Abstract
Background
Prior studies indicate that the biochemical alterations of depressive episodes result in decreased serum zinc concentrations. Given these findings, it is plausible that consistently low dietary zinc intakes contribute to depressive symptoms, yet epidemiological data are lacking. The authors tested the hypothesis that low zinc intake is associated with depressive symptoms using cross-sectional data from the population-based Boston Area Community Health survey (2002–2005).
Methods
Dietary and supplement use data were collected by validated food frequency questionnaire. Current depressive symptoms were assessed by the abridged validated Center for Epidemiologic Studies Depression scale and analyzed using multivariate logistic regression, adjusting for sociodemographic, health and lifestyle characteristics.
Results
Results showed an interaction (P=0.03) with gender, whereby zinc was associated with depressive symptoms in women (N=2,163), but not men (N=1,545). Women with low dietary or supplemental zinc intake were more likely to have depressive symptoms (e.g., dietary zinc quartile 1 vs. 4, OR=1.76, 95% CI: 1.26, 2.45; P-trend=0.004; supplemental zinc P-trend=0.03). Associations were stronger among women using antidepressant medications (e.g., total zinc OR=4.75, 95% CI: 1.98, 11.4; P-trend=0.0005).
Limitations
The cross-sectional, observational nature of the study leaves uncertain whether the observed associations represent actual causal relationships between zinc intake and depressive symptoms.
Conclusions
These findings suggest: (1) gender-specific pathophysiological mechanisms of depression, (2) inadequate dietary zinc intake contributes to depressive symptoms in women, and (3) supplemental zinc is a beneficial adjunct to antidepressant therapy in women. Additional research on both men and women is needed to verify these novel findings. If confirmed by other studies, the potential importance of adequate zinc intake is underscored by the recognized limitations of pharmacotherapy for depression.
Keywords: Diet, Dietary Supplements, Zinc, Depression, Antidepressive Agents, Epidemiology
INTRODUCTION
Depression is a critical problem and a leading cause of disability worldwide (WHO 2001). In the U.S. alone, over 40 million people – roughly 1 in 5 adults – have depressive symptoms, the majority of whom are not receiving treatment (Shim et al. 2011). Even among those on antidepressants, a substantial proportion fail to achieve remission of depressive symptoms (Mauskopf et al., 2009; Rush et al., 2006). A growing body of evidence indicates a role for zinc in depression and the mechanisms of antidepressant medications. Initially, clinical studies revealed that major depressive disorder was accompanied by decreased serum zinc concentrations, which corresponded to the severity of depressive symptoms, suggesting that depression alters zinc homeostasis (Little et al., 1989; Maes et al., 1994; Maes et al., 1997; McLoughlin et al., 1990). Since then, various studies have confirmed that serum zinc was significantly lower during acute depressive episodes, and furthermore that levels were normalized after successful antidepressant pharmacotherapy (Nowak et al., 2003; Siwek et al., 2010).
There are numerous mechanisms by which zinc may play a role in depression. Zinc is an essential trace element found in abundance in the human brain where it acts as a neuromodulator, and zinc is required to regulate numerous aspects of cellular metabolism, including immune, antioxidant, transcription and replication functions (Bitanihirwe et al., 2009). Plausible pathways that zinc may affect depression and be capable of antidepressant function include NMDA receptor antagonism, inhibition of glycogen synthase-3β activity (Ilouz et al., 2002), and increasing levels of brain-derived neurotrophic factor (BNDF) (Bitanihirwe et al., 2009; Nowak et al., 2004). The mechanism most strongly supported by experimental evidence for decreased serum zinc during depressive episodes is the activation of inflammatory processes (Marcellini et al., 2008; Siwek et al., 2010; Szewczyk et al., 2010). Significant associations between treatment-resistant depression, lower serum zinc concentrations, and markers of the immune/inflammatory response (increased CD4+/CD9+ T-cell ratio, lower TSP, and lower serum Alb and Tf) suggest that lower serum zinc in depressive disorders is a marker of a (sub)chronic immune/inflammatory response (Maes et al., 1997).
To date, the presumed direction of the association is that biochemical alterations of depressive episodes result in decreased serum zinc concentrations. However, it is plausible that consistently low dietary zinc intakes could contribute to depressive symptoms by further lowering available zinc and thereby adversely affecting numerous relevant biochemical processes. A reasonable hypothesis is that zinc consumed through diet helps prevent or mediate depressive symptoms. The latter notion has been supported by preliminary clinical trials showing benefits of zinc supplementation on depressive symptoms. In small trial (n=14) of patients with major depression beginning antidepressant treatment, zinc supplements significantly promoted a reduction in depression rating scores (Nowak et al., 2003). A study of mood states among young women found that zinc supplementation (7 mg/d) plus multivitamin led to a significantly improved depression-dejection scores compared to multivitamin alone (Sawada et al. 2010).
