Abstract
Rationale
Hydrogen peroxide (H2O2) serves as a key endothelium-derived hyperpolarizing factor mediating flow-induced dilation in human coronary arterioles (HCAs). The precise mechanisms by which H2O2 elicits smooth muscle hyperpolarization are not well understood. An important mode of action of H2O2 involves the oxidation of cysteine residues in its target proteins, including protein kinase G (PKG)-Iα, thereby modulating their activities.
Objective
Here we hypothesize that H2O2 dilates HCAs through direct oxidation and activation of PKG-Iα leading to the opening of the large-conductance Ca2+-activated K+ (BKCa) channel and subsequent smooth muscle hyperpolarization.
Methods and Results
Flow and H2O2 induced pressure gradient/concentration-dependent vasodilation in isolated endothelium-intact and -denuded HCAs, respectively. The dilation was largely abolished by iberiotoxin, a BKCa channel blocker. The PKG inhibitor Rp-8-Br-PET-cGMP also markedly inhibited flow- and H2O2-induced dilation, whereas the soluble guanylate cyclase inhibitor ODQ had no effect. Treatment of coronary smooth muscle cells (SMCs) with H2O2 elicited dose-dependent, reversible dimerization of PKG-Iα, and induced its translocation to the plasma membrane. Patch-clamp analysis identified a paxilline-sensitive single-channel K+ current with a unitary conductance of 246-pS in freshly isolated coronary SMCs. Addition of H2O2 into the bath solution significantly increased the probability of BKCa single-channel openings recorded from cell-attached patches, an effect that was blocked by the PKG-Iα inhibitor DT-2. H2O2 exhibited an attenuated stimulatory effect on BKCa channel open probability in inside-out membrane patches.
Conclusions
H2O2 dilates HCAs through a novel mechanism involving protein dimerization and activation of PKG-Iα and subsequent opening of smooth muscle BKCa channels.
Keywords: endothelium-derived hyperpolarizing factor, hydrogen peroxide, protein kinase G, Ca2+-activated K+ channel, vasodilation
Introduction
Hydrogen peroxide (H2O2), a membrane permeable and relatively stable reactive oxygen species (ROS), has emerged as an important signaling molecule in the regulation of physiological and pathophysiological processes in vascular cells.1–3 Our recent studies indicate that, in human coronary arterioles (HCAs), H2O2 serves as a key endothelium-derived hyperpolarizing factor (EDHF) responsible for both flow-mediated dilation (FMD) and to a lesser extent agonist-induced dilation.4–6 As an EDHF, H2O2 elicits vasodilation through a mechanism involving smooth muscle Ca2+-activated K+ channel (KCa) activation and subsequent membrane hyperpolarization.6 Other studies have also demonstrated that H2O2 induces potent KCa-mediated smooth muscle hyperpolarization and relaxation in porcine coronary,7–9 human10 and mouse mesenteric arteries.11 Intriguingly, the role of H2O2 as a vasodilator factor in HCAs is more prominent in disease states [e.g., in coronary artery disease (CAD)], whereas other traditional factors [i.e., nitric oxide (NO) and prostacyclin (PGI2)] play a more important role in vasodilation in the absence of CAD or its risk factors.6 Despite extensive data demonstrating the importance of H2O2 in mediating smooth muscle hyperpolarization and dilation, the precise mechanisms of action of H2O2 as an EDHF remain poorly understood. The lack of detailed analysis on the mechanism of action of H2O2 has raised some concerns regarding the proposed role of H2O2 as an EDHF.12
Accumulating evidence indicates that a conserved mechanism by which H2O2 activates intracellular signaling in cells involves the oxidation of key cysteine residues in its target proteins.13 A more recent study suggests that, via this mechanism, H2O2 induces dimerization and subsequent activation of protein kinase G (PKG)-Iα in vascular smooth muscle cells.14 PKG-Iα is an important regulator of activity of various smooth muscle K+ channels, including the large-conductance KCa (BKCa) channel.15 We hypothesized that H2O2 induces relaxation of HCAs through direct oxidation and activation of PKG-Iα leading to the opening of BKCa channels and subsequent smooth muscle hyperpolarization. Using an integrated approach comprising isolated vascular reactivity measurement, immunohistochemical and molecular biological analysis, and patch-clamp technique, we performed in-depth analysis of cellular mechanisms responsible for H2O2-induced dilation in HCAs. Our results demonstrate that BKCa channel activation plays a key role in flow- and H2O2-induced dilation of HCAs. We also demonstrate that the opening of BKCa channels requires an intermediate signaling event involving H2O2-induced protein dimerization of PKG-Iα.
Methods
A detailed Methods section is available in the Online Supplement at http://circres.ahajournals.org.
Tissue Acquisition
Fresh human right atrial appendages were obtained as discarded surgical specimens from patients undergoing cardiopulmonary bypass procedures as described previously.6 Patient demographic data are summarized in Online Table I.
Cell Culture
Human coronary artery smooth muscle cells (HCASMCs) were obtained from Lonza (Walkersville, MD), and cultured in full growth medium according to the manufacturer’s instructions. Cells between passages 4 and 6 were used for experiments.
Isometric Tension Recording
Coronary arterioles (≈100–200 µm) were carefully dissected from the endocardial surface of the atria, and mounted in multi-chamber wire myograph (model 610M, Danish Myo Technology) as previously described.16,17 After contraction with endothelin-1, relaxation responses to cumulative concentrations of H2O2 (10−6–3×10−4 mol/L), spermine NONOate (10−7–3×10−5 mol/L), a NO donor, or 8-pCPT-cGMP (10−6–10−4 mol/L), a membrane-permeable analogue of guanosine 3’,5’-cyclic monophosphate (cGMP), were determined in the absence or presence of following inhibitors: iberiotoxin (IbTX; 100 nmol/L), a specific inhibitor of BKCa channels; catalase (1000 U/ml), a H2O2 metabolizing enzyme; ODQ (10 µmol/L), a selective inhibitor of soluble guanylate cyclase (sGC); Rp-8-Br-PET-cGMP (100 µmol/L), a competitive PKG inhibitor; or DT-2 (10 µmol/L), a specific, peptide inhibitor of PKG-Iα. To examine the role of smooth muscle hyperpolarization in H2O2-induced dilation, arteries were pre-constricted with high-K+ (80 mmol/L K+) Krebs (K-PSS). Vasodilator responses are expressed as percent maximal relaxation relative to endothelin-1 or K-PSS constriction, with 100% representing full relaxation to basal tension.
Videomicroscopy
Coronary arterioles (≈100–200 µm) were cannulated with 2 glass micropipettes, and the internal diameter of arterioles was measured with a video system.6,16 Vessels were constricted with endothelin-1 to 30–50% of the baseline internal diameter. In studies of flow-induced dilation, flow was produced by changing the heights of two syringe reservoirs in equal and opposite directions to generate a pressure gradient.6,16 Flow-mediated responses (5–100 cm H2O) were examined before and after 30 min incubation with ODQ (10 µmol/L), Rp-8-Br-PET-cGMP (100 µmol/L), or iberiotoxin (IbTX, 100 nmol/L). At the end of each experiment, papaverine (10−4 mol/L), an endothelium-independent vasodilator, was added to determine the maximal internal diameter for normalization of dilator responses.
