Scholar]
- 33.Verdaguer, B., de Kochko, A., Beachy, R. N. & Fauquet, C. (1996) Plant Mol. Biol. 31, 1129–1139. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, Q., Ordiz, M. I., Dabi, T., Beachy, R. N. & Lamb, C. (2002) Plant Cell 14, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Schumann, S., Ringli, C., Heierli, D., Amrhein, N. & Keller, B. (1996) Plant J. 9, 283–296. [DOI] [PubMed] [Google Scholar]
- 36.Fujii, Y., Shimizu, T., Toda, T., Yanagida, M. & Hakoshima, T. (2000) Nat. Struct. Biol. 7, 889–893. [DOI] [PubMed] [Google Scholar]
- 37.Konig, P. & Richmond, T. J. (1993) J. Mol. Biol. 233, 139–154. [DOI] [PubMed] [Google Scholar]
- 38.Foster, R., Izawa, T. & Chua, N. H. (1994) FASEB J. 8, 192–200. [DOI] [PubMed] [Google Scholar]
- 39.Terzaghi, W. B., Bertekap, R. L., Jr. & Cashmore, A. R. (1997) Plant J. 11, 967–982. [DOI] [PubMed] [Google Scholar]
- 40.Kircher, S., Wellmer, F., Nick, P., Rugner, A., Schafer, E. & Harter, K. (1999) J. Cell Biol. 144, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igarashi, D., Ishida, S., Fukazawa, J. & Takahashi, Y. (2001) Plant Cell 13, 2483–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rook, F., Weisbeek, P. & Smeekens, S. (1998) Plant Mol. Biol. 37, 171–178. [DOI] [PubMed] [Google Scholar]
- 43.Finkelstein, R. R. & Lynch, T. J. (2000) Plant Cell 12, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindler, U., Terzaghi, W., Beckmann, H., Kadesch, T. & Cashmore, A. R. (1992) EMBO J. 11, 1275–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler, U., Beckmann, H. & Cashmore, A. R. (1992) Plant Cell 4, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]