Abstract
This study assessed acceptability of the candidate microbicide VivaGel® and two placebo gels among 61 sexually active young US and Puerto Rican women at three sites. Participants were randomly assigned to use one of the gels twice per day for 14 days. At trial completion, 59% of the women in the VivaGel® group reported being likely to use the gel in the future, whereas 23% were unlikely to use it and 18% were undecided. Participants reported problems with all three gels, including the “universal” placebo containing hydroxyethyl cellulose (HEC). The most frequent complaints were leakage, interference with sexual behavior, and decreased sexual satisfaction. Some of the complaints are not new but remain unresolved. Women’s perceived risk of HIV infection may determine whether the gels are used. Users also may want a choice of viscosity. Poor acceptability of vaginal microbicide formulations may result in poor adherence to gel use during efficacy trials and compromise validity of results.
Keywords: Microbicide acceptability, HIV prevention, HEC, Leakage
Introduction
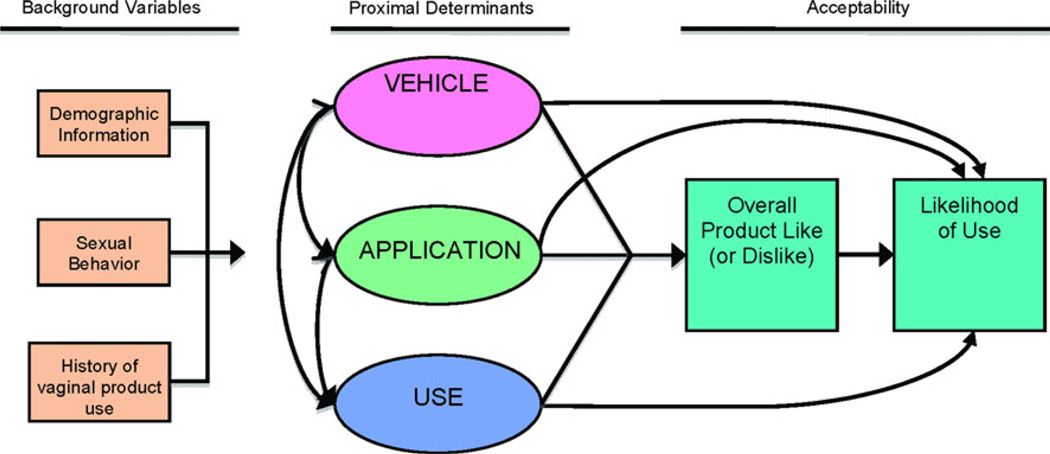
CAPRISA 004 has given proof-of-concept that a topical microbicide applied vaginally can decrease the chances of HIV transmission [1]. Yet, effectiveness of a microbicide in the “real world,” outside the structure of a clinical trial, will be contingent on product acceptability [2, 3]. Acceptability has been defined as the “voluntary and sustained use of the method in the context of alternatives” [4, p. 122]. Acceptability research can be tailored to different phases of microbicide development. For Phase 1 trials, Morrow and Ruiz [5] propose the study of four acceptability factors: (1) vehicle-associated variables, such as texture and viscosity, product scent, color, taste, and other desirable/appealing elements of formulation; (2) application-associated variables, such as ability to adhere to instructions, ease of product preparation (e.g., filling applicator with product), ease of application, portability, applicator design and disposal preferences; (3) use-associated variables, such as frequency and timing of product use, partner-specific issues, lubrication, product consistency post-use, desirable/appealing elements of use, use with/without condoms, changes in hygiene practices secondary to use, and changes in sexual pleasure secondary to use; and (4) related covariates (or background factors), such as history of vaginal and anal product use, frequency of vaginal and anal sex, relationship “harmony,” relationship communication, and demographic variables. Although Phase 1 trials do not allow for the actual measurement of voluntary and sustained use of the method in the context of alternatives, these trials do allow for the identification of factors that may threaten acceptability, and they also provide an early measurement of participants’ expectations about likelihood of future product use.
Previous findings concerning microbicide acceptability from Phase 1 vaginal microbicide trials have indicated that the above mentioned factors can influence overall acceptability, especially use-associated variables. For example, qualitative assessments showed women’s potential discomfort with using a product that is drippy, sticky, or that leaks [6, 7]. In addition, women may have varying reactions to increased vaginal lubrication, sometimes perceiving it as enhancing and other times as detracting from sexual pleasure [7–11]. Studies have also demonstrated that while quantitative acceptability ratings may be high, women’s likelihood of future use may be much lower [12]. Qualitative assessments may be a very useful complement to quantitative assessments by allowing for insights into contextual factors that affect acceptability [5, 7].
