Abstract
The voltage-gated K+ channel Kv2.1 is expressed as a highly phosphorylated protein in most central neurons, where it plays a key role in regulating neuronal membrane excitability. Previous studies have shown that Kv2.1 channel activity is upregulated by Src-mediated phosphorylation through an unknown mechanism. However, a systematic analysis of the molecular mechanism of Kv2.1 channel phosphorylation by Src is lacking. Here we show that tyrosine phosphorylation by Src plays a fundamental role in regulating Kv2.1-mediated K+ current enhancement. We found that the level of expression of the Kv2.1 protein is increased by Src kinase. Using mass spectrometric proteomic techniques, we identified two novel phosphotyrosine sites, Y686 and Y810, in the cytoplasmic domains of Kv2.1. We found that Src-dependent phosphorylation at these sites affects Kv2.1 through distinct regulatory mechanisms. Whereas phosphorylation at Y686 regulates Kv2.1 activity similarly to the known site Y124, phosphorylation at Y810 plays a significant role in regulating the intracellular trafficking of Kv2.1 channels. Our results show that these two novel tyrosine phosphorylation sites of Kv2.1 are crucial to regulating diverse aspects of Kv2.1 channel function, and provide novel insights into molecular mechanisms for the regulation of Src-dependent modulation of Kv2.1 channels.
Keywords: delayed-rectifier Kv channel, Kv2.1, Src, tyrosine phosphorylation, mass spectrometry
INTRODUCTION
The delayed rectifier K+ channel Kv2.1 plays an important role in membrane excitability in most central mammalian neurons, where it accounts for the majority of the delayed-rectifier K+ current1, 2. Kv2.1 channels are localized in distinct clusters restricted to the cell membrane of the soma, proximal dendrites2, 3, and axon initial segment4. Kv2.1 is highly phosphorylated on numerous Ser and Thr residues in mammalian neurons3, 5.
Mammalian neurons contain a wide variety of protein kinases and phosphatases that respond to synaptic activity and other forms of neuromodulation to yield changes in intrinsic membrane excitability via changes in ion channel function6. A number of reports have suggested the importance of phosphorylation in regulating the gating and localization of Kv2.1 channels in neurons. Increased excitatory synaptic activity, ischemia, and epileptic seizures3, 7, 8 lead to rapid calcineurin-mediated channel dephosphorylation and disruption of Kv2.1 clusters and to dramatic changes in the channel's voltage-dependent gating properties3. Kv2.1 channels expressed in HEK293 and COS-1 cells exhibit similar hyperpolarizing shifts in voltage dependence of activation and inactivation and loss of clustering upon dephosphorylation by alkaline phosphatase or calcineurin9. Neuronal apoptosis is induced by the enhancement of voltage-dependent K+ channel activity10, and it has been shown that the apoptotic K+ current surge in neurons is mediated at least in part through direct p38 MAPK phosphorylation of the Kv2.1 channel11, 12.
In a previous study, we used mass spectrometry to identify 16 Ser and Thr phosphorylation sites in Kv2.1 channels immunopurified from mammalian cells and brain and showed that 7 of these sites are regulated through dephosphorylation by calcineurin5. Kv2.1-mediated K+ currents are also known to be enhanced through direct tyrosine phosphorylation of the channel by Src13, 14. These changes occur in the absence of the changes in voltage-dependent gating seen for phosphorylation at Ser and Thr residues. While the molecular mechanisms of Src regulation of Kv2.1 channel activity are not fully known, it is likely that specific phosphorylation sites on the Kv2.1 channel mediate the physiologically important regulation of channel functions. While one Src phosphorylation site (Y124) has been defined previously, it is possible that other phosphorylation sites exist among the 13 cytoplasmic Tyr residues in Kv2.1, and that are also important in mediating phosphorylation-dependent physiological regulation of Kv2.1 function.
Here, we provide evidence for a fundamental role of tyrosine phosphorylation by Src in the regulation of Kv2.1 activity. We find that protein expression levels of Kv2.1 are affected by its Src-dependent phosphorylation. Using a mass spectrometry-based approach that has recently allowed us to unambiguously identify sites chemically modified with phosphate on numerous voltage-dependent ion channels purified from brain and mammalian cells5, 15–18, we identified two novel Src-dependent tyrosine phosphorylation sites, Y686 and Y810, on the Kv2.1 channel. Functional biochemical and biophysical analysis of these phosphorylation sites shows that Kv2.1 activity is regulated through changes in channel biophysical properties that occur as a result of Src-mediated phosphorylation, and that Kv2.1 tyrosine phosphorylation plays an important role in regulating the surface expression levels of the Kv2.1 channel.
MATERIALS and METHODS
Cell culture and plasmid transfection
HEK293 cells were grown in Dulbecco's modified Eagle's medium (Welgene, Korea), supplemented with 10% fetal bovine serum (Invitrogen, NY), 100 U/ml penicillin, and 100 μg/mL streptomycin. Transient transfections were carried out using the FuGENE 6 (Roche Molecular Biochemicals) or Lipofectamine 2000 (Invitrogen, NY) systems.
