Abstract
Background
Venous thromboembolism (VTE) and cardiovascular disease (CVD) share some risk factors, including obesity, yet it is unclear how dietary patterns associated with reduced risk of CVD relate to risk of VTE.
Objective
To compare relationships of adherence to a DASH-style diet with risks of CVD and VTE.
Patients/Methods
We confirmed by medical record review 1094 incident cases of CVD and 675 incident VTEs during mean follow-up of 14.6 years in 34,827 initially healthy participants in the Women’s Health Study who completed at baseline a 133-item food frequency questionnaire scored for adherence to a DASH diet. We compared estimated associations of dietary patterns with CVD and VTE from proportional hazards models in a competing risk framework.
Results
Initial analyses adjusted for age, energy intake, and randomized treatments found 36–41% reduced hazards of CVD among women in the top two quintiles of DASH score relative to those in the bottom quintile (Ptrend<0.001). In multivariate analysis, women in the top two quintiles had 12–23% reduced hazards of CVD relative to women in the bottom quintile (Ptrend=0.04). Analyses restricted to coronary events found more variable 10–33% reduced hazards in the top two quintiles (Ptrend=0.09). In contrast, higher DASH scores were unrelated to risk of VTE with a 1% reduced hazard for the top vs. bottom quintile (Ptrend=0.95).
Conclusion
An apparently strong association of adherence to the DASH diet with incidence of CVD was attenuated upon control for confounding variables. Adherence to the DASH diet was not associated with risk of VTE in women.
INTRODUCTION
Dietary patterns influence cardiometabolic risk factors including obesity, hypertension, hyperglycemia, and hyperlipidemia. In particular, randomized trials have shown that the Dietary Approaches to Stop Hypertension (DASH) diet, which is high in fruits, vegetables, and low-fat dairy products, but low in saturated and total fat, reduces blood pressure and lipid levels.1–3 Other pathways whereby the DASH diet may reduce cardiovascular risk have been described,4 and several cohort studies have found that individuals who adhere to the DASH diet have reduced risk of both coronary heart disease (CHD) and total cardiovascular disease (CVD).5–7 However, the extent to which apparent benefits are mediated by weight loss is unclear.8,9
Venous and arterial thrombosis share several pathways, as CVD and VTE commonly occur together, and both are strongly related to older age and obesity.10,11 These overlaps in risk factors raise the hypothesis that lifestyle modification including adoption of heart healthy diets can lower risk of VTE.12 Also, the reduced rate of VTE seen among women who received randomized treatment with vitamin E supplements evokes the specific question of whether dietary patterns consistent with high vitamin E intake would provide similar benefits.13 However, some primary risk factors for CVD including smoking, hypertension, diabetes, and elevated cholesterol have little or equivocal associations with VTE.14–21 While diet strongly influences obesity, observational evidence of association between dietary factors and VTE has been somewhat inconsistent.22–24
To our knowledge, no study has directly evaluated whether established dietary patterns known to be associated with CVD also predict VTE. Such associations would imply viable strategies for reducing occurrence of VTE, and indicate substantial added benefits for adherence to such heart healthy lifestyles. Also, formal comparisons between relative risks for these endpoints that adjust for competing risks would help clarify the extent of shared pathways between venous and arterial events. We used prospectively collected data from the 34,827 Women’s Health Study (WHS) participants who completed a detailed food frequency questionnaire at baseline and were followed for a mean 14.6 years to compare relative risks of atherosclerotic and venous thrombotic events associated with established dietary patterns.
METHODS
Study Participants
The WHS was a randomized trial testing whether low-dose aspirin, beta-carotene, and vitamin E prevented CVD and cancer in 39 876 women. The randomized findings have been described previously.25–27 Briefly, women health professionals aged 45 or older were enrolled into the trial from 1992–1994 and were initially free of coronary heart disease, cerebrovascular disease, and cancer (except non-melanoma skin cancer). Before randomization, 26 779 of the participants gave a blood sample which was used to identify factor V Leiden and prothrombin mutations.13 At the end of the 10-year randomized treatment period, follow-up for morbidity was complete for 97.2% of participants, and was complete for mortality for 99.4% of participants.27 Upon conclusion of the trial in 2004, observational follow-up of 33,796 women (89% survivors) began and continues to present day. The response rate to the 5-year observational follow-up questionnaire was 95%. All women provided written informed consent to participate, and the study was approved by the institutional review board of Brigham and Women’s Hospital in Boston, MA.
Assessment of Diet and Other Variables
Baseline diet was assessed using the 133-item Willett semi-quantitative food frequency questionnaire (FFQ).28 Participants reported routine intake of each food on a scale ranging from never or less than 1 time per month to 6 servings or more per day. We calculated a dietary pattern score based on the DASH diet.1 The DASH score, developed by Fung et al., targets 8 elements: high intakes of fruits, vegetables, nuts, low-fat dairy products, and whole grain, and low intakes of sodium, sweetened beverages, and red and processed meats.6 We did not consider fruit juices as a component of fruit as high intakes have been previously shown to be associated with increased risk of CVD.29–31 We constructed a score by first assigning women into quintiles based on their intake of a specific food. Scores for fruits, vegetables, nuts, low-fat dairy products, and whole grain were assigned based on the woman’s quintile ranking. For sodium, sweetened beverages, and red and processed meats, lower intakes were desired so women in the lowest quintile received a score of 5 whereas women in the highest quintile received a score of 1. We summed scores for each of the foods to obtain an overall DASH score for each participant which ranged from 8 to 38, with a mean of 23.8.
