Abstract
These experiments were completed as part of an NIH-NINDS contract entitled “Facilities of Research Excellence – Spinal Cord Injury (FORE-SCI) – Replication”. Our goal was to replicate pre-clinical data from Simard et al. (2007) showing that glibenclamide, an FDA approved anti-diabetic drug that targets sulfonylurea receptor 1 (SUR1)-regulated Ca2+ activated, [ATP]i-sensitive nonspecific cation channels, attenuates secondary intraspinal hemorrhage and secondary neurodegeneration caused by hemicontusion injury in rat cervical spinal cord. In an initial replication attempt, the Infinite Horizons impactor was used to deliver a standard unilateral contusion injury near the spinal cord midline. Glibenclamide was administered continuously via osmotic pump beginning immediately post-SCI. The ability of glibenclamide to limit intraspinal hemorrhage was analyzed at 6, 12 and 24 hours using a colorimetric assay. Acute recovery (24 hours) of forelimb function also was assessed. Analysis of data from these initial studies revealed no difference between glibenclamide and vehicle-treated SCI rats. Later, it was determined that differences in primary trauma affect the efficacy of glibenclamide. Indeed, the magnitude and distribution of primary intraspinal hemorrhage was greater when the impact was directed to the dorsomedial region of the cervical hemicord (as in our initial replication experiment), as compared to the dorsolateral spinal cord (as in the Simard et al. experiment). In three subsequent experiments, injury was directed to the dorsolateral spinal cord. In each case, glibenclamide reduced post-traumatic hemorrhage 24-48 hours post-injury. In the third experiment, we also assessed function and found that acute reduction of hemorrhage led to improved functional recovery. Thus, independent replication of the Simard et al. data was achieved. These data illustrate that the injury model and type of trauma can determine the efficacy of pre-clinical pharmacological treatments after SCI.
Keywords: cervical spinal cord injury, hemorrhage, secondary injury, sulfonylurea receptor, glibenclamide, injury models
Introduction
Traumatic spinal cord injury (SCI) activates a series of interrelated cellular and biochemical processes universally referred to as “secondary” injury. Several mechanisms of secondary injury have been identified including ischemia/hypoxia, oxidative stress and inflammation and all have been implicated in causing lesion expansion, in part through progressive hemorrhagic necrosis (PHN).
Minutes after SCI, small (petechial) hemorrhagic lesions form in the capillary-rich central gray matter (primary hemorrhage). Over the next 24 hours, additional small (secondary) hemorrhages emerge in nearby rostral and caudal segments, eventually coalescing into a single zone of necrosis (Balentine, 1978). In human SCI, the presence of intraparenchyal hemorrhage, as detected by T2-weighted MRI at 72 hours, predicts poor neurological recovery at 1 year (Flanders, et al., 1996). In contrast, for those SCI patients that present with intraspinal edema but without intraparenchymal hemorrhage, the probability for spontaneous functional recovery is high (Flanders, et al., 1996). Therefore, management of parenchymal hemorrhage is critical for promoting neurological recovery after SCI.
Recent studies provide evidence that the magnitude of secondary hemorrhage and PHN can be reduced by blocking sulfonylurea receptor 1 (SUR1)-regulated Ca2+ activated, [ATP]i-sensitive nonspecific cation channels (Simard, et al., 2007, Simard, et al., 2010). SUR1 channels are not constitutively expressed but become transcriptionally upregulated in capillary endothelial cells following hypoxic or ischemic insult. Importantly, the channels only become active when intracellular ATP is depleted, with activation leading to cytotoxic edema and oncotic cell death.
To assess the robustness and reproducibility of pre-clinical therapies such as glibenclamide, the NINDS created the “Facilities of Research Excellence – Spinal Cord Injury” (FORE-SCI) contracts. The goal of FORE-SCI is to independently replicate promising pre-clinical research studies. As part of that initiative, we attempted to replicate the initial experiments in which glibenclamide was found to limit PHN in a model of rat cervical contusion injury (Simard, et al., 2007). As shown herein, those data were replicated. In addition, drug efficacy was found to be dependent on complex variables associated with primary injury and tissue biomechanics. Specifically, injury location (relative to the dorso-ventral axis) and spine/spinal cord anisotropy seem to influence the pattern of primary hemorrhage, the magnitude of PHN and therefore the responsiveness to glibenclamide therapy. Since glibenclamide is an FDA approved antidiabetic drug with minimal risk, hypoglycemia being the only significant adverse reaction, low dose treatment may be considered for treating patients with acute SCI.
