Abstract
Notch signaling mediates cell to cell interactions that are critical for embryonic development and tissue renewal. In the canonical signaling pathway, the Notch receptor is cleaved following ligand binding, resulting in the release and nuclear translocation of the Notch intracellular domain (NICD). NICD induces gene expression by forming a ternary complex with the DNA binding protein CBF1/Rbp-Jk, Suppressor of Hairless, Lag1 (CSL) and Mastermind-Like (Maml). Hairy Enhancer of Split (Hes) and Hes related with YRPW motif (Hey) are classic Notch target genes. Notch canonical signaling plays a central role in skeletal development and bone remodeling by suppressing the differentiation of skeletal cells. The skeletal phenotype of mice misexpressing Hes1 phenocopies partially the effects of Notch misexpression, suggesting that Hey proteins mediate most of the skeletal effects of Notch. Dysregulation of Notch signaling is associated with diseases affecting human skeletal development, such as Alagille Syndrome, Brachydactyly and Spondylocostal Dysostosis. Somatic mutations in Notch receptors and ligands are found in tumors of the skeletal system. Overexpression of NOTCH1 is associated with osteosarcoma, and overexpression of NOTCH3 or JAGGED1 in breast cancer cells favors the formation of osteolytic bone metastasis. Activating mutations in NOTCH2 cause Hajdu Cheney syndrome, which is characterized by skeletal defects and fractures, and JAG1 polymorphisms are associated with variations in bone mineral density. In conclusion, Notch is a regulator of skeletal development and bone remodeling and abnormal Notch signaling is associated with developmental and postnatal skeletal disorders.
Endochondral bone formation is the developmental process that generates the appendicular skeleton and selected elements of the axial skeleton. Skeletal elements that form through this mechanism are preceded by a template of hyaline cartilage, which arises from the condensation and chondrogenic differentiation of mesenchymal cells. Chondrocytes in the hyaline cartilage proliferate, undergo hypertrophic differentiation, and deposit a mineralized matrix before becoming apoptotic. This sequence of events generates a calcified cartilage scaffold that is vascularized and colonized by skeletal precursor cells, which replace the hyaline cartilage with bone [38]. Following completion of endochondral ossification, bone is modeled during growth by bone resorbing and bone forming cells. Adult bone is remodeled by the coordinated activity of osteoblasts, the bone matrix producing cells, and osteoclasts, the multinucleated bone matrix resorbing cells [5]. Skeletal development and bone remodeling are regulated by a network of signaling pathways, and Notch has emerged as a key component of these networks.
Notch Canonical Signaling
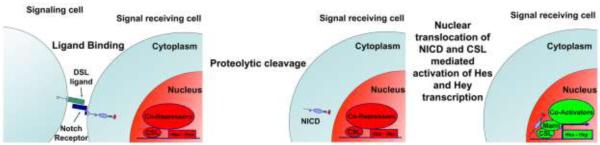
The four receptors termed Notch1 to 4, and five Delta/Serrate/Lag-2 (DSL) ligands named Jagged (Jag) 1 and 2, and Delta-like (Dll) 1, 3 and 4, are single-pass transmembrane proteins that mediate cell to cell interactions. Additional proteins able to bind to Notch receptors have been identified, but whether they activate Notch signaling in mammalian cells is not known [7]. Following binding to DSL ligands, Notch undergoes a series of proteolytic cleavages, which lead to the release of the Notch intracellular domain (NICD) in the cytoplasm. The γ-secretase complex contains the proteases Presenilin1 and Presenilin2, which are critical for the cleavage of the transmembrane portion of Notch and for activation of signaling. NICD translocates to the nucleus and forms a protein complex with Epstein-Barr virus latency C promoter binding factor (CBF)1 / Suppressor of Hairless / Lag1 (CSL), also known as RBP-Jκ in mice, and with Mastermind-Like (Maml). CSL or RBP-Jκ allows binding of the active transcriptional complex to DNA [23] (Fig.1). Proper duration of the Notch signal is ensured by the proline (P)-, glutamic acid (E)-, serine (S)-, threonine (T)-rich (PEST) motif, which is located at the C terminus of the receptor and it is necessary for the proteasomal degradation of the NICD [14;23]. Hairy Enhancer of Split (Hes), and Hes related with YRPW motif (Hey) transcription factors are established targets of Notch signaling. Seven Hes proteins (Hes1 to 7) and three Hey proteins (Hey1, Hey2, and HeyL) have been identified, and all but Hes2 and Hes3 are targets of Notch canonical signaling [20]. NICD may interact with cytosolic proteins and regulate gene expression in a CSL/ RBP-Jκ independent manner [17]. Although a role for CSL/RBP-Jκ independent Notch signaling in the regulation of osteoblast function and chondrogenesis is not apparent, it is not clear whether this is also the case for osteoclastogenesis [1;9;53].