Western blotting
Cultured cells were washed with ice-cold phosphate-buffered saline and lysed in buffer containing 1% Triton X-100, 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1 mM sodium orthovanadate, 5 mM NaF, 5 mM sodium pyrophosphate, 1 mM PMSF, aprotinin (1.5 μg/ml), antipain (10 μg/ml), leupeptin (10 μg/ml.), and benzamidine (0.1 mg/ml). Lysates were cleared by centrifugation at 16,100 × g for 30 min at 4°C, separated by 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with monoclonal anti-Kv2.1 (K89/34, UC Davis/NIH NeuroMab Facility, Davis, CA) and anti-phosphotyrosine (PY20, Upstate Biotechnology, Billerica, MA). The membranes were incubated with goat anti-mouse IgG HRP-conjugated secondary antibody (Assay Designs, Ann Arbor, MI) in 4% non-fat milk/TBS. The protein bands of Kv channels were detected using enhanced chemiluminescence reagent (Pierce, Rockford, IL). The density of protein bands was measured with ImageJ software (National Institutes of Health, Bethesda, MD).
Immunopurification of Kv2.1 from HEK293 cells and in-gel digestion
Cultured cells were pre-treated with vanadate, washed with ice-cold phosphate-buffered saline and lysed. The lysates were cleared by centrifugation at 16,100 × g for 30 min at 4°C and incubated with monoclonal anti-Kv2.1 antibody (D4/11)19 for 4 hr at 4°C; Protein G beads were then added. After 2 hr of incubation at 4°C, immunoprecipitation reaction products were collected and washed three times in lysis buffer; the beads were then resuspended in SDS-sample buffer and boiled at 100°C for 10 min to elute the precipitated proteins. The eluent was separated by 7.5% SDS-PAGE. The Kv2.1 band was directly cut out of gels, destained with 50% acetonitrile in 50 mM ammonium bicarbonate, and dried in a speed vacuum concentrator. Dried gel pieces were re-swollen in 50 mM ammonium bicarbonate containing 100 μg/ml trypsin and incubated at 37°C for 16–24 hr. Supernatant peptide mixtures were extracted with 50% acetonitrile in 5% formic acid and dried in a speed vacuum concentrator.
Mass spectrometry
A HPLC system (Paradigm MG4, Michrom Bio Resources, Auburn CA) directly coupled to an ion-trap mass spectrometer (LCQ Deca XP plus, Finnigan, San Jose, CA) was used for microcapillary LC-MS/MS data acquisition. A homemade fritless reverse-phase (RP) microcapillary column (0.1 mm × 180 mm) was packed with C18 (Phenomenex, Aqua: 5 μm 300 Å). Tryptic peptide mixtures were desalted and concentrated using the RP trap column (0.15 mm × 30 mm) before chromatographic separation on the microcapillary RP column at a flow rate of 300 nl/min. Eluted peptides were directly sprayed into the mass spectrometer. MS/MS spectra were acquired for the most intense peptide ion from the previous MS spectra with dynamic exclusion for 3 min. The two buffers used for RP chromatography were 5% acetonitrile / 0.1% formic acid (buffer A) and 80% acetonitrile / 0.1% formic acid (buffer B). A 2.5-hour gradient (0–10% B for 10 min, 10–45% B for 110 min, 45–100% B for 20 min, 100% B for 10 min) was used for maximum separation of the peptides. SEQUEST searches for LC-MS/MS data were performed for the MS/MS data set against the rat UniProt/Swiss-Prot database with differential phosphorylation on Tyr, Thr, and Ser and oxidation on Met. Peptide sequences from the search result were filtered using DTASelect software with slightly lower criteria than typically used for protein identification so as not to miss any possible phosphopeptides. The correlation values (Xcorr) were > 1.5, 2.0, and 3.0 for singly, doubly, and triply charged tryptic peptide ions, respectively, and ΔCn (the difference in correlation from the next highest Xcorr in each set) was higher than 0.08. Each filtered MS/MS spectrum that represented a possible phosphorylated peptide was manually checked and validated, and any ambiguity in phosphorylation sites was carefully examined.
Site-directed mutagenesis and transient transfection
Mutagenesis of recombinant rat Kv2.1 cDNA in the pRBG4 vector and was performed using the quick-change site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. HEK293 cells were plated in 35-cm plastic tissue culture dishes at ≈5–10% confluence and transiently transfected using the lipofectamine method with wild-type (WT) or mutant pRBG4-rKv2.1 plasmid (0.1 μg) along with a reporter plasmid expressing the eukaryotic green fluorescent protein (pEGFP-c1; 0.5 μg) and pRBG4 plasmid without any cDNA insert (0.4 μg). The transfected cells were used for electrophysiological and biochemical analyses within 24–56 hours of transfection.
Whole-cell patch clamp recording
To record Kv2.1 currents, day 1–2 (24–40 hours) post-transfection HEK293 cells with green fluorescence were selected for patch clamp studies. Kv2.1 currents were recorded using the whole-cell patch clamp technique with an Axopatch-200B amplifier (Axon Instruments, USA). K+ currents were evoked with a series of 500-ms voltage steps from a holding potential of −60 mV to +50 mV in 10-mV increments. The current amplitude was measured at +30 mV and normalized to the measured cell capacitance in each cell studied. HEK293 cells ranged in capacitance from approximately 5 to 15 pF. The pipettes had a resistance of 2–4 M when filled with internal solution, which contained 120 mM K+-aspartate, 20 mM KCl, 10 mM NaCl, and 10 mM HEPES (N-[2-hydroxyethyl] piperazine-N'-[2-ethanesulphonic acid]) adjusted to a pH of 7.2 using KOH. The external solution contained 135 mM NaCl, 5 mM KCl, 10 mM HEPES, 10 mM glucose, 2 mM CaCl2, and 1 mM MgCl2 and was adjusted to a pH of 7.4 using NaOH. For data acquisition and the application of command pulses, pCLAMP software v.10.2 and Digidata 1440A (Axon Instruments) were used. Data were filtered at 5 kHz, displayed on a computer monitor, and analyzed using pCLAMP and Origin software (Microcal origin v.7.0, USA).