Materials and Methods
Our replication efforts were focused on confirming two key in vivo observations described in Figs. 3A&B and Fig. 4 of Simard et al. (2007). First, that continuous systemic delivery of glibenclamide (200 ng/hr) via osmotic mini-pump, beginning 2-3 min post-injury, decreased intraspinal hemorrhage 6, 12 or 24 hrs after unilateral cervical spinal contusion injury. Second, that attenuation of intraspinal bleeding by glibenclamide was associated with less pathology at the injury site by 7 days and improved recovery of motor function at 1, 3 and 7 days post-injury (dpi). Following the mandate of the FORE-SCI contract, replication of these data was attempted after detailed consultation (via email and phone) with the original authors.
Figure 3.

Flat bed scans illustrating the ability of glibenclamide to attenuate acute hemorrhage after SCI. (A&B) Pilot data generated by OSU personnel during site visit to University of Maryland. Comparisons of two matched sets of injured spinal cords from rats treated with glibenclamide (Glib) or vehicle (Veh) (n=2/group). (C) Data generated during reverse site visit (UM to OSU). A representative scan (from Glib and Veh-treated rat is shown; n=3 rats/treatment). All specimens are “open-book” preparation where the spinal cord cut was down the middle at the injury site then reflected back.
Figure 4.
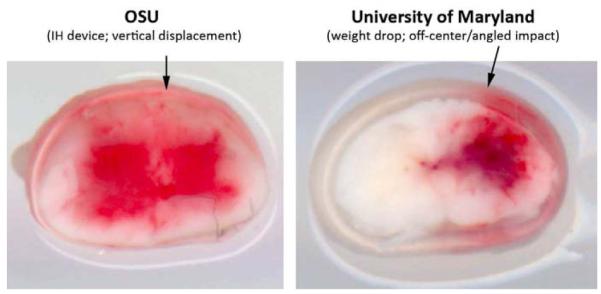
Ex vivo examples of injured spinal cords (15 minutes post-injury) showing differences in primary hemorrhage caused by injury devices used at Ohio State (OSU; used the IH impactor with vertical (arrow) contusion centered just off mid-line) or University of Maryland (UM; 10g × 25mm weight drop with contusion centered over dorsolateral sulcus at an angle (arrow) of ~5° (n=1 rat/injury device).
Pilot Studies to Replicate Biomechanics and Pathology of a Unilateral Model of Cervical SCI (Figure 1)
Figure 1.

Anatomical and behavioral characterization of unilateral cervical spinal contusion injury using the Infinite Horizons (IH) impactor. The anatomical and behavioral consequences of SCI, as defined by Simard et al. (2007), were matched before starting formal replication experiments. A 175kdyn unilateral contusion yielded unilateral necrosis and myelin sparing (A), overall lesion volume (B&C) and behavioral impairments (D; ipsilateral paw placement and rearing) that were indistinguishable from those described by Simard et al. (n=3).
Simard et al. (2007) described the use of a SCI model in which a blunt 1.3 mm impact probe driven by a 10g weight dropped vertically from a 25 mm height was used to deliver a hemi-contusion injury to the cervical spinal cord. Two different references from independent laboratories were cited that described different blunt weight drop hemi-cervical cord contusion models (Gensel, et al., 2006, Soblosky, et al., 2001). Based on the description and our knowledge of those citations, we initially operated under the assumption that any form of calibrated blunt hemi-contusion would suffice as long as the impact parameters were titrated to produce histological and forelimb deficits that resembled those achieved at 7 dpi in the control SCI group as described by Simard et al. (2007). Specifically, they described near complete destruction of gray and white matter ipsilateral to the contusion injury with evidence of contralateral damage in the medial gray and dorsal/ventral white matter by 7dpi in untreated SCI rats. These histological changes were associated with severe forelimb deficits --control SCI rats were immobile and showed little or no ipsilateral paw placement or vertical exploration in the cylinder test (see below). To ensure that a similar type of SCI was used, pilot studies were completed with the goal of replicating the anatomical and behavioral data in the control groups described by Simard et al. (see Fig. 4C-E in Simard et al., 2007).
Unilateral cervical spinal cord injury model
An Infinite Horizons (IH) impactor (1.5 mm impact probe), was used to deliver a unilateral contusion to the C5 spinal cord. All rats were anesthetized with a ketamine/xylazine cocktail (80/10 mg/kg, i.p.). To minimize variability, the same person performed all injuries. The skin overlying the cervical spinal cord was shaved then swabbed with a sequence of Betadine scrub, 80% ethanol then Betadine solution. The C2-7 verterbrae were exposed via a midline incision then a laminectomy was performed at C5. The vertebral column was stabilized rostral (C4) and caudal (C6) to the laminectomy site using clamps. After aligning the impact probe perpendicular to the animal’s midline, the probe tip was moved ~0.3 mm from the midline after which rats received a moderate unilateral cervical spinal cord contusion (175 kdyn) injury. As shown in Fig. 1, the anatomical and behavioral characteristics (see detailed methods in subsequent experiments below) of this injury mimic those described by Simard et al. (2007). Next, power analyses were completed using raw intraspinal hemorrhage data provided by Dr. Simard (α=0.05). It was determined that balanced group sizes of n=6-8/group would produce >80% power; i.e., detect a decrease in intraspinal hemorrhage of ~50% with glibenclamide treatment at 6 hours post-injury.