Participants reported body weight and height at baseline. From these we calculated body mass index (BMI, kg/m2), categorized via clinical cutpoints as <22, 22–23, 23–25, 25–29, and ≥30 kg/m2. They also provided information at baseline on potential confounders including age, education, smoking status, use of hormone therapy and postmenopausal status.10–21 Information on hormone therapy and menopausal status was updated by follow-up questionnaires and included as a time-varying covariate in analyses. Physical activity was assessed at baseline and participants were categorized into approximate quartiles corresponding to activity levels of ≤200, 200–600, 600–1500, and >1500 kcal/week based on the method of Mora et al.32
End Point Definition
Incident CVD was the primary cardiovascular end point of the WHS and included fatal and non-fatal myocardial infarction (MI), fatal and non-fatal stroke (including ischemic and hemorrhagic types), and cardiovascular death. Coronary heart disease (CHD) was a prospectively evaluated secondary endpoint that included non-fatal MI and death attributed to CHD. The occurrence of VTE was also prospectively assessed as a secondary endpoint.13,33 Women completed health questionnaires every 6 months during the first year of the trial and annually each subsequent year of the trial and observational period. Individuals reporting events, including next-of-kin for decedents, were asked to authorize release of medical records to an end points committee of physicians who reviewed them in a blinded manner.
We counted only CVD and VTE events confirmed by the end points committee on the basis of medical record indications. Unprovoked VTE was defined as deep vein thrombosis or pulmonary embolism which occurred without the presence of cancer (diagnosed either before or up to 3 months following VTE), or immobilization, trauma, or surgery within 3 months before diagnosis.
Statistical Analysis
Of the 39 876 participants, we excluded from the analysis those with missing dietary data and calorie information, those with food frequency questionnaires having > 70 blank items, those with energy intakes <600 kcal and >3500 kcal, those with missing information on covariates and those with a report of prior VTE and CVD at baseline. Consistent with prior analyses of these dietary patterns and CVD, we also excluded diabetics as this diagnosis may affect diet.4,6,7 Hieararchical numbers excluded were: 13 women with prerandomization stroke or CHD event, 1140 women with baseline VTE, 1084 women with baseline diabetes, 530 women with missing dietary information, 790 with outlying levels of dietary intake, and 1492 women with missing covariate information. Post-exclusion, 34 827 women were eligible for the analyses. Age was the time scale for proportional hazards models, and person years of follow-up were calculated from the date of randomization to the minimum of time to first incident VTE or CVD event, death, loss to follow-up or March 2010 with a mean follow-up time of 14.6 years. We did not consider VTE occurring after CVD or CVD occurring after VTE due to concern that the first event could influence diet.
DASH diet scores were categorized into quintiles, and proportional hazards regression models were stratified by event type using the approach detailed by Lunn and McNeil to assess the association between increasing score and incident CVD or VTE.34 Hazard ratios and their associated 95% confidence intervals were calculated with Quintile 1 forming the referent group; tests for trend used integer scores for quintile categories. Each diet score was evaluated in a separate model, and we tested and verified the validity of the proportional hazards assumption for all primary analyses. A power calculation indicated greater than 90% power to detect a 25% reduction in risk associated with the top quintile of intake for each outcome.
Our model assumes different associations of each covariate with VTE and CVD. A simpler model assuming equal trends across categories of diet scores for incident CVD and VTE is nested within this model, and we tested for equal association using a likelihood ratio test. Similar analyses were performed using VTE and CHD as the two endpoints.
All analyses controlled for age, total energy intake, and randomized treatment assignments. A basic model additionally adjusted for potential confounders including educational level, alcohol intake, smoking status, number of cigarettes smoked, time-varying post menopausal status and hormone therapy use, and physical activity. Preliminary analyses compared the relative hazards of CVD and VTE associated with higher baseline levels of BMI. Models were further adjusted for parental history of MI before age 60 (yes/no) but concerns that 3494 women did not provide information for this variable prevented its inclusion in the basic model. Subsequent models further adjusted for intermediates of various physiological pathways including BMI, history of hypertension – medication or blood pressure ≥140/90 mmHg (yes, no), and history of high cholesterol – cholesterol levels ≥240 mg/dL (yes, no).
Additional analyses considered the subscales of the DASH score using quintiles of each component. Analyses were also stratified by provoked and unprovoked VTE to assess associations between diet score and VTE event type, censoring participants with incident CVD. Subgroup analyses evaluated the relationship between diet and incident VTE in women considered to be at high risk for VTE including those with factorV Leiden or the prothrombin gene mutation. However, no significant effect modification by these baseline factors was found so results are not presented
To address the effects of each potential confounder on the relationship between CVD or VTE with the DASH diet, we added the following variables one a time to an initial model controlling for age, energy intake, randomization status, and time-varying hormone therapy and postmenopausal status: smoking status, highest level of education attained, alcohol intake, physical activity, body mass index, history of high cholesterol, history of hypertension, and familial history of CHD,
RESULTS
Women who adhered strongly to a DASH style diet tended to be older, postmenopausal, hormone therapy users, hypercholesterolemic, frequent exercisers, and have a higher education level (Table 1). They were also less likely to be overweight, and current smokers. Women tended to consume more calories and sodium as the DASH score increased.
Table 1.