Figure 1.
Transactivation of Notch canonical signaling. Under unstimulated conditions, co-repressors of transcription are bound to Epstein-Barr virus latency C promoter binding factor (CBF)1 / Suppressor of Hairless / Lag1 (CSL)/ RBP-Jκ and recruit suppressors of transcription. Upon binding to Delta/Serrate/Lag-2 (DSL) ligands the Notch receptor is cleaved, resulting in the release of the Notch intracellular domain (NICD). The NICD translocates to the nucleus and forms a ternary complex with CSL and Mastermind-Like (MamL). This complex displaces the transcriptional co-repressors, resulting in recruitment of activator of transcription and expression of Notch target genes, such as Hairy Enhancer of Split (Hes) and Hes related with YRPW motif (Hey).
Notch1 and Notch2 are necessary for embryonic survival and their global inactivation results in embryonic or perinatal mortality [32;52]. Notch3 is structurally similar to Notch1 and Notch2 ; Notch3 null mice are viable, and induction of Notch3 causes a phenotype reminiscent of the cerebral autosomal-dominant arteriopathy with subcortical infarcts (CADASIL) human syndrome [2;37]. Notch4 plays a role in embryonic vascular morphogenesis, but is dispensable for development since its function can be carried out by Notch1 [25]. Notch1, 2 and 3 are expressed by skeletal cells. With the exception of Dll3, individual inactivation of DSL ligands in mice is developmentally lethal, indicating that each DSL ligand has unique functions required for survival [7]. Dll3 null mice are viable and present vertebral and rib deformities secondary to patterning defects during development [7].
Notch Suppresses Skeletal Development and Chondrogenesis
Notch signaling in vitro and mouse models where Notch is misexpressed in the limb bud reveal a suppressive role in chondrogenesis [30;60]. Inactivation of Presenilin1 and Presenilin2 in the limb bud or conditional deletion of Notch1 and Notch2 cause skeletal malformations due to enhanced hypertrophic chondrocyte differentiation. This phenotype is recapitulated by the conditional ablation of Notch2, suggesting that the inhibitory effects of Notch on skeletal development are mediated by Notch2 [18]. In accordance with these findings, conditional overexpression of NICD in the limb bud impairs endochondral ossification due to suppressed chondrocyte differentiation. Deletion of Csl/Rbpjκ in chondrocytes enhances proliferation, and reverses the effects of Notch in limb buds, demonstrating that Notch regulates chondrogenesis acting through the canonical pathway [9;35]. NICD overexpression governed by the Collagen Type 2 α1 (Col2a1) promoter suppresses chondrocyte proliferation, confirming an inhibitory role of Notch in chondrogenesis The inactivation of Hes1 directed by the Paired-related homeobox (Prx)1 enhancer increases femoral length and trabecular number, suggesting that selected effects of Notch during limb bud development are mediated by Hes1 [61].
