Abstract
Biosynthetic strategies for the production of recombinant elastin-like protein (ELP) triblock copolymers have resulted in elastomeric protein hydrogels, formed through rapid physical crosslinking upon warming of concentrated solutions. However, the strength of physically crosslinked networks can be limited, and options for non-toxic chemical crosslinking of these networks are not optimal. In this report, we modify two recombinant elastin-like proteins with aldehyde and hydrazide functionalities. When combined, these modified recombinant proteins self-crosslink through hydrazone bonding without requiring initiators or producing by-products. Crosslinked materials are evaluated for water content and swelling upon hydration, and subject to tensile and compressive mechanical tests. Hydrazone crosslinking is a viable method for increasing the mechanical strength of elastin-like protein polymers, in a manner that is likely to lend itself to the biocompatible in situ formation of chemically and physically crosslinked ELP hydrogels.
1. Introduction
Recombinant elastin-like protein polymers (ELP) represent a promising new class of biomaterials that may be formulated as gels, films, nanofibers, or micelles with potential applications in drug delivery, tissue engineering, or as components of implanted medical devices [1–8]. We have recently described elastin-like protein polymers with hydrophilic, elastomeric midblock sequences flanked by self-associating, hydrophobic endblocks in an ABA triblock format [1, 9, 10]. Designs with individual block sizes in excess of 35 kDa have resulted in protein-based biomaterials demonstrating structural polymorphism, providing the opportunity to broadly tune mechanical responses and drug elution rates [11–15]. Notably, due to the self-association of endblock sequences, triblock ELPs form physical, non-covalent crosslinked gel networks in aqueous, physiologic environments (pH 7.4, 37°C), as reviewed elsewhere [1]. Physical crosslinking is reversible and, in principle, eliminates the need for inflammatory or cytotoxic compounds associated with chemical crosslinking schemes. ELP properties, including physical crosslinking, depend upon repeat sequences of the pentapeptide [(Val/Ile)-Pro-Xaa-Yaa-Gly]. The polarity of the fourth residue (Yaa) dictates the coascervation or inverse temperature transition (Tt) of the polypeptide in aqueous solution. The identity of the third residue (Xaa) influences block mechanical properties, with the consensus glycine enhancing elasticity and the substitution of alanine contributing to plastic mechanical behavior. Consequently, ELP endblocks with a significant fraction of (VPAVG) sequences tend towards plastic behavior and lower Tt while midblocks containing (VPGEG) repeats contribute to elasticity and elevated Tt. Sequences are designed so that cold, aqueous ELP solutions transition to elastomeric gels through endblock self-assembly as they are warmed to physiologic temperature, with Tt in the range of 10–15°C.
Despite the advantages of physical crosslinking, self-assembled domains can be disrupted at lower mechanical stresses than covalent crosslinks. The majority of reported methods for covalent crosslinking ELPs rely upon amino groups, and employ either chemical or enzymatic approaches. Chemical methods including isocyanates, NHS-esters, phosphines, aldehydes, or genipin have been reported [16–23], whereas enzymatic approaches have consisted of tranglutaminase and lysyl oxidase [24]. In addition, we have investigated solid-state crosslinking of recombinant elastin using both UV and visible light activated photoinitiators [25].
Strategies for combined chemical and physical crosslinking may result in significant reinforcement of properties arising from self-assembled, multiphase polymer network structure. In an ELP designated LysB10, lysine residues were included at the block interfaces, and crosslinked with glutaraldehyde (GTA) after endblock self-assembly [10]. This modification successfully stabilized the physically crosslinked multiblock domain structure with covalent crosslinks, and significantly enhanced mechanical properties. As a result of this combination of physical and chemical crosslinks, LysB10 served as an elastomeric component in protein-based biomaterials that matched the mechanical behavior of native artery, heart valve leaflet, and abdominal wall connective tissue [26–28]. However, due to toxicity, use of GTA precludes in situ crosslinking, otherwise readily feasible through the injection of cooled ELP solutions that rapidly gel when warmed. Moreover, although subcutaneous and peritoneal implants of GTA-treated ELPs in mice elicited minimal inflammatory responses [10], in many instances the cytotoxic effects of GTA and by-products from other chemical crosslinkers have been firmly established.
In this report, we describe covalently modified ELPs capable of physical and chemical self-crosslinking. This approach relies upon hydrazone bonding, and required the addition of aldehyde or hydrazide functional groups to glutamic acid residues in the ELP polymer backbone. Hydrazone crosslinking proceeds without initiators or by-products, and circumvents toxicity typical of chemical crosslinking. Moreover, the self-crosslinking character of hydrazone bonding creates the potential for in situ covalent stabilization of triblock domain structure through the coinjection of cooled, modified ELP components. Here, the approach is applied to the ELP LysB10 [10], permitting a comparison between hydrazone and GTA crosslinking. In addition, the hydrazone crosslinking is applied to B9 [13], an ELP that cannot be crosslinked with GTA due to an absence of amine functional groups. In comparison to LysB10, B9 has a significantly lower molecular weight, but presents the potential for almost twice the number of aldehyde and hydrazide modifications for hydrazone crosslinking.
2. Materials and Methods
2.1. Materials
All solvents and reagents were purchased from commercial sources and were used as received, unless otherwise noted. The biosynthetic strategy for the expression and purification of the recombinant ELP triblock polymers B9 [13] and LysB10 [10] has been described previously. The ABA triblock amino acid sequence of both ELPs is depicted in scheme 1.
Scheme 1.

Amino-acid sequence of protein-based block copolymers. Glutamic acid (E) residues employed for chemical modification are located in the ELP midblocks.
2.2. Nuclear magnetic resonance
1H NMR spectra was used to confirm the chemical modification of ELPs. Typically the modified samples were dissolved in D2O (8 mg/mL) and the spectrum was recorded at 4°C using a Varian INOVA 600 spectrometer with magnetic field strengths of 600 MHz.
2.3. Synthesis of hydrazide-modified ELPs
To solutions of B9 (600 mg, 34.8×10−4 mmol) in 20 mM HEPES buffer (pH 6.0) was added 10 equivalents of amino-dPEG4-t-boc-hydrazide (Quanta Biodesigns, Powell, OH; 632.95 mg, 1.71 mmol), hydroxysulfosuccinimide (Sulfo-NHS) (36 mg, 0.17 mmol), and 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) (132 mg, 0.69 mmol) at 4°C. The reaction mixture was stirred for 72 h at 4°C and then purified by dialysis (MWCO 10000) against ddH2O for 3 days (two water changes a day) at 4°C to afford B9-tBoc-hydrazide (570 mg, 50%) after lyophilization.