Experiment 1: (attempt to replicate therapeutic effects of glibenclamide on intraspinal hemorrhage @ 6, 12, 24 hrs post-injury and 24 hr forelimb function)
Unilateral cervical SCI and drug delivery
A total of 54 female Long-Evans rats (~10 weeks old; 247±8g) (Harlan, Indianapolis, IN) were entered into the protocol for the formal replication study. Animals were randomized evenly across surgeons and within treatment groups. Seven animals were replaced for various reasons: two received faulty injuries (forces outside of range), two developed subdural bleeding prior to injury and three died peri-operatively for unknown reasons. Two additional animals died during recovery from surgery and were not replaced. Overall, mortality rate was <10% and was not related to treatment. All rats received SCI as described above. Body temperature was maintained pre- and post-operatively using a homeothermic blanket (World Precision Instruments Model ATC1000). After injury, osmotic pumps containing glibenclamide or vehicle were placed caudal to the injury site in a subcutaneous pocket. Muscle was sutured and skin incision closed with wound clips. Animals received 4 ml subcutaneous 0.9% NaCl and were placed on 37°C warming plates during recovery.
Drug Preparation and Infusion
Glibenclamide and vehicle were prepared fresh daily. Glibenclamide (Sigma # G-2539, St. Loius, MO) was prepared for infusion by diluting 800 μl of a stock solution (5.0 mg/ml glibenclamide dissolved in DMSO [Sigma #D-2438, St. Loius, MO]) in 4.2 ml sterile 0.9% NaCl. The diluted formulation was pH adjusted to 8.5 with 0.1N NaOH to dissolve any white precipitate that formed during dilution. Vehicle-treated animals received equivalent volumes of DMSO diluted in 0.9% NaCl. Before they were loaded into pumps (Alzet #2002), vehicle and glibenclamide were filter sterilized by an individual not directly involved with the study. Pumps were loaded as per Alzet instructions and were primed overnight at 37°C. The infusion rate was 0.5μl/hr, which yielded 200ng/hr of glibenclamide.
Behavioral Analyses
Two individuals that were unaware of treatment performed all behavioral testing at 24 hours post-injury. Inclined plane performance was evaluated by placing the animal on the inclined plane (starting angle = 30°) and increasing the angle at a rate of ~ 1°/second. The test was stopped and the angle recorded at which time the animal was unable to maintain its established stationary position. Each animal was tested twice in both the “head up” and the “head down” positions. Rearing and paw placement was evaluated by placing the animal in a clear cylinder and video recording for 3 minutes (Gensel, et al., 2006). The cylinder was placed in front of a mirror so that both forelimbs could be observed at all times. The total number of paw placements (recorded separately for limb contralateral and ipsilateral to the injury) and duration of rearing were analyzed via video playback.
Spectrophotometric Analysis of Intraspinal Hemorrhage
Anesthetized rats were euthanized via transcardial perfusion with ~60 ml of 0.9% heparinized saline (1 unit heparin/ml) for 3 minutes at 6, 12 or 24 hours post-injury (after completing behavioral analyses). The spinal cord was exposed from C2-T2 and the vertebral level of injury was confirmed. A 5 mm segment of spinal cord centered on the impact site was removed then placed in pre-weighed 1.8 ml Eppendorf tubes then stored at −20°C until used in the hemoglobin assay (Choudhri, et al., 1997). Wet weight (grams) of each segment was determined. A volume of distilled water equal to 9x the wet weight was added to tubes prior to homogenizing on ice for 1 minute (Tissue Tearor, BioSpec Products, Inc.; Model 985370-395). Homogenate was centrifuged at 15,500 x g for 30 min then supernatant was removed. 20μl of clear supernatant was added to 80μl Drabkin’s reagent (Sigma # D5941 and #B4184) in replicate wells of a 96 well plate. Samples were incubated for 15 min at room temperature then absorbance was read at 560nm using a SpectraMax190 plate reader (Molecular Probes). A standard curve was generated using uninjured spinal cord homogenates spiked with known volumes of blood. Sample values were normalized using the amount of water added as a dilution factor.