In this study, we used both quantitative and qualitative research methods to evaluate the acceptability of SPL7013 Gel, known as VivaGel®, relative to that of two different placebo gels among Puerto Rican and US, healthy, sexually-active, HIV-negative women aged 18–24 years. Our conceptual model, which follows Morrow and Ruiz [5], is depicted in Fig. 1. From a quantitative perspective, acceptability was operationalized as the proportion of participants who, after having had the chance to use the product for 14 days, reported via questionnaire that they would be very likely to use the candidate microbicide during sexual intercourse in the future. The qualitative data helped us contextualize the quantitative results.
Fig. 1.
Conceptual model of microbicide acceptability for a Phase 1 trial (after Morrow and Ruiz [5])
Methods
Studies
Acceptability data were collected in two complementary studies: (1) MTN 004, entitled “Phase I Study of the Safety and Acceptability of 3% w/w/SPL7013 Gel [VivaGel®] Applied Vaginally in Sexually Active Young Women” [13]; and (2) ATN 062 (“Tell Juliana”), entitled “Microbicide-Use Adherence, Acceptability, and Attitudes among Sexually Active Young Women Participating in a Phase I Microbicide Trial (MTN 004).” MTN 004 was a three-arm, three-site, randomized, double-blind, controlled trial comparing VivaGel®, VivaGel® placebo (SP placebo), or hydroxyethyl cellulose (HEC) gel, also known as the universal placebo, that is widely used as an inert, vaginal placebo in tests of safety and efficacy. In this study, participants were asked to apply vaginally 3.5 ml of their assigned gel twice daily using the standard applicator developed by HTI Technologies for 14 days; use condoms during sexual intercourse; and avoid cunnilingus. Participants were also asked to respond to a Web-based computer-assisted self interview (CASI), available both in English and Spanish, inquiring about issues related to product acceptability. ATN 062 utilized the same MTN 004 participants and was conducted simultaneously, allowing us to expand the behavioral investigation by using additional qualitative methods (in-depth interviews) and quantitative methods (interactive voice response system, or “phone diary”).
Participants
Participants were recruited at three study sites: Tampa, Florida; San Juan, Puerto Rico; and Pittsburgh, Pennsylvania between August 2007 and November 2009. Various recruitment approaches were used, including student health centers and STI clinics, lists of former research study participants who agreed to be contacted for future studies, and through media advertisements and flyers targeted to potential participants. To be eligible for the study, women had to be HIV-negative by laboratory testing, 18–24 years old, non-pregnant, sexually active, with a normal genital tract, and using adequate contraception. Out of 61 women who participated in MTN 004, 59 chose also to enroll in ATN 062. The studies were reviewed and approved by the Institutional Review Boards of all participating institutions. Behavioral data were collected over a 3-week period with a 100% follow-up rate.
Procedures
Enrollment was handled by staff at each study site. The behavioral assessment consisted of three Web-based questionnaires, three teleconferences, and the use of an interactive voice reporting system, which are described below. Upon enrollment, participants completed the Baseline Behavioral Questionnaire, a structured Web-based CASI that assessed background variables (see Fig. 1). After completing the questionnaire, participants were introduced to an interviewer, “Juliana,” via teleconference using a webcam with Windows Live Messenger for video; a regular telephone landline was used for voice to increase privacy. The purpose of the video interviewing was to establish a personal relationship between participants and a young, bilingual, engaging Research Assistant with whom they could share the experiences they had using the microbicide. Juliana explained to participants that she lived in a different city (New York) from those in which participants lived, that they would not “run into each other in the mall,” and that all the information participants shared would be kept confidential with only aggregate or masked information being shared with other investigators. During the first teleconference, Juliana highlighted the importance of participants’ opinions for the development of a product that would fit young women’s needs. She also reinforced the instruction participants previously had received about reporting adverse events directly to the clinical staff monitoring the safety of the product and not to limit such reports to the behavioral assessments. Juliana provided instructions to participants on how to use an interactive voice response system to report use of the study gel and sexual behavior and explained that two additional video calls would take place in the future as part of the study.
After the 14 days of product use, participants attended the clinic to undergo a clinical evaluation and respond to the Product Acceptability Questionnaire, a Web-based CASI focusing on proximal determinants (of acceptability) and acceptability proper (see Fig. 1). Upon completing the assessment, participants had a second videoconference consisting of an in-depth interview on the experience of using the product.
Finally, after a 7-day washout period, participants completed the Study Burden Questionnaire, also administered via Web-based CASI, and participated in a third and final videoconference seeking feedback on study procedures and the use of technological tools to collect behavioral data. Results of this final assessment and those of the interactive voice response system will be reported in separate articles (M. Mabragaña, MD, et al., in preparation, 2011; A. Ventuneac, PhD, et al., in preparation, 2011).