Notch Suppresses Osteoblast Differentiation and Function
During post-natal life, osteoblasts arise from mesenchymal precursors that reside in the bone microenvironment [4]. Although Notch may act either as a suppressor or inducer of osteoblast differentiation in vitro, studies in transgenic murine models established that activation of Notch signaling arrests commitment of pluripotent precursors to the osteoblastic lineage and suppresses osteoblast differentiation [60]. Conditional inactivation of Notch1;Notch2 or of Csl/Rbpjκ under the control of the Prx1 enhancer depletes the bone marrow from osteoblast progenitors [18]. Accordingly, NICD overexpression directed by the Prx1 enhancer induces proliferation and suppresses the differentiation of mesenchymal precursor cells in the adult mouse. Concurrent inactivation of Csl/Rbpjκ restores normal osteoblastic differentiation, demonstrating that Notch canonical signaling suppresses the commitment of mesenchymal cells to the osteoblastic lineage [9]. NICD overexpression controlled by a 3.6 kilobase (kb) fragment of the Collagen Type 1 α1 (Col1a1) promoter, which is expressed during the early stages of osteoblast differentiation, causes osteopenia due to decreased number of osteoblasts, confirming an inhibitory effect of Notch on osteoblast differentiation [63]. Overexpression of NICD under the control of a 2.3 kb fragment of the Col1a1 promoter, which is active in mature osteoblasts, causes an increase in osteoblasts synthesizing woven bone, possibly because impaired terminal osteoblast differentiation, an effect mediated by the Notch canonical pathway [13;53]. The different timing of activation of the 3.6 kb and 2.3 kb Col1a1 promoter fragments are probably responsible for the differences in phenotype observed, although both models support a role of Notch as a suppressor of osteoblast differentiation [22].
The inhibitory effects of Notch on osteoblastogenesis are due to interactions of Notch with transcription factors or with other intracellular signaling pathways. NICD and Hey1 bind and inhibit the transactivation of Runt related transcription factor (Runx)2, a transcription factor necessary for osteoblast differentiation [18]. Notch also suppresses Wnt/β-catenin signaling, which is required for osteoblastogenesis, and interacts with Nuclear Factor of Activated T cells (NFAT) [8;63]. Five isoforms of NFAT are known, and NFATc1 through NFATc4 are expressed by cells of the osteoblastic lineage, and in these cells Notch suppresses the transcription of NFATc1 and enhances the expression of NFATc2 by post transcriptional mechanisms. CSL/RBP-Jκ and NFATc1 form a protein complex, and compete for DNA consensus sequences, resulting in a reciprocal inhibition of Notch and NFATc1 transactivation [62]. In vitro NFATc1, like Notch, inhibits expression of osteoblast gene markers suggesting that their interaction serve as a local regulatory mechanism to control osteoblast differentiation [47;62].
Overexpression of Hes1 under the control of the 3.6 kb Col1a1 promoter causes osteopenia in female mice due to reduced osteoblast number, and inactivation of Hes1 in osteoblasts increases trabecular bone volume due to enhanced mineral apposition rate in male mice [61]. Accordingly, Hes1 binds to the osteocalcin promoter and suppresses its transactivation [65], However, interaction of Hes1 with Runx2 induces osteocalcin and osteopontin promoter activity, indicating that under selected conditions Hes1 carries skeletal functions that differ from those described for Notch signaling [28;34;44]. Global Hey1 transgenic mice display a modest skeletal phenotype, but preliminary results, showing increased bone mass in Hey1 heterozygous null mice in a Heyl null background, suggest that Hey proteins mediate the inhibitory effects of Notch in the skeleton [42;56]. However, data from animal models of global misexpression have to be interpreted with caution, since the skeletal phenotype observed could be secondary to systemic non-specific effects, and a definitive role for Hey genes in skeletal development and bone remodeling has not been established.