Experiment 2 (Ohio State → University of Maryland site visit)
Methods were the same as for Experiment 1 with the following exceptions. Ketamine and xylazine were injected into the hindlimb muscle on the right side (contralateral to the injury site). The animal was placed in a Stoelting stereotaxic frame with head held in place via mouth bar and the T2 spinous process was secured with clamps. On the left side, the entire lamina of C5, the articular process and dorsal half of the pedicle were removed using a 1.3-mm diamond burr and high-speed drill (DiamondBurs; Tucker, GA). The laminectomy site was flooded with sterile 0.9% frequently to prevent thermal damage to spinal cord and surrounding tissues. Surgeons were vigilant during drilling to ensure that no damage occurred to the underlying dorsal roots and adjacent spinal cord or dura. The weight drop device was based on that of Soblosky et al (Soblosky, et al., 2001). The impact rod (1.5 mm diameter, 3.95 grams) was positioned at vertebral level C6 with the medial edge aligned with the dorsal route entry zone of C6 and the caudal edge directly across from C7 axilla but at a slight angle (~5°) relative to the dorso-ventral axis of the spinal cord. The impact rod was carefully lowered onto the intact dura and within 5s a 10g weight was released from a height of 25mm.
Drug preparation and infusion (applies to Experiments 2-3)
During the visit to UM, it was learned that in newer experiments (Simard, et al., 2010), a “loading” dose (10μg/kg) of glibenclamide (or vehicle alone) was injected (i.p.) immediately after SCI, and this was supplemented with infusion via osmotic mini-pumps as described in Experiment 1. The pump model for Exp. 2-5 was changed from Alzet #2002 (0.5 ul/hr) to #2001 (1.0 ul/hr). To adjust for the difference in infusion volume and maintain a rate of 200 ng glibenclamide/hr, the glibenclamide stock concentration was reduced to 2.5mg/ml.
Assessment of hemorrhage at lesion site (Experiments 2-3)
Based on recommendations from UM, the hemoglobin assay was replaced with a simpler and more reliable, but equally sensitive method. After transcardiac perfusion as in Experiment 1, the spinal cord was carefully removed then lightly fixed via immersion in 4% paraformaldehyde (prepared in 0.1M phosphate buffer, pH 7.2) for 15-30 minutes. The fixed spinal cord was cut in cross section through the site of impact then reflected in an “open book” fashion. Opposing “book-ends” were placed on a flat bed scanner and scanned at 1200 dpi to grossly visualize the magnitude and distribution of intraspinal hemorrhage.
Experiment 3 (modified SCI at Ohio State)
Upon returning to OSU, attempts were made to independently replicate the pilot data obtained at University of Maryland (UM; see Fig. 3). Initially, we experienced problems using the UM SCI model. Since this weight drop device does not provide biomechanical feedback, there is no way to assess the quality of injury immediately post-injury. Instead, behavioral and anatomical analyses are needed at 24 hours. Using this approach, we found variable forelimb deficits ipsilateral to the injury site and inconsistencies in the magnitude and contralateral spread of acute intraspinal hemorrhage (n=15 rats). Thus, it was decided that two researchers from UM (O.T. and V.G.) would travel to Columbus to “cross-train” OSU personnel and ensure consistency and reproducibility of the UM injury model.
The UM team coached the OSU surgeon (C.A.T.) in real-time using a microscope equipped with video camera and monitor. All instruction from the UM team was verbal, i.e., they did not perform surgery. From this interaction it became clear that OSU was not removing enough pedicle to ensure consistent rod placement (relative to the nerve root axilla and dorsal/medial axis of the spinal cord) or minimize the potential for the impact rod to contact bone during the injury. Also, it became clear that flexion responses in the trunk and the ipsilateral lower extremities could predict a “successful” injury. Animals that fail to elicit a strong trunk flexor reaction (>45 degrees from starting point) at the time of injury should be immediately excluded from the experiment. The flexion response can be rated as 0, 0.5 (some or moderate) or 1 (moderate to extensive). Any animal not scoring at least 0.5 should be excluded. Details of this surgical protocol and outcomes assessment have subsequently been published (Simard, et al., 2010).
After the UM team departed, a total of n=8 rats were used to test efficacy of glibenclamide: n=2 glibenclamide and n=2 vehicle treated rats at 24 hour and 7 days post-injury (n=4/group). Animals were assessed at 24 hours, 3 and 7 days for gross motor function (open-field Basso-Beattie-Bresnahan locomotor scores), inclined plane (3 trials each for head up and head down starting position) and cylinder. Even though the BBB scale is not an accepted method for monitoring cervical SCI, it provided a quick and simple means to detect gross deficits in hindlimb function ipsi-and contralateral to the site of injury.