Epidemiological data in this research area are lacking. While laboratory studies elucidate mechanisms and small trials support the utility of zinc supplements for depressive symptoms, no large population-based studies have examined the role of zinc intake in relation to depressive symptoms in the general adult population. One study of older European adults aged 60–84 y found that those with low dietary zinc intakes had lower serum zinc concentrations and were more likely to have depressive symptoms (Marcellini et al., 2006). A study of 46 female students aged 20–25 y in Iran showed that dietary zinc intake, which was significantly correlated with serum zinc levels, was inversely correlated with depressive symptoms (Amani et al., 2010). Neither study accounted for antidepressant treatment, zinc supplement use, or other medical, lifestyle, or sociodemographic factors that may have had a role in explaining the observed associations.
Our objective was to examine the association between dietary and supplemental zinc intake and depressive symptoms in a large sample of women and men from the general population. We use data from a racially/ethnically diverse population-based random sample survey considering numerous lifestyle and medical characteristics as well as antidepressant and zinc supplement use.
METHODS
Study Design and Population
We analyzed cross-sectional, observational epidemiological data from the Boston Area Community Health (BACH) Survey, a population-based, random stratified cluster sample survey (McKinlay et al., 2007). From 2002–2005, BACH recruited 2,301 men and 3,201 women aged 30–79 y from three racial/ethnic groups (Hispanic, non-Hispanic black, and non-Hispanic white) to be representative of those in Boston, MA, USA. Data were obtained during a 2-hour, in-person home interview by a trained phlebotomist-interviewer. All participants provided written informed consent. The study was approved by the New England Research Institutes’ Institutional Review Board.
The final sample size for this analysis was 3,708 total (2,163 women, 1,545 men). Excluded were those who did not return the FFQ (615 women, 430 men), reported an implausible daily energy intake (women: <600 or >3,500 kcal/day; men: <800 or >4,200 kcal/d) or omitted ≥60 of the 103 dietary questions (423 women, 326 men). Compared to the larger BACH sample, the resulting analytic sample had fewer Hispanics and more whites, but there were no appreciable differences in gender, age, or BMI.
Dietary Assessment
Participants completed the English or Spanish SWAN 01/02 version of the 1995 Block FFQ designed to ascertain diet from multiethnic populations (Block et al., 1986; Huang et al., 2002). Both versions have been validated in various settings with moderate-to-high validity and reliability (Block et al., 1986; Block et al., 2006; Boucher et al., 2006).
The FFQ asks respondents to provide data on their usual eating habits over the past year, by marking frequency of consumption of over 100 core foods listed and their average serving sizes, given photographs of food models. The FFQ also obtains data on vitamin and mineral supplement use. Nutrient contents were computed based on values of the US Dept. of Agriculture nutrient database for standard reference and data provided by manufacturers of processed foods and supplements.
Depressive Symptoms Assessment
Depressive symptoms were assessed using the abridged Center for Epidemiologic Studies depression scale (CES-D) (Turvey et al., 1999). The abridged CES-D consists of eight yes/no questions. The presence of ≥5 depressive symptoms is an indicator of moderate-to-severe depression (Turvey et al., 1999). This measure has been independently validated in a number of studies, including the Asset and Health Dynamics (AHEAD) study (Turvey et al., 2009), and was found to have high internal consistency and convergent validity in the BACH Survey data (unpublished data).
Ascertainment of Antidepressant Medication Use
A complete inventory of prescribed and over-the-counter medications was taken during the home interview. Participants gathered all medications used within the past four weeks and described any additional medications to the interviewer to record. Medication labels and/or responses were coded using the Slone Drug Dictionary (Kelley et al., 2003), which classifies into therapeutic class using a modification of the American Hospital Formulary Service Drug Pharmacologic Therapeutic Classification System. Antidepressant drugs of interest for this analysis included selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine), selective serotonin norepinephrine reuptake inhibitors (SNRIs) (e.g., venlafaxine), serotonin modulators (trazadone and nefazodone), buproprion, and tetracyclic antidepressants. An indicator variable for “any antidepressant use” was created to identify participants pharmacologically treated for depression; tricyclic antidepressants were not included because they are often directly prescribed to treat urinary symptoms.
Statistical Analysis
Nutrient intakes were adjusted for total energy intake using residuals (Willett et al., 1997). Participants were grouped into quartiles of daily intake of zinc. To minimize the influence of outliers, linear tests for trend were assessed using the median values of deciles of intake to represent the exposure of all participants in the same decile. Supplement use of zinc in the past year was considered in three categories, reflecting doses assumed from multivitamins (0.1–15 mg/d) or individual zinc supplements (>15 mg/d), with non-users as the reference category.
We used logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI) for the associations between zinc intakes and the primary outcome of depressive symptoms. Preliminary analyses examined effect modification and interactions by gender, race/ethnicity, antidepressant use, and polyunsaturated fat or magnesium intake. Multivariate models were constructed by manually adding/removing factors that were associated with both zinc and depressive symptoms, and retaining those that changed the estimate of association >10% (see Table 2 footnote). We also considered the following factors in the multivariate models, but did not include them because they did not affect the final results: marital status, cancer, stroke, asthma, menopausal status, intakes of polyunsaturated fat, magnesium and vitamin B6, and interactions between dietary zinc and polyunsaturated fat or magnesium.