Statistical analysis
Data are expressed as the mean ± S.E.M. of three independent experiments or as specified for each figure. The significance of differences among means was evaluated using Student's t test. A value of P < 0.05 was considered statistically significant.
RESULTS
Identification of tyrosine phosphorylation sites on the Kv2.1 channel
To investigate the tyrosine phosphorylation of the Kv2.1 channel, we used HEK293 cells transiently expressing the rat Kv2.1 channel. Cells were treated with DMSO, ionomycin (a calcium ion ionophore), carbachol (a muscarinic receptor activator), and sodium pervanadate (PV) (a tyrosine phosphatase inhibitor). Consistent with the previous results5, 9, ionomycin and carbachol treatments showed the decreased electrophoretic mobility of Kv2.1 band due to dephosphorylation of the channels20, whereas PV did not (Fig. 1A). The Kv2.1 channel proteins expressed in HEK293 cells were immunopurified with anti-Kv2.1 (D4/11) monoclonal antibody (mAb) and immunoblotted with anti-Kv2.1 (K89/34) mAb. Tyrosine phosphorylation of the Kv2.1 channel was detected using an anti-phosphotyrosine (PY-20) mAb. One signal with a molecular weight corresponding to that of Kv2.1 could be detected in Kv2.1 immunopurified from sodium-pervanadate-treated HEK293 cells, but no such signal was seen for Kv2.1 purified from control cells, or cells treated with other reagents (Fig. 1A). We next examined the dose-dependent effects of PV (25 – 300 μM) on the tyrosine phosphorylation of the Kv2.1 channel. The tyrosine phosphorylation level of the Kv2.1 channel was increased by pervanadate in a dose-dependent manner (Fig. 1B). Taken together, these results suggest that pervanadate-mediated inhibition of tyrosine phosphatase activity is largely responsible for the increased tyrosine phosphorylation of the Kv2.1 channel.
Fig. 1.
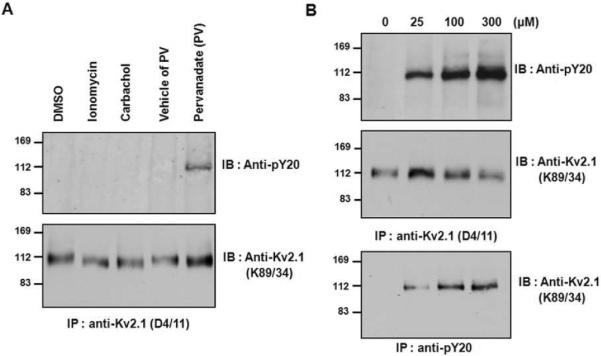
Tyrosine phosphorylation of the Kv2.1 channel is induced by pervanadate treatment. (A) After ionomycin, carbachol, or pervanadate treatment, tyrosine phosphorylation of Kv2.1 was detected only in the pervanadate-treated sample. HEK293 cells were transfected with Kv2.1 expression plasmid; cells were treated with DMSO, ionomycin, carbachol, or pervanadate, lysed and immunoprecipitated with anti-Kv2.1 mAb. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 and anti-PY-20 mAbs. (B) The effect of pervanadate concentration on Kv2.1 tyrosine phosphorylation. HEK293 cells were transfected with Kv2.1 expression plasmid. Cells treated with pervanadate (0, 25, 100, or 300 μM) were lysed and immunoprecipitated with anti-Kv2.1 (top and middle) and anti-PY20 (bottom). The immunoprecipitates were separated by 7.5% SDS-PAGE and immunoblotted with anti-PY20 (top) and anti-Kv2.1 mAbs (middle and bottom).
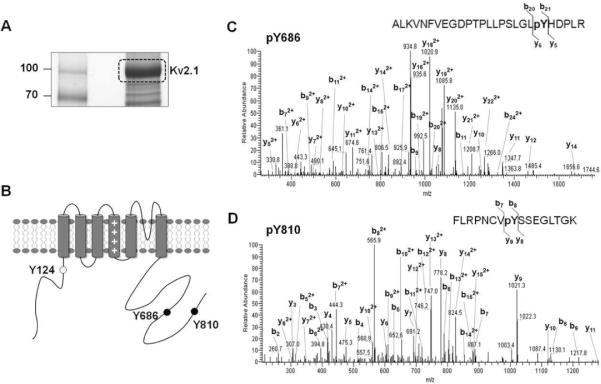
To identify the tyrosine phosphorylation sites of the Kv2.1 channel, we used a mass-spectrometry-based proteomic approach similar to that used in our previous studies of the phosphorylation of ion channels immunopurified from mammalian cells and brain [Kv2.15, 18, Kv1.215, BKα16, and Nav1.217]. Kv2.1 channels expressed in HEK293 cells that had been treated with pervanadate were immunopurified as above, size-fractionated on SDS-PAGE, and stained with colloidal Coomassie blue (Fig. 2A). The Kv2.1 gel band at ~120 kDa was excised and subjected to in-gel trypsin digestion, and Kv2.1 tryptic peptides were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) and identified using the SEQUEST database search engine. This approach led to the identification of two novel tyrosine phosphorylation sites located in the C-terminal cytoplasmic intracellular region of the channel; these sites correspond to Y686 and Y810 of the amino acid sequence (Fig. 2B). The previously reported phosphorylation site Y124 was not detected because of the unfavorable mass of the resultant tryptic peptides in this study (the molecular weights of detectable peptide fragments ranged between 740 and 3000)16. A manual check and validation of the MS/MS spectra showed an unambiguous assignment of the phosphorylation sites in the tryptic peptides to the Y686 and Y810 sites of the Kv2.1 channel (Fig. 2C and 2D). These data suggest that Y686 and Y810 of the Kv2.1 channel protein are phosphorylated by endogenous tyrosine kinases in HEK293 cells.