Mean or Frequency of Baseline Health and Lifestyle Characteristics across Quintiles of DASH Dietary Pattern Score
|
Quintiles based on DASH Score |
||||||
|---|---|---|---|---|---|---|
| 1 (n=8606) | 2 (n=7757) | 3 (n=5525) | 4 (n=7140) 5 | (n=5799) | Mean, (5th, 95th percentiles) or Prevalence (%) | |
| Participant Characteristics* | ||||||
| Age (y) | 53 | 54 | 55 | 56 | 56 | 55 (46, 68) |
| Whites, (%) | 94 | 95 | 96 | 96 | 96 | 95 |
| Current smokers, (%) | 23 | 14 | 10 | 8 | 5 | 13 |
| Randomization status, vitamin E (%) | 49 | 50 | 50 | 50 | 50 | 50 |
| Family history of CHD (%) | 13 | 13 | 13 | 11 | 13 | 13 |
| History of hypertension medication or ≥140/90 | 25 | 25 | 24 | 24 | 23 | 24 |
| mmHg | ||||||
| (%) | ||||||
| History of high cholesterol ≥240mg/dL (%) | 27 | 28 | 29 | 30 | 31 | 29 |
| Licensed practical nurse/licensed veterinary nurse education level (%) | 19 | 14 | 13 | 10 | 8 | 14 |
| Kilocalories of energy expenditure (kcal/week) | 623 | 844 | 981 | 1134 | 1456 | 972 (14, 3178) |
| Clinical Characteristics | ||||||
| BMI, kg/m2 | 26.4 | 26.1 | 25.8 | 25.5 | 25.0 | 25.8 (19.9, 35.4) |
| Baseline hormone therapy use, (%) | 36 | 40 | 43 | 44 | 46 | 41 |
| Post Menopausal Status (%) | 47 | 52 | 54 | 59 | 61 | 54 |
| Dietary Intake | ||||||
| Energy Intake, kcal | 1652 | 1675 | 1719 | 1785 | 1849 | 1728 (947, 2710) |
| Alcohol intake (g/day) | 4.6 | 4.6 | 4.5 | 4.5 | 4.3 | 4.5 (0, 19.6) |
| Components of Dash Scores† | ||||||
| Sodium, mg‡ | 1793 | 1819 | 1860 | 1915 | 1950 | 1861 (935, 3056) |
| Cola‡ | 0.56 | 0.28 | 0.19 | 0.14 | 0.07 | 0.27 (0.00, 1.20) |
| Fruits | 0.75 | 1.21 | 1.53 | 1.89 | 2.51 | 1.50 (0.21, 3.49) |
| Vegetables | 2.51 | 3.25 | 3.75 | 4.35 | 5.45 | 3.74 (1.09, 7.84) |
| Low-Fat Dairy | 0.52 | 0.87 | 1.13 | 1.34 | 1.69 | 1.06 (0.00, 2.93) |
| Nuts | 0.24 | 0.32 | 0.38 | 0.46 | 0.64 | 0.39 (0.00, 1.14) |
| Red Meat ‡ | 1.02 | 0.80 | 0.68 | 0.57 | 0.35 | 0.71 (0.07, 1.71) |
| Whole Grain | 0.78 | 1.21 | 1.49 | 1.77 | 2.33 | 1.45 (0.13, 3.77) |
All values are presented as servings per day with the exception of sodium, which is presented as mg/day.
For the DASH diet, higher intakes are given lower scores
Event Rates and Relationship with BMI
Over a mean follow-up period of 14.6 ± 2.4 years (the 5th, 25th, 50th, 75th, and 95th percentiles of follow-up time were 9.4, 14.4, 15.3, 15.8, and 16.1 years, respectively), 1769 confirmed cases of incident CVD or VTE occurred, including 1094 women who had CVD first and 675 women who had VTE first. Overall incidence rates of CVD, CHD, and VTE were 216, 85, and 133 events per 100 000 person-years, respectively. Higher levels of BMI were associated with increased hazard of both CVD and VTE (supplemental table). For example, relative to women with BMI < 22 kg/m2, women with BMI ≥30 kg/m2 had a relative hazard of CVD of 1.7, and a relative hazard of VTE of 2.9, adjusted for potential confounding variables.
DASH Diet Score
After adjustment for age, randomization status, and energy intake, hazard ratios of incident CVD and CHD decreased linearly across quintiles of the DASH score (both Ptrend<0.001). For CVD, significant hazard reductions of 20%, 25%, 41%, and 36% were observed with respective quintiles 2, 3, 4, and 5 relative to quintile 1 (Table 2). However, no such pattern was detected for VTE (Ptrend= 0.95). Likelihood ratio tests revealed significantly different associations between CVD or CHD and VTE with the DASH score (CVD: P<0.001, CHD: P = 0.001).
Table 2.
Multivariable Hazard Ratios (95% CIs) of VTE, CVD, and CHD across Quintiles of DASH Score of the 34 827 WHS participants
|
Quintiles of Dash Score |
Ptrend | P value for equal association with VTE€ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Average Mean Score (range) | 17.7 (8–20) | 22.1 (21–23) | 24.5 (24–25) | 26.9 (26–28) | 30.7 (29–38) | ||
| CVD (1094 Cases) | 292 | 245 | 174 | 194 | 189 | ||
| Incidence rate (per 100 000 person-years) | 234 | 217 | 215 | 186 | 224 | ||
| Age, energy, and randomization status | 1.00 | 0.80 (0.68–0.95) | 0.75 (0.62–0.91) | 0.59 (0.49–0.70) | 0.64 (0.53–0.78) | <0.001 | <0.001 |
| Basic Model* | 1.00 | 0.94 (0.79–1.11) | 0.94 (0.78–1.14) | 0.77 (0.63–0.93) | 0.88 (0.72–1.07) | 0.04 | 0.21 |
| Basic Model plus each set of risk factors, added one group at a time† | |||||||
| BMI | 1.00 | 0.94 (0.79–1.12) | 0.95 (0.78–1.15) | 0.78 (0.64–0.95) | 0.91 (0.74–1.10) | 0.08 | 0.11 |
