Abstract
Previous studies have demonstrated that moderate hypothermia reduces histopathological damage and improves behavioral outcome after experimental traumatic brain injury (TBI). Further investigations have clarified the mechanisms underlying the beneficial effects of hypothermia by showing that cooling reduces multiple cell injury cascades. The purpose of this study was to determine whether hypothermia could also enhance endogenous reparative processes following TBI such as neurogenesis and the replacement of lost neurons. Male Sprague-Dawley rats underwent moderate fluid-percussion brain injury and then were randomized into normothermia (37°C) or hypothermia (33°C) treatment. Animals received injections of 5-bromo-2′-deoxyuridine (BrdU) to detect mitotic cells after brain injury. After 3 or 7 days, animals were perfusion-fixed and processed for immunocytochemistry and confocal analysis. Sections were stained for markers selective for cell proliferation (BrdU), neuroblasts and immature neurons (doublecortin), and mature neurons (NeuN) and then analyzed using non-biased stereology to quantify neurogenesis in the dentate gyrus (DG). At 7 days after TBI, both normothermic and hypothermic TBI animals demonstrated a significant increase in the number of BrdU-immunoreactive cells in the DG as compared to sham-operated controls. At 7 days post-injury, hypothermia animals had a greater number of BrdU (ipsilateral cortex) and doublecortin (ipsilateral and contralateral cortex) immunoreactive cells in the DG as compared to normothermia animals. Because adult neurogenesis following injury may be associated with enhanced functional recovery, these data demonstrate that therapeutic hypothermia sustains the increase in neurogenesis induced by TBI and this may one of the mechanisms by which hypothermia promotes reparative strategies in the injured nervous system.
Keywords: Dentate gyrus, Doublecortin, Fluid-percussion, Hypothermia, Neurogenesis, Traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major clinical problem in the United States affecting both civilian and military populations (Faul, et al., 2010, Maas, et al., 2008, Martin, et al., 2008). Although a significant amount of information is now known regarding the pathophysiology of brain injury (Bigler and Maxwell, 2011, Bramlett and Dietrich, 2004), the successful translation to the clinic of therapeutic interventions shown to be promising in animal models has yet to be achieved (Maas, et al., 2010). One potential therapy that improves outcome in specific patient populations is therapeutic hypothermia (Bernard, et al., 2002, Eicher, et al., 2005, Holzer, et al., 2005, Marion and Bullock, 2009, Shankaran, et al., 2005). Therapeutic hypothermia reduces histopathological damage caused by brain injury by targeting multiple, specific secondary injury mechanisms such as elevations in intracranial pressure and inflammation (Bratton, et al., 2007, Dietrich and Bramlett, 2010, Jiang, et al., 2006, Marion and Bullock, 2009, Polderman, 2008, Qiu, et al., 2007). To facilitate the translation of hypothermia therapy to specific TBI patient populations, it is important to understand not only the secondary injury mechanisms targeted by hypothermia, but also the potentially reparative mechanisms that may be regulated by hypothermia (Clifton, et al., 2011, Dietrich, et al., 2009).
In the adult brain, stem cells reside in specific anatomical regions such as the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) (Doetsch, et al., 1997, Eriksson, et al., 1998, Gage, et al., 1998, Gould, et al., 1999, Johansson, et al., 1999). The potential for neurogenesis to occur in the adult nervous system has stimulated investigations into whether this cellular process plays a role in functional recovery after adult brain injury (Emsley, et al., 2005). Indeed, various studies have reported evidence for increased neurogenesis in animal models of global and focal cerebral ischemia, epilepsy and TBI (Arvidsson, et al., 2002, Braun, et al., 2002, Covolan, et al., 2000, Felling and Levison, 2003, Jessberger, et al., 2007, Jin, et al., 2001, Kee, et al., 2001, Liu, et al., 1998, Parent, et al., 1997). In the area of TBI, stimulation of neurogenesis has been observed in both the SVZ and SGZ using cell proliferation markers such as 5-bromo-2′-deoxyuridine (BrdU) (Chirumamilla, et al., 2002, Dash, et al., 2001, Emery, et al., 2005, Kernie, et al., 2001, Sun, et al., 2005). In a study by Urrea et al. (2007), BrdU and NeuN double-staining provided evidence that some of these newly generated cells develop neuronal phenotypes as soon as 5 days after injury. Other studies have provided evidence that some of these proliferating cells become neurons using doublecortin (DCX), which selectively marks migrating neuroblasts and immature neurons (Barha, et al., 2011, Brown, et al., 2003, Couillard-Despres, et al., 2005, Francis, et al., 1999, Gleeson, et al., 1999, Rao and Shetty, 2004, Rola, et al., 2006, Verwer, et al., 2007). Neurogenesis and glial proliferation in the SVZ have been reported to persist for up to a year after brain trauma, but whether this is sustained in the SGZ of the hippocampus is unclear (Atkins, et al., 2010, Chen, et al., 2003, Gao, et al., 2008). Taken together, these data indicate the potentially important role of neurogenesis in the endogenous reparative response of the brain after TBI (Blaiss, et al., 2011).