Enzymatic Isolation of Vascular Cells
Vascular endothelial cells (ECs) and SMCs were enzymatically dissociated from arteries as previously described.16,18 Cells were placed on ice or at 4°C and used the same day.
Patch-Clamp Recording of K+ Currents
Single-channel K+ currents were recorded from cell-attached and excised inside-out membrane patches of freshly isolated coronary SMCs using the patch-clamp method as previously described.18 For both cell-attached and inside-out patches, the pipette solution contained (in mmol/L) 125 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA), and 5 HEPES (pH 7.2), and the bath solution contained (in mmol/L) 125 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 glucose, 10 EGTA, and 5 HEPES (pH 7.2). Channel currents were recorded for at least 3–6 min under control conditions and after treatment with H2O2 (10–100 µmol/L) in the absence or presence of paxilline (100 nmol/L, a specific and cell-permeable inhibitor of BKCa channels). Unless otherwise stated, all chemicals were applied to the bath through perfusion. To determine the role of PKG-Iα in H2O2-induced BKCa channel activation, cells were preincubated for 45–60 min with DT-2 (10 µmol/L) and the effect of H2O2 determined. Experiments were performed at room temperature.
RNA Extraction and RT-PCR
Total RNA from vascular tissues was extracted with TRIzol, and cDNA was synthesized. For freshly isolated vascular cells, RNA extraction was performed as previously reported.16 The cDNA was subjected to PCR amplification using a 38-cycle touch-down protocol with gene-specific primers.
Immunoblot Analysis
Protein samples (10–20 µg) were subjected to 10% SDS-PAGE. Membranes were blotted with a monoclonal mouse antibody specific to a conserved epitope of Slo1 (1:1000 dilution; NeuroMab L6/60 clone) or with a polyclonal rabbit anti-human PKG-I (1: 400 dilution; Abcam), followed by horseradish peroxidase-conjugated secondary antibodies.
Immunohistochemistry
Freshly dissected coronary arterioles were fixed with 10% formalin, embedded in paraffin wax, and cut into 4-µm sections. Sections were blocked for endogenous peroxidase and non-specific protein binding, and probed with a polyclonal rabbit antibody specific to human PKG-I (1:100 dilution; Abcam). For immunodetection, sections were incubated with a HRP-conjugated antibody, and then with a peroxidase-substrate solution.
Immunocytochemistry
Freshly isolated SMCs were fixed with 4% paraformaldehyde for 10 min, and then permeabilized with 0.1% saponin for 10 min. Cells were then incubated either with a polyclonal rabbit antibody specific to human PKG-I (1:50 dilution; Abcam), or a monoclonal mouse antibody specific to a conserved epitope of Slo1 (1:200 dilution; NeuroMab L6/60 clone), followed by an appropriate goat secondary antibody conjugated with Alexa Fluor 488 (Invitrogen). Images were immediately taken using an epi-fluorescence microscope (model Eclipse TE-200, Nikon) with a 60x (NA 1.40) oil objective.
Statistical Analysis
Data are presented as means ± SE. Significant differences between mean values were evaluated by Student t test or ANOVA followed by the Student-Newman-Keuls multiple-comparison test. To compare concentration-response between treatment groups, a two-way repeated measures ANOVA was used. P values of p<0.05 were considered statistically significant.
Results
Role of BKCa channels in H2O2-induced dilation of human coronary arterioles
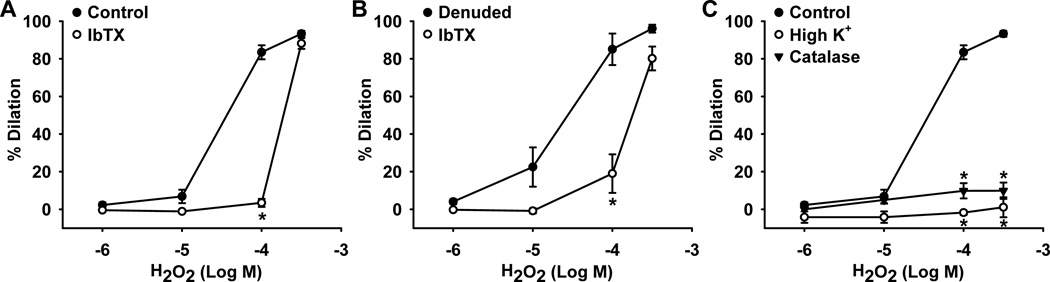
To examine the mechanism of H2O2-induced dilation in HCAs, graded doses of H2O2 (10−6–3×10−4 mol/L) were applied to endothelium-intact and -denuded HCAs. H2O2 induced similar concentration-dependent dilation of both endothelium-intact (Figure 1A; dilation at 10−4 mol/L, 84±4%, n=13) and -denuded (Figure 1B; 85±8%, n=4) HCAs, indicating an endothelium-independent smooth muscle mechanism of dilation. The dilation was markedly inhibited by the specific BKCa channel blocker iberiotoxin (100 nmol/L) in both endothelium-intact (Figure 1A; 3±2%, n=9, P<0.05 versus control) and -denuded (Figure 1B; 19±10%, n=4, P<0.05 versus control) HCAs, implicating BKCa channels as the smooth muscle end-effectors of H2O2-induced relaxation. Consistent with previous data from our laboratory,6 dilation to H2O2 was completely abolished by high K+ (1±5%, n=7, P<0.05 versus control) as well as by 1,000 U/ml exogenous catalase (10±4%, n=3, P<0.05 versus control), a H2O2-metabolizing enzyme, confirming that this relaxation response is elicited through a K+ channel end-effector and is a H2O2-sensitive effect (Figure 1C).
Figure 1. Role of BKCa channels in H2O2-mediated dilation of human coronary arterioles.
H2O2 induced similar dose-dependent dilation of both endothelium-intact (A) and -denuded (B) arterioles. These relaxations were inhibited by 100 nmol/L iberiotoxin (IbTX), a BKCa channel blocker, and eliminated by high K+ (80 mmol/L) and exogenous catalase (1,000 U/ml) (C). n=3–13 vessels/each group; *P<0.05 vs. control.