To solutions of LysB10 (600 mg, 28.8×10−4 mmol) in 20 mM HEPES buffer (pH 6.0) was added 10 equivalents of amino-dPEG4-t-boc-hydrazide (300 mg, 0.81 mmol), hydroxysulfosuccinimide (Sulfo-NHS) (21.54 mg, 0.102 mmol), and 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) (79.2 mg, 0.414 mmol) at 4°C. The reaction mixture was stirred for 72 h at 4°C and then purified by dialysis (MWCO 10000) against ddH2O for 3 days (two water changes a day) at 4°C to afford LysB10-tBoc-hydrazide (576 mg, 54%) after lyophilization.
For both modified LysB10 and B9, cleavage of the acid-labile Boc protection group was carried out in a mixture of trifluoroacetic acid (TFA), water, and triisopropylsilane (TIS) (TFA:H2O:TIS, 95:2.5:2.5). Excess TFA was removed under high vacuum and the crude mixture was diluted with water and exhaustively dialyzed (MWCO 10000) against ddH2O for 3 days. The solutions were finally lyophilized to yield either B9-hydrazide or LysB10-hydrazide as a white product in 85–90% yield.
The conjugation efficiency was determined by 1H NMR spectroscopy based on comparison of the integration of methyl protons of the t-Boc group with the β-methyl protons of alanine in Boc protected ELP-hydrazides. B9 and LysB10 have 164 and 465 alanine groups, and 48 and 28 glutamic acid groups, per macromolecule, respectively. Using the intensity of the alanine peak as a reference, the number of methyl protons of t-Boc group per macromolecule of ELP was determined from the 1H NMR spectrum. The percent of modified glutamic acid residues was then calculated as a = (b/(9·c)) ·100%, where a is the percent modification, b is the number of methyl protons of t-Boc, 9 is the number of methyl protons per t-Boc group, and c is the number of glutamic acid residues per macromolecule.
2.4. Synthesis of aldehyde-modified ELPs
B9 (630 mg, 36.54×10−4 mmol) was dissolved in distilled, deionized water and aminoacetaldehyde dimethylacetal (40 equivalents, 565 μL) was added. The pH of the reaction mixture was adjusted to pH 7.5 with 0.1M NaOH/0.1M HCl and hydroxysulfosuccinimide (Sulfo-NHS) (37.8 mg, 0.179 mmol), and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC) (138.6 mg, 0.725 mmol) were added. The reaction mixture was stirred for 72 h at 4°C and then purified by dialysis (MWCO 10000) against ddH2O for 3 days (two water changes a day) at 4°C to afford B9-dimethylacetal (605 mg, 58%) after lyophilization.
For aldehyde modification of LysB10, the ELP (600 mg, 28.8×10–4 mmol) in 20 mM HEPES buffer (pH 6.0) was added aminoacetaldehyde dimethylacetal (240 ul, 40 equiv.), hydroxysulfosuccinimide (Sulfo-NHS) (21.54 mg, 0.102 mmol), and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC) (79.2 mg, 0.414 mmol). The reaction mixture was stirred for 48 h at room temperature and then purified by dialysis (MWCO 10000) against ddH2O for 3 days (two water changes a day) at 4°C to afford LyB10-dimethylacetal (576 mg, 52%) after lyophilization.
For both ELPs, cleavage of the acid-labile acetal protection group was carried out in a mixture of TFA-H2O (95:5). Excess of TFA was removed under high vacuum and the crude mixture was diluted with water and exhaustively dialyzed (MWCO 10000) against ddH2O for 3 days. The solutions were lyophilized to yield B9- or LysB10-aldehyde as a white product in 83% yield.
The conjugation efficiency was again determined by 1H NMR spectroscopy based on comparison of methyl protons of the dimethylacetal group with the β-methyl protons of alanine residues, similar to the calculation above. Percent modification was calculated as a = (b/(6·c)) ·100%, where a is the percent modification, b is the number of methyl protons of the dimethylacetal group, 6 is the number of methyl protons per dimethylacetal group, and c is the number of glutamic acid residues per macromolecule of ELP. Complete deprotection was validated by an absence of detectable signal corresponding to t-Boc or dimethylacetal in the NMR spectra of the products.
2.5. Rheological analysis of chemically modified ELP solutions
Rheological tests were performed on an Advanced Rheological Expansion System III rheometer (ARES III, TA Instruments, NJ) in a parallel plate configuration (plate diameter 25 mm). Proteins were dissolved in ddH20, 10 wt%, 4°C, and positioned between parallel plates with a gap width of 0.2 to 0.35 mm. Dynamic mechanical experiments were performed in shear deformation mode. Initial strain amplitude measurements collected at 4°C and 37°C at a frequency of 1 Hz ensured that all studies were conducted within the linear viscoelastic regime, with dynamic storage (G′) and loss modulus (G″) independent of strain amplitude. Gelation temperature was determined by heating from 4°C to 37°C at a rate of 1°C/min. Representative results are presented.
2.6. Fabrication of ELP films
Lyophilized, unmodified B9 and LysB10 were dissolved at 100 mg/mL in 4°C ddH20 and then poured into Te on casting molds for regulated solvent evaporation performed at 23°C for 48 h. After complete solvent evaporation, some LysB10 films were further processed by GTA vapor phase crosslinking (termed LysB10-GTA). Specifically, films were enclosed in a 2-L chamber containing 10 mL of 25% GTA solution. Films were placed on a platform 4 cm above the GTA solution and exposed to vapor for 24 h. Subsequently, lms were rinsed in PBS for 48 h with a change of PBS at 24 h.
Hydrazone crosslinked films were prepared from modified ELP solutions (hydrazide and aldehyde modified B9 or LysB10, 100 mg/mL). Solutions prepared in 4°C ddH20 and loaded in separate pre-cooled 1 mL syringes. By precision syringe pump (Harvard Apparatus, Holliston, MA), 200 μL of the hydrazide or aldehyde modification of a given ELP solution were dispensed into a Teflon mold. The hydrazide or aldehyde modified ELP solutions were thoroughly mixed, placed in a humidifying chamber at 37°C for 24 h, and dried at 23°C for 48 h. Specimens are referred to as LysB10- or B9-hydrazone.