Notch Modulates Osteoclastogenesis
Osteoclastogenesis is modulated by the ratio of Receptor Activator of Nuclear Factor κ-B Ligand (RANKL), which induces osteoclast formation, and osteoprotegerin, a soluble RANKL receptor that binds and inhibits RANKL [54]. The role of Notch signaling in osteoclastogenesis is cell context dependent. Notch can suppress osteoclastogenesis in vitro by inhibiting the differentiation of the mononuclear precursors and by enhancing osteoprotegerin expression in osteoblasts [1;59]. Accordingly, conditional inactivation of Presenilin1 and Presenilin2 in osteoblasts causes an increase in osteoclast number due to suppressed osteoprotegerin expression [13]. Under selected conditions, the Notch2 intracellular domain and nuclear factor κ-B interact in osteoclasts to induce the activity of the Nfatc1 promoter and as a consequence promote the terminal phases of osteoclast differentiation [15]. In addition, skeletal metastasis of breast cancer tumor cells with enhanced Notch signaling enhance osteoclastogenesis by inducing Interleukin 6 in osteoblasts and by direct effects on osteoclast precursors [43]. Studies in mice misexpressing Hes1 in osteoblasts suggest that Hes1 promotes osteoclastogenesis, indicating that Hes1 does not mediate the reported inhibitory effects of Notch on osteoclastogenesis [61].
Notch and Developmental Disorders of the Skeleton
Recessive Brachydactyly is characterized by aberrant patterning of the hands and feet, micrognatia, and reduced growth. An inactivating frameshift mutation of Chondroitin Sulfate Synthase (CHSY)1, a transmembrane protein containing a fringe domain, is associated with this disease. Upregulation of JAG1 and increased activation of Notch signaling, which is reversed in the presence of a functional CHSY1 protein, are documented in cultured fibroblasts from affected individuals, suggesting that increased Notch activity causes Recessive Brachydactyly [55]. Accordingly, mutations of the Notch ligand Jag2 cause syndactyly in mice, indicating that impairment of Notch signaling causes patterning defects of the hand and feet [21;45].
Alagille Syndrome is a disease associated with several systemic defects, such as impaired bile duct formation leading to liver failure, aberrant vertebral segmentation, which results in butterfly vertebrae or hemivertebrae, and cardiovascular defects including Fallot’s syndrome [16]. Loss of function of JAG1 alleles containing splicing site mutations, missense base substitutions, premature termination codons leading to truncated proteins, or complete gene deletions, are associated with Alagille Syndrome [24]. Rarely, mutations in both NOTCH2 and JAG1 are found in this disease [33]. Jag1 inactivation in mice is lethal, and Jag1 heterozygous mice with a Notch2 hypomorphic allele display growth defects recapitulating those found in Alagille Syndrome, confirming a role for Notch signaling in the pathogenesis of this disease [31].
Spondylocostal Dysostosis is characterized by a series of vertebral segmentation defects and rib anomalies that lead to trunk dwarfism, which have been associated to either dominant or recessive mutations in various components of the Notch signaling pathway [16;57]. The pudgy Dll3 allele, and global inactivation of Dll3 in mice, causes developmental defects that are reminiscent of Spondylocostal Dysostosis, suggesting that mutations in DLL3 cause this disease in humans [11;27]. Notch signaling induces the expression of Mesoderm posterior 2 (Mesp2), a transcription factor that is critical for somite patterning during development, since its inactivation in mice causes vertebral fusions [41]. Accordingly, Spondylocostal Dysostosis type 2, which presents with segmentation abnormalities of the thoracic vertebrae, is associated with inactivating mutations of MESP2 [6;58]. Post-translational modifications of the extracellular domain of Notch modulate affinity for specific ligands, and glycosylation of Notch by fringe proteins promotes Notch-Dll1 interactions while decreasing the affinity of Notch for Jag1 [51]. Lunatic Fringe (Lfng) null mice display rib cage and vertebral abnormalities, and mutations of LFNG are found in individuals affected by Spondylocostal Dysostosis type 3 [10;48]. Global inactivation of Hes7 in mice disrupts the pattern of Lfng expression during somite segmentation, resulting in a shortened rib cage [3]. Accordingly, Spondylocostal Dysostosis type 4 in humans is associated with inactivating mutations of HES7, providing additional evidence that dysregulation of Notch signaling causes this disease [49;50].