New findings indicate that some anti-inflammatory treatments may actually improve recovery by promoting neurogenesis (Barha, et al., 2011, Pedersen, et al., 2009, Whitney, et al., 2009). Because hypothermia is a potent anti-inflammatory agent (Chatzipanteli, et al., 2000, Globus, et al., 1995, Goss, et al., 1995, Kinoshita, et al., 2002, Vitarbo, et al., 2004, Whalen, et al., 1997), this suggests that TBI-induced neurogenesis may potentially be affected by temperature manipulations In global ischemia, hypothermia has been found to either increase or have no effect on the survival of newly generated dentate granule cells (Lasarzik, et al., 2009, Silasi and Colbourne, 2011), but whether hypothermia affects TBI-induced neurogenesis is unknown. Thus, we determined whether posttraumatic hypothermia would sustain the increase in neurogenesis observed in the SGZ of the hippocampus after TBI using a trauma model known to be sensitive to mild hypothermia (Atkins, et al., 2007, Bramlett, et al., 1997, Dietrich, et al., 1994, Lotocki, et al., 2006).
Materials and Methods
Animals
Male Sprague-Dawley rats (n=42, 276-395 g, Charles Rivers Laboratories, Wilmington, MA, USA) were used in all experiments. Animal care was in accordance with the guidelines set forth by the University of Miami Animal Care and Use Committee and the NIH Guide for the Care and Use of Laboratory Animals. Animals were housed in a constant temperature-controlled room (22°C) for at least 7 days before the study and exposed to a 12-hour light-dark cycle. Rats were allowed free access to water and food, but food was withheld overnight before the brain injury surgery.
Fluid-percussion injury surgery
The parasagittal fluid-percussion (FP) injury surgery was performed as previously described (Atkins, et al., 2010, Lotocki, et al., 2009). Rats were anesthetized with 3% isoflurane, 70% N2O, and 30% O2 and received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) to anchor a modified plastic syringe hub (3.5 mm inner diameter, PrecisionGlide Needle; Becton Dickinson, Franklin Lakes, NJ, USA) over the exposed dura of the right parietal cortex.
Twenty-four hr after the craniotomy, animals were anesthetized with 3% isoflurane, 70% N2O, and 30% O2 and then intubated and mechanically ventilated (Stoelting, Wood Dale, IL, USA) with 0.5-1.0% isoflurane, 70% N2O, and 30% O2. To ensure consistent physiological responses among animals, the femoral artery was cannulated to monitor blood gases (pO2 and pCO2), blood pH, and mean arterial blood pressure (MABP), which were maintained within standard ranges from 15 min prior to TBI and for up to 4 hr after TBI. Pancuronium bromide (1.0 mg/kg) was intravenously administered to facilitate mechanical ventilation. A subgroup of sham-operated (n=6) and TBI rats (n=6) received an intraperitoneal (IP) injection of BrdU (50 mg/kg) a marker for DNA replication at both 24 hr and 30 min before injury (Gratzner, 1982, Miller and Nowakowski, 1988). Additional IP injections of BrdU were given every day after surgery until sacrifice.
To induce TBI, a moderate FP pulse (1.8-2.1 atm) was delivered to the right parietal cortex. Sham animals underwent all surgical procedures except for the FP pulse. Rectal and temporalis muscle thermistors measured core and brain temperatures, respectively, using self-adjusting feedback warming lamps (Atkins, et al., 2010, Lotocki, et al., 2009). Moderate hypothermia was induced by blowing cold air over the animals’ heads starting 30 min after TBI to maintain a brain temperature between 33.0-33.6°C. Sham animals received normothermia treatment identical to the normothermia TBI animals. Normothermic animals were maintained at a brain temperature of 36.6-37.2°C. After TBI or sham injury, hypothermia was maintained for 4 hr followed by a slow rewarming period at ambient temperatures of 22-23°C.
Immunohistochemical analysis
At 3 (n=21) or 7 days (n=21) after TBI or sham procedures, animals were anesthetized using 3% isoflurane, 70% N2O and 30% O2 and transcardially perfused with saline (80 ml) and then with 4% paraformaldehyde (4°C, 350 ml). The brains were cryoprotected with 30% sucrose in phosphate-buffered saline (PBS) and sectioned in PBS (60 μm thick) with a sliding microtome (LEICA SM200R, Leica Microsystems, Inc., Buffalo Grove, IL, USA). Serial sections spaced 360 μm apart were immunostained with anti-doublecortin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as previously described (Atkins, et al., 2010) or with mouse anti-BrdU antibody (1:100, Roche, Indianapolis, IN, USA). Development of the immunostaining was done with anti-goat IgG (1:200, Vector Laboratories, Burlingame, CA, USA), anti-mouse IgG or anti-rat IgG at a concentration of 1:200, ABC Elite (Vector Laboratories) and 2.5% nickel ammonium sulfate 0.05% 3,3′-diaminobenzidine, and 0.001% H2O2 in acetate-imidazole buffer (NiDAB, Vector Laboratories). All of the sections were then dehydrated and cover-slipped. A subgroup of sections were double-labeled using mouse anti-BrdU antibody (1:10, Roche, Indianapolis, IN, USA) and goat anti-doublecortin (1:150, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-NeuN antibody (1:150, Chemicon Inc., Billerica, MA, USA) in combination with anti-mouse and anti-goat Alexa Fluor Dye (488 and 594, Invitrogen, Eugene, OR, USA) as secondary antibodies for confocal imaging. Control sections without primary antibody were evaluated for non-specific labeling.