Role of sGC and PKG-Iα in H2O2- and NO-mediated dilation of human coronary arterioles
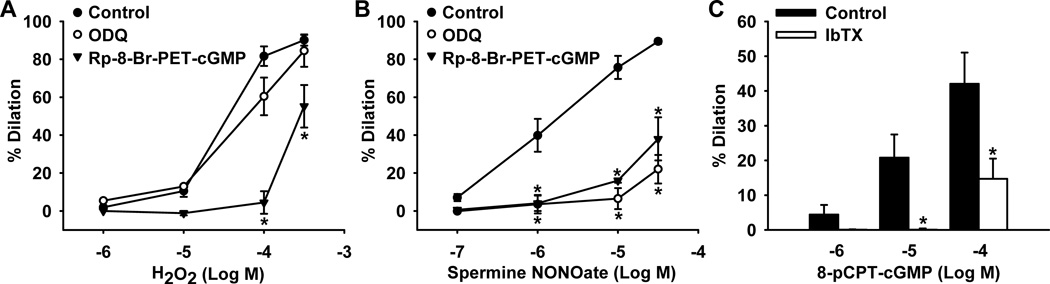
To further examine the mechanism by which H2O2 induces smooth muscle hyperpolarization through BKCa channel activation, the role of the sGC-cGMP-PKG pathway in H2O2- and NO-mediated dilation was investigated. As illustrated in Figure 2A, H2O2 dose-dependently dilated HCAs (dilation at 10−4 mol/L, 82±3%, n=15). The dilation was attenuated by the PKG inhibitor Rp-8-Br-PET-cGMP (100 µmol/L) (5±6%, n=6, P<0.05 versus control) but not by ODQ (10 µmol/L), an inhibitor of sGC (61±8, n=9), indicating that H2O2 acts downstream of sGC at the level of PKG. DT-2, a peptide inhibitor of PKG-Iα, further confirmed the downstream action of this transferrable dilator agent, as the H2O2 -induced relaxation (81±6%, n=3) was significantly reduced by administration of 10 µmol/L DT-2 (13±9%, n=3, P<0.05 versus control) (data not shown). In contrast, the NO donor spermine NONOate-induced relaxation (dilation at 10−5 mol/L, 76±6%, n=11) were inhibited by both Rp-8-Br-PET-cGMP (16±1%, n=4, P<0.05 versus control) and ODQ (7±6%, n=4, P<0.05 versus control), signifying NO action upstream of both sGC and PKG (Figure 2B). Further, direct PKG activation by the stable cell-permeable cGMP analogue 8-pCPT-cGMP (10−6–10−4 mol/L) dilated HCAs in a concentration-dependent manner (dilation at 10−4 mol/L, 42±9%, n=7) as shown in Figure 2C. This response was blocked by iberiotoxin (15±6%, n=5, P<0.05 versus control), confirming a role for BKCa channels in PKG-induced smooth muscle hyperpolarization and relaxation. As summarized in Online Table II, no significant effect on baseline vascular tone was observed for ODQ, Rp-8-Br-PET-cGMP, and iberiotoxin, and similar dilation to the smooth muscle relaxant papaverine (100 µmol/L) was found in arterioles with or without pretreatment with these inhibitors.
Figure 2. Role of guanylate cyclase and PKG in H2O2- and NO-mediated relaxation of human coronary arterioles.
A: H2O2-induced relaxation responses were inhibited by the PKG inhibitor Rp-8-Br-PET-cGMP (100 µmol/L) but not by the soluble guanylate cyclase inhibitor ODQ (10 µmol/L). B: The NO donor spermine NONOate-induced relaxations that were inhibited by both Rp-8-Br-PET-cGMP and ODQ. C: The stable cGMP analogue 8-pCPT-cGMP dose-dependently dilated coronary arterioles, a response inhibited by the BKCa channel blocker iberiotoxin (100 nmol/L). n=4–15 vessels/each group; *P<0.05 vs. control.
H2O2-induced protein dimerization of PKG-Iα in human coronary artery smooth muscle cells
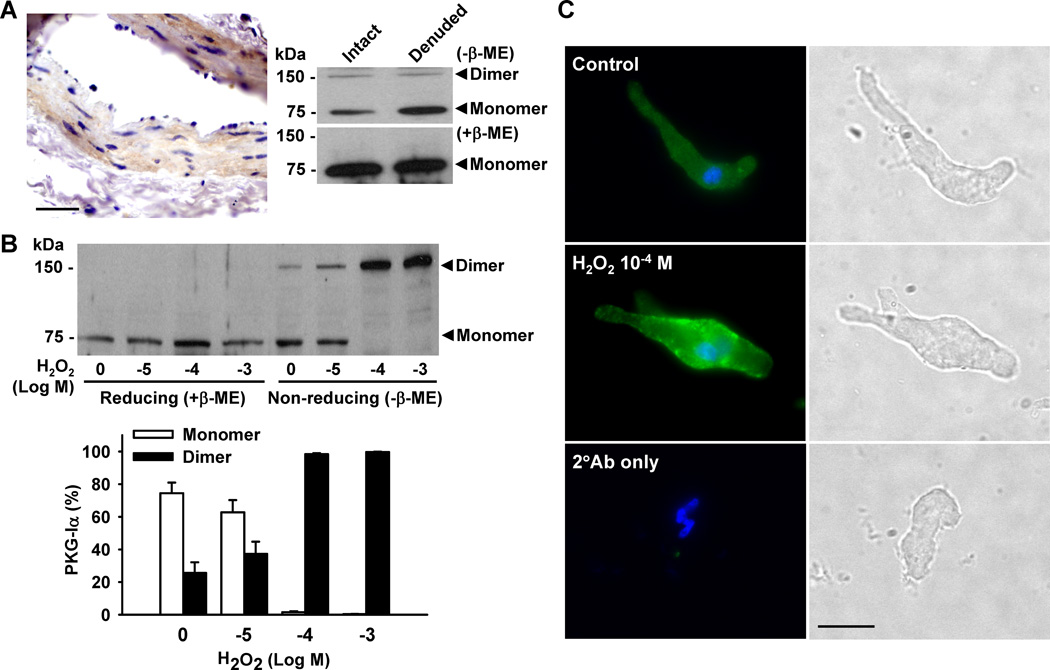
Recent evidence suggests that H2O2 directly activates the PKG isoform PKG-Iα but not PKG-Iβ by oxidizing a specific cysteine residue of PKG-Iα, leading to disulfide dimerization of this isoform.14 To elucidate the role of H2O2 in the dimerization and activation of PKG-Iα in HCAs, immunohistochemistry and Western blotting were used to assess protein expression of this kinase. Immunohistochemical analysis demonstrated expression of PKG-I protein in coronary arterioles, in particular within the smooth muscle cell layer (Figure 3A, left). This was further verified by Western blot, which revealed that under basal conditions, most of PKG-I exists as a 75 kDa monomer form (Figure 3A, right). Treatment of cultured coronary SMCs with H2O2 induced concentration-dependent dimerization of PKG-I, an effect which was abolished in the presence of the reducing agent β-mercaptoethanol. Given that only PKG-Iα undergoes disulfide dimerization in the presence of H2O2, these results indicate that PKG-I proteins detected in coronary SMCs are mostly the 1α isoform. Immunoblotting studies of cultured human vascular SMCs from multiple vascular beds using PKG-Iα- and PKG-Iβ-specific antibodies also revealed that PKG-Iα is the predominant isoform of PKG-I.19
Figure 3. H2O2-induced protein dimerization of PKG-I in human coronary artery smooth muscle cells.
A: The protein expression of PKG-I in HCAs was detected by immunohistochemical analysis (left panel; scale bar = 50 µm) and Western blot (right panel). Under basal conditions, PKG-I was primarily in monomeric form. B: Treatment of HCASMCs with H2O2 induced concentration-dependent dimerization of PKG-I which was blocked by the reducing agent β-mercaptoethanol (β-ME). Data are representative of 3 independent experiments. C: Immunofluorescence detected diffuse cytosolic expression of PKG-I under control conditions (upper). H2O2 (100 µmol/L) induced punctate expression of PKG-I along the plasma membrane (middle). Scale bar = 20 µm. Data are representative of 3 independent experiments with 5–10 cells/group/experiment.