Notch and Osteoporosis
Hajdu-Cheney Syndrome is an autosomal dominant disease characterized by localized osteolysis at the distal ends of the phalanges, bone loss and fractures [19]. This disorder is associated with single point mutations in NOTCH2 that allow the mutated mRNA to escape non-sense mediated transcriptional decay. The mutations generate a truncated NOTCH2 protein capable of forming the Notch transcriptional complex, but lacking the PEST domain required for proteolytic degradation of the intracellular domain [46]. The truncated protein is stable, and increased levels for NOTCH2 intracellular domain are present in fibroblasts harvested from affected individuals. These observations suggest that the manifestations of Hajdu-Cheney syndrome are due to sustained NOTCH2 activation [46]. It is of interest that a genome wide association study demonstrated a correlation between bone mineral density and JAG1 polymorphisms in a Chinese population and in a population of mixed European and Chinese ancestry [26].
Notch and Malignancies Affecting the Skeleton
Notch signaling plays a critical role in the immune system by influencing the lineage specification of lymphoid and myeloid cells [39]. Activation of Notch1 is associated with T-cell acute lymphoblastic leukemia and lymphoma, and aberrant Notch activation is involved in multiple myeloma, possibly by inducing cell proliferation or by inhibiting apoptosis [29]. The invasive potential of osteosarcoma is associated with increased Notch signaling, and elevated expression of JAG1, NOTCH1 and HES1 is found in human osteosarcoma [12;64]. The tumor burden of athymic mice inoculated with human osteosarcoma cell lines expressing a dominant negative form of Maml was reduced confirming the role of canonical Notch signaling in osteosarcoma. Accordingly, γ-secretase inhibitors diminished tumor burden, demonstrating that Notch plays a role in the tumorigenic potential of osteosarcoma [12].
Bone metastases are a major complication of carcinoma of the breast, and Notch appears to mediate selected interactions between osteoblastic and metastatic cells. Human bone marrow stromal cells induce the expression of NOTCH3 in carcinoma of the breast cell lines. Systemic inoculation of breast carcinoma cells, or direct injection of the cells in the bone marrow, induces osteolytic bone metastases in athymic mice, and downregulation of NOTCH3 reduces the skeletal metastatic potential suggesting a role of NOTCH3 in the invasiveness of breast cancer cells [66]. Expression of JAG1 in mammary tumor cells correlates with the tumor load and with the ability to form bone metastasis in mice. Tumor cells expressing JAG1 activate Notch signaling and induce the expression of interleukin 6 in osteoblasts, and as a consequence promote osteoclastogenesis and the formation of osteolytic bone metastasis. In addition, Notch induces osteoclastogenesis acting directly in osteoclast precursors, and the lytic lesions release transforming growth factor β from bone, which in turn upregulates JAG1. Administration of γ-secretase inhibitors or downregulation of JAG1 by RNA interference decreases the osteolytic potential of the carcinoma of the breast, demonstrating a role for Notch signaling in the formation of osteolytic metastases [43].
Conclusions
In conclusion, canonical Notch signaling is a regulator of skeletal development and bone remodeling. Although the effects of Notch are cell context dependent, under most conditions Notch inhibits osteoblastogenesis by interacting with other intracellular signaling networks. The effects of Notch on osteoclastogenesis are also complex and both stimulatory and inhibitory effects have been reported, possibly related to Notch interactions with intracellular proteins or to its ability to induce osteoprotegerin. Alterations in components of canonical Notch signaling lead to developmental skeletal disorders and gain of function mutations lead to bone loss. Notch plays a role in the development and invasiveness of osteosarcoma and in the metastatic potential of carcinoma of the breast. From a therapeutic perspective, Notch signaling can be targeted by the use of antibodies to Notch ligands, to the extracellular domain of Notch, or to the site of initial cleavage [40]. In addition, soluble Maml like peptides capable of penetrating the cell have been developed to interfere with Notch signaling with the hope of treating disorders associated with excessive Notch activity [36].