Stereological cell counting
Prior to stereology, antibody penetration was verified to be complete in all sections at 63x magnification. The dentate granule cell layer was contoured using a BX51TRF Olympus microscope (Olympus America, Center Valley, PA, USA) at 10x magnification in combination with StereoInvestigator software v9.10.2 (MicroBrightfield, Inc., Williston, VT, USA). The observer was blinded for all analyses. Quantitative assessment of the BrdU and DCX immunoreactive cells were conducted according to methods previously described by Lotocki and colleagues (Lotocki, et al., 2011). Sections between bregma levels −3.6 and −4.8 mm, near the epicenter of the injury (bregma −3.8mm), were chosen for analysis. A counting grid of 70×70 μm was placed over the dentate granule cell layer. Section thickness was determined to be 15 μm, the optical dissector height was set at 9 μm, and guard zones were set at 3 μm. At 63x magnification (NA 1.42), a 35×35 μm counting frame was used for counting 38-145 randomly placed sampling sites. Q values for the DCX cell counts ranged between 197-677, the CE range was 0.04-0.07 and the CV range was 0.073-0.237. The CE2/CV2 values for the DCX cell counts were 0.07 for the 3 day normothermia group, 0.11 for the 3 day hypothermia group, 0.06 for the 7 day hypothermia group and 0.27 for the 7 day hypothermia group and 0.32 for the sham group. Images were taken with a BX51TRF Olympus microscope (Olympus America) and a Microfire A/R camera (Optronics Goleta, CA, USA) at 20x and montaged using the virtual slice module in the Neurolucida software program (MicroBrightfield, Inc.).
Statistical analyses
Physiological parameters were analyzed using one-way ANOVA test with Tukey’s multiple comparison post test. BrdU counts were analyzed using a one way ANOVA with Fisher LSD procedure and DCX counts were analyzed using a two-way ANOVA followed by a Fisher LSD posthoc test. Significance was defined when p<0.05. All analyses were performed using SigmaStat 3.01 Software (Systat Software Inc.San Jose, Ca, USA).
Results
Physiological studies
All physiological variables were within normal ranges (pH, pO2, pCO2, MABP) before and following the traumatic insult (Table 1). Significant differences were seen in the animals’ temperature recordings within the hypothermia group compared to normothermia. No other significant differences in physiological measurements were seen between the experimental groups.
Table 1.
Physiologic parameters (mean ± SEM)
| Normothermia |
Hypothermia |
|||||
|---|---|---|---|---|---|---|
| Sham 3d | Sham 7d | TBI 3d | TBI 7d | TBI 3d | TBI 7d | |
| Pretrauma | ||||||
| pH | 7.46 ± 0.02 | 7.47 ± 0.02 | 7.47 ± 0.01 | 7.47 ± 0.01 | 7.48 ± 0.01 | 7.47 ± 0.01 |
| pCO2 | 40.03 ± 0.97 | 39.70 ± 1.56 | 37.87 ± 0.83 | 38.09 ± 0.95 | 38.42 ± 1.13 | 38.49 ± 1.19 |
| pO2 | 166.00 ± 7.37 | 168.33 ± 9.00 | 170.17 ± 9.02 | 161.44 ± 9.94 | 169.33 ± 2.74 | 157.00 ± 7.99 |
| MABP | 129.60 ± 9.63 | 120.30 ± 2.02 | 125.72 ± 4.62 | 124.49 ± 3.05 | 124.52 ± 4.90 | 127.73 ± 2.97 |
| Brain temperature | 36.70 ± 0.06 | 36.70 ± 0.08 | 36.77 ± 0.03 | 36.72 ± 0.05 | 36.75 ± 0.07 | 36.77 ± 0.04 |
| Rectal temperature | 36.60 ± 0.10 | 36.53 ± 0.02 | 36.57 ± 0.11 | 36.71 ± 0.08 | 36.67 ± 0.11 | 36.71 ± 0.07 |
| Post-trauma | ||||||
| pH | 7.43 ± 0.02 | 7.45 ± 0.01 | 7.43 ± 0.01 | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.44 ± 0.01 |
| pCO2 | 36.57 ± 0.99 | 37.15 ± 0.61 | 39.05 ± 0.58 | 37.70 ± 0.65 | 38.02 ± 1.16 | 37.16 ± 0.56 |
| pO2 | 150.33 ± 19.37 | 126.00 ± 5.63 | 127.33 ± 4.61 | 136.56 ± 5.94 | 145.17 ± 7.25 | 152.22 ± 5.78 |
| MABP | 115.67 ± 6.48 | 117.67 ± 4.14 | 114.22 ± 6.64 | 120.37 ± 3.75 | 115.07 ± 4.87 | 114.41 ± 3.06 |
| Brain temperature | 36.77 ± 0.07 | 36.78 ± 0.05 | 36.68 ± 0.03 | 36.70 ± 0.05 | 33.02 ± 0.05* | 33.29 ± 0.1* |
| Rectal temperature | 36.73 ± 0.03 | 36.83 ± 0.04 | 36.77 ± 0.05 | 36.83 ± 0.06 | 33.92 ± 0.03* | 33.71 ± 0.12* |
All physiolog ical variables were within norm al range throughout the experiments.
p<0.001 compared with normothermia
BrdU findings
At both 3 and 7 days after TBI, BrdU immunoreactive cells were observed within the ipsilateral and contralateral dentate gyrus of the hippocampus (Fig. 1). These immunoreactive cells frequently resided in the subgranular zone at the junction between the granular cell layer and the hilus of the hippocampal dentate gyrus. In the hypothermic animals, clusters of BrdU positive cells could be frequently seen in the dentate gyrus.