| History of high cholesterol | 1.00 | 0.94 (0.79–1.11) | 0.94 (0.77–1.14) | 0.77 (0.63–0.93) | 0.87 (0.71–1.07) | 0.03 | 0.20 |
| History of hypertension | 1.00 | 0.93 (0.79–1.11) | 0.95 (0.78–1.16) | 0.79 (0.65–0.95) | 0.91 (0.74–1.11) | 0.09 | 0.29 |
| Family history of CHD‡ | 1.00 | 0.88 (0.73–1.06) | 0.97 (0.79–1.19) | 0.77 (0.62–0.94) | 0.83 (0.67–1.04) | 0.04 | 0.24 |
| All of the above¥ | 1.00 | 0.88 (0.73–1.06) | 0.98 (0.80–1.21) | 0.79 (0.64–0.96) | 0.87 (0.70–1.09) | 0.11 | 0.18 |
| CHD (430 Cases) | 123 | 104 | 68 | 64 | 71 | ||
| Incidence rate (per 100 000 person-years) | 98 | 92 | 84 | 61 | 84 | ||
| Age, energy, and randomization status | 1.00 | 0.82 (0.63–1.06) | 0.71 (0.52–0.95) | 0.47 (0.35–0.64) | 0.59 (0.44–0.80) | <0.001 | 0.001 |
| Basic Model* | 1.00 | 1.00 (0.77–1.30) | 0.94 (0.69–1.28) | 0.67 (0.49–0.92) | 0.90 (0.65–1.24) | 0.09 | 0.19 |
| Basic Model plus each set of risk factors, added one group at a time† | |||||||
| BMI | 1.00 | 1.00 (0.77–1.30) | 0.94 (0.69–1.28) | 0.68 (0.50–0.94) | 0.95 (0.69–1.31) | 0.16 | 0.13 |
| History of high cholesterol | 1.00 | 1.00 (0.77–1.30) | 0.93 (0.69–1.27) | 0.66 (0.48–0.91) | 0.89 (0.65–1.23) | 0.07 | 0.17 |
| History of hypertension | 1.00 | 1.00 (0.76–1.30) | 0.95 (0.70–1.29) | 0.69 (0.50–0.94) | 0.93 (0.68–1.29) | 0.14 | 0.25 |
| Family history of CHD‡ | 1.00 | 0.90 (0.67–1.20) | 0.95 (0.69–1.31) | 0.66 (0.48–0.93) | 0.83 (0.58–1.17) | 0.07 | 0.19 |
| All of the above¥ | 1.00 | 0.89 (0.67–1.19) | 0.96 (0.70–1.32) | 0.68 (0.49–0.95) | 0.87 (0.61–1.22) | 0.13 | 0.15 |
| VTE (675 Cases) | 142 | 148 | 111 | 152 | 122 | ||
| Incidence rate (per 100 000 person-years) | 114 | 131 | 137 | 146 | 145 | ||
| Age, energy, and randomization status | 1.00 | 1.06 (0.84–1.34) | 1.07 (0.83–1.37) | 1.06 (0.84–1.34) | 1.00 (0.78–1.28) | 0.95 | |
| Basic Model* | 1.00 | 1.05 (0.83–1.33) | 1.05 (0.82–1.35) | 1.05 (0.82–1.33) | 0.99 (0.76–1.28) | 0.95 | |
| Basic Model plus each set of risk factors, added one group at a time† | |||||||
| BMI | 1.00 | 1.06 (0.84–1.34) | 1.08 (0.84–1.39) | 1.09 (0.86–1.39) | 1.08 (0.83–1.40) | 0.54 | |
| History of high cholesterol | 1.00 | 1.05 (0.83–1.33) | 1.05 (0.81–1.35) | 1.05 (0.82–1.33) | 0.99 (0.76–1.28) | 0.94 | |
| History of hypertension | 1.00 | 1.05 (0.83–1.33) | 1.05 (0.82–1.36) | 1.05 (0.83–1.33) | 1.00 (0.77–1.29) | 1.00 | |
| Family history of CHD‡ | 1.00 | 1.06 (0.83–1.35) | 1.02 (0.77–1.34) | 1.01 (0.78–1.31) | 1.00 (0.76–1.31) | 0.87 | |
| All of the above¥ | 1.00 | 1.07 (0.84–1.37) | 1.04 (0.79–1.37) | 1.05 (0.81–1.36) | 1.08 (0.82–1.43) | 0.67 | |
Values are HR (95% CIs)
Adjusted for randomization status (aspirin, vitamin E, and beta-carotene), age (continuous), smoking (never, past, current), time-varying postmenopausal status (premenopausal, postmenopausal, not sure), time varying hormone therapy use (never, past, current), alcohol intake (rarely/never, 1–3 drinks per month, 1–6 drinks per week, and ≥1 drink per day), energy intake (quintiles), physical activity (quartiles), cigarettes per day (continuous; approximately as 10 cigarette per day increment) and highest education level (LPN/LVN, associate’s degree, bachelor’s degree, advanced degree).
Models were adjusted for all covariates in the basic model and BMI (<22, 22.1–23.0, 23.1–24.9, 25.0–29.9, and ≥30), history of high cholesterol, history of hypertension, and family history of CHD added one at a time
Family history was not included in the basic model due to concerns about missing data and the loss of incident events
Models were adjusted for all covariates in the basic model and BMI (<22, 22.1–23.0, 23.1–24.9, 25.0–29.9, and ≥30), history of high cholesterol, and history of hypertension, and family history of CHD.
p value associated with null hypothesis that the trend across DASH score quintiles is the same for VTE and CVD or CHD; likelihood ratio test with 1 df.
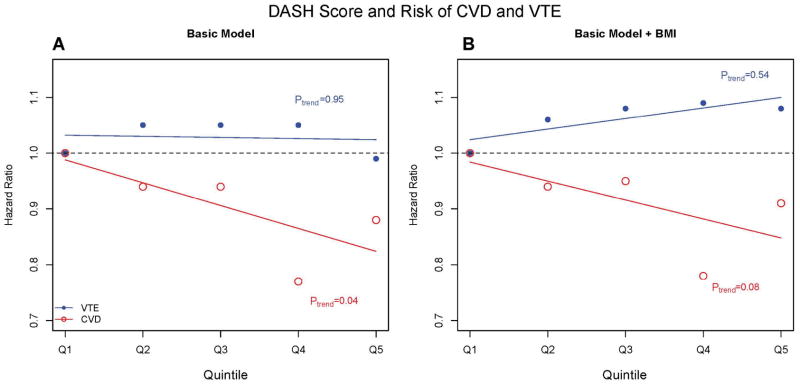
A basic model adjusting for standard CVD risk factors attenuated the association between higher DASH scores and incident CVD and CHD. The trend test across quintiles of the DASH score remained significant for CVD (P=0.04), but was no longer significant for CHD (P=0.09), and the relative hazard reductions for CVD in quintiles 2 through 5 relative to 1 now ranged from 6% to 22%. No association was observed for increasing DASH score and VTE in the basic model (Ptrend=0.95; Figure).
Figure.