Fig. 2.
Identification of Kv2.1 tyrosine phosphorylation sites Y686 and Y810. (A) Immunopurification of Kv2.1. Coomassie brilliant blue-stained SDS gel of a large-scale immunopurification showing the yield of Kv2.1 channel. (B) Cartoon of the membrane topology of Kv2.1 showing tyrosine phosphorylation sites identified by LC-MS/MS analyses. Black dots mark the novel tyrosine phosphorylation sites identified in this study; white dots mark tyrosine phosphorylation sites identified in a previous study. (C) MS/MS spectrum of Kv2.1 tyrosine phosphopeptide pY686. Shown is the product ion spectrum of a triply charged, singly phosphorylated tryptic peptide at m/z 982.20. (D) MS/MS spectrum of Kv2.1 tyrosine phosphopeptide pY810. Shown is the product ion spectrum of a triply charged, singly phosphorylated tryptic peptide at m/z 637.34.
Increased expression of the Kv2.1 channel is mediated by Src phosphorylation
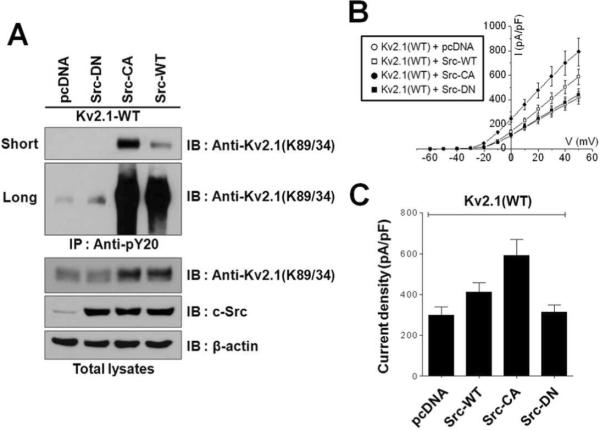
Previous studies have reported that Kv2.1 activity is up-regulated through direct tyrosine phosphorylation by Src kinase and that such up-regulation of activity results in enhanced K+ current13, 14. To further characterize how Src-mediated tyrosine phosphorylation increases Kv2.1 channel activity, the Kv2.1 channel was co-expressed with wild type mouse Src (Src-WT), constitutively active (Y529F) Src (Src-CA), or dominant negative (K297M) Src (Src-DN) in HEK293 cells. Tyrosine-phosphorylated proteins were immunopurified with PY20 antibodies and detected by immunoblotting with anti-Kv2.1 (K89/34) mAb. Whereas co-expression of Src-CA and Src-WT led to a clear increase in the tyrosine phosphorylation signal of the Kv2.1 channel protein, Kv2.1 purified from cells expressing Src-DN and or empty vector showed no phosphotyrosine signal (short exposure) or a weak phosphotyrosine signal (long exposure) (Fig. 3A). These results confirm previous reports that the tyrosine residues of Kv2.1 protein are directly phosphorylated by Src13, 14, Whereas, under control conditions, Kv2.1 channels are weakly phosphorylated at the level of tyrosine residues, thus, Kv2.1 channels purified from transfected in HEK293 cells and from rat brain do not exhibit any detectable tyrosine phosphorylation sties by LC-MS/MS5, 18. Interestingly, co-expression of Kv2.1 channel protein with Src-WT or Src-AC, but not with Src-DN or vector, resulted in increased expression of Kv2.1 channel protein in HEK293 cells (Fig. 3A), suggesting that Src-dependent tyrosine phosphorylation of the Kv2.1 channel may be involved to increased expression of Kv2.1.
Fig. 3.
Src-induced increases in the expression level and activity of the Kv2.1 channel. (A) Kv2.1 protein levels are increased by Src-dependent tyrosine phosphorylation. Kv2.1-WT was co-transfected with Src variants (Src-DN, Src-CA and Src-WT) in HEK293 cells. Cells were lysed and immunoprecipitated using anti-pY20. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 mAb. Short, short exposure; Long, long exposure. (B) Kv2.1-mediated K+ currents are increased by Src-dependent tyrosine phosphorylation. Whole cell patch recordings obtained from HEK293 cells co-expressing Kv2.1-WT with Src variants (Src-DN, Src-CA and Src-WT) are shown. Data are from HEK293 cells. (C) Mean ± S.E.M. current densities from HEK293 cells co-expressing Kv2.1-WT and Src variants (Src-DN (n=7), Src-CA (n=10), and Src-WT (n=10) or pcDNA (n=8)). Data are from HEK293 cells, asterisks represent samples that are significantly different (p< 0.05) from pcDNA.