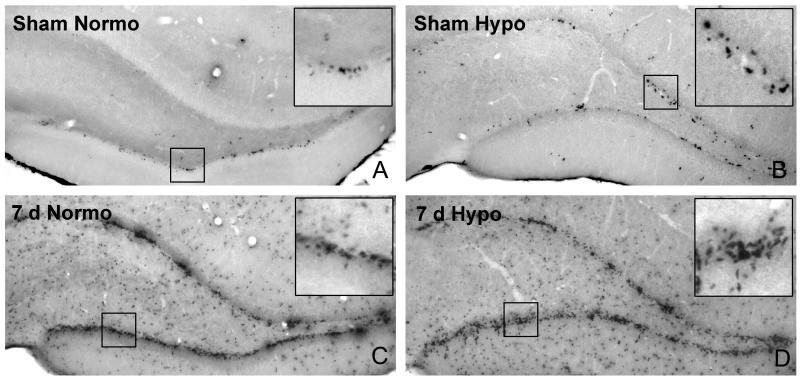
Fig. 1.
Representative BrdU immunostained micrographs of the ipsilateral dentate gyrus 7 days post-injury. A. Non-injured sham-operated normothermic (37°C) dentate gyrus shows few BrdU positive cells. B. Induced hypothermia (33°C) in sham-operated control animals also results in few BrdU positive cells. C. At 7 days after moderate fluid percussion brain injury, an abundant proliferation of BrdU positive cells is observed. D. Post-traumatic hypothermia appears to increase numbers of BrdU stained cells compared to normothermic TBI. Insert shows local aggregates of positive cells in dentate gyrus.
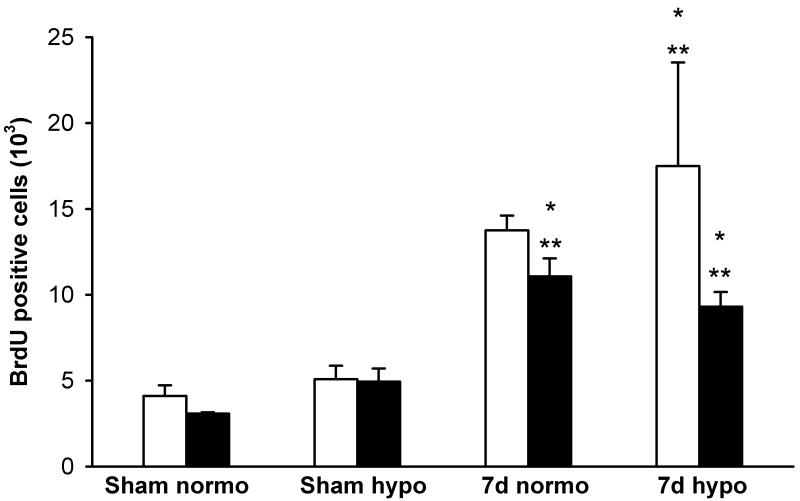
Importantly, TBI animals that received posttraumatic hypothermia had an increase in a number of BrdU-positive cells in the granular cell layer and SGZ of the dentate gyrus compared to normothermic animals at seven days posttrauma. Quantitative data supported the concept of increased cellular proliferation in the contralateral dentate gyrus at 7 days after TBI in both normothermic and hypothermic animals compared to sham-operated groups (*p<0.03 vs sham-normo, **P<0.03 vs sham-hypo, Fig. 2). At 7 days, the frequency of BrdU positive cells was significantly increased in the ipsilateral TBI-hypothermic group compared to both sham-operated groups (*p<0.03 vs sham-normo, **P<0.03 vs sham-hypo).
Fig. 2.
Mean (±SEM) bar graph summarizing unbiased stereological quantitative cell counts of BrdU-positive cells in sham-operated and F-P injured rats. White bar represents ipsilateral or injured hemisphere and black bar represents contralateral hemisphere. At 7 days after normothermic TBI, contralateral numbers of BrdU positive cells are increased compared to sham-operated rats (*p<0.03 vs sham-normo, **P<0.03 vs sham-hypo). At 7 days after F-P injury, post-traumatic hypothermia leads to increased numbers of positive cells within the ipsilateral and contralateral dentate gyrus compared to normothermia treated animals (*p<0.03 vs sham-normo, **P<0.03 vs sham-hypo).
Doublecortin results
Similar to that seen with BrdU, a high frequency of DCX immunoreactive cells were observed at 3 and 7 days after TBI. Visualization of cell bodies, axons and dendrites of the stained cells are clearly seen within the ipsilateral hemisphere (Fig. 3). At 7 days after injury, a high frequency of DCX/NeuN stained cells were present within the subgranular zone of the hippocampus.