Gross analysis of hemorrhage and histological analysis
At 24 hours post injury, flat bed scans were taken of lesion centers as described above. On day 7, animals were anesthetized and transcardially perfused with cold 0.1M phosphate buffered saline pH 7.4, followed by 4% paraformaldehyde pH 7.4. Spinal cords were removed and a 6 mm length of spinal cord centered on the lesion was blocked and post fixed for 2 hours in 4% paraformaldehyde. The tissue was placed in 0.2M phosphate buffer overnight at 4°C followed by immersion in 30% sucrose in phosphate buffer until tissue sank. After cryoprotection the tissue was snap frozen and stored at −80°C. Tissue blocks were later embedded in OCT compound (VWR Scientific Products, Bridgeport, NJ) then cut in cross-section at 10μm on a Microm HM505E cryostat. Sections were mounted onto slides (ColorFrost/Plus; Fisher, Pittsburgh, PA) then were stained with eriochrome cyanine and cresyl violet. Unbiased stereological quantitation of lesion volume was performed on equally spaced (~400μm apart) digital images of spinal cord sections that spanned the rostrocaudal extent of the lesion.
Results
Experiment 1 (initial replication)
Gross visual inspection of whole spinal cords at 6, 12 or 24 hours post-injury revealed similar amounts of hemorrhage at the site of impact with some visible blood extending into rostral and caudal spinal segments in both vehicle and glibenclamide-treated animals (Figure 2A).
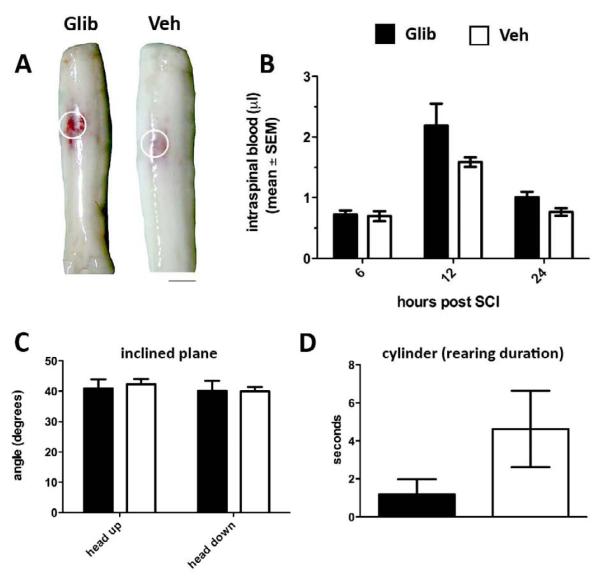
Figure 2.
Acute glibenclamide treatment has no effect on intraspinal hemorrhage or functional recovery after spinal hemicontusion injury produced by the IH injury device (175kdyn). (A) Representative whole spinal cords 24 hours post-injury provide gross assessment of unilateral hemorrhage between glibenclamide and Ctr (vehicle)-treated rats. (B) Spectrophotometric measurements of spinal cord homogenates at 6, 12 and 24 hours post-injury comparing glibenclamide and vehicle controls. (C&D) Inclined plane (C) and vertical exploration/rearing (D) at 24 hours post-injury also were unaffected by glibenclamide (p>0.05 for all comparisons in B-D). (n=6-8/group)
To quantify intraspinal hemorrhage, spinal cords were homogenized then prepared for spectrophotometric analysis as described by Simard et al. (Simard, et al., 2007). Maximum extravasation occurred ~12 hours post-injury in both vehicle and glibenclamide-treated animals but there was no differences in the magnitude of intraspinal hemorrhage between groups (Figure 2B).
Previously, glibenclamide was found to reduce hemorrhage and improve performance on inclined plane and vertical exploration tasks at 24 hours post-injury (Simard, et al., 2007). However, consistent with the above data showing no between-group differences in hemorrhage, glibenclamide did not affect acute functional recovery (Fig. 2C&D).
As a result of extensive discussions between Drs. Popovich and Simard, we suspected that our initial failed replication experiment was a result of using SCI models that produced different forms of primary trauma. At OSU, the Infinite Horizons (IH) impactor was used to deliver hemi-contusion injuries that were perpedicular to the spinal cord axis and just medial to the dorsolateral sulcus on the right side of the C5 spinal cord (Fig. 1). This causes pathology and behavioral impairment at 7 dpi that was indistinguishable from that caused by the weight drop technique described by Simard et al. (compare Fig. 1 above with Fig. 4C-E in Simard et al.); however, differences in the location of injury (relative to the midline and axis of the spinal cord) and the subsequent forces generated on the microvascular network might be distinct between the two models. Indeed, unlike injuries delivered via the IH device and most other standardized contusion devices (e.g., OSU, NYU/MASCIS) (Gruner, 1992, Scheff, et al., 2003, Stokes, et al., 1992) where impact occurs perpendicular to the dorsoventral axis of the spinal cord, the standard Simard injury is delivered at a slight angle (~5°) to this axis with the impact centered lateral to the dorsolateral sulcus. In effect, this latter injury occurs directly over the right dorsal horn with the momentum of the impact carried directly into the gray matter.
Experiment 2 (Ohio State → University of Maryland site visit)
To test the hypothesis that differences in primary trauma affected our failure to replicate, a research technician (C.A.T.) from OSU traveled to the Simard laboratory at University of Maryland (UM) to learn their surgical approach and injury technique. As described in Materials and Methods, the UM surgical approach and method of SCI differ from those used at OSU in Experiment 1.