Immunofluorescence was used to visualize the translocation of PKG-Iα in freshly isolated coronary artery smooth muscle cells. Diffuse cytosolic staining of PKG-Iα was observed under control conditions (Figure 3C, upper panel). Administration of 100 µmol/L H2O2 changed the staining pattern to one punctuated by expression along the plasma membrane (Figure 3C, middle panel), suggesting translocation of the protein from the cytosol to the plasma membrane upon activation by H2O2. Determination of plasma membrane/cytoplasmic ratio of PKG-Iα fluorescence confirmed the plasma membrane localization of this protein in H2O2-treated cells (1.30±0.04 versus 0.88±0.02 of control; P<0.05). The lack of signal in the preparations without primary antibodies (Figure 3C, lower) shows that this is an immunospecific response.
Expression of BKCa channels in coronary smooth muscle cells
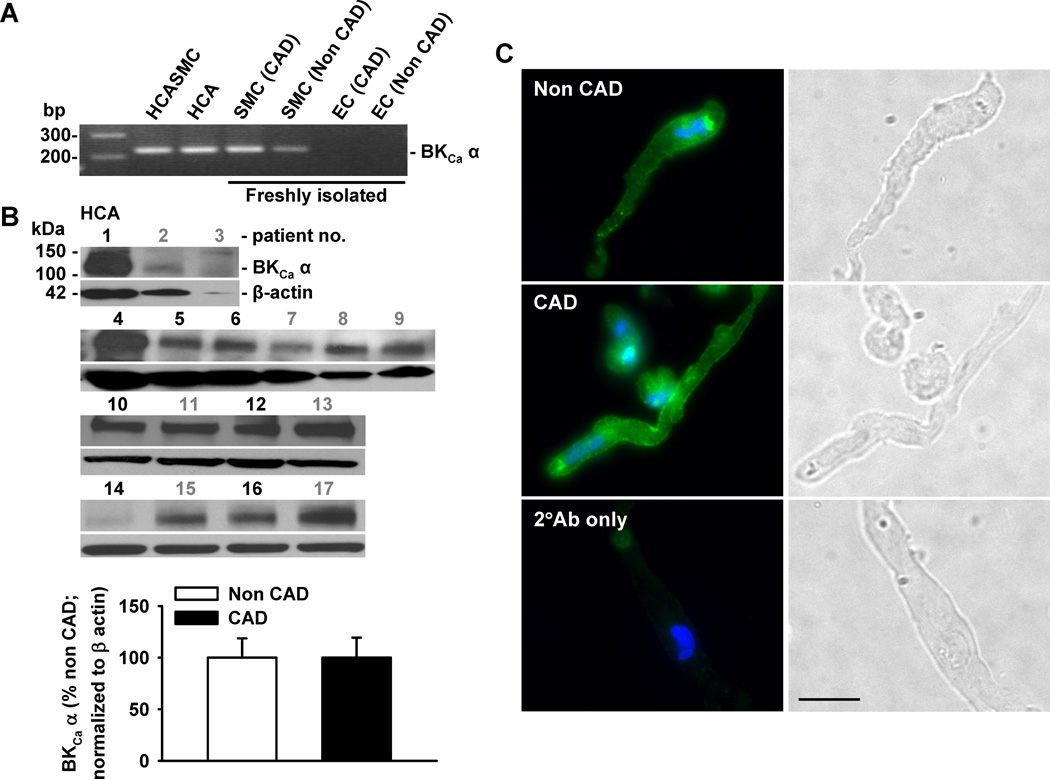
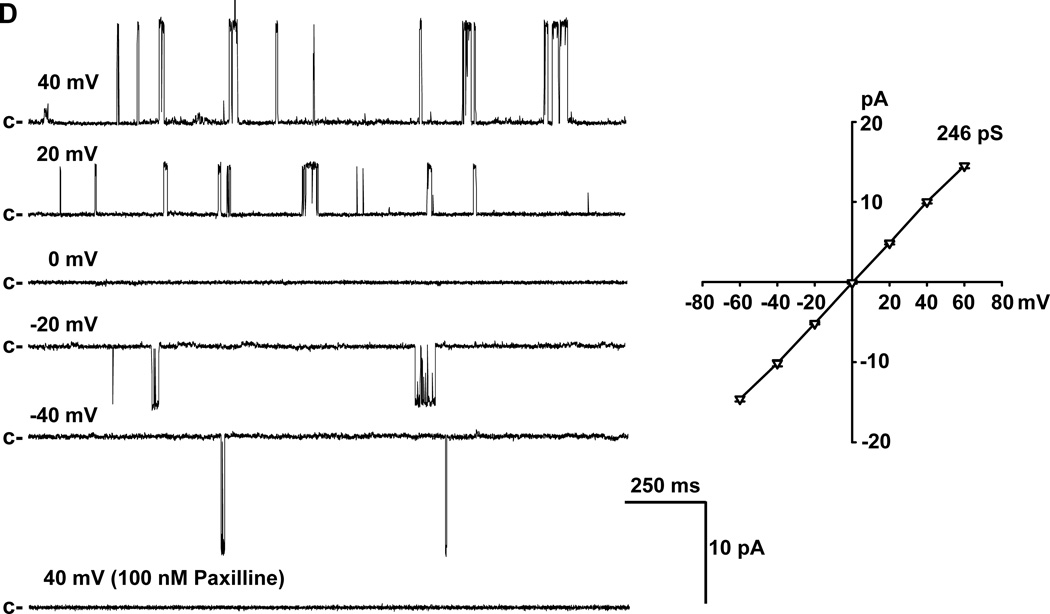
BKCa channel expression is known to be modulated by disease,20,21 but its expression in HCAs from patients with CAD is not clear. Therefore, the expression of BKCa channels in coronary SMCs of patients with and without CAD was examined. Expression of BKCa α-subunit was detected at both transcript (mRNA) (Figure 4A) and protein (Figure 4B) levels from patients with and without CAD. The average protein expression level of BKCa α-subunits showed no significant difference between patients with CAD and those without, although in 1–2 samples (out of 8) from CAD patients the level of BKCa channel expression was much higher than average. The expression of BKCa channels on the plasma membrane was further verified via immunofluorescence, which demonstrated BKCa channel immunoreactivity (in green) in smooth muscle cells of patients with CAD (Figure 4C, middle) or without CAD (Figure 4C, upper). Cell nuclei were stained in blue. Analysis of plasma membrane/cytoplasmic ratio of BKCa fluorescence showed a similar plasma membrane distribution of this protein in smooth muscle cells from patients with and without CAD (1.25±0.03 and 1.23±0.03, respectively; P>0.05). To examine the functional status of BKCa channels, single-channel activity and conductance were measured at different patch potentials using inside-out patches. As illustrated in Figure 4D (left), the channel open state probability (NPo) was enhanced with each sequential increase in patch potential. Channel openings were abolished by the addition to the bath of 100 nmol/L paxilline, a selective and cell-permeable BKCa channel blocker. The current-voltage relationship determined over the voltage range of −60 and +60 mV in 20-mV increments revealed a unitary conductance of 246 pS, and a reversal potential of 0 mV when recorded in symmetrical K+ (145 mmol/L) solutions (Figure 4D, right). These findings indicate that the 246-pS single-channel K+ currents recorded from HCA smooth muscle cell membranes display electrophysiological and pharmacological properties consistent with those of BKCa single-channel currents recorded from conduit coronary SMCs.22
Figure 4. Expression of BKCa channels in coronary smooth muscle cells.