Quantitative Assessment
Background Variables
The following background variables were included in the quantitative assessment:
Demographics
These included age, income, country of residence, race/ethnicity, education level, and employment status.
Sexual behavior
At baseline, participants were asked to report on their past sexual behavior including age at first vaginal and anal sex, lifetime number of male sex partners, number of male sex partners in the past 3 months, and number of vaginal and anal sex occasions with and without condoms in the past 3 months.
History of vaginal product use
Questions included ever use of vaginal products including tampons, lubricants, vaginal moisturizers, topical yeast infection medications, vaginal douches, spermicides, female condoms, cervical caps, and desiccants. Participants were asked to specify the number of times they had used vaginal douches or lubricants in the past 3 months.
Proximal Determinant Variables
The following proximal determinant variables were included in the assessment:
Vehicle-associated variables
Attitudes about the gel
Questions included, “How much did you like the color/taste/smell of the gel?” “How much did you like the consistency of the gel (how thick or thin it was)?” “How much did you like how the gel felt inside your vagina immediately after inserting it?” “How much did you like how the gel felt inside your vagina 30 min after inserting it?” (1: Disliked very much to 10: Liked very much). In these and other 10-point scales, average ratings falling between 1 and 3.33 were interpreted as dislike; those between 3.33 and 6.66 as neither liked nor disliked; and those above 6.66 as liked.
Application-associated variables
Application process and applicator
Participants were asked to rate, “Overall, how much did you like putting the gel inside your vagina?” “How easy was it to put the gel inside your vagina?” “How much did you like the gel applicator (the device you used to deliver the gel inside your vagina)?” “How easy would it be to carry this gel around if you needed to?” and “How easy would it be for you to store this gel?” (1: Disliked very much to 10: Liked very much, and 1: very difficult to 10: very easy).
Problems with gel
Participants were asked whether they experienced any problems using the gel (yes/no) or any leakage after using the gel (none/some/a lot). If a participant reported leakage, she was asked, “How much were you bothered by leakage?” (1: Not at all to 10: Very much).
Sexual behavior during the 14 days of the trial
Participants were asked, “How many times did you have vaginal intercourse using the gel with condoms? With gel without condoms? With condoms without gel? Without condoms or gel?”
Sexual pleasure
Participants were asked, “How much did you like vaginal intercourse when using the gel?” (1: Disliked very much to 10: Liked very much) and “Overall, how sexually satisfied were you with vaginal sex when using the gel?” (1: Not at all to 10: A lot). In addition, participants were asked to compare, “Thinking about your experience having vaginal sex after using this specific gel, was this better, worse, or no different from other occasions when you did not use this gel?”. Finally, participants reported on whether or not vaginal penetration was easier after using the gel (not easier, somewhat easier, much easier).
Partner’s reaction
Participants reported on partners’ reactions by partner type (main, casual, and one-time). Since the overwhelming majority of participants (58/61) reported using the gel only with a main partner, we examined responses to the item, “Overall, how much did your most recent main partner like the gel?” (1: Disliked very much to 10: Liked very much).
Acceptability Variables
The following microbicide acceptability variables were included in the assessment:
Overall product like (or dislike)
participants were asked via Web-based CASI, “Overall (i.e., considering all the episodes in which you used this gel), how much did you like the gel?” (1: Disliked very much to 10: Liked very much).
Likelihood of gel use
The Web-based CASI assessment included the following question: “If a gel were available that provided some protection against HIV, and it looked like the one you have used in this study, how likely would you be to use it every time you have vaginal intercourse?” (1: Extremely unlikely to 10: Extremely likely). Finally, participants reported on their likelihood of gel use on occasions when they did not use condoms.
Qualitative Assessment
Juliana conducted a semi-structured interview following an interview guide. It included open-ended questions and follow-up probes of participants’ experiences using the gel, including any problems they had while using the gel, and sexual activity, the participant’s description of her partner’s reaction to gel use during sex, and the participant’s assessment of her likelihood of using the gel in the future. Participants were encouraged to speak freely and to elaborate on specific experiences they had using the gel with or without sexual intercourse. The interviewer sought to explore the context in which young women were using the gel with sex by asking about the participant’s relationship with her partner, their usual sexual behavior, and the effect of the gel on sexual intimacy and enjoyment. The interview lasted approximately 1 h. Juliana developed rapport with participants during an initial teleconference at each participant’s first study visit, and she was gender- and age-matched with participants so that they could feel they were talking with a peer in whom they could confide.