ACKNOWLEDGMENTS
This work was supported by grant DK045227 (EC) from the National Institute of Diabetes, Digestive and Kidney Diseases and by a Research Fellowship from the Arthritis Foundation (SZ).
Footnotes
The authors have stated that they have no conflict of interest.
REFERENCE LIST
- 1.Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J.Biol.Chem. 2008;283:6509–6518. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- 2.Bellavia D, Checquolo S, Campese AF, Felli MP, Gulino A, Screpanti I. Notch3: from subtle structural differences to functional diversity. Oncogene. 2008;27:5092–5098. doi: 10.1038/onc.2008.230. [DOI] [PubMed] [Google Scholar]
- 3.Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15:2642–2647. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canalis E. The fate of circulating osteoblasts. N.Engl.J.Med. 2005;352:2014–2016. doi: 10.1056/NEJMe058080. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E, Giustina A, Bilezikian JP. Mechanisms of Anabolic Therapies for Osteoporosis. N.Engl.J.Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 6.Cornier AS, Staehling-Hampton K, Delventhal KM, Saga Y, Caubet JF, Sasaki N, Ellard S, Young E, Ramirez N, Carlo SE, Torres J, Emans JB, Turnpenny PD, Pourquie O. Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. Am.J Hum.Genet. 2008;82:1334–1341. doi: 10.1016/j.ajhg.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 Overexpression Inhibits Osteoblastogenesis by Suppressing Wnt/beta-Catenin but Not Bone Morphogenetic Protein Signaling. J.Biol.Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, O’Keefe RJ, Hilton MJ. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137:1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunwoodie SL. Mutation of the fucose-specific beta1,3 N-acetylglucosaminyltransferase LFNG results in abnormal formation of the spine. Biochim.Biophys.Acta. 2009;1792:100–111. doi: 10.1016/j.bbadis.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Dunwoodie SL, Clements M, Sparrow DB, Sa X, Conlon RA, Beddington RS. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development. 2002;129:1795–1806. doi: 10.1242/dev.129.7.1795. [DOI] [PubMed] [Google Scholar]
- 12.Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum.Mol.Genet. 2009;18:1464–1470. doi: 10.1093/hmg/ddp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat.Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev.Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K. The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol.Cell Biol. 2008;28:6402–6412. doi: 10.1128/MCB.00299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gridley T. Notch signaling and inherited disease syndromes. Hum.Mol.Genet. 2003;12:R9–13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- 17.Heitzler P. Biodiversity and noncanonical Notch signaling. Curr.Top.Dev.Biol. 2010;92:457–481. doi: 10.1016/S0070-2153(10)92014-0. [DOI] [PubMed] [Google Scholar]
- 18.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat.Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isidor B, Lindenbaum P, Pichon O, Bezieau S, Dina C, Jacquemont S, Martin-Coignard D, Thauvin-Robinet C, Le MM, Mandel JL, David A, Faivre L, Cormier-Daire V, Redon R, Le CC. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat.Genet. 2011;43:306–308. doi: 10.1038/ng.778. [DOI] [PubMed] [Google Scholar]