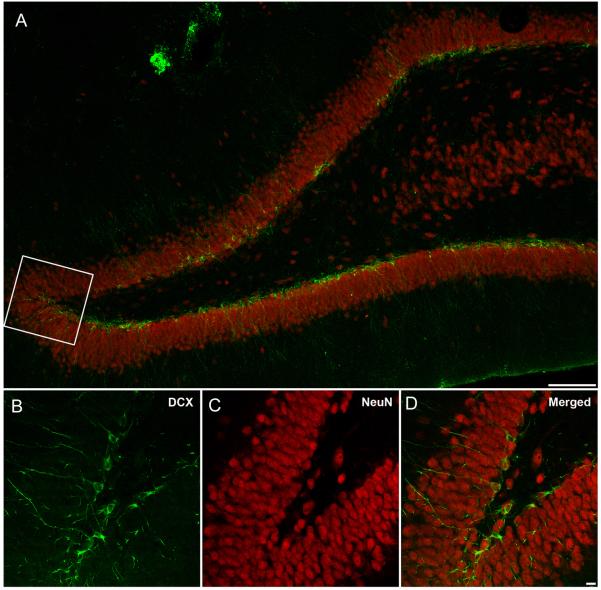
Fig. 3.
Confocal microscopy images double-labeled with NeuN (red) and DCX (green) at 7 days after F-P injury. A. DCX immunopositive cells are primarily located throughout the granular layer of the dentate gyrus on the injured hemisphere (Bar 100 μm). B-D. Higher magnification of the inset area showing DCX (B), NeuN (C) and double-labeled cell bodies and processes (D). Note the cellular processes extending throughout the granular cell layer of the dentate gyrus (Bar 10 μm) .
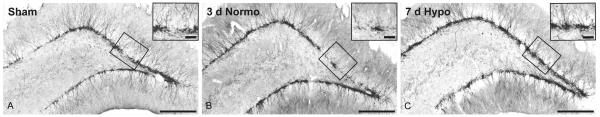
To quantify the number of DCX immunoreactive cells in the dentate gyrus, immunostaining using DCX and DAB was performed. As shown in Fig. 4, a high frequency of immunoreactive cells were observed throughout the subgranulayer of the dentate gyrus. Immunoreactive cells displayed extensive processes extending throughout the cellular layers of the DG. As seen in Fig. 4C, there was an apparent increase in the frequency of DCX stained cells in animals where posttraumatic hypothermia was produced. Frequently, an aggregation of immunoreactive cells could be visualized in hypothermic TBI rats.
Fig. 4.
Immunocytochemical stained images show DCX positive cells in the dentate gyrus of representative animals 3 and 7 days after TBI. A. Sham-operated animal, B. Normothermic TBI animal and C. Hypothermic TBI animal. An apparent increased frequency of DCX stained cells is observed with post-traumatic hypothermic treatment (Bar 250 μm). Insert shows local aggregates of positive cells in the dentate gyrus (Bar 50 μm).
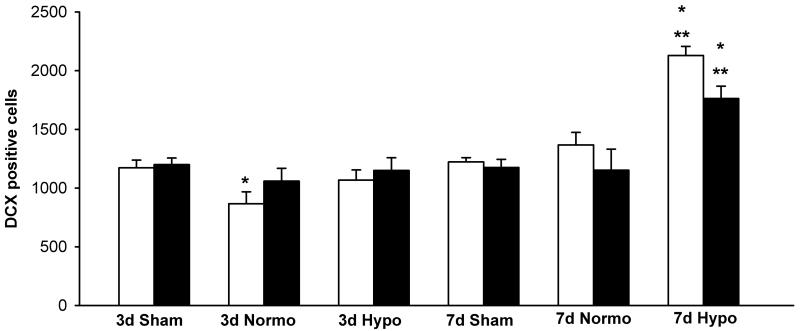
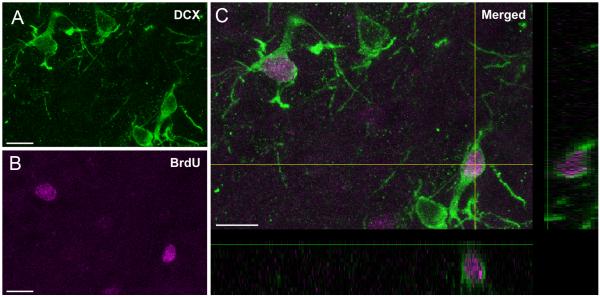
Quantitative assessment supported an increased frequency of DCX stained cells at 7 days as compared to sham-operated and normothermic TBI animals. Two-way ANOVA was significant (p<0.05) for group, time and group x time for both ipsilateral and contralateral hemispheres (Fig. 5). Posthoc analysis for the ipsilateral, injured hemisphere indicated that 3 day normothermic animals were significantly different from 3 day sham values (*p<0.05). Furthermore, ipsilateral and contralateral hemispheres for the 7 day DCX immunostained cells were significantly elevated in TBI-hypothermic animals compared to 7 day sham (*p<0.05) and TBI-normothermic (**p<0.05) groups. Confocal images showed double-labeling of DCX positive cells with BrdU, indicating evidence for local neurogenesis and cell survival at 7 days after TBI (Fig. 6)
Fig. 5.
Bar graphs summarizing nonbiased stereological DCX-positive cell counts at 3 and 7 days after injury (mean ± SEM). White bars represent ipsilateral or injured hemispheres and black bars represent contralateral hemispheres. Three day data were only statistically significant for the ipsilateral hemisphere of the TBI-normothermic group. These TBI animals had a small but significant reduction of DCX-positive cells compared to 3 day sham (*p<0.05 vs 3d Sham). In contrast to the 3 day data, there were more robust findings for the 7 day TBI-hypothermic animals. These data were statistical significant (*p<0.05 vs 7d sham, **p<0.05 vs 7d Normo) for both sham and normothermic animals in both ipsilateral and contralateral hemispheres. Therefore, hypothermia increases the number of DCX positive cells after TBI.