After observing then documenting details of the surgery and injury procedures, a pilot study was completed on-site at UM to test the efficacy of glibenclamide. All surgeries were completed by UM personnel; however, all SCI animals were randomized for treatment and all vehicle and glibenclamide was prepared and administered by OSU personnel (C.A.T). Because UM personnel had no knowledge of group designations, we were able to test and interpret drug efficacy without bias.
During this visit, it also was learned that an immediate post-injury loading dose (within 5 minutes of injury) of glibenclamide (10μg/kg) had become a standard part of the treatment protocol in the Simard lab. All subsequent experiments described in this report used a loading dose with continuous infusion via osmotic pump (as in Fig. 2). Using the new surgical approach, acute delivery of glibenclamide was found to limit intraspinal hemorrhage (vs. vehicle).
These data confirmed the efficacy of glibenclamide as described in Simard’s original publication. Moreover, the data suggested that subtle differences in primary trauma, perhaps related to the rate of endothelial injury and vascular compromise, can predict the neuroprotective potential of glibenclamide. To test the hypothesis that the standardized IH contusion injury produced more severe contralateral hemorrhage (as compared to the off-center weight drop), we compared the magnitude of intraspinal hemorrhage created by the two models at 15 min post-injury SCI. As shown in Fig. 4, the IH injury produced rapid bilateral intraspinal hemorrhage. In contrast, hemorrhage was unilateral and was largely restricted to the gray matter immediately beneath the site of injury using the weight drop model.
Experiment 3 (University of Maryland → Ohio State site visit)
A UM injury device was transported back to OSU for follow-up replication studies. Initially, this device produced variable results in our hands. Indeed, even though all major aspects of the surgical and injury approach learned at UM were replicated at OSU, the magnitude and distribution of acute intraspinal hemorrhage (24-48 hrs post-injury) were variable as were overt deficits in ipsilateral forelimb function. Thus, some assumptions were likely made during training at UM that precluded translation of the technique at OSU. To rectify this, a “reverse site-visit” occurred. UM personnel traveled to OSU to oversee the surgical approach and use of the injury device in a small group of rats. OSU personnel performed all experimental procedures. During that visit, it was learned that placement of the injury probe relative to the dorsal root entry zone and pedicle affected injury severity and therefore the rate of onset and magnitude of hemorrhage. Once this variable was accounted for, all subsequent spinal cord injuries performed at OSU were identical to those created at UM (based on rate of spread of hemorrhage, acute histology and rate of forelimb recovery; data not shown). Again, a pilot study was completed in which n=6 rats were treated with glibenclamide or vehicle (n=3/group) then intraspinal hemorrhage was assessed 24 hours later (Fig. 3C). We observed a reduction in intraspinal hemorrhage that was qualitatively similar to that observed during the OSU-to-UM site visit (compare Fig. 3A&B with 3C).
With our approach matched to that of UM, we again tested the effects of glibenclamide in a small cohort of rats. Importantly, this experiment was completed after UM personnel returned home and a subset of rats survived until 7dpi. Similar to the data generated at UM, we found significant attenuation of intraspinal hemorrhage early after SCI in glibenclamide-treated rats (24 hours). These acute changes were predictive of reduced pathology at 7 dpi and improved neurological function from 1-7 dpi (Figs. 5&6). Using the BBB open field locomotor rating scale as a crude measure of overground locomotion, significant preservation of hind limb function was evident in glibenclamide-treated rats one day post-injury (Fig. 6). Similar improvements were detected on the inclined plane and cylinder tasks (Fig. 6). All rats were analyzed on inclined plane at 1, 3 and 7 days; however, rearing and paw placement (cylinder) were analyzed only at 3 and 7 days (unable to rear on day 1). Between 3 and 7 days, 22 rearing events were recorded for glibenclamide-treated rats as compared to 3 events in vehicle-treated rats. The average duration of rearing was ~15 sec for glibenclamide-treated rats versus ~1 sec for vehicle-treated rats. The overall variability between groups was large but the mean difference between groups was consistent with the anatomical data (Fig. 5) and other measures of behavioral recovery (Fig. 6).
Figure 5.
Glibenclamide reduces acute intraspinal hemorrhage and lesion expansion after SCI. (A&B) Fresh specimens of injured spinal cord (24 hours post-injury) show enhanced petechial hemorrhage (arrows) and distribution of intraspinal hemorrhage in vehicle (A) compared to glibenclamide (B) treated rats. By 7 days, the extent of hemorrhagic necrosis is reduced over the rostral-caudal extent of the injured spinal cord (histological insets; red shading indicates areas of necrosis). (C&D) Glibenclamide significantly reduces lesion pathology across the rostro-caudal extent of the contusion lesion at 7dpi (p<0.05 via 2-way ANOVA; n=2 rats/treatment).