A: Expression of BKCa channel α-subunits was detected at mRNA level in freshly isolated SMCs from patients with and without CAD, as indicated by a representative image of RT-PCR analysis. B: BKCa α-subunit protein was expressed in coronary arterioles from patients with (patient no. in black) and without CAD (patient no. in gray). Lower, summarized data; n=8 and 9 for CAD and non CAD, respectively. C: Presence of BKCa channel α-subunit protein (green) is confirmed with immunocytochemistry using freshly dispersed SMCs. Cell nuclei are stained in blue. Scale bar = 20 µm. Data are representative of 3 independent experiments with 5–10 cells/ group/ experiment. D: In inside-out patches of freshly isolated SMCs from human coronary arterioles, an increase in membrane potential enhanced BKCa channel open probability (left) that was abolished by 100 nmol/L paxilline, a specific BKCa channel inhibitor. The current-voltage relationship revealed a unitary conductance of 246 pS with a reversal potential of 0 mV in symmetrical (145 mmol/L) K+ solutions (right). c, closed state. n=4 patches.
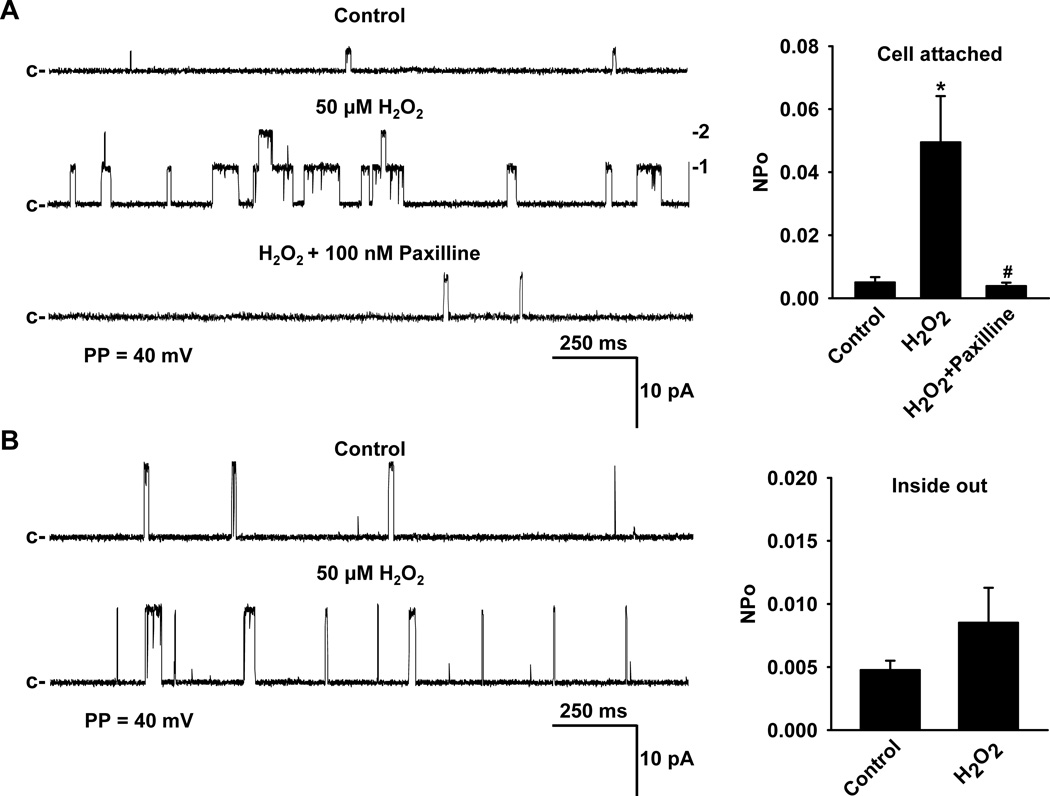
Effect of H2O2 on BKCa channel currents in isolated human coronary artery smooth muscle cells
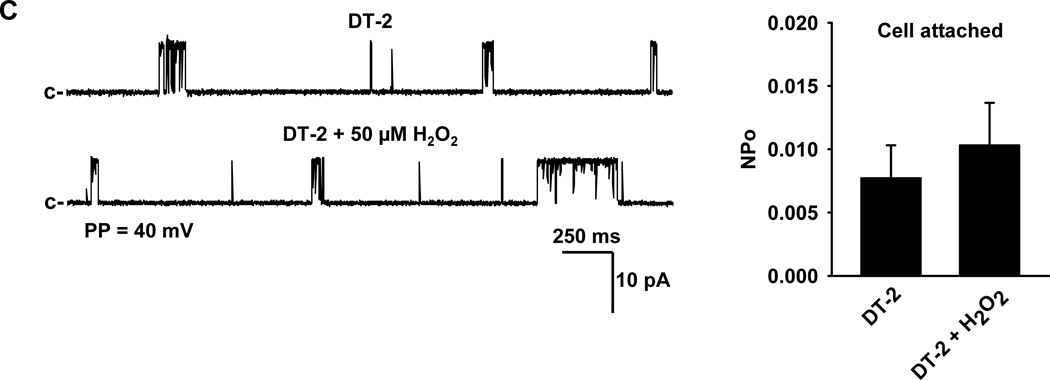
To investigate the activation of BKCa channel currents by exogenously applied H2O2, BKCa single-channel currents were recorded in cell-attached or inside-out membrane patches of freshly isolated smooth muscle cells of HCAs using the patch clamp technique. In cell-attached patches, addition of 50 µmol/L H2O2 to the bath induced activation of BKCa single-channel currents (NPo, 0.0050±0.0017 before versus 0.0495±0.0147 after H2O2; n=11, respectively; P<0.05) that was subsequently blocked by the addition of 100 nmol/L paxilline to the bath (NPo, 0.0039± 0.0011, n=10, P<0.05 versus H2O2) (Figure 5A), indicating that H2O2 activates smooth muscle BKCa channels. The activation of BKCa channels by H2O2 was concentration-dependent in the concentration range of 10 to 100 µmol/L (data not shown). Compared to cell-attached patches, the effect of H2O2 on BKCa single-channel opening in inside-out membrane patches was markedly reduced (NPo, 0.0048±0.0007 before and 0.0085±0.0028 after; n=12, respectively; P > 0.05). Together, these results suggest that H2O2 activation of BKCa channels requires an intracellular second messenger signaling pathway. To determine whether PKG participates as an intracellular signaling molecule in the H2O2-induced BKCa activation, the effects of H2O2 on BKCa single-channel currents were measured in cell-attached patches in the presence of 10 µmol/L DT-2, a PKG-Iα inhibitor. As demonstrated in Figure 5C, inhibition of PKG-Iα by DT-2 attenuated the increase in BKCa single-channel activity elicited by H2O2 (NPo, 0.0077± 0.0026 before versus 0.0103 ± 0.0034 after; n=10, respectively; P > 0.05) to a level similar to those observed in the inside-out membrane patches lacking intracellular signaling systems.
Figure 5. Effect of H2O2 on BKCa channel currents in freshly isolated coronary arteriolar smooth muscle cells.
Using cell-attached patches (A), H2O2 (50 µmol/L) increased BKCa single-channel currents in a paxilline (100 nmol/L)-sensitive fashion. However, H2O2-induced BKCa activation was greatly diminished in single-channel recordings from inside-out patches which lack intracellular constituents (B). H2O2-activated BKCa single-channel currents recorded from cell-attached patches were reduced in the presence of 10 µmol/L DT-2, a PKG-Iα inhibitor (C). c, closed state; PP, patch potential. n=6–12 patches/each group; *P<0.05 vs. control, #P<0.05 vs. H2O2.