Table 2.
Associations between Zinc Intake and Current Depressive Symptoms1
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Women | Stratified by SSRI Use2 | N=1,545 245 cases |
||||||||
| N=2,163 508 cases |
No n=1,893 390 cases |
Yes n=270 118 cases |
||||||||
| Zinc Intake | Median intake, mg/d | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Dietary, quartile | Women | Men | ||||||||
| 4 (High) | 13.1 | 13.3 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 3 | 10.9 | 11.0 | 1.48 (1.07, 2.06) | 0.02 | 1.36 (0.94, 1.95) | 2.30 (1.02, 5.17) | 0.04 | 1.41 (0.88, 2.23) | ||
| 2 | 9.3 | 9.5 | 1.62 (1.17, 2.45) | 0.004 | 1.44 (1.00, 2.07) | 0.05 | 2.99 (1.31, 6.83) | 0.01 | 1.22 (0.75, 2.00) | |
| 1 (Low) | 7.6 | 7.7 | 1.76 (1.26, 2.45) | 0.001 | 1.61 (1.12, 2.32) | 0.01 | 3.48 (1.34, 9.07) | 0.01 | 1.08 (0.66, 1.74) | |
| P-trend | 0.004 | P-trend | 0.02 | P-trend | 0.02 | P-trend | 0.80 | |||
| Supplemental | ||||||||||
| None | 0 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 0.1–15 mg/d | 15.0 | 15.0 | 0.89 (0.69, 1.14) | 0.94 (0.71, 1.23) | 0.79 (0.39, 1.57) | 0.003 | 0.86 (0.59, 1.28) | |||
| >15 mg/d | 50.0 | 50.0 | 0.65 (0.36, 1.17) | 0.93 (0.51, 1.68) | 0.11 (0.03, 0.46) | 0.01 | 1.68 (0.93, 3.03) | |||
| P-trend | 0.03 | P-trend | 0.58 | P-trend | 0.001 | P-trend | 0.47 | |||
| Total, quartile | ||||||||||
| 4 (High) | 26.8 | 26.8 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 3 | 14.8 | 14.9 | 1.13 (0.80, 1.60) | 0.94 (0.64, 1.36) | 2.90 (1.15, 7.34) | 0.02 | 0.80 (0.48, 1.32) | |||
| 2 | 10.5 | 11.0 | 1.22 (0.87, 1.71) | 0.98 (0.68, 1.42) | 3.29 (1.38, 7.86) | 0.007 | 1.12 (0.72, 1.76) | |||
| 1 (Low) | 8.7 | 8.6 | 1.47 (1.06, 2.05) | 0.02 | 1.18 (0.83, 1.69) | 4.75 (1.98, 11.4) | 0.0005 | 1.11 (0.69, 1.78) | ||
| P-trend | 0.008 | P-trend | 0.26 | P-trend | 0.0005 | P-trend | 0.51 | |||
Multivariate models adjusted for age (5-year categories), race/ethnicity, socioeconomic status, BMI (categorical), physical activity, smoking status (never, former, current), total energy intake (quintiles) and any antidepressant/antipsychotic medication use. Models for women additionally adjusted for cardiac disease and arthritis/rheumatism. Models for men additionally adjusted for diabetes, prostatitis, American Urological Association Symptom Index score for lower urinary tract symptoms, and alcohol intake (g/d). P-values for zinc intake categories are listed if P≤0.05.
The association between supplemental or total zinc intake and depression was significantly stronger among women using SSRI medications (supplemental zinc P-interaction=0.008; total zinc P-interaction=0.01). Including the interaction term between supplemental zinc and SSRIs in the model for dietary zinc among all women did not appreciably alter the association between dietary zinc and depression. No statistically significant interactions were observed for the association between dietary zinc and antidepressant medications in women or men (e.g., SSRI P-interaction=0.4).
In additional analyses, we aimed to create treatment arms of a theoretical trial of women or men with depression. For this purpose, we classified participants as having depression if they had current symptoms (CES-D≥5) or currently used antidepressant medications. We then created subgroups: (a) antidepressant use with low-to-moderate zinc intake (b) antidepressant with moderate-to-high zinc intake (c) no antidepressant use with moderate-to-high zinc intake, (d) no antidepressant use with low-to-moderate zinc intake. We repeated these subgroups considering dietary, supplemental, and total zinc intakes. Median intake levels were used to define moderate-to-high dietary (≥10.1 mg/d) and total (≥12.8 mg/d) zinc, while standard doses in supplements were used to define moderate-to-high supplemental zinc (≥15 mg/d). These subgroups were then analyzed as a class variable in multivariate generalized linear regression models for the outcome of CES-D score.