Fig. 6.
Confocal micrographs demonstrate double-labeled neurons with A. BrdU (pink) and B. DCX (green) at 7 days after F-P injury in a hypothermic rat. C. A subpopulation of BrdU and DCX immunostained cells were observed thoughout the subgranular layer of the DG. Images generated from xz and xy planes further demonstrate BrdU labeling within the DCX labeled cell. (Bar 10 μm).
Discussion
TBI produces long-term neurodegenerative changes and a spectrum of behavioral abnormalities resulting from regional patterns of cell death and circuit dysfunction (Bramlett, et al., 1997, Dixon, et al., 1999, Pierce, et al., 1998, Smith, et al., 1997). Recent experimental studies have concentrated on developing therapeutic strategies that protect vulnerable brain regions from secondary injury mechanisms as well as promote endogenous reparative strategies (Hallbergson, et al., 2003, Picard-Riera, et al., 2004, Raghupathi and McIntosh, 1998). Ultimately, an approach whereby the therapeutic benefit is both neurorestorative and neuroprotective may be most beneficial in terms of promoting functional recovery in patients with severe TBI (Lu, et al., 2011, Urrea, et al., 2007). Our data demonstrate that early cooling after moderate TBI leads to a sustained increase in neurogenesis in the dentate gyrus as compared to normothermic injured animals. These findings suggest that in addition to protecting the traumatized brain from structural damage, early cooling may promote cellular reparative strategies important for memory functioning (Blaiss, et al., 2011, Kitamura, et al., 2009).
Previous studies have reported increases in cellular proliferation in the dentate gyrus and SVZ after TBI and other CNS insults (Blaiss, et al., 2011, Dash, et al., 2001, Kernie, et al., 2001, Parent, et al., 1997, Ramaswamy, et al., 2005, Richardson, et al., 2007, Whitney, et al., 2009, Yu, et al., 2008). These studies have found that several cell types proliferate after TBI, including microglia-macrophages and astrocytes, as well as neurons (Chirumamilla, et al., 2002, Urrea, et al., 2007). Chen and colleagues (2003) used markers of cell proliferation, including Ki-67 and the proliferative cell nuclear antigen, to detect metabolically active cells and neurogenesis for up to 1 year following trauma in the SVZ. Thus, data from a variety of studies provide evidence for neurogenesis being activated after trauma, which may contribute to the spontaneous behavioral recovery seen after TBI (Kernie, et al., 2001). However, a concern of adult neurogenesis after TBI or other CNS insults is that the increase in cell proliferation and survival of immature neurons does not last, with more chronic studies showing decreases in immature neuronal cell markers at 12-16 weeks post-injury (Atkins, et al., 2010, Gao, et al., 2008, Potts, et al., 2009, Rola, et al., 2006, Takasawa, et al., 2002, Whitney, et al., 2009). Thus, although there appears to be an initial burst in cellular proliferation and neurogenesis in the SGZ after trauma, these early events may not be adequate to maintain this population of cells (Dash, et al., 2001, Urrea, et al., 2007). This is an important consideration since newly generated cells in the DG need at least four weeks to become functional neurons (Kempermann, et al., 2004, Koch, et al., 2008).
In this regard, despite the burst of neurogenesis in the early recovery stages after TBI, many studies have documented chronic hippocampal learning deficits (Bramlett, et al., 1997, Dash, et al., 1995, Pierce, et al., 1998, Smith, et al., 1991). Similarly, studies using genetic ablation or pharmacological interventions that inhibit neurogenesis indicate that neurogenesis is critical for specific aspects of spatial memory formation (Doetsch, et al., 1999, Lau, et al., 2009, Noonan, et al., 2010). In a study by Blaiss and colleagues (2011), the selective ablation of dividing stem cell progenitors with ganciclovir resulted in a reduction in the progenitor pool and impaired cognitive recovery in a nestin-HSV-TK transgenic trauma model. Thus, evidence currently supports a functional role for adult neurogenesis in memory formation during normal adult brain maturation and after CNS injury, suggesting that therapies that sustain the increase in neurogenesis after TBI may promote recovery of hippocampal functioning (Deng, et al., 2010).
Recent data indicate that immature neurons generated after TBI or other brain insults may be particularly sensitive to environmental factors as well as secondary injury consequences, again questioning the long-term benefits of this cellular response to trauma (Whitney, et al., 2009). In a study by Back and colleagues (2002), progenitor cells were shown to be highly vulnerable to hypoxic ischemic injury. The release of inflammatory factors from resident microglia and activated astrocytes as well as the recruitment of monocytes and lymphocytes from the blood circulation may also contribute to the suppression of long-term neurogenesis after TBI (Liu, et al., 2005, Peng, et al., 2008, Whitney, et al., 2009). Interestingly, evidence for blocking inflammatory events after CNS injury may augment neurogenesis (Whitney, et al., 2009). In one study, interleukin-6 blockage alone appeared sufficient to restore neurogenesis, a consequence associated with decreased microglial activation (Vallieres, et al., 2002). Nitric oxide (NO) is produced by multiple cell types after brain injury, and previous studies have reported that NO produced by nNOS inhibits neurogenesis (Moreno-Lopez, et al., 2004, Packer, et al., 2003). Other investigations emphasize the vulnerability of immature neurons to cell death through apoptotic mechanisms (Ekdahl, et al., 2003). In this regard, excitotoxic damage, the production of oxygen free radicals and inflammation has been reported to be sensitive to posttraumatic temperature manipulations (Chatzipanteli, et al., 2000, Globus, et al., 1995, Kinoshita, et al., 2002, Lotocki, et al., 2006, Vitarbo, et al., 2004). In the present study, early cooling after TBI increased cellular proliferation and the frequency of DG DCX-immunoreactive cells at the 7 day posttraumatic period. Taken together, these findings indicate that posttraumatic cooling could target early progenitor cell death mechanisms involving excitotoxicity or inflammatory events, resulting in longer survival and the subsequent differentiation of immature cells.