Figure 6.
Glibenclamide reduces functional deficits caused by unilateral cervical spinal contusion injury. (A) Glibenclamide preserves open field locomotor ability at 24 hours post-injury (***p<0.001 vs. Veh; n=4 rats/treatment). (B&C) Open field scores at 24 hours post-injury predict improved recovery of glibenclamide-treated rats (vs. vehicle) on the inclined plane between 1-7 days post-injury (*p<0.05; 2-way ANOVA).
Discussion
The present study assessed the reproducibility and robustness of the findings of Simard et al. who showed that acute post-injury delivery of glibenclamide attenuates secondary intraspinal hemorrhage and progressive necrosis caused by hemicontusion injury in rat cervical spinal cord. Although our initial attempts to replicate those data were unsuccessful, we eventually confirmed their observations when specific details of their injury model were reproduced. This was possible as a result of thorough discussion and extensive “hands-on” training from the Simard laboratory. Also, new data were generated showing that the primary hemorrhage, the magnitude of post-traumatic progressive hemorrhagic necrosis (PHN) and the success of intervention using pharmacological therapies (e.g., glibenclamide) that target PHN are likely to be affected by differences in the primary trauma.
Physical damage to the spinal cord causes vascular rupture and intraspinal bleeding (primary hemorrhage). Secondary hemorrhage evolves as a function of time with the volume of extravasated blood increasing >2-fold within 24 hours due to delayed compromise of the microvascular endothelia. Recent data indicate that secondary hemorrhage and post-hemorrhagic necrosis (PHN) are active processes that are conserved across species and involve Sp1-mediated transcription of Abcc8, which in turn regulates de novo expression of SUR1-regulated NCCa-ATP channels in capillary endothelial cells, neurons and oligodendrocytes (Simard, et al., 2010).
Glibenclamide (Glyburide) is an FDA-approved drug that is widely used to treat type 2 diabetes mellitus. The drug can be given orally or intravenously and the risk profile of treatment is minimal, making it an ideal candidate for use in treating acute SCI. Glibenclamide likely limits PHN by blocking the interaction between SUR1 and the pore-forming subunits of NCCa-ATP channels in endothelia. This reduces ion flow down an electrochemical gradient thereby preventing cytotoxic edema. Although endothelia are protected, glibenclamide also may confer neuroprotection via direct actions on neurons and glia. Indeed, expression of Abcc8/SUR1 is increased in neurons and oligodendrocytes in the ischemic penumbra of traumatically injured brain and spinal cord (Gerzanich, et al., 2009, Simard, et al., 2009, Simard, et al., 2010). Since transcriptional regulation of Abcc8 controls SUR1expression, it is likely that more specific molecular therapeutics can be developed to attenuate PHN.
Using controlled models of mid-line dorsal spinal contusion injury (targeting mostly thoracic spinal cord level), impact velocity invariably predicts the magnitude and spread of axonal and vascular pathology in white matter (Sparrey, et al., 2008). However, gray matter vasculature is less tolerant to compressive forces than vessels in white matter (Ichihara, et al., 2001, Maikos, et al., 2008, Maikos and Shreiber, 2007, Sparrey, et al., 2009). This enhanced vulnerability of gray matter microvasculature is magnified in injuries that occur “off-center” from the dorsal midline, presumably because the spinal cord is anisotropic and the modulus (elasticity) of gray and white matter are different. Using a finite element model to predict patterns of tissue stress and strain, it was shown that the magnitude and rate of spinal cord compression for injuries located “off-center” were identical to those created by standard “mid-line” contusion injuries; however, patterns of stress and strain were shifted laterally and the magnitude of distortion was increased (Maikos, et al., 2008). Accordingly, the distribution and magnitude of intraparenchymal hemorrhage will vary as a function of the loading parameters (e.g., impact velocity, force) and location of injury.
Our present data suggest that the efficacy of any drug that targets mechanisms of PHN will vary as a function of the primary trauma. Using a widely available model of spinal contusion injury, with the primary trauma directed vertically near the midline of the dorsal spinal cord, bilateral hemorrhage was observed throughout the gray and white matter within 15 minutes. The distribution and magnitude of this primary hemorrhage changed when the impact was delivered “off-center” at a slight angle. In general, the SCI research community has adopted one or more variations of the former type of injury, i.e., high velocity vertically oriented mid-line thoracic or cervical contusion injury models (e.g., the IH impactor, MASCIS/NYU weight drop, OSU ESCID), because they reproduce with high fidelity and consistency, the gross pathological consequences of most forms of traumatic human SCI (Gruner, 1992, Scheff, et al., 2003, Stokes, et al., 1992). In fact, most pre-clinical neuroprotective strategies have been tested using these models. However, most human SCI clinical trials have failed, perhaps because the complexity and heterogeneity of human injuries cannot be adequately studied using a single injury model (Tator, 2006). Indeed, subtle differences in the mechanical variables associated with injury and intrinsic differences in the biomechanical properties of gray and white matter can influence the onset, magnitude and distribution of tissue damage.