All statistical tests were two-sided, performed at alpha=0.05, and conducted in SUDAAN v.10.0 (Research Triangle Park, NC).
RESULTS
Preliminary analyses revealed a statistically significant interaction by gender (P-interaction=0.028). Therefore, all subsequent analyses were stratified by gender. Table 1 describes characteristics of men and women, overall and by depressive symptoms status. Depressive symptoms were present in 15.9% of men and 23.5% of women, with a common mean score of 6.3 (SEM=0.1) among those with ≥5 symptoms on the abridged CES-D. Overall, 16.9% of women and 11.3% of men currently used antidepressant medications. Among these antidepressant users, 43.5% of women and 42.5% of men had ongoing depressive symptoms. SSRIs were the most commonly used antidepressants (used by 74.0% of female antidepressant users, 66.1% of male).
Table 1.
Characteristics of Women and Men, Overall and by Presence of Depressive Symptoms
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Total Women | Depression Symptoms | Total Men | Depression Symptoms | |||
| N=2,163 | No n=1,655 |
Yes n=508 |
N=1,545 | No n=1,300 |
Yes n=245 |
|
| Age, y, mean (se) | 51.1 (0.3) | 51.3 (0.3) | 50.5 (0.6) | 50.1 (0.3) | 49.8 (0.4) | 51.8 (0.7) |
| Race/ethnicity | N (%) | % | % | N (%) | % | % |
| Black, non-Hispanic | 720 (33.3) | 43.1 | 29.2 | 437 (28.3) | 27.3 | 33.5 |
| Hispanic | 582 (26.9) | 25.7 | 44.3 | 426 (27.6) | 26.7 | 32.2 |
| White, non-Hispanic | 861 (39.8) | 31.2 | 26.5 | 682 (44.1) | 46.0 | 34.3 |
| Socioeconomic status2 | ||||||
| Low | 904 (41.8) | 48.1 | 64.8 | 573 (37.1) | 32.4 | 61.9 |
| Medium | 905 (41.8) | 39.9 | 31.1 | 674 (43.6) | 45.9 | 31.5 |
| High | 354 (16.4) | 12.0 | 4.1 | 298 (19.3) | 21.7 | 6.5 |
| Cigarette smoker | ||||||
| Never | 1,092 (50.5) | 50.7 | 47.5 | 582 (37.7) | 39.7 | 27.0 |
| Former | 571 (26.4) | 26.8 | 21.5 | 461 (29.8) | 30.9 | 24.0 |
| Current | 500 (23.1) | 22.6 | 31.1 | 502 (32.5) | 29.4 | 49.0 |
| Body mass index, mean (se), kg/m2 | 30.5 (0.2) | 29.9 (0.2) | 32.5 (0.4) | 28.6 (0.2) | 28.4 (0.2) | 29.4 (0.4) |
| Alcohol intake, mean (se), g/d | 1.9 (0.02) | 1.9 (0.03) | 1.7 (0.05) | 2.5 (0.04) | 2.5 (0.04) | 2.5 (0.10) |
| Physical activity3 | ||||||
| Low | 764 (35.3) | 40.7 | 50.4 | 482 (31.2) | 26.4 | 56.7 |
| Medium | 1,068 (49.4) | 45.3 | 41.2 | 698 (45.2) | 47.6 | 32.3 |
| High | 331 (15.3) | 14.1 | 8.4 | 365 (23.6) | 26.0 | 11.0 |
| Medical history | ||||||
| Arthritis or rheumatism | 737 (34.1) | 36.6 | 42.4 | 312 (20.2) | 17.7 | 33.6 |
| Asthma | 439 (20.3) | 22.2 | 28.0 | 215 (13.9) | 12.2 | 23.3 |
| Cardiac disease | 214 (9.9) | 10.7 | 17.4 | 170 (11.0) | 9.8 | 17.6 |
| Diabetes | 275 (12.7) | 10.5 | 17.7 | 191 (12.4) | 10.5 | 22.5 |
| Depression symptoms | 508 (23.5) | 0 | 100 | 245 (15.9) | 0 | 100 |
| Antidepressant medication use1 | 365 (16.9) | 13.9 | 31.3 | 174 (11.3) | 7.7 | 30.2 |
| Antipsychotic medication use1 | 74 (3.4) | 3.7 | 7.7 | 56 (3.6) | 2.0 | 12.2 |
| Any vitamin/mineral supplement use | 1,002 (46.3) | 43.2 | 33.7 | 560 (36.3) | 37.8 | 28.1 |
| Individual zinc supplement use | 132 (6.1) | 4.8 | 4.0 | 96 (6.2) | 5.7 | 9.0 |
| Dietary Intakes, mean (se) servings/d | ||||||
| Fruits and vegetables | 2.5 (0.03) | 2.6 (0.04) | 2.2 (0.06) | 2.4 (0.04) | 2.4 (0.04) | 2.2 (0.09) |
| Meat, fish, poultry, beans, and eggs | 1.8 (0.02) | 1.8 (0.03) | 1.9 (0.05) | 2.4 (0.04) | 2.4 (0.04) | 2.6 (0.10) |
| Total energy, kcal | 1,592 (13) | 1,579 (15) | 1,633 (30) | 1,936 (18) | 1,915 (19) | 2,044 (46) |
| Polyunsaturated fat, g | 13.3 (0.1) | 13.4 (0.1) | 13.1 (0.2) | 12.6 (0.1) | 12.6 (0.1) | 12.4 (0.3) |
| Vitamin B6, mg | 1.8 (0.01) | 1.8 (0.01) | 1.7 (0.02) | 1.8 (0.01) | 1.8 (0.02) | 1.7 (0.03) |
| Magnesium, mg | 300 (1.5) | 304 (1.8) | 286 (3.1) | 289 (1.7) | 291 (1.9) | 280 (4.3) |
| Zinc, mg | 10.3 (0.05) | 10.3 (0.1) | 10.1 (0.1) | 10.4 (0.06) | 10.4 (0.07) | 10.3 (0.2) |
Antidepressants included: selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors, serotonin modulators (5-HT2 blockers), mirtazapine, and buproprion. Antipsychotics included atypical antipsychotics and other antipsychotics/neuroleptics. Antidepressant and antipsychotic medication categories were not mutually exclusive.