Analyses
Quantitative data were analyzed using SPSS [14]. Participants who used VivaGel® were compared separately to those who used the SP and HEC placebos. Chi-square tests were used for dichotomous and trichotomous variables, and t tests were used for continuous variables. Mann–Whitney tests were used for sexual behavior variables as several of them had skewed distributions. Since each comparison involved two statistical tests, we considered P values of less than 0.025 to be statistically significant.
Due to the small sample size, testing the model in Fig. 1 was limited to three linear regression analyses. In each regression, likelihood of future use was the dependent variable, and product types (VivaGel®, SP placebo, or HEC) were included as covariates. The first regression included background variables as independent variables (age, education, income, employment status, country of residence, lifetime number of partners [ranked], frequency of vaginal sex in past 3 months [ranked], number of vaginal products used, and frequency of lubricant use for vaginal sex in past 3 months). The second regression included three summary scores representing the proximal determinants: vehicle, application, and use. Finally, a third regression included any independent variables associated with likelihood of use at the 0.05 level in either of the first two regressions to evaluate whether or not the proximal determinants explained significant variance in the outcome measure above and beyond background variables.
Qualitative interviews were audio recorded and transcribed, and the transcripts were verified for accuracy. No translations were required because the members of the analysis team who processed the Spanish transcripts were fully bilingual. Based on the interview guide, categories and themes were identified to develop a codebook with primary and secondary code levels. The codebook included definitions, inclusion and exclusion criteria, and examples. To validate and finalize the codebook, three researchers coded an initial set of three transcripts independently and then compared them to assess convergence. Discrepancies were discussed until consensus was reached. The codebook was modified where necessary, and researchers then coded the remaining transcripts independently using QSR NVivo 8.0 software for qualitative data analysis. Coding reports were then reviewed by a team of seven researchers who summarized the main findings.
Results
This section is organized following the theoretical model of Fig. 1 from left to right. We start with background factors, then discuss proximal determinants of product acceptability, and finally present the acceptability results.
Background Factors
Demographics
Table 1 presents the demographic characteristics of the sample. The 61 participants in the study (of which only 59 were interviewed) were on average 20.85 years old (SD = 1.66), range 18–24. They had an average yearly income of USD 8,562 per year (SD = 6,644). One-third lived in Puerto Rico. In terms of ethnicity, 49% of the participants were white/European American, 39% Latina (100% of participants from Puerto Rico identified as Latina or Hispanic), 8% African American, and 3% Asian/Pacific Islander. Some college education was reported by 66% of participants, and 16% had college degrees. Two-thirds were employed. Pairwise comparisons between VivaGel® and SP placebo versus VivaGel® and HEC placebo showed no statistically significant differences on demographic characteristics.
Table 1.
Demographics
| Total N = 61 Mean (SD) |
VivaGel n = 22 Mean (SD) |
SP placebo n = 21 Mean (SD) |
HEC placebo n = 18 Mean (SD) |
VivaGel vs. SP | VivaGel vs. HEC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2/t | df | Pa | χ2/t | df | Pa | |||||
| Age | 20.85 (1.66) | 20.77 (1.54) | 21.00 (1.64) | 20.78 (1.90) | 0.47 | 41 | 0.642 | 0.01 | 38 | 0.993 |
| Income | 8,562 (6,644) | 6,964 (5,780) | 11,383 (7,707) | 7,518 (5,786) | 2.01 | 36 | 0.052 | 0.30 | 36 | 0.770 |
| Residence | 0.04 | 1 | 1.000 | 0.33 | 1 | 0.737 | ||||
| Puerto Rico (%) | 33 | 36 | 33 | 28 | ||||||
| United States (%) | 67 | 64 | 67 | 72 | ||||||
| Ethnicity/Raceb | 0.66 | 1 | 0.330 | 0.09 | 1 | 1.000 | ||||
| White/European American (%) | 49 | 55 | 33 | 61 | ||||||
| Hispanic/Latina (%) | 39 | 36 | 48 | 33 | ||||||
| African-American (%) | 8 | 9 | 10 | 6 | ||||||
| Asian/Pacific Islander (%) | 3 | 0 | 10 | 0 | ||||||
| Education levelc | −0.14 | 41 | 0.892 | −0.19 | 38 | 0.853 | ||||
| Partial high school (%) | 2 | 0 | 5 | 0 | ||||||
| High school graduate (%) | 8 | 5 | 5 | 17 | ||||||
| Partial college (%) | 66 | 73 | 62 | 61 | ||||||
| College graduate (%) | 16 | 14 | 24 | 11 | ||||||
| Partial graduate school (%) | 5 | 9 | 0 | 6 | ||||||
| Graduate school degree (%) | 3 | 0 | 5 | 6 | ||||||
| Employed (full or part-time) | 67 | 77 | 57 | 67 | 1.98 | 1 | 0.203 | 0.56 | 1 | 0.498 |
Since each contrast involves two statistical tests, P values less than 0.025 are significant
χ2 tests = White/European American versus Hispanic/Latino
Statistical tests = t tests of education as a continuous variable
Sexual Behavior
Table 2 summarizes the participants’ past sexual behavior as reported at baseline. On average, participants had their initial vaginal sexual experience at age 16 (SD = 1.97). Those who also had anal sex (N = 31, 50.8% of the sample) reported that it first occurred on average at age 18.4 (SD = 2.20). Participants had had an average of 6.5 (SD = 7.64) sexual partners in their lifetime although most of them had had only one sexual partner in the 90 days prior to enrolling in the study. The subsample that had had anal sex in the 90 days prior to enrollment (N = 10, 16.3% of the sample) had an average of 4.9 anal sex occasions, most of them unprotected by condoms. Only one pairwise comparison was statistically significant: participants who used the SP placebo reported significantly less vaginal sex in the past 3 months than participants who used VivaGel® (25.94 [SD = 13.17] vs. 48.19 [SD = 26.39], P = 0.009).