2.7. Evaluation of water content in ELP films
Hydrazone-crosslinked ELP films were fully dehydrated under vacuum. Films were subsequently incubated in PBS (37°C, 24 h) and fully hydrated weights were obtained. The equilibrium water content and equilibrium swelling ratio were determined and expressed as mean ± standard deviation. Equilibrium water content (EC) was defined as EC = (WH – WD) / WH, where WH and WD are the hydrated and dehydrated weights, respectively. Equilibrium swelling ratio (ES ) was defined as ES = WH / WD.
2.8. Non-crosslinked extractables in ELP films
Disk-shaped hydrazone-crosslinked ELP films (d = 1 cm) were cast in Teflon molds as described above. Hydrated films were incubated below the inverse transition temperature in PBS (4°C, 5–7 days). Every 48 h films were dehydrated and dry weight was monitored for material losses. The percent extractable (X) was defined as X = DF / DI · 100%, where DI and DF and are the initial and final dehydrated weights, respectively. All calculations were expressed as a mean ± standard deviation.
2.9. Tensile analysis
ELP film samples were cut into a dumbbell shape using a stainless steel die with gauge dimensions of 13 mm × 4.75 mm. Uniaxial stress-strain properties of ELP films were determined using a dynamic mechanical thermal analyzer (DMTA 2000, Rheometric Scientific) with a 15 N load cell, with test specimens immersed in 37°C PBS. All specimens were preconditioned by cyclically stretching to 50% strain for one cycle followed by 20 cycles of 30% strain with off loading periods of 5 minutes between each cycle. Resilience was calculated from the final loading cycle as R = (AUL / AL) · 100%, where R is resilience, AUL is area under the unloading curve, and AL is area under the loading curve. Resilience is a measure of elasticity, or the percentage of energy that is stored during material deformation and returned during the unloading cycle. Due to the limited maximum strain of the DMTA, uniaxial tensile failure responses were also characterized using a miniature materials tester (Minimat 2000, Rheometric Scientific) at a rate of 5 mm/min until failure. Failure tests were conducted in air at room temperature (22°C) with all samples fully hydrated with PBS and coated with a thin layer of mineral oil to prevent specimen drying. Low and high-strain elastic moduli were derived from the slope of linear regressions applied from 0% – 40% and 100% – 200% strain, respectively.
2.10. Compression Tests
Compression tests were performed on hydrogels rather than solvent-cast films. Cylindrical gels for compression testing were prepared by drawing solutions of protein polymers (100 mg/mL) into 1mL syringes (1mL BD syringes (d=4.67mm)) at 4°C. After 24 hr equilibration at 4°C syringes were transferred to 37 °C for 24 h to gel the material through endblock self-association. Cylindrical slices (diameter, 4.67 mm, height, 2 mm) were cut from the molded cylinders and incubated in PBS (37°C, 24 h). Some samples (LysB10-GTA) were GTA crosslinked. To avoid drying that occurs during the vapor-phase protocol that was applied to dehydrated LysB10-GTA films, gels were crosslinked in a solution of 0.25% GTA in PBS (37°C, 24 h), and then rinsed for 48 h in PBS with a change of PBS at 24 h. Dimensions of all samples were measured by optical microscopy. After rinsing, specimens were stored in PBS containing sodium azide (0.2 mg/mL) and tested within 48 hours. Compression measurements were acquired on a Bose ELF system with a 1.1 lb load cell and a hydration chamber containing PBS at 37°C. Samples were preconditioned by cyclic compression ranging from 1%–20% strain for 10 cycles. Compression stress-strain testing was performed at 0.025 mm/sec. Compression stress-relaxation testing was preformed by compressing to a strain of 75% at 0.025 mm/sec followed by monitoring change in compressive stress over time. Cotangent moduli were calculated from the compression stress-strain plots at 10% intervals from 10% to 80%. Specifically, the cotangent was calculated by performing a linear regression on the points at a given percent strain ± 1% strain.
2.11. Statistical Methods
Tests for statistically significant differences between the means of two groups were conducted with the Student’s t-test (two-tailed, homoscedastic). Tests between three or more groups were conducted with one-way ANOVA followed by the Tukey HSD test.
3. Results and Discussion
3.1. Chemical modification of ELP triblocks
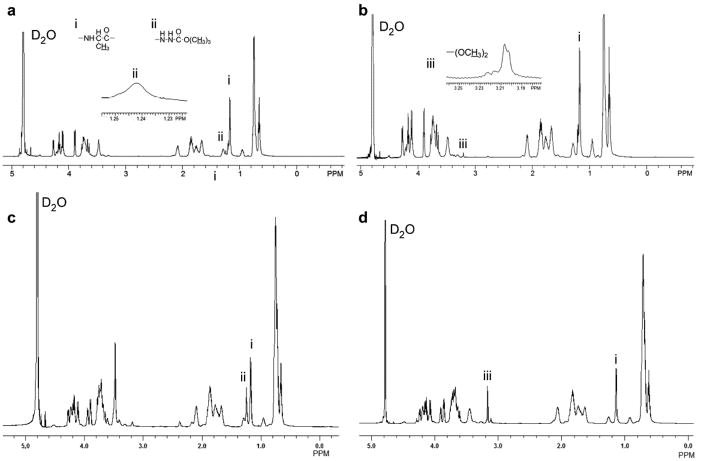
The carboxyl moieties in the midblock region of both B9 and LysB10 served as the synthetic handle for the incorporation of aldehyde or hydrazide groups (Scheme 2). Hydrazide functionalization proceeded through reaction with amine-PEG4-t-Boc-hydrazide using carbodiimide chemistry. Following reaction with water-soluble carbodiimide, EDC, the transient O-acylisourea intermediate was formed and converted to the more stable sulfo-NHS. This intermediate was reacted with excess amino-dPEG4-t-Boc-hydrazide at to obtain the hydrazide functionalized polypeptide polymer. Conjugation efficiency was analyzed by 1H NMR spectroscopy based on selective peak assignments for the polypeptide backbone and the Boc-protected hydrazide-polypeptide linker (Fig. 1). Quantification of the integrated signals from protons of the t-Boc group with the β-methyl protons of alanine in B9-hydrazide yielded coupling efficiencies of 50% and 52–54% for B9 and LysB10 respectively.