- 20.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J.Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 21.Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J.Bone Miner.Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krantz ID, Piccoli DA, Spinner NB. Clinical and molecular genetics of Alagille syndrome. Curr.Opin.Pediatr. 1999;11:558–564. doi: 10.1097/00008480-199912000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 26.Kung AW, Xiao SM, Cherny S, Li GH, Gao Y, Tso G, Lau KS, Luk KD, Liu JM, Cui B, Zhang MJ, Zhang ZL, He JW, Yue H, Xia WB, Luo LM, He SL, Kiel DP, Karasik D, Hsu YH, Cupples LA, Demissie S, Styrkarsdottir U, Halldorsson BV, Sigurdsson G, Thorsteinsdottir U, Stefansson K, Richards JB, Zhai G, Soranzo N, Valdes A, Spector TD, Sham PC. Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am.J Hum.Genet. 2010;86:229–239. doi: 10.1016/j.ajhg.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusumi K, Sun ES, Kerrebrock AW, Bronson RT, Chi DC, Bulotsky MS, Spencer JB, Birren BW, Frankel WN, Lander ES. The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat.Genet. 1998;19:274–278. doi: 10.1038/961. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Thomas DM, Gutierrez G, Carty SA, Yanagawa S, Hinds PW. HES1 cooperates with pRb to activate RUNX2-dependent transcription. J Bone Miner Res. 2006;21:921–933. doi: 10.1359/jbmr.060303. [DOI] [PubMed] [Google Scholar]
- 29.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 30.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 31.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 32.McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- 33.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am.J Hum.Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLarren KW, Lo R, Grbavec D, Thirunavukkarasu K, Karsenty G, Stifani S. The mammalian basic helix loop helix protein HES-1 binds to and modulates the transactivating function of the runt-related factor Cbfa1. J.Biol.Chem. 2000;275:530–538. doi: 10.1074/jbc.275.1.530. [DOI] [PubMed] [Google Scholar]
- 35.Mead TJ, Yutzey KE. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc.Natl.Acad.Sci.U.S.A. 2009;106:14420–14425. doi: 10.1073/pnas.0902306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moellering RE, Cornejo M, Davis TN, Del BC, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monet M, Domenga V, Lemaire B, Souilhol C, Langa F, Babinet C, Gridley T, Tournier-Lasserve E, Cohen-Tannoudji M, Joutel A. The archetypal R90C CADASIL-NOTCH3 mutation retains NOTCH3 function in vivo. Hum.Mol.Genet. 2007;16:982–992. doi: 10.1093/hmg/ddm042. [DOI] [PubMed] [Google Scholar]
- 38.Olsen BR, Reginato AM, Wang W. Bone development. Annu.Rev.Cell Dev.Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 39.Radtke F, Fasnacht N, MacDonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Ryeom SW. The cautionary tale of side effects of chronic Notch1 inhibition. J Clin.Invest. 2011;121:508–509. doi: 10.1172/JCI45976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 42.Salie R, Kneissel M, Vukevic M, Zamurovic N, Kramer I, Evans G, Gerwin N, Mueller M, Kinzel B, Susa M. Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone. 2010;46:680–694. doi: 10.1016/j.bone.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J.Biol.Chem. 2005;280:40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 45.Sidow A, Bulotsky MS, Kerrebrock AW, Bronson RT, Daly MJ, Reeve MP, Hawkins TL, Birren BW, Jaenisch R, Lander ES. Serrate2 is disrupted in the mouse limb-development mutant syndactylism. Nature. 1997;389:722–725. doi: 10.1038/39587. [DOI] [PubMed] [Google Scholar]
- 46.Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, Kim KH, Pauli RM, Aftimos S, Stewart H, Kim CA, Holder-Espinasse M, Robertson SP, Drake WM, Trembath RC. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat.Genet. 2011;43:303–305. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- 47.Sitara D, Aliprantis AO. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol.Rev. 2010;233:286–300. doi: 10.1111/j.0105-2896.2009.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparrow DB, Chapman G, Wouters MA, Whittock NV, Ellard S, Fatkin D, Turnpenny PD, Kusumi K, Sillence D, Dunwoodie SL. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am.J.Hum.Genet. 2006;78:28–37. doi: 10.1086/498879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparrow DB, Guillen-Navarro E, Fatkin D, Dunwoodie SL. Mutation of Hairy-and-Enhancer-of-Split-7 in humans causes spondylocostal dysostosis. Hum.Mol.Genet. 2008;17:3761–3766. doi: 10.1093/hmg/ddn272. [DOI] [PubMed] [Google Scholar]