Role of sGC, PKG, and BKCa channels in human coronary arteriolar relaxation to flow
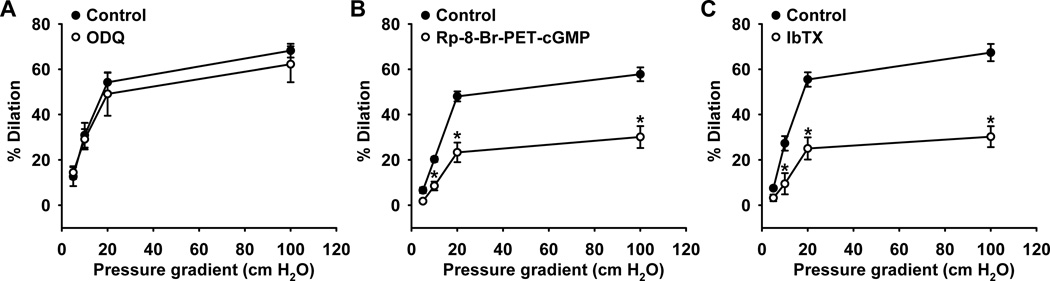
To verify the functional significance of the proposed signaling pathway, the roles of guanylate cyclase, PKG, and BKCa in FMD was examined. H2O2 has been demonstrated to be a key mediator of FMD in HCAs.4,6 Pretreatment with the sGC inhibitor ODQ had no effect on FMD (dilation at 100-cm H2O gradient, 62±8% versus 68±3% of control; n=3, respectively; P > 0.05) indicating no role for sGC (Figure 6A). In contrast, inhibition of PKG by Rp-8-Br-PET-cGMP significantly attenuated flow-mediated dilation (30±5% versus 58±3% of control; n=5, respectively; P<0.05), suggesting the involvement of downstream PKG in the relaxation response to increased flow. Furthermore, flow-induced dilation was significantly impaired in the presence of iberiotoxin (30±5% versus 67±4% of control; n=6, respectively; P<0.05), implicating a role for BKCa channel activity as an initiating mechanism of smooth muscle hyperpolarization and dilation. Treatment of arterioles with ODQ, Rp-8-Br-PET-cGMP and iberiotoxin did not significantly affect baseline vessel diameters (Online Table II).
Figure 6. Role of guanylate cyclase, PKG, and BKCa channels in human coronary arteriolar relaxation to flow.
Fluid flow induced dilation of human coronary arterioles was attenuated by the PKG inhibitor Rp-8-Br-PET-cGMP (B) and by the BKCa channel blocker iberiotoxin (C). The guanylate cyclase inhibitor ODQ, had no effect (A). n=3-6 vessels/each group; *P<0.05 vs. control.
Discussion
This study uncovers a novel mechanism of H2O2-mediated smooth muscle hyperpolarization and dilation in the human coronary microcirculation. The major new findings are threefold. First, exogenous H2O2-induced dilation of HCAs results from the opening of smooth muscle BKCa channels. Second, the opening of BKCa channels requires intracellular signaling through H2O2-induced dimerization and translocation of PKG-Iα. Third, shear stress, which releases endogenous endothelium-derived H2O2, dilates HCAs by activating a similar signaling pathway as exogenous H2O2. In sum, these data indicate that H2O2-induced protein dimerization and activation of PKG-Iα and subsequent opening of BKCa channels serves as an important mechanism responsible for H2O2-mediated dilation of HCAs. The findings of this study provide further support for the proposed role of H2O2 as a diffusible EDHF in the human coronary microvessels4 and may apply to other coronary stimuli that induce a H2O2-mediated dilation, such as metabolic coronary dilation.23
PKG in H2O2-induced dilation
The role of cGMP as a second messenger for NO-induced vasodilation has been well established.24 This mediator is synthesized in SMCs by sGC, a cytosolic enzyme activated by NO donors and endogenous NO released from ECs in response to vasodilators such as acetylcholine and bradykinin. Once generated, cGMP activates its downstream target PKG (type Iα and Iβ) in SMCs, followed by the initiation of a variety of cellular processes leading to smooth muscle relaxation.25 Consistent with this general mechanism, both sGC and PKG inhibitors markedly reduced NO-mediated dilation in HCAs. In contrast, inhibition of PKG (specifically PKG-Iα) but not sGC markedly reduced H2O2-mediated dilation in HCAs, indicating that H2O2 acts downstream of sGC signaling and at the level of PKG-Iα. Such lack of inhibition by the sGC inhibitor ODQ has also been reported previously by our laboratory.6
The PKG-Iα and -Iβ are two splice-variant isoforms of PKG, differing in their N-terminus by ~100 amino acids. However, only the Iα isoform is redox sensitive and activated by H2O2 through disulfide dimerization.14 The activation of different targets within the cGMP-PKG pathway by H2O2 and NO is further supported by the findings that H2O2-mediated dilation was abolished by high K+, whereas NO-mediated dilation was only partially inhibited by high K+ (data not shown). It is generally agreed that PKG-I mediates smooth muscle relaxation through both Ca2+-dependent (e.g., K+ channel activation) and Ca2+-independent (e.g., changes in the activity of myosin light chain phosphatase) mechanisms, but two PKG-I isoforms may differ in their specific mechanisms of relaxation.25 Therefore, it is possible that the selective activation of PKG-Iα but not PKG-Iβ contributes, at least partially, to H2O2-induced high K+-sensitive dilation. It is also interesting to note that H2O2 activates PKG-Iα through a marked (>10-fold) increase in the affinity (indicated by a decrease in Km) for substrate, whereas cGMP activates PKG-Iα primarily by increasing its maximum velocity (Vmax) without changes in the Km for substrate.14 Thus, an increase in PKG-Iα affinity for selected substrates (e.g., K+ channels) may serve as another potential mechanism underlying high K+-sensitive dilation to H2O2. The (patho)physiological significance of the specific signaling initiated by H2O2 versus NO in vascular smooth muscle remains to be clarified.
It has been well demonstrated that H2O2 relaxes bovine pulmonary and coronary arteries primarily through sGC activation.26–28 The mechanism by which H2O2 stimulates sGC activity has been associated with the formation of compound I, a short-lived oxidized intermediate form of catalase that occurs during the metabolism of H2O2 by this enzyme.26 Pretreatment of the reaction mixture with the catalase inhibitor 3-amino-1,2,4-triazole (3-AT) reduces the stimulatory effect of catalase in the presence of H2O2 on sGC activity, further suggesting the role of catalase in H2O2-induced activation of sGC.26 Despite the stimulatory effect on sGC by H2O2, H2O2-induced relaxation is resistant to the sGC inhibitor ODQ in both pulmonary and coronary arteries.27 Therefore, the possible contribution of sGC in H2O2-induced dilation of human coronary arterioles can not be completely excluded. However, the effectiveness of ODQ as an inhibitor of sGC activation by H2O2 may depend on vascular beds studied; for example, ODQ inhibits a portion of H2O2-induced dilation in porcine coronary arteries.9 In addition, 3-AT does not affect H2O2-induced dilation in HCAs (authors’ unpublished observations), indicating that catalase or its derived compound I is not involved in the vasodilatory responses of H2O2.