Scheme 2.
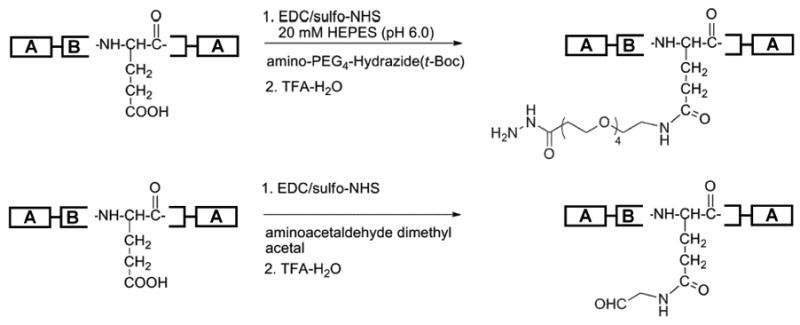
Chemical modification of protein-based block coploymers to incorporate hydrazide and aldehyde groups in the midblock (B).
Figure 1.
1H NMR spectrum (600 MHz, 4 oC) of (a) LysB10-tBoc-hydrazide, (b) LysB10-dimethylacetal, (c) B9-tBoc-hydrazide, and (d) B9-dimethylacetal in D2O (8 mg/mL). Signals from (i) alanine, (ii) the t-Boc group, and (iii) the dimethyacetal group are indicated.
In parallel, B9 and LysB10 glutamic acid units were modified to incorporate the aldehyde groups on the polymer backbone (Scheme 2). Following reaction with excess aminoacetaldehyde dimethylacetal, dialysis, and lyophilization, the product was quantified by 1H NMR analysis. Comparison of the relative ratio of integration of methyl signal of the dimethyacetal with the β-methyl protons of alanine confirmed 58% and 52% aldehyde modification for B9 and LysB10 respectively (Fig. 1). Removal of the aldehyde protection group provided B9- and LysB10-aldehydes.
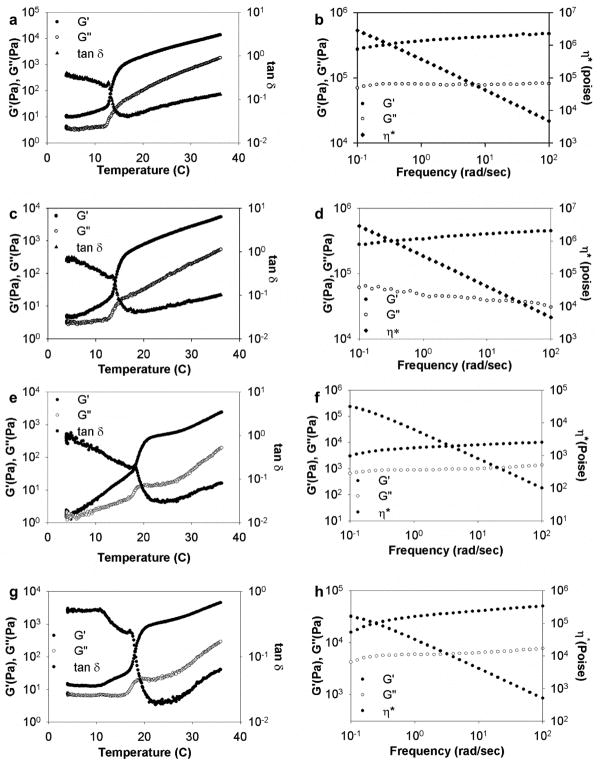
The gelation point of modified protein polymer solutions was determined by measurement of G′ and G″ as a function of increasing temperature at a fixed frequency (Fig. 2). Above transition temperatures of 13°C and 15°C for modified LysB10 and B9, the shear storage (G′) and loss (G″) modulus of concentrated solutions of the modified protein polymers increased, while tan δ (G″/G′) decreased, consistent with the formation of a viscoelastic gel. The modified LysB10 and B9 transition temperatures were in accordance with observations for the unmodified protein polymers [9, 10], suggesting that additions of functional groups to the midblock did not interfere with endblock self-assembly kinetics.
Figure 2.
Rheological behavior of modified triblock protein polymers in water. Dynamic shear storage (G′), loss modulus (G″) and tan δ are plotted as a function of temperature (γ 2%, ω 1 Hz) for (a) LysB10-hydrazide, (c) LysB10-aldehyde, (e) B9-hydrazide, and (g) B9-aldehyde. Dynamic storage and loss modulus (G′, G″) and complex viscosity (η*) are plotted as a function of frequency (γ 2%, 37°C) for (b) LysB10-hydrazide, (d) LysB10-aldehyde, (f) B9-hydrazide, and (h) B9-aldehyde.
3.2. Hydrazone crosslinking
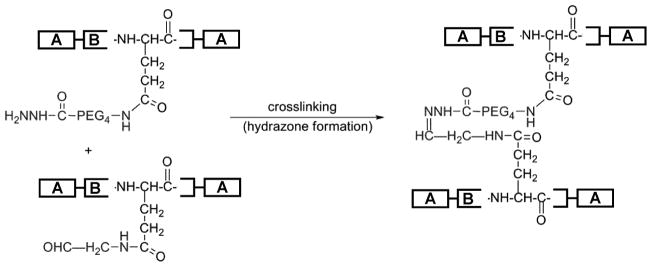
Hydrozone bonds, consisting of a double bond between carbon and nitrogen atoms, occur spontaneously in aqueous solvents between carbonyl groups, typically aldehyde or ketone moieties, and amine functional groups. Reaction equilibrium favors bonding under aqueous conditions although water is a reaction by-product. Hydrazone formation between hydrazide- and aldehyde-modified B9 is shown in Scheme 3. To determine the extent of crosslinking, the percent of extractable protein was examined by incubating the sample at temperatures below Tt for 7 days. When incubated at 4°C, the physical crosslinks will be disrupted causing non-crosslinked films to dissolve immediately. After 7 days, B9-hydrazone and LysB10-hydrazone films retained approximately 85 ± 10% and 71 ± 4% of their mass, respectively. The equilibrium water contents and swelling ratios are shown in Table 2. Greater water content in B9 films is consistent with a larger hydrophilic midblock relative to LysB10 films. In both ELPs, hydrazone crosslinking tended to decrease water content and swelling, but much more of an effect was observed in the B9 system.