Figure A Basic model is adjusted for randomization status (aspirin, vitamin E, beta carotene), age (continuous), smoking (never, past, current), time varying postmenopausal status (premenopausal, postmenopausal, not sure), time varying hormone therapy use (never, past, current), alcohol intake (rarely/never, 1–3 drinks per month, 1–6 drinks per day, ≥1 drink per day), energy intake (quintiles), physical activity (quartiles), cigarettes per day (continuous; approximately 10 cigarettes per day), and highest education level attained (LPN/LVN, associates degree, bachelor’s degree, advanced degree), Figure B adjusts for all basic model covariates and BMI (<22, 22–23, 25-25, 25–30, and ≥30).
We further adjusted for intermediates on various physiological pathways and family history of CHD separately by adding each variable one at a time to the basic model. For both CVD and CHD, these inclusions resulted in slight additional attenuations of the apparent associations of categories of the DASH score with CVD and CHD.
No significant relationship was observed between higher DASH scores and reduced risk of VTE in either individually or fully adjusted models (all Ptrend >0.50, Figure). Tests of the null hypothesis that the trend across quintiles of the DASH score was equal for VTE and CVD or CHD were also insignificant (Table 2).
In analysis stratified by unprovoked (266 cases) and provoked (409 cases) event type, no relationship between categories of the DASH score and reduced risk of VTE was observed in any model (Table 3).
Table 3.
Multivariable Hazard Ratios of Subtypes of VTE across Quintiles of Diet Score
|
Quintiles of Dash Score |
Ptrend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Unprovoked VTE (266 Cases) | 53 | 64 | 42 | 60 | 47 | |
| Incidence rate (per 100 000 person-years) | 42 | 57 | 52 | 58 | 56 | |
| Age, energy, and randomization status | 1.00 | 1.25 (0.87–1.80) | 1.10 (0.73–1.65) | 1.15 (0.79–1.67) | 1.05 (0.70–1.57) | 0.96 |
| Basic Model* | 1.00 | 1.19 (0.82–1.72) | 1.01 (0.67–1.53) | 1.03 (0.70–1.52) | 0.91 (0.59–1.38) | 0.46 |
| Basic Model + BMI‡ | 1.00 | 1.21 (0.83–1.74) | 1.04 (0.69–1.58) | 1.08 (0.73–1.59) | 1.00 (0.65–1.52) | 0.79 |
| Provoked VTE (409 Cases) | 89 | 84 | 69 | 92 | 75 | |
| Incidence rate (per 100 000 person-years) | 71 | 74 | 85 | 88 | 89 | |
| Age, energy, and randomization status | 1.00 | 0.95 (0.71–1.28) | 1.05 (0.76–1.43) | 1.01 (0.75–1.36) | 0.97 (0.71–1.33) | 0.98 |
| Basic Model* | 1.00 | 0.96 (0.71–1.30) | 1.07 (0.78–1.48) | 1.05 (0.78–1.43) | 1.05 (0.75–1.46) | 0.61 |
| Basic Model + BMI‡ | 1.00 | 0.98 (0.72–1.32) | 1.10 (0.80–1.52) | 1.10 (0.81–1.49) | 1.14 (0.82–1.59) | 0.32 |
Values are HRs (95% CIs).
Adjusted for randomization status (aspirin, vitamin E, and beta-carotene), age (continuous), smoking (never, past, current), time-varying postmenopausal status (premenopausal, postmenopausal, not sure), time-varying hormone therapy use (never, past, current), alcohol intake (rarely/never, 1–3 drinks per month, 1–6 drinks per week, and ≥1 drink per day), energy intake (quintiles), physical activity (quartiles), cigarettes per day (continuous; approximately as 10 cigarette per day increment) and highest education level (LPN/LVN, associate’s degree, bachelor’s degree, advanced degree).
Categorical Adjustment for BMI (<22, 22.1–23.0, 23.1–24.9, 25.0–29.9, and ≥30)
Components of the DASH diet score were analyzed for potential relationships with VTE where foods with high vitamin E content (i.e. nuts, vegetables) were of specific interest (Table 4). We observed a 25% reduced risk of VTE among those with the highest fruit intake (≥2.1 servings/day) and a 23% reduced risk among those with the highest nut consumption (≥0.58 servings/day). No other component was significantly associated with VTE. After adjustment for BMI, we observed no significant relationship between any component and reduced risk of total VTE, or of unprovoked or provoked VTE in stratified models.
Table 4.