We next explored the functional regulation of the Kv2.1 channel by Src. Whole-cell electrophysiological recordings were performed in HEK293 cells transiently co-expressing Kv2.1 channels and Src-WT, Src-CA, or Src-DN. Similar to the results of a previous study14, HEK293 cells that co-expressed Kv2.1 and Src-WT or Src-CA showed a significant increase in whole-cell K+ current amplitude (pA/pF = 412.285 ± 44.708 and pA/pF = 591.395 ± 79.125, respectively), compared to cells in which Kv2.1 was co-expressed with Src-DN or pcDNA (pA/pF = 314.434 ± 35.893 and pA/pF = 299.270 ± 39.392, respectively). Moreover, the increase in Kv2.1 K+ current was dependent on Src activity; Src-CA increased Kv2.1 K+ current to levels ~1.4-fold higher than seen in cells expressing Src-WT (Fig. 3B and 3C). Our electrophysiological and immunochemical analyses reveal similar patterns of changes in K+ current and Src-activity-dependent Kv2.1 protein expression levels in these cells (Fig. 3A). These results suggest that tyrosine phosphorylation-dependent increases in Kv2.1 activity may result from Src activity-dependent increases in Kv2.1 expression.
Functional modulation of the Kv2.1 channel by Src
To determine whether Src-mediated modulation of tyrosine phosphorylation at Y686 and Y810 regulates the increased levels of Kv2.1 expression and current, we constructed three non-phosphorylatable mutants, Kv2.1-Y124F14, Kv2.1-Y686F, and Kv2.1-Y810F, and expressed Kv2.1-WT and these mutants in HEK293 cells. Cells were pretreated with or without Src kinase inhibitor PP2 before PV treatments. Tyrosine-phosphorylated Kv2.1 channels were immunopurified with PY-20 antibodies and analyzed by immunoblotting with anti-Kv2.1 (K89/34) mAb. PV treatments induced tyrosine phosphorylation of Kv2.1-WT, Kv2.1-Y124F, Kv2.1-Y686F, and Kv2.1-Y810F with different levels of phosphorylation status, whereas pretreatment with PP2 showed no phosphotyrosine signal (short exposure) or a weak phosphotyrosine signal (long exposure). Moreover, the tyrosine phosphorylation levels of these mutants compared to Kv2.1-WT were reduced (Fig. 4A). These results suggest that PV mediates tyrosine phosphorylation of Y124, Y686, and Y810 residues on Kv2.1 channel via Src, and these sites might be phosphorylated by other tyrosine kinases.
Fig. 4.
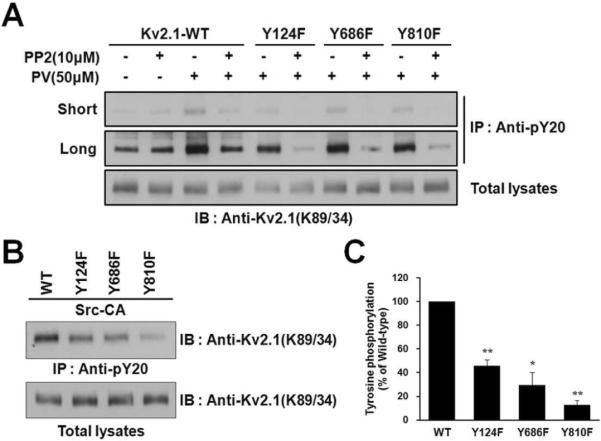
The Y686 and Y810 residues of the Kv2.1 channel are phosphorylated by Src. (A) PV phosphorylates the Y124, Y686, and Y810 residues of Kv2.1 via Src kinase. HEK293 cells were transfected with Kv2.1-WT or mutants (Y124F, Y686F, and Y810F). Cells were pretreated with or without Src kinase inhibitor PP2 before PV treatments. Cells were lysed and immunoprecipitated with anti-pY20 mAb. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 mAb. Short, short exposure; Long, long exposure. (B) Mutating Y124, Y686 and Y810 decreases tyrosine phosphorylation of Kv2.1. HEK293 cells were co-transfected with Kv2.1-WT or mutants (Y124F, Y686F, and Y810F) and Src-CA. Cells were lysed and immunoprecipitated with anti-pY20 mAb. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 mAb. (C) Kv2.1 tyrosine phosphorylation levels, normalized to Kv2.1 protein expression, are shown. Data are expressed as the mean ± S.E.M. (n=3, * P < 0.05, ** P < 0.01, Student's t test).
We next co-expressed these mutants with Src-CA in HEK293 cells. Cells were lysed and subjected to immunoprecipitation with PY-20). We verified that there are no differences in the protein expression level between Kv2.1-WT and mutants in cells co-expressing Src-CA (Fig. 4B). The Src-mediated tyrosine phosphorylation levels of the Kv2.1-Y124F, Kv2.1-Y686F, and Kv2.1-Y810F mutants were reduced compared to Kv2.1-WT (Figs. 4B and 4C). Immunoprecipitation with another anti-phosphotyrosine (4G10) also showed similar results (Supplemental Fig. 1). These results suggest that the Y686 and Y810 residues of the Kv2.1 channel protein are directly phosphorylated and are the major sites of phosphorylation of Kv2.1 channel by Src.