Another mechanism that hypothermia may be working through to sustain neurogenesis is through inhibiting apoptosis of the newly generated neurons. In a study by Atkins and colleagues (2007), posttraumatic hypothermia potentiated extracellular signal-regulated kinase 1/2 (ERK1/2) activation and its downstream effectors including the transcription factor cAMP response element-binding protein (CREB). In that study, ERK1/2 was selectively activated within dentate granular cells particularly at the SGZ. In culture, neural progenitor cell proliferation has been reported to occur through fibroblast growth factor activating the ERK1/2 pathway (Kalluri, et al., 2007). In the non-injured brain, infusion of brain-derived neurotrophic factor (BDNF), an activator of ERK1/2 signaling, induces hippocampal neurogenesis and neuronal differentiation and survival (Kirschenbaum and Goldman, 1995, Leventhal, et al., 1999, Scharfman, et al., 2005, Suh, et al., 2009). Conditions like physical exercise and enriched environments increase endogenous BDNF levels as well as stimulate SGZ neurogenesis and improve outcome after TBI (Gaulke, et al., 2005, Kempermann, et al., 1997, Komitova, et al., 2005, Lee, et al., 2000, Passineau, et al., 2001, Rossi, et al., 2006, Russo-Neustadt, et al., 2004). Thus, the effects of hypothermia on the ERK1/2 pathway and pro-survival signaling in immature neurons could explain, in part, the beneficial effects of early cooling on cognitive recovery after TBI.
Posttraumatic epilepsy (PTE) is commonly observed in patients with moderate to severe TBI and promotes secondary injury processes and long-term cognitive deficits (Bao, et al., 2011, Vespa, et al., 2010). In models of epilepsy, evidence for acute increases in neurogenesis, similar to that seen after TBI have been reported (Parent, et al., 1997, Scott, et al., 2000). Although severe seizures caused higher levels of cell proliferation in the DG than mild seizures, less new cells differentiated into neurons after severe seizures (Bonde, et al., 2006). In a recent study by Atkins and colleagues (2010) posttraumatic cooling reduced the incidence and severity of PTEs. However, numbers of DG DCX-positive cells remained reduced in both normothermic and hypothermic PTE animals at 12 weeks compared to sham controls. Based on these and other published data from the ischemia field regarding chronic neuronal protection (Corbett, et al., 2000, Dietrich, et al., 1993), it may be interesting to suggest that extended post-traumatic cooling durations may also be required to promote the long-term survival of immature neurons after TBI (Silasi and Colbourne, 2011). Also, the possibility of combining early cooling with pharmacological agents that induce neurogenesis may also be important to consider for future investigations (Pieper, et al., 2011).
In summary, our data show that early cooling may sustain early endogenous reparative strategies after TBI. These studies emphasize that therapeutic hypothermia, in addition to reducing early injury cascades, may also promote cell proliferation and neurogenesis within vulnerable brain regions. These findings may help clarify the mechanisms by which hypothermia promotes cognitive function in the early stages after TBI. Additional work is necessary to clarify the molecular processes by which temperature is affecting this important cellular response to injury. It is anticipated that this line of research could promote the successful translation of therapeutic hypothermia to the clinical arena for specific TBI patient populations to provide better functional recovery.
Research Highlights.