Multivariable Hazard Ratios (95% CIs) of CVD and VTE across quintiles of components of the DASH diet score in 34 827 WHS participants
|
Quintiles of Food Intake |
Ptrend | Pequal association€ | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Fruit (servings/day) | <0.63 | 0.63–1.05 | 1.06–1.55 | 1.56–2.20 | ≥2.21 | ||
| CVD (1094 Cases), HR (95% CI) | 1.00 | 0.94 (0.78–1.13) | 0.80 (0.65–0.97) | 0.84 (0.69–1.03) | 0.82 (0.67–1.01) | 0.04 | 0.76 |
| VTE (675 Cases), HR (95% CI) | 1.00 | 0.98 (0.76–1.26) | 0.93 (0.73–1.20) | 0.94 (0.73–1.21) | 0.75 (0.57–0.99) | 0.04 | |
| Vegetables (servings/day) | <1.95 | 1.95–2.82 | 2.83–3.76 | 3.77–5.19 | ≥5.20 | ||
| CVD HR (95% CI) | 1.00 | 0.94 (0.78–1.14) | 0.88 (0.73–1.07) | 0.97 (0.80–1.18) | 0.89 (0.72–1.09) | 0.41 | 0.22 |
| VTE HR (95% CI) | 1.00 | 0.88 (0.68–1.13) | 1.07 (0.83–1.39) | 1.08 (0.84–1.39) | 1.04 (0.79–1.36) | 0.37 | |
| Low-fat dairy (servings/day) | <0.20 | 0.20–0.56 | 0.57–1.06 | 1.07–1.57 | ≥1.58 | ||
| CVD HR (95% CI) | 1.00 | 0.96 (0.80–1.15) | 0.89 (0.75–1.06) | 0.90 (0.73–1.11) | 0.91 (0.75–1.10) | 0.24 | 0.04 |
| VTE HR (95% CI) | 1.00 | 0.93 (0.72–1.21) | 1.15 (0.91–1.45) | 1.13 (0.86–1.47) | 1.18 (0.92–1.52) | 0.09 | |
| Red and processed meat (servings/day) | <0.28 | 0.28–0.48 | 0.49–0.70 | 0.71–1.06 | ≥1.07 | ||
| CVD HR (95% CI) | 1.00 | 0.92 (0.77–1.12) | 1.03 (0.86–1.25) | 1.00 (0.83–1.21) | 0.99 (0.80–1.22) | 0.84 | 0.67 |
| VTE HR (95% CI) | 1.00 | 0.90 (0.71–1.15) | 0.99 (0.78–1.26) | 1.19 (0.94–1.50) | 0.78 (0.59–1.03) | 0.70 | |
| Whole grains (servings/day) | <0.50 | 0.50–0.93 | 0.94–1.36 | 1.40–2.20 | ≥2.21 | ||
| CVD HR (95% CI) | 1.00 | 1.01 (0.84–1.21) | 1.03 (0.86–1.24) | 0.86 (0.71–1.05) | 0.96 (0.79–1.17) | 0.35 | 0.29 |
| VTE HR (95% CI) | 1.00 | 1.05 (0.81–1.35) | 1.31 (1.03–1.67) | 1.03 (0.79–1.33) | 1.12 (0.86–1.45) | 0.54 | |
| Nuts (servings/day) | <0.13 | 0.13–0.20 | 0.21–0.34 | 0.35–0.57 | ≥0.58 | ||
| CVD HR (95% CI) | 1.00 | 0.93 (0.75–1.15) | 0.99 (0.83–1.17) | 0.94 (0.79–1.13) | 1.02 (0.85–1.23) | 0.94 | 0.15 |
| VTE HR (95% CI) | 1.00 | 0.89 (0.68–1.17) | 0.91 (0.73–1.13) | 0.94 (0.75–1.18) | 0.77 (0.61–0.98) | 0.08 | |
| Sugar sweetened beverages (servings/day)† | <0.07 | 0.07–0.12 | 0.13–0.43 | ≥0.44 | |||
| CVD HR (95% CI) | 1.00 | 0.90 (0.76–1.07) | 1.06 (0.91–1.24) | 1.20 (1.01–1.43) | 0.12 | 0.12 | |
| VTE HR (95% CI) | 1.00 | 0.84 (0.68–1.05) | 0.97 (0.79–1.18) | 0.94 (0.74–1.18) | 0.45 | ||
Values are HR (95% CIs)
Adjusted for randomization status (aspirin, vitamin E, and beta-carotene), age (continuous), smoking (never, past, current), time-varying postmenopausal status (premenopausal, postmenopausal, not sure), time-varying hormone therapy use (never, past, current), alcohol intake (rarely/never, 1–3 drinks per month, 1–6 drinks per week, and ≥1 drink per day), energy intake (quintiles), physical activity (quartiles), cigarettes per day (continuous; approximately as 10 cigarette per day increment), and highest education level (LPN/LVN, associate’s degree, bachelor’s degree, advanced degree)
p value associated with null hypothesis that the trend across DASH score quintiles is the same for VTE and CVD; likelihood ratio test with 1 df.
Values collapsed into quartiles as more than 20% of the population consumed 0 servings per day.
To evaluate the impact of individual confounding variables, we added potential confounders one at a time to a model adjusting for age, randomization status, energy intake, and time varying hormone therapy and postmenopausal status (Table 5). Control for cigarette smoking (including number of cigarettes smoked per day) had the strongest influence on estimates of the association of the DASH diet with the hazard of CVD, and control for BMI had the strongest influence on estimates of the association of the DASH diet with the hazard of VTE.
Table 5.
Hazard Ratios (95% CIs) of CVD and VTE across quintiles of DASH diet score in 34 827 WHS participants for covariates added one at a time
| Quintiles of Dash Score | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ptrend | P value for equal association with VTE€ | |
| CVD (1094 Cases) | |||||||
| Age, energy, randomization status, hormone therapy use and post menopausal status | 1.00 | 0.80 (0.67–0.95) | 0.75 (0.62–0.91) | 0.59 (0.49–0.70) | 0.64 (0.53–0.77) | <0.001 | <0.001 |
| Age, energy, randomization status, hormone therapy use and post menopausal status plus each of the following added one at a time | |||||||
| Smoking† | 1.00 | 0.90 (0.76–1.07) | 0.90 (0.74–1.09) | 0.73 (0.60–0.88) | 0.82 (0.68–1.00) | 0.005 | 0.11 |
| Alcohol Intake | 1.00 | 0.81 (0.68–0.96) | 0.76 (0.63–0.91) | 0.59 (0.49–0.71) | 0.65 (0.53–0.78) | <0.001 | <0.001 |
| Education level | 1.00 | 0.82 (0.69–0.97) | 0.78 (0.64–0.94) | 0.61 (0.51–0.74) | 0.68 (0.56–0.82) | <0.001 | 0.001 |
| Physical Activity | 1.00 | 0.83 (0.70–0.98) | 0.79 (0.65–0.95) | 0.62 (0.51–0.75) | 0.69 (0.57–0.84) | <0.001 | 0.002 |
| Body mass index | 1.00 | 0.80 (0.68–0.95) | 0.76 (0.63–0.92) | 0.60 (0.50–0.72) | 0.67 (0.55–0.80) | <0.001 | <0.001 |
| History of hypertension | 1.00 | 0.80 (0.68–0.95) | 0.76 (0.63–0.92) | 0.60 (0.50–0.72) | 0.67 (0.55–0.81) | <0.001 | <0.001 |
| History of high cholesterol | 1.00 | 0.80 (0.68–0.95) | 0.75 (0.62–0.91) | 0.58 (0.49–0.70) | 0.64 (0.53–0.77) | <0.001 | <0.001 |
| Family history of CHD | 1.00 | 0.75 (0.62–0.90) | 0.78 (0.64–0.95) | 0.59 (0.48–0.71) | 0.61 (0.50–0.75) | <0.001 | <0.001 |
| All of the above in one model | 1.00 | 0.88 (0.73–1.06) | 0.98 (0.80–1.21) | 0.79 (0.64–0.96) | 0.87 (0.70–1.09) | 0.11 | 0.18 |
| VTE (675 Cases) | |||||||