To examine the roles of the Y686 and Y810 residues in the modulation of Kv2.1 activity by Src, we co-expressed Kv2.1-Y124F, Kv2.1-Y686F, and Kv2.1-Y810F mutants with and without Src-CA in HEK293 cells and analyzed the physiological properties of the cells by whole-cell voltage clamp. In the absence of Src-CA expression, cells expressing Kv2.1-WT, Kv2.1-Y124F, Kv2.1-Y686F, or Kv2.1-Y810F channels showed no differences in K+ current amplitude (Fig. 5). Thus, these mutations themselves have little effect on Kv2.1 channel function. On the other hand, co-expression of the Kv2.1-WT channel with Src-CA led to increased K+ current compared to that in cells expressing Kv2.1-WT without Src-CA (Fig. 5A). Cells expressing the Kv2.1-Y124F mutant did not exhibit an increase in K+ current density in the presence of Src-CA (Fig. 5B), consistent with previous results14. Co-expression of Src-CA also did not lead to an increase in current in cells expressing Kv2.1-Y686F (Fig. 5C). This result suggests that, like the Y124 residue, the Y686 residue of the Kv2.1 channel protein modulates the induction of Kv2.1-mediated K+ currents through direct phosphorylation by Src. Unlike the other mutants, co-expression of Src-CA with Kv2.1-Y810F led to an approximately 40% increase in K+ current in comparison to cells expressing Kv2.1-Y810F in the absence of Src-CA (Fig. 5D). This result suggests that the mutation at Y810 is not sufficient to prevent the enhancement of channel gating that result in an increase in Kv2.1-mediated K+ current and that the phosphorylation of Y810 residue of the Kv2.1 channel by Src has a role distinct from that at Y124 and Y686.
Fig. 5.
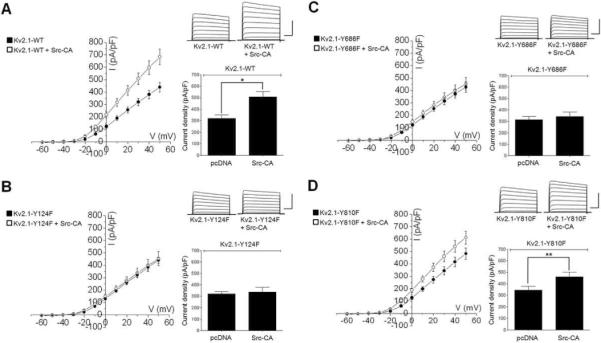
Effects of the constitutively active form of Src, Src-CA, on outward K+ currents in Kv2.1-expressing HEK cells using the whole-cell patch clamp technique. (A) (Left) Current-voltage relations of WT Kv2.1 channels expressed in the absence (n = 14, filled symbols) or presence of Src-CA (n = 15, open symbols). (Right) Representative whole-cell K+ currents and mean ± S.E.M. current densities from HEK cells expressing Kv2.1-WT or Kv2.1-WT and Src-CA (B) (Left) Current-voltage relations of Kv2.1-Y124F channels expressed in the absence (n = 7, filled symbols) or presence of Src-CA (n = 13, open symbols). (Right) Representative whole-cell K+ currents and mean (± S.E.M.) current densities in HEK cells expressing Kv2.1-Y124F or Kv2.1-Y124F and Src-CA. (C) (Left) Current-voltage relations of Y686F Kv2.1 channels expressed in the absence (n = 9, filled symbols) or presence of Src-CA (n = 12, open symbols). (Right) Representative whole-cell K+ currents and mean (± S.E.M.) current densities in HEK cells expressing Kv2.1-Y686F or Kv2.1-Y686F and Src-CA. (D) (Left) Current-voltage relations of Kv2.1-Y686F channels expressed in the absence (n = 20, filled symbols) or presence of Src-CA (n = 19, open symbols). (Right) Representative whole-cell K+ currents and mean (± S.E.M.) current densities in HEK cells expressing Kv2.1-Y686F or Kv2.1-Y686F and Src-CA. (*P < 0.01, **P < 0.05; Student's t-test). Current density was calculated as current amplitude at +30 mV (normalized to cell capacitance) of the K+ current evoked by sequential 10 mV voltage steps to +50 mV from a holding potential of −60 mV. Calibration: 300 pA, 100 ms.
Regulation of Kv2.1 channel membrane trafficking by Src
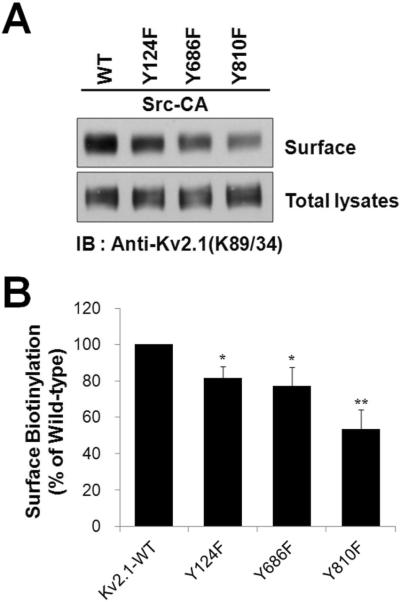
To further investigate whether Src-mediated phosphorylation of Y124, Y686, and Y810 residues regulates the cell-surface expression of Kv2.1, we selectively labeled the cell-surface pool of Kv2.1 channel protein by a cell surface biotinylation assay, purified biotinylated and non-biotinylated fractions, and performed immunoblotting with anti-Kv2.1 mAb. Fig. 6 shows that a large fraction of the Kv2.1-WT channel protein is present in the biotinylated fraction (plasma membrane fraction) in the presence of Src-CA. The biotinylated fraction of Kv2.1-Y124F, Kv2.1-Y686F, and Kv2.1-Y810F mutant channel proteins co-expressed with Src-CA show a decrease compared to that of the Kv2.1-WT channel. Interestingly, the biotinylated fraction of the Kv2.1-Y810F mutant channel protein expressed in the presence of Src mainly decreased compared to that of the Kv2.1-WT channel. Taken together, our results suggest that Y124, Y686, and Y810 phosphorylation by Src may be involved in the Kv2.1 channel protein to reach the plasma membrane.
Fig. 6.