Scheme 3.
Crosslinking of modified protein copolymers by hydrazone bond formation.
Table 2.
Water Content of Hydrazone-Crosslinked ELPs
Hydrazone crosslinking of hyaluronic acid, alginate, dextran, and other materials has emerged as a promising technology for a wide range of biomaterials applications, and has been studied in vivo in several models (Table 1) [29–52]. Hydrazone conjugation is a traceless reaction, free from side products and the use of potentially toxic reagents. Moreover, the hydrazone bond demonstrates favorable resistance to hydrolysis when compared to strategies employing amine and aldehyde groups for the formation of Schiff’s bases. Additionally, the lower pKa of hydrazides (4 to 5) compared to the pKa of primary amines (10 to 11), permits reaction at neutral to slightly acidic conditions.
Table 1.
Hydrazone Crosslinked Materials
| Biomaterial | Aldehyde Component | Hydrazide Component | Application | In vivo | Ref. |
|---|---|---|---|---|---|
| Hyaluronan | HA | HA | bone tissue engineering (BMP-2 delivery) | rat | [29] |
| vocal fold (multicomponent gels with collagen) | pig | [30] | |||
| anti-inflammatory delivery (dexamethasone) | mouse | [31] | |||
| nerve blockade (bupivacaine delivery) | rat | [32] | |||
| surgical adhesion prevention, drug delivery (tPA) | rabbit | [33] | |||
| surgical adhesion prevention | rabbit | [34] | |||
| HA | BP | drug delivery (bisphosphonate) | -- | [35] | |
| HA | PVA | bone tissue engineering (BMP-2 delivery) | rat | [36] | |
| HA or PEG | HA | vocal fold regeneration | -- | [38] | |
| PEG | HA | drug delivery (anti-bacterial, anti-inflammatory) | -- | [39] | |
| DX or HA | HA | tissue regeneration, drug delivery | rat | [40] | |
| PG | ADH or PAH | cell and drug delivery | -- | [42] | |
| Alginate | PG | ADH | cell delivery | -- | [43] |
| Alg | ADH | bone tissue engineering | mouse | [44] | |
| PG | ADH | cell adhesion, tissue engineering | -- | [45] | |
| Alg | ADH | drug delivery (methotrexate, doxorubicin, mitoxantrone) | -- | [46] | |
| PG | ADH | drug delivery (daunomycin) | -- | [47] | |
| Dextran | DX | CMC | drug delivery (amphotericin) | -- | [48] |
| DX or CMC | CMD | surgical adhesion prevention | rabbit | [49] | |
| DX | ADH | ocular cell therapy | -- | [50] | |
| DX | ADH | drug delivery, tissue engineering | -- | [51] | |
| Misc. | PVA | PVA | cell delivery | -- | [37] |
| CP | ADH | drug delivery (doxorubicin) | -- | [52] |
Abbreviations: HA, hyaluronan; BP, aminomethylene-bisphosphonate; PVA, poly(vinyl alcohol); PEG, polyethylene glycol; DX, Dextran; CS, chondroitin sulfate; Alg, sodium alginate; ADH, adipic acid dihydrazide; PG, polyguluronate; CMC, carboxymethylcellulose; PAH, poly(acrylamide-co-hydrazide); CMD, carboxymethyldextran; CP, citrus pectin; tPA, tissue-type plasminogen activator; BMP-2, bone morphogenic protein 2.
3.3. Mechanical Responses of crosslinked protein polymers
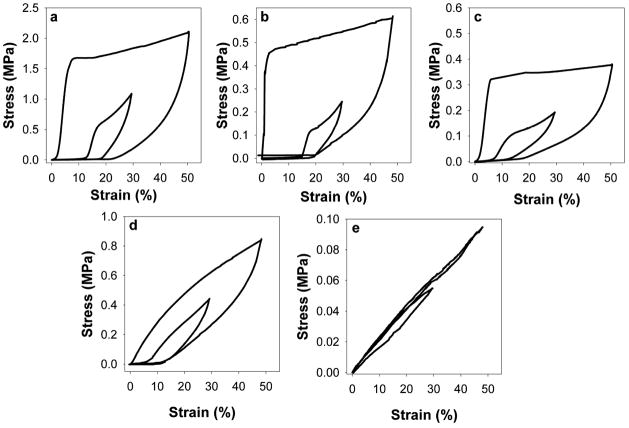
Analysis of ELP films demonstrated reinforcement of mechanical strength and stiffness upon hydrazone crosslinking. Stress-strain data for sequential preconditioning cycles indicated an initial accumulation of residual deformation and decline in peak stress for all samples. Additionally, hysteresis between loading and unloading cycles decreased, most significantly following the first loading cycle (Fig. 3). After several cycles, the mechanical response became consistent, presumably as initial load-induced alterations to biopolymer chain interrelationships and entanglements stabilized [10]. Responses were highly consistent after 20 cycles, yielding representative stress-strain plots and the calculated resilience values in Fig. 4 and Table 3. The greater resilience of uncrosslinked B9 relative to LysB10 (82 ± 4% compared to 52 ± 2%) is consistent with the substantially larger ratio of midblock size to endblock size in the B9 sequence. Crosslinking tended to lower resilience, by approximately 10–12% for LysB10-glutaraldehyde and LysB10-hydrazone, and 30% for B9-hydrazone. Moreover, hydrazone crosslinking dramatically increased the modulus of B9, while the modulus change for LysB10 after hydrazone crosslinking was relatively minor. Because B9 is smaller than LysB10 (165 kDa vs 209 kDa), but has a larger midblock with nearly twice as many glutamic acid residues available for modification and subsequent crosslinking, it is not surprising that hydrazone crosslinking had a greater relative effect on the resilience and modulus of B9 compared to LysB10.
Figure 3.
Mechanical preconditioning of crosslinked LysB10 films. LysB10-GTA, LysB10-hydrazone, and LysB10 are shown in (a, b, c). B9 films included B9-hydrazone and B9 (d, e). Representative initial cycles to 50% strain are shown along side the 20th loading cycle, to 30% strain.
Figure 4.