Socioeconomic status was calculated using the method by Green (Green, 1970), incorporating both education and household income normalized to income in the Northeast United States (US Census 2000), and categorized as follows based on the distribution of the derived variable: low (lower 25th%), middle (middle 50th%), high (upper 25th%).
Physical activity was measured by means of the validated Physical Activity Scale for the Elderly (Washburn et al., 1993); scores were classified as follows: <100=low, 100-249=medium, ≥ 250=high.
Depressive symptoms were more common among Hispanics and participants with lower socioeconomic status, higher BMI, lower physical activity, and other medical conditions (chi-square P<0.05). Depression was not associated with total daily caloric intake, or carbohydrates, fat, protein, or sodium intakes. Women and men consuming low levels of dietary zinc were more likely to be of lower socioeconomic status, black race, and less likely to use vitamin/mineral supplements.
Among women, dietary, supplemental and total zinc were significantly associated with the presence of depressive symptoms (Table 2). Women in the lowest dietary intake quartile were ~ 80% more likely to have depressive symptoms than those in the highest quartile, and the odds of depression increased linearly with decreasing dietary zinc intake (Ptrend=0.004). Similarly, women using supplemental zinc were less likely to have depressive symptoms than non-users (Ptrend=0.03). Among men, there were no associations between dietary or supplemental zinc intake and depressive symptoms, nor interactions between antidepressant use and zinc in the likelihood of depression (P-interaction=0.44).
Interactions with Antidepressant Medication among Women
Among women, there was a statistically significant interaction between total zinc intake and use of SSRIs (P-interaction=0.01) in the likelihood of depressive symptoms. As anticipated, overall, SSRI users were more likely to have depressive symptoms than non-users (by indication). However, the odds of ongoing depressive symptoms among SSRI users was cut in half (OR=0.44, 95% CI: 0.24, 0.80; P=0.007) among users with moderate-to-high total zinc intake (above the median 12.8 mg/day, OR=2.05, 95% CI: 1.28, 3.28; P=0.003), compared to users with lower zinc intake (<12.8 mg/day OR= 4.01, 95% CI: 2.56, 6.29; P<0.0001, data not shown). This interaction was driven by supplemental zinc intake (P-interaction=0.008) more so than dietary zinc intake (P-interaction=0.36, see Table 2). No statistically significant interactions were observed for use of SNRIs, tricyclic antidepressants, antipsychotic medications, or any other antidepressants.
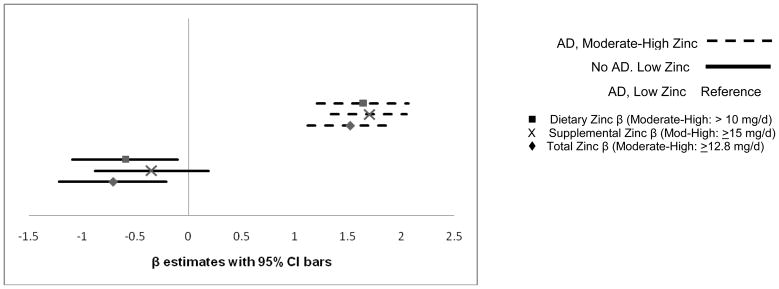
Figure 1 depicts the estimated change in depression symptom score by category of antidepressant and/or zinc intake, among women with depression. Antidepressant users, regardless of zinc intake, had lower CES-D scores (indicating fewer depression symptoms) compared to women classified with depression but not using antidepressants. However, compared to antidepressant users with lower zinc intake, antidepressant users with moderate-to-high dietary or total zinc intake had significantly lower CES-D scores (total zinc, β=−0.71, SEE=0.26, 95% CI: −1.22, −0.21; P=0.006).