Tyrosine phosphorylation of the Kv2.1 channel at Y810 is critical for the membrane trafficking of the channel by Src. (A) HEK293 cells were cotransfected with Kv2.1-WT or mutants (Y124F, Y686F, and Y810F) and Src-CA. Cells were then incubated with sulfo-NHS-SS-biotin, and biotinylated proteins were analyzed by immunoblotting with anti-Kv2.1. Eluates from avidin beads and total lysates were separated by 7.5% SDS-PAGE and compared. (B) The intensity of surface Kv2.1 levels, normalized to total Kv2.1 protein expression, is shown. Data are expressed as the mean ± S.E.M. (n=4, * P < 0.05, ** P < 0.01, Student's t test).
DISCUSSION
The activity, intracellular trafficking, and surface expression of Kv channels are regulated through their direct phosphorylation by members of the Src kinase family21, 22. In the present work, we further define the role of Src-mediated tyrosine phosphorylation of Kv2.1 channels in the modulation of channel activity. Our data support a complex model in which tyrosine phosphorylation of the Kv2.1 channel by Src regulates the dynamic functions of the channel, increases the expression of channel proteins, enhances K+ current density, and regulates surface expression through direct phosphorylation.
Previous studies have reported that the Y124 residue of the Kv2.1 channel is directly phosphorylated and that Kv2.1-mediated K+ currents are enhanced through Y124 phosphorylation by Src13, 14. But it is still possible that other phosphorylation sites exist among the 13 cytoplasmic Tyr residues in Kv2.1, and that are also important in mediating phosphorylation-dependent physiological regulation of Kv2.1 function. Using the same mass spectrometry-based proteomic approach that we used for the identification of novel phosphorylation sites of ion channels in previous studies5, 6, 15, 16, 18, we identified two novel tyrosine phosphorylation sites, Y686 and Y810, in the Kv2.1 channel purified from HEK293 cells. By mutation of these two phosphorylation sites, we demonstrated that both Y686 and Y810, like Y124, are directly phosphorylated by Src and that Src-mediated phosphorylation of these sites regulates the surface expression and activity of Kv2.1 channels. Our observations suggest that phosphorylation of the Kv2.1 channel by Src at Y686 and Y810 may play an important role in the dynamic regulation of Kv2.1 function.
Despite the important role of Src in Kv2.1 channel-mediated K+ current enhancement13, 14, the molecular mechanism of this effect is not fully known. An earlier study proposed a mechanism in which conformational changes in Kv2.1 channels resulting from the phosphorylation of specific tyrosine sites such as Y124 regulate the properties of its membrane–spanning region14; however, this model is insufficient to describe the molecular mechanism of the dynamic regulation of Kv2.1 channel properties by Src. Such dynamic regulation of channel properties could, however, occur as a result of changes in Kv2.1 protein expression or regulation of the plasma membrane trafficking of Kv2.1 channels by Src. In the present work, we found that the expression of Kv2.1 channel proteins is dramatically increased through direct phosphorylation by Src-WT and Src-CA, whereas Src-DN did not increase the expression of Kv2.1 proteins. Furthermore, whole-cell patch clamp recording revealed that Kv2.1-mediated K+ currents are enhanced proportionally to increases in Src activity. Our results suggest that Kv2.1 channel expression is regulated in a Src activity-dependent manner and that an increase in Kv2.1 channel expression may be one of the molecular mechanisms of Kv2.1-mediated K+ current enhancement.
When Kv2.1 channel mutants were co-expressed with Src-CA, we found that the Y686F and Y810F mutants exhibited distinct properties. Similar to the Y124F mutant, the Y686F mutant did not exhibit the enhancement of K+ current in response to Src-CA co-expression. This finding suggests that Y686 may regulate the functions of the channel by Src-dependent phosphorylation through a similar mechanism of Y124F. The Y124 residue is located within the T1 domain of the intracellular N-terminal of the Kv2.1 channel, whereas Y686 is localized within its intracellular C-terminal region14, 23, yet each mutation can completely block Kv2.1-mediated K+ current enhancement. Previous studies have shown that the intracellular trafficking and surface expression of Kv2.1 are regulated through N- and C-terminal interactions of the channel23, 24. Moreover, these N- and C-terminal interactions regulate the phosphorylation-dependent modulation of the channel24. Phosphorylation at Y124 and at Y686 presumably acts through a complementary mechanism via the N- and C-terminal interaction, such that mutation of either residue eliminates the impact of the mutating the other. The relationship of the N- and C-terminal interactions and phosphorylation of Y124 and Y686 in Kv2.1-mediated K+ current enhancement by Src remain interesting questions for future study.
The present work demonstrates that the Y124, Y686, and Y810 residues of the Kv2.1 channel may be involved in the surface expression of the channel via Src-dependent phosphorylation. Previous studies have also shown that tyrosine phosphorylation regulates the intracellular trafficking and surface expression of Kv channels. For example, Kv1.2 channel currents are suppressed upon tyrosine kinase activation25, and it has been suggested that the phosphorylation of Y132, which is located in the N-terminal region, results in rapid endocytosis of the channel22. Similarly, it was suggested that the tyrosine phosphorylation of Kv1.5 may regulate its endocytosis through proline-rich SH-binding-domain-dependent internalization26. Compared with other Kv channels, phosphorylation of the Kv2.1 channel by Src shows a functionally distinct regulation of channel trafficking that presumably occurs through a distinct mechanism. Mutation of Tyr to Phe decreases the enhancement of K+ current that is normally observed in the presence of Src. Surface biotinylation results obtained with the Kv2.1 mutants, which show that biotinylated channel proteins are decreased, are consistent with these observation and support the idea that the these sites are related with surface expression levels of Kv2.1 channel through phosphorylation by Src.