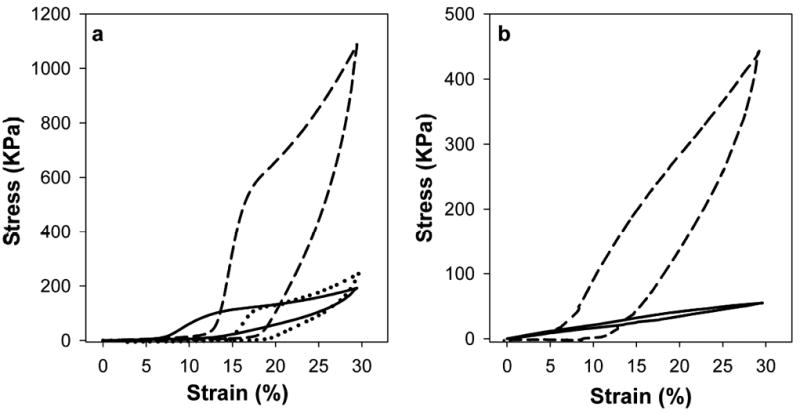
Stress-strain data for crosslinked and non-crosslinked films after mechanical preconditioning. The response is representative of multiple data sets and illustrates the 20th cycle to 30% strain. (a) LysB10-glutaraldehyde (dashed), LysB10-hydrazone (dotted), and uncrosslinked LysB10 (solid). (b) B9-hydrazone (dashed) and uncrosslinked B9 (solid).
Table 3.
Summary of Mechanical Parameters in Crosslinked and Non-Crosslinked Films
| Sample | Resilience (%) | Low-strain tensile modulus (Mpa) | High-strain tensile modulus (Mpa) | UTS (Mpa) | Strain at failure (%) |
|---|---|---|---|---|---|
| B9 | 82 ± 4 | 0.14 ± 0.02 | 0.14 ± 0.02 | 0.49 ± 0.03 | 384 ± 29 |
| B9-Hyd | 51 ± 2 | 2.16 ± 0.36 | N.A. | 1.50 ± 0.21 | 96 ± 6 |
| LysB10 | 52 ± 2 | 0.74 ± 0.11 | 0.35 ± 0.06 NS | 2.39 ± 0.27 NS | 437 ± 39 |
| LysB10-Hyd | 41 ± 1NS | 1.53 ± 0.14 | 0.49 ± 0.02 NS | 2.14 ± 0.13 NS | 308 ± 27 |
| LysB10-glut | 39 ± 1 NS | 3.16 ± 0.10 | 1.33 ± 0.10 | 4.12 ± 0.33 | 204 ± 26 |
Denotes non-significant differences. For comparisons within a given ELP (B9 or LysB10), all differences were significant except between the denoted entries (p<0.05).
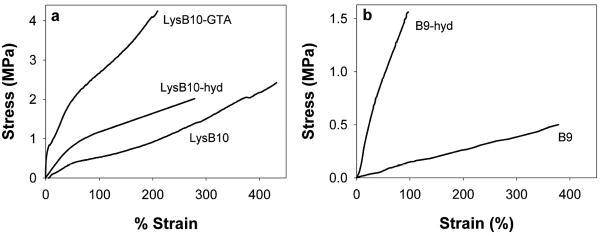
When tested to tensile failure, crosslinked materials displayed decreased strain-to-failure (Table 3, Figure 5). LysB10-hydrazone displayed a minor increase in stiffness and insignificant change in ultimate tensile strength, with glutaraldehyde crosslinking resulting in significant modulus and strength increases (Fig. 5a). Relative to LysB10-hydrazone, GTA crosslinking substantially increased the stiffness and strength of LysB10 films (Fig. 5b). All told, tensile properties were consistent with or in excess of a variety of soft tissues, such as the blood vessel wall (modulus: 0.1 to 2.0 MPa, UTS: 0.4 to 2.0 MPa) and human urinary bladder (UTS: 0.270 ± 0.140 MPa) [53–55].
Figure 5.
Representative uniaxial stress-strain response for pre-conditioned films, including (a) LysB10, LysB10-hydrazone, and glutaraldehyde crosslinked LysB10, as well as (b) B9 and B9-hydrazone. Films were strained to failure.
Reduction of strain-to-failure upon crosslinking may be due to reduced mobility of the crosslinked midblocks. Speculatively, the larger effect of GTA compared to hydrazone crosslinking on LysB10 properties may be due to the multicomponent nature of glutaraldehyde solutions, which crosslink proteins through several distinct mechanisms [56]. Additionally, the positioning of the crosslinkable residues within the multiphase network differed in LysB10-GTA and LysB10-hydrazone. Specifically, although marginally fewer sites were available for glutaraldehyde crosslinking in LysB10 (8 lysine residues per macromolecule compared to approximately half of the 28 glutamic acid residues modified with aldehyde or hydrazide groups), the lysine residues may have been in closer physical proximity because they resided either within the collapsed, hydrophobic domains or at the domain interfaces. In contrast, the hydrazide and aldehyde functional groups were located within the more mobile, hydrated midblocks.
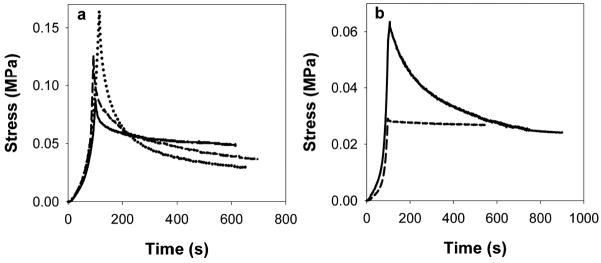
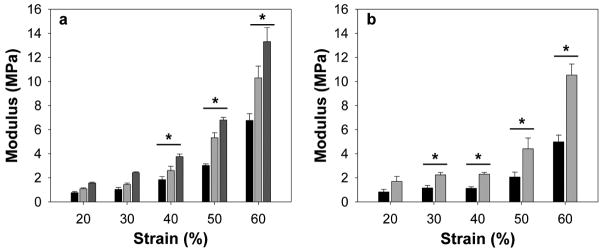
In compression, crosslinked ELPs showed an increase in the cotangent compressive modulus relative to non-crosslinked materials, indicating mechanical reinforcement at high strains (Fig. 6). In contrast to tensile test results, in compression the modulus of LysB10-hydrazone exceeded that of LysB10-GTA. It is likely that the solution-phase GTA crosslinking applied to specimens for compressive analysis was less extensive than the vapor-phase crosslinking of films used in tensile testing. Compressive stress relaxation demonstrated the greatest stress developed in LysB10-hydrazone, followed by LysB10-GTA and LysB10 (Fig. 7). This result again suggests a greater initial stiffness in LysB10-hydrazone gels compared to LysB10-GTA. Hydrazone crosslinking also increased the compressive stress initially developed in B9 gels (Fig. 7b). However, stress relaxation over time in LysB10-hydrazone and B9-hydrazone was increased compared to non-crosslinked specimens, corresponding to somewhat reduced elasticity also observed in crosslinked ELP films in tension.