Table 2.
Sexual behavior
| Total N = 61 |
VivaGel n = 22 |
SP placebo n = 21 |
HEC placebo n = 18 |
VivaGel vs. SP |
VivaGel vs. HEC |
|||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Ua | Pb | Ua | Pb | |
| Age at first vaginal sex | 16.00 (1.97) | 16.55 (2.04) | 15.65 (1.79) | 15.72 (2.02) | 162.0 | 0.137 | 152.0 | 0.205 |
| Age at first anal sex (N = 31) | 18.35 (2.20) | 18.13 (2.30) | 18.33 (1.80) | 18.86 (2.67) | 66.0 | 0.928 | 47.0 | 0.695 |
| Lifetime number of male sex partners | 6.50 (7.64) | 6.27 (4.90) | 6.40 (8.94) | 6.89 (9.13) | 188.5 | 0.425 | 178.5 | 0.593 |
| Number of male sex partners in past 90 days | 1.07 (0.31) | 1.09 (0.43) | 1.10 (0.31) | 1.00 (0.00) | 209.0 | 0.535 | 189.0 | 0.366 |
| Total number of vaginal sex occasions in past 90 days | 40.43 (24.83) | 48.19 (26.39) | 25.94 (13.17) | 45.62 (26.70) | 90.0 | 0.009 | 159.5 | 0.693 |
| Number of vaginal sex occasions in past 90 days without condoms | 27.41 (23.50) | 27.43 (22.28) | 17.00 (14.73) | 38.44 (28.39) | 131.0 | 0.162 | 131.5 | 0.262 |
| Total number of anal sex occasions in past 90 days | 0.82 (2.54) | 1.00 (2.43) | 0.50 (1.47) | 0.94 (3.54) | 202.0 | 0.508 | 176.5 | 0.377 |
| Number of anal sex occasions in past 90 days without condoms | 0.78 (2.53) | 1.00 (2.43) | 0.40 (1.35) | 0.94 (3.54) | 200.5 | 0.473 | 176.5 | 0.377 |
U = Mann–Whitney test
Since each contrast involves two statistical tests, P values less than 0.025 are significant
History of Vaginal Product Use
Table 3 presents participants’ prior experience (ever) using vaginal products. (Questions on product use during the past 3 months do not appear in the table but are presented in the text.) Almost all of the participants had used tampons; 67% had used lubricants during vaginal sex (mean number of times using vaginal lubricants in the prior 3 months was 1.66); 59% had used vaginal moisturizers; 38% had used vaginal medication to treat yeast infections, and almost one-third had used vaginal douches (mean number of times using vaginal douches among the six women who used them in the prior 3 months was 2.5). Only one pairwise comparison was statistically significant: participants in the SP placebo condition were less likely to have used lubricants during vaginal sex than those in the VivaGel® condition (48% vs. 86%, P = 0.010).
Table 3.