The present data suggest that acute neuroprotective therapies, specifically those that target mechanisms of PHN, will be largely ineffective when primary hemorrhage in white matter is extensive. In contrast, in a subset of injuries, it should be feasible to limit the spread of hemorrhagic necrosis from gray matter into white matter, thereby minimizing secondary white matter injury and preserving indices of neurological function. Since hemorrhage is an early and pivotal trigger of secondary white matter injury, it is important to understand the biological variables associated with specific types of SCI and how these help predict treatment efficacy.
Our experiments points to the importance of studying diverse models of SCI when evaluating the pre-clinical efficacy of potential therapeutic compounds, similar to the approach taken in studies on ischemic and traumatic brain injury in which multiple models are routinely used for evaluation. Even though glibenclamide can limit the anatomical and functional consequences of PHN, the drug may not be efficacious for all forms of SCI. Regardless, it is FDA approved and has been used in humans for decades with minimal risk, hypoglycemia being the only significant adverse reaction. As such, low dose glibenclamide could be given to treat individuals with acute SCI. Future studies are needed to determine whether discrete forms of primary trauma can be linked to unique radiological signatures or biomarker panels. This information can help inform post-injury care and stratification of injury populations for clinical trials where glibenclamide or related compounds may be better targeted to a wider demographic of less severely injured patient populations (e.g., ASIA B, C or D) instead of the more common practice of enrolling individuals who suffer the most severe types of SCI (e.g., ASIA A).
Research Highlights.
 Independent replication of a reported pre-clinical therapy for SCI was achieved
Independent replication of a reported pre-clinical therapy for SCI was achieved Documenting pre-clinical drug efficacy depends on the injury model
Documenting pre-clinical drug efficacy depends on the injury model Glibenclamide (FDA-approved anti-diabetic drug) could be used for acute SCI therapy
Glibenclamide (FDA-approved anti-diabetic drug) could be used for acute SCI therapy
Acknowledgments
Thanks to Drs. J. Marc Simard, Orest Tsymaluk and Volodymyr Gerzanich for thoughtful and invaluable discussions throughout these experiments. Thank you also to Drs. Dana McTigue, Michele Basso, Lyn Jakeman, John Buford, Sandra Kostyk and the technical staff and trainees of the Center for Brain and Spinal Cord Repair for discussions related to manuscript selection and experimental design. Sponsored by NIH-NINDS contract HHSN271200800040C to P.G.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. [PubMed] [Google Scholar]
- 2.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 3.Flanders AE, Spettell CM, Tartaglino LM, Friedman DP, Herbison GJ. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–655. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- 4.Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- 5.Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, Freichel M, Simard JM. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15:185–191. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126-128. [DOI] [PubMed] [Google Scholar]
- 7.Ichihara K, Taguchi T, Shimada Y, Sakuramoto I, Kawano S, Kawai S. Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J Neurotrauma. 2001;18:361–367. doi: 10.1089/08977150151071053. [DOI] [PubMed] [Google Scholar]
- 8.Maikos JT, Qian Z, Metaxas D, Shreiber DI. Finite element analysis of spinal cord injury in the rat. J Neurotrauma. 2008;25:795–816. doi: 10.1089/neu.2007.0423. [DOI] [PubMed] [Google Scholar]
- 9.Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma. 2007;24:492–507. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- 10.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 11.Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J Neurotrauma. 2009;26:2257–2267. doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simard JM, Woo SK, Norenberg MD, Tosun C, Chen Z, Ivanova S, Tsymbalyuk O, Bryan J, Landsman D, Gerzanich V. Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci Transl Med. 2010;2:28ra29. doi: 10.1126/scitranslmed.3000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- 15.Sparrey CJ, Choo AM, Liu J, Tetzlaff W, Oxland TR. The distribution of tissue damage in the spinal cord is influenced by the contusion velocity. Spine. 2008;33:E812–819. doi: 10.1097/BRS.0b013e3181894fd3. [DOI] [PubMed] [Google Scholar]
- 16.Sparrey CJ, Manley GT, Keaveny TM. Effects of white, grey, and pia mater properties on tissue level stresses and strains in the compressed spinal cord. J Neurotrauma. 2009;26:585–595. doi: 10.1089/neu.2008.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes BT, Noyes DH, Behrmann DL. An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma. 1992;9:187–195. doi: 10.1089/neu.1992.9.187. [DOI] [PubMed] [Google Scholar]
- 18.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-957. [DOI] [PubMed] [Google Scholar]