Posttraumatic hypothermia increases the frequency of BrdU positive cells in the dentate gyrus
This posttraumatic temperature manipulation also increases frequency of immature neurons
Doublecortin immunoreactive cells are visualized in the subgranular layer of the dentate gyrus
Cooling may both protect and promote reparative strategies after TBI
Acknowledgements
The authors wish to thank Jeremy Lytle for editorial support. This study was supported by the National Institutes of Health grants NS030291 and NS42133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr., Alonso OF, Chen S, Bramlett HM, Hu BR, Dietrich WD. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007;26:810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Truettner JS, Lotocki L, Sanchez-Molano J, Kang Y, Alonso OF, Sick TJ, Dietrich WD, Bramlett HM. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. European Journal of Neuroscience. 2010;28:35–42. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YH, Bramlett HM, Atkins CM, Truettner JS, Lotocki G, Alonso OF, Dietrich WD. Post-traumatic seizures exacerbate histopathological damage after fluid-percussion brain injury. J Neurotrauma. 2011;28:35–42. doi: 10.1089/neu.2010.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol. 2011;231:72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Maxwell WL. Neuroimaging and neuropathology of TBI. NeuroRehabilitation. 2011;28:63–74. doi: 10.3233/NRE-2011-0633. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci. 2006;23:965–974. doi: 10.1111/j.1460-9568.2006.04635.x. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: Similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: Effects of post-traumatic hypothermia. Acta Neuropathol (Berl) 1997;93:190–199. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermia. J Neurotrauma. 2007;24(Suppl 1):S21–25. doi: 10.1089/neu.2007.9993. [DOI] [PubMed] [Google Scholar]
- Braun H, Schafer K, Hollt V. bIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: Biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Chen XH, Iwata A, Nonaka M, Browne KD, Smith DH. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J Neurotrauma. 2003;20:623–631. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, Conley A, Puccio A, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Hamilton M, Colbourne F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. 2000;163:200–206. doi: 10.1006/exnr.2000.7369. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Covolan L, Ribeiro LT, Longo BM, Mello LE. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol (Berl) 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26:301–312. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery DL, Fulp CT, Saatman KE, Schutz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22:978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths. Centers for Disease Control and Prevention; National Center for Injury Prevention and Control Atlanta, GA: 2010. [Google Scholar]
- Felling RJ, Levison SW. Enhanced neurogenesis following stroke. J Neurosci Res. 2003;73:277–283. doi: 10.1002/jnr.10670. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulke LJ, Horner PJ, Fink AJ, McNamara CL, Hicks RR. Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res Mol Brain Res. 2005;141:138–150. doi: 10.1016/j.molbrainres.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: Effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW, DeKosky ST. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma. 1995;12:159–167. doi: 10.1089/neu.1995.12.159. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414–418. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr., Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Xu W, Li WP, Gao GY, Bao YH, Liang YM, Luo QZ. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci. 2007;25:1041–1048. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1b messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: Importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci U S A. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Koch JD, Miles DK, Gilley JA, Yang CP, Kernie SG. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Lasarzik I, Winkelheide U, Thal SC, Benz N, Lorscher M, Jahn-Eimermacher A, Werner C, Engelhard K. Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth Analg. 2009;109:1632–1639. doi: 10.1213/ANE.0b013e3181bab451. [DOI] [PubMed] [Google Scholar]
- Lau BW, Yau SY, Lee TM, Ching YP, Tang SW, So KF. Intracerebroventricular infusion of cytosine-arabinoside causes prepulse inhibition disruption. Neuroreport. 2009;20:371–377. doi: 10.1097/WNR.0b013e328324edcd. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari JP, Perez E, Sanchez J, Alonso O, Bramlett HM, Dietrich WD. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of posttraumatic hypothermia. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0802. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotocki G, de Rivero Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane RW, Dietrich WD. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. Eur J Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Lotocki G, Vaccari Jde R, Alonso O, Molano JS, Nixon R, Safavi P, Dietrich WD, Bramlett HM. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neuroscience letters. 2011;499:143–148. doi: 10.1016/j.neulet.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Sun CL, Wo PY, Yen HH, Tang TH, Ng MC, Huang ML, Yang YL. Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J Neurotrauma. 2011;28:441–450. doi: 10.1089/neu.2010.1473. [DOI] [PubMed] [Google Scholar]
- Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma. 2009;26:455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- Martin EM, Lu WC, Helmick K, French L, Warden DL. Traumatic brain injuries sustained in the Afghanistan and Iraq wars. Am J Nurs. 2008;108:40–47. doi: 10.1097/01.NAJ.0000315260.92070.3f. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp Neurol. 2001;168:373–384. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- Pedersen MO, Larsen A, Pedersen DS, Stoltenberg M, Penkova M. Metallic gold treatment reduces proliferation of inflammatory cells, increases expression of VEGF and FGF, and stimulates cell proliferation in the subventricular zone following experimental traumatic brain injury. Histol Histopathol. 2009;24:573–586. doi: 10.14670/HH-24.573. [DOI] [PubMed] [Google Scholar]
- Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, Zheng J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008;56:903–916. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76:223–231. doi: 10.1002/jnr.20040. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, Capota M, Britt JK, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2011;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Potts MB, Rola R, Claus CP, Ferriero DM, Fike JR, Noble-Haeusslein LJ. Glutathione peroxidase overexpression does not rescue impaired neurogenesis in the injured immature brain. J Neurosci Res. 2009;87:1848–1857. doi: 10.1002/jnr.21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W, Chen K, Zhou J, Xu Z. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22:229–235. doi: 10.1016/j.jcrc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, McIntosh TK. Pharmacotherapy for traumatic brain injury: a review. Proc West Pharmacol Soc. 1998;41:241–246. [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169–181. doi: 10.1016/j.nec.2006.10.007. xi. [DOI] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Silasi G, Colbourne F. Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. J Cereb Blood Flow Metab. 2011;31:1725–1735. doi: 10.1038/jcbfm.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Pierce JE, Wolf JA, Trojanowski JQ, Graham DI, McIntosh TK. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22:299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Sluiter AA, Balesar RA, Baayen JC, Noske DP, Dirven CM, Wouda J, van Dam AM, Lucassen PJ, Swaab DF. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007;130:3321–3335. doi: 10.1093/brain/awm264. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur DL, Xu Y, Eliseo M, Etchepare M, Dinov I, Alger J, Glenn TP, Hovda D. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor a expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Clark RS, Marion DW, DeKosky ST, Heineman S, Schiding JK, Memarzadeh F, Kochanek PM. The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J Neurotrauma. 1997;14:561–572. doi: 10.1089/neu.1997.14.561. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]