Vaginal product use
| Have you ever used…? | Total (% yes) |
VivaGel (% yes) |
SP placebo (% yes) |
HEC placebo (% yes) |
VivaGel vs. SP | VivaGel vs. HEC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | Pa | χ2 | df | Pa | |||||
| Tampons | 97 | 100 | 100 | 89 | NA | 2.57 | 1 | 0.196 | ||
| Lubricants during vaginal sex | 67 | 86 | 48 | 67 | 7.35 | 1 | 0.010 | 2.20 | 1 | 0.253 |
| Vaginal moisturizers (e.g., Moist Again, Replens) | 59 | 77 | 43 | 56 | 5.32 | 1 | 0.031 | 2.13 | 1 | 0.185 |
| Yeast infection medicines | 38 | 36 | 38 | 39 | 0.01 | 1 | 1.000 | 0.03 | 1 | 1.000 |
| Vaginal douches | 30 | 23 | 43 | 22 | 1.98 | 1 | 0.203 | 0.00 | 1 | 1.000 |
| Spermicides | 15 | 9 | 29 | 6 | 2.69 | 1 | 0.123 | 0.18 | 1 | 1.000 |
| Female condoms | 3 | 9 | 0 | 0 | 2.00 | 1 | 0.488 | 1.72 | 1 | 0.492 |
| Cervical cap | 3 | 0 | 10 | 0 | 2.10 | 1 | 0.488 | NA | ||
| Dessicants | 0 | 0 | 0 | 0 | NA | NA | ||||
NA = not applicable due to no variance
Since each contrast involves two statistical tests, P values less than 0.025 are significant
In summary, the women who participated in this study were young, mainly white and Latina, well-educated, employed, sexually experienced, with frequent sexual activity (not always condom protected) at time of enrollment; they had experience using vaginal products, including two-thirds having used vaginal lubricants during intercourse, an important precedent for the use of a microbicidal or placebo gel during the trial.
Proximal Determinants of Product Acceptability
We report below the results both of the quantitative and qualitative assessments of proximal determinants of product acceptability. Table 4 presents pairwise comparisons showing no statistically significant differences between VivaGel® and either placebo except for two items (participant’s sexual satisfaction with gel and partner’s sexual satisfaction with gel), in which participants liked VivaGel® more than the SP placebo. The qualitative data illustrates likes and dislikes expressed by participants about all three products. Qualitative comments are presented for all three products to underscore that the HEC placebo elicited similar reactions to those of the experimental condition and its matched placebo.
Table 4.
Product acceptability and related factors
| Total N = 61 M (SD) |
VivaGel® n = 22 M (SD) |
SP placebo n = 21 M (SD) |
HEC placebo n = 18 M (SD) |
VivaGel®vs. SP | VivaGel®vs. HEC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | df | Pa | t | df | Pa | |||||
| Vehicle-associatedb | ||||||||||
| Color | 8.51 (2.09) | 8.32 (2.32) | 8.43 (2.23) | 8.83 (1.65) | −0.16 | 41 | 0.874 | 0.79 | 38 | 0.433 |
| Smellc | 7.05 (2.22) | 7.00 (2.42) | 7.60 (1.96) | 6.64 (2.25) | −0.66 | 24 | 0.516 | −0.39 | 25 | 0.697 |
| Consistency | 6.28 (2.62) | 6.27 (1.98) | 5.52 (3.25) | 7.17 (2.33) | 0.91 | 33 | 0.371 | 1.31 | 38 | 0.198 |
| Application-associated | ||||||||||
| Overall insertion process | 5.82 (2.18) | 5.59 (1.50) | 5.90 (2.28) | 6.00 (2.79) | −0.54 | 41 | 0.595 | 0.56 | 25 | 0.581 |
| Ease of insertion process | 9.69 (0.70) | 9.73 (0.70) | 9.81 (0.51) | 9.50 (0.86) | −0.44 | 41 | 0.664 | −0.92 | 38 | 0.362 |
| Like/dislike of applicator | 8.33 (1.79) | 8.36 (1.59) | 8.24 (1.95) | 8.39 (1.91) | 0.23 | 41 | 0.818 | 0.05 | 38 | 0.964 |
| Ease of portability | 8.41 (2.01) | 8.77 (1.85) | 8.24 (2.17) | 8.17 (2.07) | 0.87 | 41 | 0.388 | −0.98 | 38 | 0.334 |
| Ease of storage | 8.75 (1.85) | 9.09 (1.51) | 8.57 (2.14) | 8.53 (1.91) | 0.93 | 41 | 0.360 | −1.03 | 37 | 0.311 |
| Use-associated | ||||||||||