Figure 6.
The cotangent compressive modulus is shown for protein polymer gels, including (a) LysB10, black; LysB10-GTA, light gray; and LysB10-hydrazone, dark gray. The moduli of the three LysB10 variants were significantly different at compressive strains of 40% and higher (p<0.05). (b) Moduli of B9, black, and B9-hydrazone, gray, were significantly different at compressive strains of 30% and higher (p<0.05).
Figure 7.
Compressive stress-relaxation of protein polymer gels. (a) Representative data plotted for LysB10 (solid line), LysB10-GTA (dashed), and LysB10-hydrazone (dotted). (b) Stress relaxation data for B9 (dashed) and B9-hydrazone (solid line).
3.4. Conclusions
In summary, hydrazone crosslinking represents a route to covalent self-crosslinking of ELP biomaterials, in a manner that reinforces physical crosslinks arising from temperature-driven self-assembly. Water content and mechanical analysis suggested that hydrazone crosslinking had the greatest effect on the ELP B9, most likely due to the greater number of glutamic acid residues and lower molecular weight of this material. Measurement of extractable material, water content, and mechanical testing suggested that LysB10 films and hydrogels were also reinforced by hydrazone crosslinking. The effect of hydrazone crosslinking was less pronounced than the effect of vapor phase GTA crosslinking on protein film mechanics. However, hydrazone-crosslinked LysB10 gels displayed greater stiffness than solution-phase crosslinked LysB10-GTA. These observations and promising in vivo data from several other reports suggest that hydrazone crosslinking is a viable method for the formation of biocompatible in situ chemically and physically crosslinked ELP gels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliv Rev. 2002;54:1057–73. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 2.Herrero-Vanrell R, Rincon AC, Alonso M, Reboto V, Molina-Martinez IT, Rodriguez-Cabello JC. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J Control Release. 2005;102:113–22. doi: 10.1016/j.jconrel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5:497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 5.Richman GP, Tirrell DA, Asthagiri AR. Quantitatively distinct requirements for signaling-competent cell spreading on engineered versus natural adhesion ligands. J Control Release. 2005;101:3–12. doi: 10.1016/j.jconrel.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Jordan SW, Haller CA, Sallach RE, Apkarian RP, Hanson SR, Chaikof EL. The effect of a recombinant elastin-mimetic coating of an ePTFE prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials. 2007;28:1191–7. doi: 10.1016/j.biomaterials.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Woodhouse KA, Klement P, Chen V, Gorbet MB, Keeley FW, Stahl R, et al. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials. 2004;25:4543–53. doi: 10.1016/j.biomaterials.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Sallach RE, Cui W, Balderrama F, Martinez AW, Wen J, Haller CA, et al. Long-term biostability of self-assembling protein polymers in the absence of covalent crosslinking. Biomaterials. 2009;31:779–91. doi: 10.1016/j.biomaterials.2009.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagapudi K, Brinkman WT, Thomas BS, Park JO, Srinivasarao M, Wright E, et al. Viscoelastic and mechanical behavior of recombinant protein elastomers. Biomaterials. 2005;26:4695–706. doi: 10.1016/j.biomaterials.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL. Elastin-mimetic protein polymers capable of physical and chemical crosslinking. Biomaterials. 2009;30:409–22. doi: 10.1016/j.biomaterials.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright E, McMillan R, Cooper A, Apkarian RP, Conticello VP. Thermoplastic elastomer hydrogels via self-assembly of an elastin-mimetic triblock polypeptide. Adv Funct Mater. 2002;12:149–54. [Google Scholar]
- 12.Sallach RE, Wei M, Biswas N, Conticello VP, Lecommandoux S, Dluhy RA, et al. Micelle density regulated by a reversible switch of protein secondary structure. J Am Chem Soc. 2006;128:12014–9. doi: 10.1021/ja0638509. [DOI] [PubMed] [Google Scholar]
- 13.Nagapudi K, Brinkman W, Leisen J, Thomas B, Wright E, Haller C, et al. Protein-based thermoplastic elastomers. Macromolecules. 2005;38:345–54. [Google Scholar]
- 14.Wu X, Sallach R, Haller CA, Caves JA, Nagapudi K, Conticello VP, et al. Alterations in physical cross-linking modulate mechanical properties of two-phase protein polymer networks. Biomacromolecules. 2005;6:3037–44. doi: 10.1021/bm0503468. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Sallach RE, Caves JM, Conticello VP, Chaikof EL. Deformation responses of a physically cross-linked high molecular weight elastin-like protein polymer. Biomacromolecules. 2008;9:1787–94. doi: 10.1021/bm800012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellingham CM, Lillie MA, Gosline JM, Wright GM, Starcher BC, Bailey AJ, et al. Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties. Biopolymers. 2003;70:445–55. doi: 10.1002/bip.10512. [DOI] [PubMed] [Google Scholar]
- 17.Vieth S, Bellingham CM, Keeley FW, Hodge SM, Rousseau D. Microstructural and tensile properties of elastin-based polypeptides crosslinked with genipin and pyrroloquinoline quinone. Biopolymers. 2007;85:199–206. doi: 10.1002/bip.20619. [DOI] [PubMed] [Google Scholar]
- 18.Girotti A, Reguera J, Rodriguez-Cabello JC, Arias FJ, Alonso M, Matestera A. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater Med. 2004;15:479–84. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 19.Martino M, Tamburro AM. Chemical synthesis of cross-linked poly(KGGVG), an elastin-like biopolymer. Biopolymers. 2001;59:29–37. doi: 10.1002/1097-0282(200107)59:1<29::AID-BIP1003>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Macosko CW, Urry DW. Elastomeric polypentapeptides cross-linked into matrixes and fibers. Biomacromolecules. 2001;2:170–9. doi: 10.1021/bm0000900. [DOI] [PubMed] [Google Scholar]
- 21.Nowatzki PJ, Tirrell DA. Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials. 2004;25:1261–7. doi: 10.1016/s0142-9612(03)00635-5. [DOI] [PubMed] [Google Scholar]