Figure 1.
Adjusted change in CES-D score among 714 women with depression (depressive symptoms and/or any antidepressant medication use). The reference group is antidepressant (AD) users with lower zinc intake, and is represented by the vertical 0 line. Horizontal lines indicate the 95% CI for β estimates for change in CES-D score by dietary, supplemental, or total zinc. Lower scores indicate fewer depressive symptoms. Statistically significantly fewer depressive symptoms were reported among women combining higher dietary (P=0.02) or total zinc (P=0.0055) with AD use, compared to women using AD alone. There was no significant difference in CES-D score comparing the non-users of ADs by zinc intake category in this sub-analysis (data not shown).
DISCUSSION
In this population-based study, low dietary zinc intake was positively associated with depression symptoms among women, but not men. Furthermore, the association was considerably stronger among women using SSRI antidepressants, whereby those with low zinc intake were almost five times as likely to have ongoing depressive symptoms while on treatment, compared to women with high zinc intake. The significant findings in women were robust to consideration of numerous relevant sociodemographic and health characteristics, and, among SSRI users, consistent for both dietary and supplemental sources of zinc. Meanwhile, the lack of similar associations in men supports existing evidence of a gender difference in both the presentation and pathophysiological mechanism of depression.
Numerous possible mechanisms have been implicated to explain associations between decreased serum zinc during depressive episodes and antidepressant-like activity of zinc in laboratory models. These include NMDA receptor antagonism, inhibition of glycogen synthase-3β activity (Ilouz et al., 2002), increasing levels of brain-derived neurotrophic factor (BNDF) (Bitanihirwe et al., 2009; Nowak et al., 2004), and mediation of inflammatory pathways and oxidative stress (Marcellini et al., 2008; Siwek et al., 2010; Szewczyk et al., 2010). As the precise mechanisms by which zinc affects mental health remain under investigation, the possibility that supplemental or dietary zinc could prevent or alleviate depressive symptoms in the general population has been largely unexamined.
Our results are consistent with findings from two prior epidemiological studies, showing inverse associations between dietary zinc and depressive symptoms in restricted populations of either older European adults (Marcellini et al., 2006) or young Iranian female students (Amani et al., 2010). Addressing an important limitation of the current study, both of these prior studies measured serum zinc concentration and found significant correlations between dietary and serum measures. However, neither study adjusted for any potential confounders, nor examined antidepressant medication use, zinc supplement use, or gender differences, which we accomplished using comprehensive random sample survey data.
Our finding of a pronounced gender difference in the association between zinc intake and depressive symptoms warrants further attention. Depressive symptoms are more prevalent in women (Shim et al., 2011; Van de Velde et al., 2010), and numerous studies indicate gender differences in clinical manifestations, treatment response, and neural or inflammatory correlates of depression (Brummett et al., 2008; Dotson et al., 2009; Ma et al., 2011; Piccinelli et al., 2000). One relevant factor may be the hormonal milieu (Kessler, 2003; Kornstein et al., 2010; Piccinelli et al., 2000). Compared to men, women have significantly lower serum zinc concentrations, which are further lowered if using oral contraceptives, hormone therapy, or during the child-bearing years (Hotz et al., 2003; Prasad et al., 1975). Preliminary clinical trials have shown that estrogen improves depressive symptoms or accelerates the response to SSRIs (Rasgon et al., 2002; Rasgon et al., 2007; Schmidt et al., 2000). Evidence suggests that estrogen facilitates serotonergic transmission in brain regions involved in mood disorders, with significant physiologic brain changes in the right frontal region (Morgan et al., 2007). Zinc administration has been shown to increase the density of serotonin receptors in the frontal cortex (Cope et al., 2010). Just as studies indicate greater efficacy of SSRIs in the presence of estrogen (Baca et al., 2004; Kornstein et al., 2000; Rasgon et al., 2007; Thase et al., 2005), it is plausible that zinc intake has a similar greater effect among women, particularly when combined with SSRI use. Thus, a possible explanation for our findings of gender differences is that women have a heightened response to antidepressant actions of zinc, which is further increased when they are also using SSRIs. Another possible pathway for gender-specific effects may involve BDNF. Zinc has been shown to increase cortical/hippocampal BDNF gene expression (Nowak et al., 2004; Sowa-Kucma et al., 2008), resulting in increased BDNF similar to the effect of antidepressants. Animal models have shown that loss of BDNF increases susceptibility for depression in females, but not males (Autry et al., 2009; Monteggia et al., 2007); thus, a zinc-BDNF pathway may be noticeably stronger in women.
Apart from these speculative biological explanations for our observed gender differences, it is possible that measurement error among men reduced our ability to assess associations with zinc in men. Although the FFQ has been validated with acceptable validity and reliability across various populations, one study found that the correlation coefficient for zinc between FFQ and 24-hour recalls was lower among men (0.42) than women (0.58) (Subar et al., 2001). Such measurement error would increase variability and dilute any associations among men.