| Feeling immediately after insertion | 6.11 (2.35) | 6.05 (2.26) | 6.33 (2.13) | 5.94 (2.78) | −0.43 | 41 | 0.670 | −0.13 | 38 | 0.900 |
| Feeling 30 min after insertion | 5.46 (2.46) | 5.05 (1.86) | 6.00 (2.59) | 5.33 (2.93) | −1.39 | 41 | 0.171 | 0.36 | 28 | 0.721 |
| Bothered by leakage | 6.69 (2.66) | 6.68 (2.48) | 7.05 (2.44) | 6.28 (3.18) | −0.49 | 41 | 0.628 | −0.45 | 38 | 0.654 |
| Bothered by leakage onto underwear or bedsheets | 5.20 (3.43) | 5.50 (3.26) | 5.33 (3.44) | 4.67 (3.76) | 0.16 | 41 | 0.871 | −0.75 | 38 | 0.457 |
| Enjoyment of vaginal sex with gel | 6.77 (2.63) | 7.10 (2.67) | 5.45 (2.40) | 7.94 (2.28) | 2.06 | 38 | 0.047 | 1.02 | 35 | 0.315 |
| Participant’s sexual satisfaction with gel | 7.05 (2.64) | 7.90 (2.14) | 5.75 (2.53) | 7.53 (2.85) | 2.95 | 39 | 0.005 | −0.46 | 36 | 0.646 |
| Participant’s sexual satisfaction with partner without gel | 9.18 (1.34) | 9.19 (1.08) | 9.32 (0.89) | 9.00 (1.97) | −0.40 | 38 | 0.692 | −0.38 | 36 | 0.707 |
| Main partners’ overall like/dislike for gel | 5.17 (2.34) | 5.55 (2.50) | 4.22 (1.99) | 5.67 (2.30) | 1.82 | 38 | 0.076 | 0.16 | 38 | 0.875 |
| Partner’s sexual satisfaction with gel | 6.90 (2.59) | 7.57 (2.34) | 5.70 (2.58) | 7.47 (2.53) | 2.44 | 39 | 0.019 | −0.13 | 36 | 0.899 |
| Product acceptability | ||||||||||
| Overall liking of product | 6.21 (2.42) | 6.14 (2.36) | 6.10 (2.66) | 6.44 (2.33) | 0.05 | 41 | 0.957 | 0.41 | 38 | 0.682 |
| Likelihood of using gel for vaginal sex (at follow-up) | 7.48 (2.86) | 7.09 (2.93) | 7.57 (2.96) | 7.83 (2.75) | −0.54 | 41 | 0.595 | 0.82 | 38 | 0.417 |
| Likelihood of using gel when no condoms | 8.41 (2.47) | 8.36 (2.19) | 7.95 (3.06) | 9.00 (1.97) | 0.51 | 41 | 0.614 | 0.96 | 38 | 0.346 |
| Covert use | ||||||||||
| Likelihood main partner would notice gel during sex | 7.00 (2.54) | 7.41 (2.46) | 7.81 (2.04) | 5.56 (2.68) | −0.58 | 41 | 0.566 | 2.28 | 38 | 0.029 |
| Likelihood casual partner would notice gel during sex | 6.08 (2.83) | 5.86 (3.12) | 7.00 (2.34) | 5.29 (2.82) | −1.32 | 40 | 0.193 | 0.59 | 37 | 0.560 |
| Likelihood one-time partner would notice gel during sex | 5.17 (3.13) | 5.18 (3.33) | 5.95 (2.78) | 4.24 (3.17) | −0.81 | 40 | 0.425 | 0.90 | 37 | 0.375 |
Since each contrast involves two statistical tests, P values less than 0.025 are significant
Most 10-point scales range from 1: disliked very much/not at all to 10: liked very much/very much
Only 37 participants reported having smelled the gel
Vehicle-Associated Variables
Overall, product color (colorless, transparent) received the highest rating (8.51). Product scent was rated 7.05 although slightly under half of respondents skipped this question (they may not have smelled the product). Consistency received an average rating of 6.28 (in the neither-liked–nor-disliked range). Only three participants had tasted the product (this was not required) and rated it 5.33. Due to missing data, neither taste nor smell ratings were used in the summary score for vehicle characteristics. No pairwise comparisons for vehicle-associated variables were statistically significant.
At times, mean ratings provide less insight into participants’ experiences with the gel than information that can be derived from qualitative interviews. For example, concerning the consistency of VivaGel®, most participants described the gel as liquidy, wet, or runny. Although some said the gel felt cool or refreshing, others complained about its consistency. One participant described VivaGel® as “like period blood, only thicker,” (#40) and another felt that it left a residue (#52).