K+ channels in H2O2-induced dilation
There is substantial evidence that H2O2 applied exogenously produces membrane hyperpolarization and relaxation of smooth muscle in several vascular beds.4–11,29–32 However, disparate results have been reported for the specific K+ channel(s) involved in H2O2-induced hyperpolarization or dilation, and the signaling cascade leading to K+ channel activation remains largely unexplored. Activation of ATP-sensitive K+ (KATP) channels has been implicated in H2O2-mediated dilation of porcine and cat cerebral arteries.29,30 A number of studies indicate that BKCa channels play an important role in H2O2-induced dilation of porcine coronary arteries and arterioles,7–9 whereas other studies show that voltage-gated K+ (Kv) channels but not BKCa channels contribute to the dilatory effect of H2O2 in canine and rat coronary arterioles.31,32 By measuring both isolated vascular reactivity and single K+-channel activity, we demonstrate that H2O2-induced dilation of human coronary arterioles depends on increased opening of BKCa channels. In particular, our patch-clamp studies showed that H2O2 activated BKCa currents recorded from the cell-attached patches (intracellular components present) but not from inside-out patches (intracellular components absent) and that the stimulatory effect of H2O2 on BKCa activity was markedly reduced by the PKG-Iα inhibitor DT-2. Taken together, these data provided strong evidence that H2O2-induced BKCa channel opening requires intracellular signaling, i.e. the activation of PKG-Iα in smooth muscle cells.
Several recent studies indicate that BKCa channels are under complex but coordinated regulation by a variety of protein kinases, including protein kinase C (PKC), protein kinase A (PKA), and PKG, as well as by protein phosphatases including protein phosphatase 1.33–35 Specifically, phosphorylation of S1151 by PKC during receptor agonist stimulation renders the channel responsive to PKG but prevents its activation by PKA, whereas PKC phosphorylation of both S1151 and S695 renders the channel insensitive to PKG and PKA. PKG sensitivity can be rescued by protein phosphatase 1, a BKCa-associated protein that is constitutively active against phosphorylation of S695. The balance between protein kinases and protein phosphatases may vary in different smooth muscle preparations, contributing to the sensitivity of BKCa channels and potentially other K+ channels to PKG and activators such as H2O2.
There are rather extensive data from experimental animal models indicating that different diseases can have divergent effects on the expression and/or channel activity of the vascular smooth muscle BKCa channel.20 For example, an upregulation of BKCa channel expression associated with an increased K+ current and vasodilation has been reported in SHR and DOCA-salt-induced hypertensive rats.20 In contrast, a loss of BKCa-mediated dilation with an unexpected increase in the expression of BKCa channel subunits has been described in the coronary microcirculation using a swine model of metabolic syndrome.21 In the present study, we found that there is no significant difference in the protein expression level of coronary smooth muscle BKCa α-subunits between patients with CAD and those without, and more importantly, the BKCa channel is functional and mediates the dilation of coronary arterioles to H2O2 and flow in disease. Thus, our data further underscore the complexity of BKCa channel regulation by cardiovascular pathologies.20
It remains to be determined whether flow- and H2O2-induced activation of BKCa channels in HCAs represents a compensatory pathway for the loss or impaired function of other K+ channels in the presence of CAD and other risk factors. As discussed above, H2O2-induced dilation is mediated by Kv channels in the coronary microcirculation of dogs and rats.31,32 Although the involvement of different K+ channels in H2O2-induced dilation in the human versus animals may simply reflect species-specific differences, there is evidence that Kv channels may be importantly involved in H2O2-induced dilation in relatively healthy subjects without preexisting CAD or many other cardiovascular disorders (author’s unpublished observations). Because functional BKCa channels are present in subjects both with and without CAD and risk factors, the activation of BKCa channel-mediated dilation in CAD may result from the modulation of signaling pathways (e.g. protein phosphorylation) rather than from the change of the number of functional BKCa channels.
Interestingly, when applied to the cytosolic side of an excised membrane patch in human embryonic kidney (HEK)-293 cells overexpressing the BKCa α-subunit, H2O2 inhibits BKCa channel currents by oxidizing the cysteine residues on the α- subunit.36–38 In the present study, H2O2 had no inhibitory effect on BKCa channel currents in inside-out patches. The reasons for these discrepancies remain unclear but could be related to variation of channels in native cells versus over-expression systems or the H2O2 concentrations (50 µmol/L used in the current study, and < 1 mmol/L H2O2 used in others).36–38 In HCAs, the dilation induced by the highest concentration of H2O2 (300 µmol/L) was blocked by high K+ but not affected by the BKCa channel blocker iberiotoxin, indicating the possible involvement of other K+ channels. The identities of such K+ channels remain to be determined.
In patch-clamp studies, we observed that, in some cell-attached patches, BKCa channels exhibit smaller single-channel conductance (e.g., 100–200 pS) even with high-K+ bath solution to nullify effects of cell resting membrane potential. Our results are comparable to those obtained in smooth muscle cells from large human coronary arteries.22 The reasons for the reduced conductance are unclear. Because the K+ currents recorded in cell-attached patches are sensitive to the specific BKCa blocker paxilline and the conductance of these currents increases to approximately 240–250 pS once the patch is detached from the cell to form an inside-out patch, we suggest that the properties of the currents studied are most consistent with those of BKCa channels.
Study limitations
Exogenous H2O2 has been widely used to study the role of H2O2 in cell signaling.2 In the present study, EC50 (the concentration of drug that produces a 50% maximal response) of exogenous H2O2-induced dilation in HCAs is approximately 3×10−5 mol/L, with a minimum concentration required to induce a catalase-sensitive vasodilation of approximately 10−5 mol/L. These concentrations, similar to or lower than those used in other studies,7–11,26–32 are higher than those reported for endogenously generated H2O2 (<10−6 mol/L) by physiological stimuli such as shear stress4 and A23187.39 This could be explained by the fact that the intracellular H2O2 may only reach 1–15% of the exogenously applied concentration,2 probably due to endogenous anti-oxidant systems that protect cells from ROS. It is also possible that H2O2 is generated locally at higher concentrations than those measured, before it diffuses to the active site.
Using immunoblotting and immunocytochemistry, we found that H2O2 induced PKG-Iα dimerization and translocation to the plasma membrane in coronary SMCs. The dimerization results in the activation of PKG-Iα associated with a decrease in the enzyme’s Km value.14 It is presumed, though not directly tested, that H2O2-induced dimerization of PKG-Iα contributes to the opening of BKCa channels and smooth muscle dilation in HCAs. Because there are no specific pharmacological agents available to inhibit the dimerization of PKG-Iα by H2O2, testing this hypothesis would require genetic manipulation of cysteine residues of PKG-Iα,14 an approach not feasible for isolated human blood vessels. Similarly, we did not directly assess PKG-Iα-mediated protein phosphorylation of BKCa channels, the result of which could provide further support for the activation of BKCa by dimerized PKG-Iα. Since specific antibodies for PKG-targeted residue in BKCa (serine 1072) are not available, the results obtained with a general anti-phosphoserine antibody may be difficult to interpret because other protein kinases (such as PKC) also phosphorylate serine residues of the BKCa channel.