| Age, energy, randomization status, hormone therapy use and post menopausal status | 1.00 | 1.06 (0.84–1.34) | 1.07 (0.83–1.37) | 1.07 (0.85–1.34) | 1.00 (0.78–1.29) | 0.94 | |
| Age, energy, randomization status, hormone therapy use and post menopausal status plus each of the following added one at a time | |||||||
| Smoking† | 1.00 | 1.05 (0.83–1.32) | 1.04 (0.81–1.34) | 1.04 (0.82–1.31) | 0.97 (0.76–1.25) | 0.84 | |
| Alcohol Intake | 1.00 | 1.06 (0.85–1.34) | 1.07 (0.83–1.37) | 1.07 (0.84–1.34) | 1.00 (0.78–1.28) | 0.96 | |
| Education level | 1.00 | 1.06 (0.84–1.34) | 1.07 (0.83–1.37) | 1.06 (0.84–1.34) | 1.01 (0.79–1.30) | 0.90 | |
| Physical Activity | 1.00 | 1.07 (0.85–1.35) | 1.08 (0.84–1.39) | 1.08 (0.85–1.36) | 1.02 (0.79–1.32) | 0.85 | |
| Body mass index | 1.00 | 1.08 (0.86–1.37) | 1.11 (0.86–1.42) | 1.12 (0.89–1.42) | 1.11 (0.87–1.43) | 0.35 | |
| History of hypertension | 1.00 | 1.07 (0.85–1.34) | 1.07 (0.84–1.38) | 1.07 (0.85–1.35) | 1.02 (0.79–1.30) | 0.86 | |
| History of high cholesterol | 1.00 | 1.06 (0.85–1.34) | 1.07 (0.83–1.37) | 1.07 (0.85–1.34) | 1.00 (0.78–1.29) | 0.94 | |
| Family history of CHD | 1.00 | 1.08 (0.84–1.37) | 1.04 (0.79–1.35) | 1.03 (0.81–1.32) | 1.01 (0.78–1.31) | 0.95 | |
| All of the –above in one model | 1.00 | 1.07 (0.84–1.37) | 1.04 (0.79–1.37) | 1.05 (0.81–1.36) | 1.08 0.82–1.43) | 0.67 | |
Also adjusted for amount of cigarettes smoked per day (continuous; approximately as 10 cigarette per day increment)
DISCUSSION
Consistent with several prior studies, in this prospective evaluation of 34,827 initially healthy women, higher DASH diet scores were associated with moderately but statistically significantly reduced risk of overall CVD and CHD in age and energy adjusted models.1,9,35,36. Control for additional confounding variables led to attenuation of these associations. In contrast, in spite of a strong association between higher BMI and risk of VTE, no associations were observed between DASH scores and VTE in any model. Overall, our study indicates potentially different relationships between diet and venous versus arterial events.
To our knowledge, there have been only three prior studies that have explored dietary factors and their relationship with venous events. The LITE study found significant associations with VTE using nutrient, food, and dietary pattern based analyses in 197 cases of incident VTE.23 Vitamin B6 and fruit and vegetable intakes were inversely associated with incident VTE whereas red and processed meat intake was positively associated with risk of VTE.23 The investigators derived prudent (healthy) and western (high fat, low fruit and vegetable intake) dietary patterns and found a western-style dietary pattern to be associated with increased risk of VTE.23 However, these results were not observed in a comparable analysis of 1950 incident VTEs using similar variables in the Iowa Women’s Health Study (IWHS).22 A hospital-based, case-control study of VTE in Thailand found greater consumption of vegetables and fish, and also greater consumption of spicy foods, in controls relative to cases.24
The current study differs from these prior studies in methodological approaches to create dietary patterns. Our analysis used a priori developed scores of the DASH dietary pattern that may have a more general application, yet are consistent with both prudent and western dietary patterns in that intake of fruit, vegetables, and fish is rewarded while intake of saturated fat and red meat is penalized. Furthermore, diet was assessed multiple times in LITE using a 66 item FFQ administered by an interviewer, which allowed for composite dietary analysis as well as greater precision through food model use during participant interviews.22 Despite the self-reported dietary assessment of the IWHS and the WHS, both studies utilized FFQ’s with more comprehensive food lists of 127 items in the IWHS and 133 items in the WHS.22 However, it is likely that dietary misclassification occurred in all three cohorts.
Our study has several noteworthy limitations in that data on risk factors and diet are self-reported, albeit in health professionals. The restriction to female health professionals limits generalizability, although enhanced validity can arise from more accurate reporting of health behaviors and risk factors. While we considered a substantial number of potential confounding variables, unmeasured confounding remains an important consideration. It is likely that changes in dietary intake occurred over the extended follow-up period. Still, the Spearman correlations between the DASH score calculated at baseline and then again 10 years later was 0.47, which is significant and consistent with correlations in cardiovascular biomarkers observed over a similar period.37 Furthermore, in creating DASH scores, each dietary component was given equal weight when it is possible that one part of the diet may contribute more heavily to disease risk than another. The use of such scores may also be problematic when they are applied to different populations where equal scores could constitute intakes of each component that are considerably variable. Nonetheless, the observed moderate associations for each of the healthy diets with total CVD and CHD provide evidence of their validity. Although relationships of dietary scores with risk of CVD versus VTE were no longer significantly different in analyses that controlled for lifestyle factors for DASH score models, the modest, non-significantly reduced risk of VTE associated with higher scores on the DASH diet was completely absent upon control for body mass index.