CONCLUSION
Reversible phosphorylation plays an important role in regulating the expression, plasma membrane trafficking, localization, and gating properties of Kv channels in neurons6. The data presented here demonstrate the fundamental role of tyrosine phosphorylation by Src in regulating Kv2.1-mediated K+ current enhancement. Kv2.1 protein expression levels are increased as a result of its phosphorylation by active Src. We identified two novel Tyr sites of Src-dependent phosphorylation in Kv2.1 channels and showed that mutation of these sites to non-phosphorylatable residues results in blockage or decrease of the K+ current enhancement that is normally observed in the presence of active Src and that these sites regulate the activities and the surface expression levels of the channel when Src is present. Although the results presented here show that functional regulation of Kv2.1 occurs though multiple sites and mechanisms, a common outcome of these mechanisms is the regulation of K+ current enhancement by Src-dependent phosphorylation. It is not known when or how Kv2.1 channels are phosphorylated at tyrosine residues by Src; from earlier studies, this process is presumed to involve pathogenic or injurious conditions. Residue Y124, a known target of Src, is critical for the apoptotic current surge12. Src activity has been shown to be increased after brain ischemia, and tyrosine phosphorylation of NMDA receptors induced through direct binding of activated Src kinases after brain ischemia enhances their activity27, 28. The multiple pathways of regulation of Kv2.1 channels by Src that appear to underlie the diverse modulation of Kv channel functions by Src-dependent tyrosine phosphorylation under injurious conditions such as neuronal ischemia represent a worthwhile subject for future study
Supplementary Material
ACKNOWLEGEMENTS
We are grateful to Dr. James. S Trimmer for his assistance and helpful comments. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology grants 20090063284, KRF-2008-313-E00075, and NIH grant NS42225. Mass spectrometry was performed at the UC Davis Proteomics Facility
Footnotes
Supporting Information Supplementary figure S1. This material is available free of charge via internet at http://pubs.acs.org.
REFERENCES
- 1.Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans. 2007;35(Pt 5):1064–8. doi: 10.1042/BST0351064. [DOI] [PubMed] [Google Scholar]
- 2.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88(4):1407–47. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misonou H, Mohapatra D, Park E, Leung V, Zhen D, Misonou K, Anderson A, Trimmer J. Regulation of ion channel localization and phosphorylation by neuronal activity. Nature neuroscience. 2004;7(7):711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 4.Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313(5789):976–9. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 6.Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology (Bethesda) 2008;23:49–57. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- 7.Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006;26(52):13505–14. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005;25(48):11184–93. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006;26(2):685–95. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70(4):363–86. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 11.Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104(9):3568–73. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J Physiol. 2009;587(Pt 18):4393–404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiran Z, Peretz A, Sines T, Shinder V, Sap J, Attali B, Elson A. Tyrosine phosphatases epsilon and alpha perform specific and overlapping functions in regulation of voltage-gated potassium channels in Schwann cells. Mol Biol Cell. 2006;17(10):4330–42. doi: 10.1091/mbc.E06-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2.1 Channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J Biol Chem. 2003;278(19):17509–14. [Google Scholar]
- 15.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104(50):20055–60. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7(11):2188–98. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J Proteome Res. 2010;9(4):1976–84. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KS, Mohapatra DP, Trimmer JS. Proteomic analyses of K(v)2.1 channel phosphorylation sites determining cell background specific differences in function. Channels (Austin) 2007;1(2):59–61. doi: 10.4161/chan.4388. [DOI] [PubMed] [Google Scholar]
- 19.Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharmacology. 1996;35(7):851–65. doi: 10.1016/0028-3908(96)00128-1. [DOI] [PubMed] [Google Scholar]
- 20.Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7(7):711–8. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 21.Nitabach MN, Llamas DA, Thompson IJ, Collins KA, Holmes TC. Phosphorylation-dependent and phosphorylation-independent modes of modulation of shaker family voltage-gated potassium channels by SRC family protein tyrosine kinases. J Neurosci. 2002;22(18):7913–22. doi: 10.1523/JNEUROSCI.22-18-07913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase-dependent suppression of a voltage-gated potassium channel. Mol Biol Cell. 2004;15(9):4073–88. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju M, Stevens L, Leadbitter E, Wray D. The Roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel. J Biol Chem. 2003;278(15):12769–78. doi: 10.1074/jbc.M212973200. [DOI] [PubMed] [Google Scholar]
- 24.Mohapatra DP, Siino DF, Trimmer JS. Interdomain cytoplasmic interactions govern the intracellular trafficking, gating, and modulation of the Kv2.1 channel. J Neurosci. 2008;28(19):4982–94. doi: 10.1523/JNEUROSCI.0186-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang XY, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75(6):1145–56. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 26.Steele DF, Zadeh AD, Loewen ME, Fedida D. Localization and trafficking of cardiac voltage-gated potassium channels. Biochem Soc Trans. 2007;35(Pt 5):1069–73. doi: 10.1042/BST0351069. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Mu D, Biran V, Faustino J, Chang S, Rincon CM, Sheldon RA, Ferriero DM. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63(5):632–41. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- 28.Du CP, Gao J, Tai JM, Liu Y, Qi J, Wang W, Hou XY. Increased tyrosine phosphorylation of PSD-95 by Src family kinases after brain ischaemia. Biochem J. 2009;417(1):277–85. doi: 10.1042/BJ20080004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.