Clinical implications
Flow-induced dilation of coronary arterioles from patients with CAD is mediated by a unique mechanism requiring the release of H2O2 from endothelial cells and subsequent smooth muscle hyperpolarization.4–6 In the absence of CAD or its risk factors, other traditional mediators (i.e., NO and PGI2) may play a more prominent role in dilation of coronary arterioles.6 In the present study, we provide evidence that the dimerization of PKG-Iα and subsequent activation of BKCa channels are two key intracellular signaling events responsible for H2O2-induced smooth muscle hyperpolarization and relaxation, thus revealing a fundamental signaling mechanism responsible for the role of H2O2 in regulation of coronary vascular tone and blood flow. With near maximal cardiac extraction of oxygen at rest, it is essential that myocardial perfusion is closely coupled to increases in myocyte metabolism.23 By serving as a potent vasodilator in the coronary microcirculation, H2O2, derived from the blood vessel itself as well as from beating myocytes, may be an important endogenous regulator of myocardial perfusion under normal conditions and in disease states.23
Novelty and Significance.
What Is Known?
Hydrogen peroxide (H2O2) is a relatively stable reactive oxygen species (ROS) that serves as a signaling molecule in diverse physiological and pathophysiological responses.
H2O2 is a key transferable endothelium-derived mediator responsible for flow-induced dilation and to a lesser extent receptor agonist-induced dilation in the human coronary microcirculation, particularly in patients with or at risk for coronary artery disease.
H2O2 dilates blood vessels through the activation of smooth muscle K+ channels, including the large-conductance Ca2+-activated K+ (BKCa) channel.
What New Information Does This Article Contribute?
Exogenous H2O2 opens arterial smooth muscle BKCa channels resulting in dilation of human coronary arterioles.
The mechanism by which H2O2 opens BKCa channels involves dimerization and translocation of protein kinase G (PKG)-Iα.
A similar signaling pathway, involving BKCa activation via PKG-Iα, mediates flow-induced and H2O2-dependent dilation of human coronary arterioles.
Accumulating evidence suggests that H2O2 serves as an important signaling molecule in the vascular system, although it is traditionally viewed as a harmful by-product of cell metabolism. Endothelial H2O2, is the key diffusible factor mediating flow-induced (and to a lesser extent bradykinin-induced) dilation in the human coronary microcirculation. However, the precise mechanisms by which H2O2 relaxes coronary arterioles remain unclear. In this study, we found that exogenous H2O2 dilates human coronary arterioles by opening smooth muscle BKCa channels through dimerization and translocation of PKG-Iα. Flow or shear stress, which releases endothelium-derived H2O2, dilates coronary arterioles by activating a signaling pathway similar to that induced by exogenous H2O2. Our findings provide further support for the proposed role of H2O2 as a diffusible endothelium-derived hyperpolarizing factor in both animals and humans. and have implications for antioxidant treatment strategies in patients with coronary disease.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Kathryn M. Gauthier for expert assistance with patch clamping. We also thank the Division of Cardiothoracic Surgery at the Medical College of Wisconsin and the VA Medical Center, the Cardiothoracic Surgery Group of Milwaukee, the Cardiovascular Surgery Associates of Milwaukee, the Midwest Heart Surgery Institute, the Wisconsin Heart Group, Froedtert Memorial Lutheran Hospital, and the Aurora St. Luke’s Medical Center in Milwaukee for providing surgical specimens.
Sources of Funding
This work was supported by the National Heat, Lung, and Blood Institute at the National Institutes of Health [R01 HL080704 and R01 HL094971 to D.D.G., R01 HL096647 to D.X.Z.]; and the American Heart Association [SDG 0830042N to D.X.Z.].
Non-standard abbreviations and acronyms
- cAMP
adenosine 3',5'-cyclic monophosphate
- 3-AT
3-amino-1,2,4-triazole
- cGMP
guanosine 3’,5’-cyclic monophosphate
- BKCa channel
large conductance Ca2+-activated K+ channel
- CAD
coronary artery disease
- EC
endothelial cell
- EDHF
endothelium-derived hyperpolarizing factor
- FMD
flow-mediated dilation
- HCA
human coronary arteriole
- HCASMC
human coronary artery smooth muscle cell
- H2O2
hydrogen peroxide
- IbTX
iberiotoxin
- KATP channel
ATP-sensitive K+ channel
- KCa channel
Ca2+-activated K+ channel
- Kv channel
voltage-gated K+ channel
- L-NAME
NG-nitro-L-arginine methyl ester
- NPo
open state probability
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PKA
protein kinase A or cAMP-dependent protein kinase
- PKC
protein kinase C
- PKG
protein kinase G or cGMP-dependent protein kinase
- PSS
physiological salt solution
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SMC
smooth muscle cell
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Perez-Vizcaino F, Cogolludo A, Moreno L. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir Physiol Neurobiol. 2010;174:212–220. doi: 10.1016/j.resp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Schröder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 7.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and - independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 8.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 9.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- 10.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 11.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 15.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. ACh-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K+ Am J Physiol Heart Circ Physiol. 2007;293:H152–H159. doi: 10.1152/ajpheart.00268.2006. [DOI] [PubMed] [Google Scholar]
- 18.Li PL, Zhang DX, Ge ZD, Campbell WB. Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H1229–H1236. doi: 10.1152/ajpheart.00736.2001. [DOI] [PubMed] [Google Scholar]
- 19.Christensen EN, Mendelsohn ME. Cyclic GMP-dependent protein kinase Ialpha inhibits thrombin receptor-mediated calcium mobilization in vascular smooth muscle cells. J Biol Chem. 2006;281:8409–8416. doi: 10.1074/jbc.M512770200. [DOI] [PubMed] [Google Scholar]
- 20.Rusch NJ. BK channels in cardiovascular disease: a complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297:H1580–H1582. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- 21.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canty JM, Jr, Iyer VS. Hydrogen peroxide and metabolic coronary flow regulation. J Am Coll Cardiol. 2007;50:1279–1281. doi: 10.1016/j.jacc.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Palmer RJM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 25.Surks HK. cGMP-dependent protein kinase I and smooth muscle relaxation: a tale of two isoforms. Circ Res. 2007;101:1078–1080. doi: 10.1161/CIRCRESAHA.107.165779. [DOI] [PubMed] [Google Scholar]
- 26.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 27.Iesaki T, Gupte SA, Kaminski PM, Wolin MS. Inhibition of guanylate cyclase stimulation by NO and bovine arterial relaxation to peroxynitrite and H2O2. Am J Physiol Heart Circ Physiol. 1999;277:H978–H985. doi: 10.1152/ajpheart.1999.277.3.H978. [DOI] [PubMed] [Google Scholar]
- 28.Neo BH, Kandhi S, Wolin MS. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2010;299:H1235–H1241. doi: 10.1152/ajpheart.00513.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- 30.Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol. 2002;283:H406–H411. doi: 10.1152/ajpheart.00007.2002. [DOI] [PubMed] [Google Scholar]
- 31.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 32.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 33.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 34.Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem. 2001;276:43239–43245. doi: 10.1074/jbc.M104202200. [DOI] [PubMed] [Google Scholar]
- 35.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2010;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 37.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. Circ Res. 2006;99:607–616. doi: 10.1161/01.RES.0000243147.41792.93. [DOI] [PubMed] [Google Scholar]
- 39.Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Lüscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest. 1998;101:1530–1537. doi: 10.1172/JCI650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.