- 22.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–80. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 23.Lim DW, Nettles DL, Setton LA, Chilkoti A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules. 2008;9:222–30. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–79. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 25.Nagapudi K, Brinkman WT, Leisen JE, Huang L, McMillan RA, Apkarian RP, et al. Photomediated solid-state cross-linking of an elastin-mimetic recombinant protein polymer. Macromolecules. 2002;35:1730–7. [Google Scholar]
- 26.Caves JM, Kumar VA, Xu W, Naik N, Allen MG, Chaikof EL. Microcrimped collagen fiber-elastin composites. Adv Mater. 2010;22:2041–4. doi: 10.1002/adma.200903612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caves JM, Kumar VA, Martinez AW, Kim J, Ripberger CM, Haller CA, et al. The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts. Biomaterials. 2010;31:7175–82. doi: 10.1016/j.biomaterials.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caves JM, Cui W, Wen J, Kumar VA, Haller CA, Chaikof EL. Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair. Biomaterials. 2011;32:5371–9. doi: 10.1016/j.biomaterials.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Sanz E, Ossipov DA, Hilborn J, Larsson S, Jonsson KB, Varghese OP. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J Control Release. 2011;152:232–40. doi: 10.1016/j.jconrel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Farran AJ, Teller SS, Jha AK, Jiao T, Hule RA, Clifton RJ, et al. Effects of matrix composition, microstructure, and viscoelasticity on the behaviors of vocal fold fibroblasts cultured in three-dimensional hydrogel networks. Tissue Eng Part A. 2010;16:1247–61. doi: 10.1089/ten.tea.2009.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito T, Fraser IP, Yeo Y, Highley CB, Bellas E, Kohane DS. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials. 2007;28:1778–86. doi: 10.1016/j.biomaterials.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Yeo Y, Bellas E, Highley CB, Langer R, Kohane DS. Peritoneal adhesion prevention with an in situ cross-linkable hyaluronan gel containing tissue-type plasminogen activator in a rabbit repeated-injury model. Biomaterials. 2007;28:3704–13. doi: 10.1016/j.biomaterials.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27:4698–705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Varghese OP, Sun W, Hilborn J, Ossipov DA. In situ cross-linkable high molecular weight hyaluronan-bisphosphonate conjugate for localized delivery and cell-specific targeting: a hydrogel linked prodrug approach. J Am Chem Soc. 2009;131:8781–3. doi: 10.1021/ja902857b. [DOI] [PubMed] [Google Scholar]
- 36.Bergman K, Engstrand T, Hilborn J, Ossipov D, Piskounova S, Bowden T. Injectable cell-free template for bone-tissue formation. J Biomed Mater Res A. 2009;91:1111–8. doi: 10.1002/jbm.a.32289. [DOI] [PubMed] [Google Scholar]
- 37.Ossipov DA, Piskounova S, Hilborn J. Poly (vinyl alcohol) cross-linkers for in vivo injectable hydrogels. Macromolecules. 2008;41:3971–82. [Google Scholar]
- 38.Jia X, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–44. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169–84. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 40.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–69. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 41.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials. 2002;23:3661–71. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, Bouhadir KH, Mooney DJ. Controlled degradation of hydrogels using multi-functional cross-linking molecules. Biomaterials. 2004;25:2461–6. doi: 10.1016/j.biomaterials.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Lee KY, Bouhadir KH, Mooney DJ. Degradation behavior of covalently cross-linked poly (aldehyde guluronate) hydrogels. Macromolecules. 2000;33:97–101. doi: 10.1002/1520-6017(200007)89:7<910::AID-JPS8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56:228–33. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Bouhadir KH, Hausman DS, Mooney DJ. Synthesis of cross-linked poly (aldehyde guluronate) hydrogels. Polymer. 1999;40:3575–84. [Google Scholar]
- 46.Bouhadir KH, Alsberg E, Mooney DJ. Hydrogels for combination delivery of antineoplastic agents. Biomaterials. 2001;22:2625–33. doi: 10.1016/s0142-9612(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 47.Bouhadir KH, Kruger GM, Lee KY, Mooney DJ. Sustained and controlled release of daunomycin from cross-linked poly(aldehyde guluronate) hydrogels. J Pharm Sci. 2000;89:910–9. doi: 10.1002/1520-6017(200007)89:7<910::AID-JPS8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Hudson SP, Langer R, Fink GR, Kohane DS. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials. 2010;31:1444–52. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito T, Yeo Y, Highley CB, Bellas E, Kohane DS. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials. 2007;28:3418–26. doi: 10.1016/j.biomaterials.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Maia J, Ferreira L, Carvalho R, Ramos MA, Gil MH. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer. 2005;46:9604–14. [Google Scholar]
- 51.Maia J, Ribeiro MP, Ventura C, Carvalho RA, Correia IJ, Gil MH. Ocular injectable formulation assessment for oxidized dextran-based hydrogels. Acta Biomater. 2009;5:1948–55. doi: 10.1016/j.actbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Takei T, Sato M, Ijima H, Kawakami K. In situ gellable oxidized citrus pectin for localized delivery of anticancer drugs and prevention of homotypic cancer cell aggregation. Biomacromolecules. 2010;11:3525–30. doi: 10.1021/bm1010068. [DOI] [PubMed] [Google Scholar]
- 53.Bergel DH. The static elastic properties of the arterial wall. J Physiol. 1961;156:445–57. doi: 10.1113/jphysiol.1961.sp006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dahms SE, Piechota HJ, Dahiya R, Lue TF, Tanagho EA. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br J Urol. 1998;82:411–9. doi: 10.1046/j.1464-410x.1998.00748.x. [DOI] [PubMed] [Google Scholar]
- 55.Mohan D, Melvin JW. Failure properties of passive human aortic tissue. I--uniaxial tension tests. J Biomech. 1982;15:887–902. doi: 10.1016/0021-9290(82)90055-0. [DOI] [PubMed] [Google Scholar]
- 56.Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37:790-6, 8–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]