The apparently different associations of adherence to a DASH diet with risk of CVD and VTE may reflect differences in underlying disease pathways. Both blood pressure and lipid levels, which are favorably impacted by the DASH diet have uncertain relationships with the risk of VTE.10,11,18
Strengths of our analysis lie in the use of competing risk survival analysis that allows for the evaluation of hazard ratios for both CVD and VTE simultaneously. Also, to our knowledge, this is the first study to evaluate if established dietary patterns, with previously demonstrated associations with CVD, are also associated with VTE. The large number of confirmed incident venous events in the WHS remains another primary strength, and our study also presents dietary analyses stratified by provoked and unprovoked event types. Other strengths include the prospective design, extended follow-up, adjustment for energy expenditure using measures previously validated within this cohort, and use of a nutritional assessment tool that has demonstrated its efficacy in assessing long term dietary intake in prior research.38
VTE occurs frequently, more often than CHD during the 14+ year follow-up of our cohort, and is associated with high treatment costs and serious clinical sequelae. Yet, effective lifestyle approaches for its primary prevention have not been demonstrated. The shared relationship of obesity with risk of both CVD and VTE suggests that dietary strategies to reduce weight are worthy of exploration as potential approaches to lower risk of both event types. While adherence to the DASH diet may provide additional protective effects independent of BMI for CVD, it had no significant relationship with VTE. Several alternative diets, tailored to the needs of individual patients, have been shown to lower weight, favorably impact CVD risk factors, and can be maintained over time.39 It remains to be seen whether clinically-meaningful weight loss, achieved and maintained through any of several such diets, is an effective approach to reduce risk of both CVD and VTE.
Supplementary Material
Acknowledgments
Supported by grant AG031061 from the National Institute on Aging
References
- 1.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Obarzanek E, Sacks FM, Vollmer WM, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74(1):80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 4.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 5.de Souza RJ, Swain JF, Appel LJ, Sacks FM. Alternatives for macronutrient intake and chronic disease: a comparison of the OmniHeart diets with popular diets and with dietary recommendations. Am J Clin Nutr. 2008;88(1):1–11. doi: 10.1093/ajcn/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 7.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obarzanek E, Vollmer WM, Lin P-H, et al. Effects of individual components of multiple behavior changes: the PREMIER trial. Am J Health Behav. 2007;31(5):545–560. doi: 10.5555/ajhb.2007.31.5.545. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20(3):225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 11.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 12.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1–7. doi: 10.1016/j.jacc.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Glynn RJ, Ridker PM, Goldhaber SZ, Zee RYL, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women’s Health Study. Circulation. 2007;116(13):1497–1503. doi: 10.1161/CIRCULATIONAHA.107.716407. [DOI] [PubMed] [Google Scholar]
- 14.Hansson PO, Eriksson H, Welin L, Svärdsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the study of men born in 1913”. Arch Intern Med. 1999;159(16):1886–1890. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277(8):642–645. [PubMed] [Google Scholar]
- 16.Grady D, Wenger NK, Herrington D, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132(9):689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008;359(19):2025–2033. doi: 10.1056/NEJMra0707993. [DOI] [PubMed] [Google Scholar]
- 18.Everett BM, Glynn RJ, Buring JE, Ridker PM. Lipid biomarkers, hormone therapy and the risk of venous thromboembolism in women. J Thromb Haemost. 2009;7(4):588–596. doi: 10.1111/j.1538-7836.2009.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomp ER, Rosendaal FR, Doggen CJM. Alcohol consumption is associated with a decreased risk of venous thrombosis. Thromb Haemost. 2008;99(1):59–63. doi: 10.1160/TH07-07-0470. [DOI] [PubMed] [Google Scholar]
- 20.Pahor M, Guralnik JM, Havlik RJ, et al. Alcohol consumption and risk of deep venous thrombosis and pulmonary embolism in older persons. J Am Geriatr Soc. 1996;44(9):1030–1037. doi: 10.1111/j.1532-5415.1996.tb02933.x. [DOI] [PubMed] [Google Scholar]
- 21.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160(22):3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 22.Lutsey PL, Steffen LM, Virnig BA, Folsom AR. Diet and incident venous thromboembolism: the Iowa Women’s Health Study. Am Heart J. 2009;157(6):1081–1087. doi: 10.1016/j.ahj.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffen LM, Folsom AR, Cushman M, Jacobs DR, Jr, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation. 2007;115(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.641688. [DOI] [PubMed] [Google Scholar]
- 24.Bhoopat L, Rojnuckarin P, Hiransuthikul N, Intragumtornchai T. Low vegetable intake is strongly associated with venous thromboembolism in Thai population. Blood Coagul Fibrinolysis. 2010;21(8):758–763. doi: 10.1097/MBC.0b013e3283403537. [DOI] [PubMed] [Google Scholar]
- 25.Cook NR, Lee I-M, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Lee I-M, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 28.Willett WC. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 29.Hansen L, Dragsted LO, Olsen A, et al. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr. 2010;104(2):248–255. doi: 10.1017/S0007114510000462. [DOI] [PubMed] [Google Scholar]
- 30.Hung H-C, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn RJ, Ridker PM, Goldhaber SZ, Buring JE. Effect of low-dose aspirin on the occurrence of venous thromboembolism: a randomized trial. Ann Intern Med. 2007;147(8):525–533. doi: 10.7326/0003-4819-147-8-200710160-00004. [DOI] [PubMed] [Google Scholar]
- 34.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 35.Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panagiotakos DB, Pitsavos C, Stefanadis C. Alpha-priori and alpha-posterior dietary pattern analyses have similar estimating and discriminating ability in predicting 5-Y incidence of cardiovascular disease: methodological issues in nutrition assessment. J Food Sci. 2009;74(7):H218–224. doi: 10.1111/j.1750-3841.2009.01268.x. [DOI] [PubMed] [Google Scholar]
- 37.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55(2):305–312. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 38.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 39.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.