- 50.Sparrow DB, Sillence D, Wouters MA, Turnpenny PD, Dunwoodie SL. Two novel missense mutations in HAIRY-AND-ENHANCER-OF-SPLIT-7 in a family with spondylocostal dysostosis. Eur.J Hum.Genet. 2010;18:674–679. doi: 10.1038/ejhg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley P. Regulation of Notch signaling by glycosylation. Curr.Opin.Struct.Biol. 2007;17:530–535. doi: 10.1016/j.sbi.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 53.Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, Lee B. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25:2175–2183. doi: 10.1002/jbmr.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am.J.Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian J, Ling L, Shboul M, Lee H, O’Connor B, Merriman B, Nelson SF, Cool S, Ababneh OH, Al-Hadidy A, Masri A, Hamamy H, Reversade B. Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling. Am.J Hum.Genet. 2010;87:768–778. doi: 10.1016/j.ajhg.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu X, Lim K, Ganss K, Surendran R, Kopan R, Gessler M, Long F. Notch inhibits early stages of osteoblastogenesis through RBP-Jk and Hey proteins. J Bone Miner Res. 2009 Sep 15;24(Suppl1) Ref Type: Abstract. [Google Scholar]
- 57.Turnpenny PD, Alman B, Cornier AS, Giampietro PF, Offiah A, Tassy O, Pourquie O, Kusumi K, Dunwoodie S. Abnormal vertebral segmentation and the notch signaling pathway in man. Dev.Dyn. 2007;236:1456–1474. doi: 10.1002/dvdy.21182. [DOI] [PubMed] [Google Scholar]
- 58.Whittock NV, Sparrow DB, Wouters MA, Sillence D, Ellard S, Dunwoodie SL, Turnpenny PD. Mutated MESP2 causes spondylocostal dysostosis in humans. Am.J Hum.Genet. 2004;74:1249–1254. doi: 10.1086/421053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi S, Sakano S. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood. 2003;101:2227–2234. doi: 10.1182/blood-2002-06-1740. [DOI] [PubMed] [Google Scholar]
- 60.Zanotti S, Canalis E. Notch and the Skeleton. Mol.Cell Biol. 2010;30:886–896. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanotti S, Smerdel-Ramoya A, Canalis E. Hairy and enhancer of split (HES)1 is a determinant of bone mass. J Biol.Chem. 2011;286:2648–2657. doi: 10.1074/jbc.M110.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanotti S, Smerdel-Ramoya A, Canalis E. Reciprocal regulation of notch and nuclear factor of activated T-cells (NFAT)c1 transactivation in osteoblasts. J Biol.Chem. 2011;286:4576–4588. doi: 10.1074/jbc.M110.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch Inhibits Osteoblast Differentiation And Causes Osteopenia. Endocrinology. 2008;149:3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin.Cancer Res. 2008;14:2962–2969. doi: 10.1158/1078-0432.CCR-07-1992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Zhang Y, Lian JB, Stein JL, van Wijnen AJ, Stein GS. The Notch-responsive transcription factor Hes-1 attenuates osteocalcin promoter activity in osteoblastic cells. J Cell Biochem. 2009;108:651–659. doi: 10.1002/jcb.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z, Wang H, Ikeda S, Fahey F, Bielenberg D, Smits P, Hauschka PV. Notch3 in human breast cancer cell lines regulates osteoblast-cancer cell interactions and osteolytic bone metastasis. Am.J Pathol. 2010;177:1459–1469. doi: 10.2353/ajpath.2010.090476. [DOI] [PMC free article] [PubMed] [Google Scholar]