A limitation of the current analysis is the cross-sectional nature, which leaves uncertainty as to whether the observed associations represent actual causal relationships between zinc and depressive symptoms. It is possible that depression was accompanied by decreased appetite and therefore decreased overall dietary intakes. However, our data showed that the total caloric intake and intakes of major nutrients were similar between depressed and non-depressed individuals. Regarding zinc supplements, their use is not accepted as standard therapy for depression, and zinc is not marketed over-the-counter as a mental health supplement. Furthermore, results of preliminary trials of zinc supplements and depression were largely unpublished at the time of our data collection. Thus, it is unlikely that women were using zinc supplements to help improve their mood, thereby alleviating concerns about reverse causation. Rather than a limitation, a potential advantage of the cross-sectional design here is that many of the hypothesized pathophysiological mechanisms are appropriately evaluated by proximate measurement of zinc intake and depressive symptoms, as has been shown by trials showing relatively immediate effects of supplemental zinc (Nowak et al., 2003; Sawada et al., 2010; Siwek et al., 2010).
Among women, the observed interaction between zinc intake and antidepressant use is consistent with preliminary trials showing the beneficial effects of zinc as an adjunct to antidepressant therapy in treatment-resistant patients (Nowak et al., 2003; Szewczyk et al., 2008). Our data indicated that among women receiving antidepressant therapy, ongoing depressive symptoms were two to four times as likely among those with low zinc intake. Among women not using SSRIs, dietary zinc remained associated with depressive symptoms, albeit to a lesser extent, whereas supplemental zinc did not. Dietary zinc was also more relevant than supplemental zinc for depressive symptom severity among the subgroup of women classified as having depression (based on current symptoms or antidepressant therapy). There, moderate-to-high dietary or total zinc intake further improved the pronounced benefits of antidepressant therapy. One possible reason for the weaker associations with supplemental zinc is measurement error; dose of supplemental zinc was derived from standard doses in multivitamins and individual zinc supplements, rather than recorded directly from the supplement label.
Strengths of this study include its racial/ethnic diversity, inclusion of a broad age range, and unique community-based recruitment, which allowed us to avoid issues of diagnostic bias and to study depressive symptoms among individuals without access to health care. Our findings suggest that in the general female population, low dietary zinc intake may contribute to depressive symptoms. Moreover, among women being treated for depression with antidepressant medication, greater zinc intake may augment treatment response. Across subgroup analyses, our results were consistent for dietary zinc, but less so for supplemental zinc. As we examined only prevalent cases, longitudinal research is needed to determine whether dietary zinc is relevant specifically for either the development or persistence of depressive symptoms, or both. Additional research on both men and women is needed to verify our findings of gender disparities. If confirmed by other studies, the potential importance of adequate zinc intake is underscored by the recognized limitations of pharmacotherapy for depression (Insel et al., 2009).
Acknowledgments
Funding/Disclosure Footnote: This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, Grant Nos. R21DK081844 and DK56842. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Abbreviations used in the manuscript
- AHEAD
Asset and Health Dynamics
- BACH
Boston Area Community Health
- BNDF
Brain-derived neurotrophic factor
- CES-D
Center for Epidemiologic Studies Depression
- SNRI
Selective serotonin norepinephrine reuptake inhibitors
- SSRI
Selective serotonin reuptake inhibitors
Footnotes
Supplemental online material: None
N. Maserejian, S. Hall, and J. McKinlay have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amani R, Saeidi S, Nazari Z, Nematpour S. Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biol Trace Elem Res. 2010;137:150–8. doi: 10.1007/s12011-009-8572-x. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca E, Garcia-Garcia M, Porras-Chavarino A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:57–65. doi: 10.1016/S0278-5846(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Cunningham MG. Zinc: the brain’s dark horse. Synapse. 2009;63:1029–49. doi: 10.1002/syn.20683. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A databased approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3:A77. [PMC free article] [PubMed] [Google Scholar]
- Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block 98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Zuchner S, Collins A, Williams RB. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope EC, Levenson CW. Role of zinc in the development and treatment of mood disorders. Curr Opin Clin Nutr Metab Care. 2010;13:685–9. doi: 10.1097/MCO.0b013e32833df61a. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beason-Held L, Kraut MA, Resnick SM. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int J Geriatr Psychiatry. 2009;24:809–19. doi: 10.1002/gps.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78:756–64. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- Huang MH, Schocken M, Block G, Sowers M, Gold E, Sternfeld B, Seeman T, Greendale GA. Variation in nutrient intakes by ethnicity: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2002;9:309–19. doi: 10.1097/00042192-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun. 2002;295:102–6. doi: 10.1016/s0006-291x(02)00636-8. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60:1466–7. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- Kelley KE, Kelley TP, Kaufman DW, Mitchell AA. The Slone Drug Dictionary: A research driven pharmacoepidemiology tool. Pharmacoepidemiol Drug Safety. 2003;12:168–9. [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Young EA, Harvey AT, Wisniewski SR, Barkin JL, Thase ME, Trivedi MH, Nierenberg AA, Rush AJ. The influence of menopause status and postmenopausal use of hormone therapy on presentation of major depression in women. Menopause. 2010;17:828–39. doi: 10.1097/gme.0b013e3181d770a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Castellanos X, Humphries LL, Austin J. Altered zinc metabolism in mood disorder patients. Biol Psychiatry. 1989;26:646–8. doi: 10.1016/0006-3223(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chiriboga DE, Pagoto SL, Rosal MC, Li W, Merriam PA, Hebert JR, Whited MC, Ockene IS. Association between Depression and C-Reactive Protein. Cardiol Res Pract. 2011:286509. doi: 10.4061/2011/286509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, D’Haese PC, Scharpe S, D’Hondt P, Cosyns P, De Broe ME. Hypozincemia in depression. J Affect Disord. 1994;31:135–40. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Meltzer HY, Altamura C, Desnyder R. Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol Psychiatry. 1997;42:349–58. doi: 10.1016/S0006-3223(96)00365-4. [DOI] [PubMed] [Google Scholar]
- Marcellini F, Giuli C, Papa R, Gagliardi C, Dedoussis G, Herbein G, Fulop T, Monti D, Rink L, Jajte J, Mocchegiani E. Zinc status, psychological and nutritional assessment in old people recruited in five European countries: Zincage study. Biogerontology. 2006;7:339–45. doi: 10.1007/s10522-006-9048-4. [DOI] [PubMed] [Google Scholar]
- Marcellini F, Giuli C, Papa R, Gagliardi C, Dedoussis G, Monti D, Jajte J, Giacconi R, Malavolta M, Mocchegiani E. Zinc in elderly people: effects of zinc supplementation on psychological dimensions in dependence of IL-6 −174 polymorphism: a Zincage study. Rejuvenation Res. 2008;11:479–83. doi: 10.1089/rej.2008.0680. [DOI] [PubMed] [Google Scholar]
- Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26:83–97. doi: 10.1002/da.20505. [DOI] [PubMed] [Google Scholar]
- McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–96. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin IJ, Hodge JS. Zinc in depressive disorder. Acta Psychiatr Scand. 1990;82:451–3. doi: 10.1111/j.1600-0447.1990.tb03077.x. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–97. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Morgan ML, Cook IA, Rapkin AJ, Leuchter AF. Neurophysiologic changes during estrogen augmentation in perimenopausal depression. Maturitas. 2007;56:54–60. doi: 10.1016/j.maturitas.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Nowak G, Legutko B, Szewczyk B, Papp M, Sanak M, Pilc A. Zinc treatment induces cortical brain-derived neurotrophic factor gene expression. Eur J Pharmacol. 2004;492:57–9. doi: 10.1016/j.ejphar.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Nowak G, Siwek M, Dudek D, Zieba A, Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol J Pharmacol. 2003;55:1143–7. [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Oberleas D, Moghissi KS, Lei KY, Stryker JC. Effect of oral contraceptive agents on nutrients: I. Minerals. Am J Clin Nutr. 1975;28:377–84. doi: 10.1093/ajcn/28.4.377. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry. 2002;63(Suppl 7):45–8. [PubMed] [Google Scholar]
- Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, Wroolie TE, Brunhuber MV, Rapkin A. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338–43. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sawada T, Yokoi K. Effect of zinc supplementation on mood states in young women: a pilot study. Eur J Clin Nutr. 2010;64:331–3. doi: 10.1038/ejcn.2009.158. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–20. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the united states: results from the national health and nutrition examination survey (NHANES), 2005–2008. J Am Board Fam Med. 2011;24:33–8. doi: 10.3122/jabfm.2011.01.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, Zieba A, Pilc A, Popik P, Nowak G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord. 2010;126:447–52. doi: 10.1016/j.jad.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Sowa-Kucma M, Legutko B, Szewczyk B, Novak K, Znojek P, Poleszak E, Papp M, Pilc A, Nowak G. Antidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm. 2008;115:1621–8. doi: 10.1007/s00702-008-0115-7. [DOI] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Kubera M, Nowak G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Poleszak E, Sowa-Kucma M, Siwek M, Dudek D, Ryszewska-Pokrasniewicz B, Radziwon-Zaleska M, Opoka W, Czekaj J, Pilc A, Nowak G. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep. 2008;60:588–9. [PubMed] [Google Scholar]
- Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt) 2005;14:609–16. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- Turvey CL, Schultz SK, Beglinger L, Klein DM. A longitudinal community-based study of chronic illness, cognitive and physical function, and depression. Am J Geriatr Psychiatry. 2009;17:632–41. doi: 10.1097/jgp.0b013e31819c498c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- Van de Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc Sci Med. 2010;71:305–13. doi: 10.1016/j.socscimed.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) [last accessed March 3, 2011];The World Health Report 2001 - Mental Health: New Understanding, New Hope. Available at http://www.who.int/whr/2001/en/index.html.