Concerning the SP placebo, the same variance in responses was observed. Many participants described the overall consistency of the gel as “slippery” and “drippy,” while a few participants described the gel’s consistency as “thick” and “sticky”, or “like Vaseline.” One participant described it as “gross”, saying that though it did not leak, “When I would go to the bathroom, I knew I would have to stay waiting there for two huge plops of goo to come out” (#31). Some liked the consistency: “It wasn’t really sticky but it wasn’t runny, it was like a good consistency” (#34) and, as in the case of VivaGel®, some participants said the gel made them feel “fresh.” A few participants expressed disgust when referring to the color of the SP placebo, despite it being a clear, colorless gel. For example, one participant described seeing a greenish-yellow discharge on the applicator (#61). One participant mentioned that the gel smelled like medicine (#37).
With respect to the HEC placebo, many participants did not like its consistency/feel, describing it as “messy,” “gross,” “disgusting,” “slimy,” or “cold,” and/or made them feel as if they were menstruating. One reported that it reminded her of “guys’ cum,” which she found unpleasant. Some noted they were self-conscious wearing certain clothes (shorts, bathing suits) while using the gel. However, other participants did not experience discomfort. Several participants noted that the gel was odorless and clear, which made it more acceptable to use. Furthermore, some participants reported that they became accustomed to the sensation of the HEC placebo over time.
Application-Associated Variables
Participants rated the application process using the HTI applicator 5.82 for the overall insertion process (neither liked nor disliked) and 9.69 for ease of insertion (very easy). Average ratings of 8.33, 8.41 and 8.75 indicated, respectively, that the applicator was liked, easy to carry around and easy to store. Whereas none of the participants in the SP placebo and HEC placebo conditions had problems with the applicator, two participants (9%) in the VivaGel® condition reported problems. No pairwise comparisons for application-associated variables were statistically significant.
In the qualitative interviews, those who had problems with the applicator mentioned difficulties with both assembly and use, including assembling the plunger (connecting it to the piston), forgetting to take the cap off, and scratching oneself with the tip. Overall, the qualitative interviews collected favorable reviews of the applicator, which was described as comfortable to use and similar to a tampon. A few participants recommended that the applicator be smaller and more discreet for the sake of portability and storage.
Use-Associated Variables
Participants’ ratings concerning the feeling of the gel inside the vagina immediately after insertion (6.11) were higher than ratings for feeling of the gel 30 min after insertion (5.46). A paired t test showed that these ratings were significantly different (t = 2.70, df = 60, P = 0.009). This may reflect the detection of leakage: only 2 participants (3%) reported no leakage, whereas 34 (56%) participants experienced some leakage, and 25 (41%) a lot of leakage. Thirty-eight (62%) reported some leakage onto underwear or bed sheets and 6 (10%) reported a lot of leakage onto underwear or bed sheets. Those experiencing leakage gave, on average, a 6.69 rating to how much they were bothered by leakage (lower limit of the bothered “very much” range).
In the qualitative interviews, many participants had a chance to discuss leakage in depth. Of those participants assigned to the VivaGel® condition, most (although less frequently among Puerto Ricans) complained about gel leaking and messiness. Almost all subjects discussed wearing panty liners and having to change them from once to four times a day. One participant went to the bathroom more frequently to wipe up the gel. Another participant took more frequent showers. Leaking happened anywhere from immediately to within an hour after gel insertion. Some participants felt VivaGel® limited their physical activity. For example, one participant reported avoiding going to the gym for the 2 weeks of the trial due to not feeling comfortable. By contrast, one participant reported going swimming while using VivaGel® and not noting any problems.
Participants in the SP placebo condition also complained that the gel leaked and was messy. The degree to which leakage bothered respondents varied greatly. For example, one respondent said, “It didn’t really cause any other aggravation, and it wasn’t a large amount” (#43). In contrast, another participant said, “I didn’t really enjoy the feeling of the gel. It was clumpy, it was runny, it was just—it wasn’t very pleasant” (#18). Most of those reporting leakage said that it started some time after application (30 min–2 h), and they typically dealt with it by using panty liners and wiping though it sometimes required changing underwear. Most of those who addressed the issue of leaking said that it was more of a problem with the morning application than the evening application (because they typically went to bed after evening use).
The HEC placebo also elicited complaints about leakage. Almost all participants had to wear a pad or panty liner during the study (one noted that a maxi pad was needed because a panty liner was not sufficient) and change them often (between every hour to every several hours). Most reported that the gel first started to leak out within an hour of inserting it.
During the 14 days of the trial, participants had an average of 6.88 (SD = 4.94) occasions of vaginal intercourse in which they used the gel with condoms. As seen in Table 4, on average, participants gave a rating of 6.77 to their enjoyment of vaginal sex using the gel with no significant differences among conditions. Participants in the SP placebo group rated their own sexual satisfaction with the gel lower than those in the VivaGel® group (5.75 [SD = 2.53] vs. 7.90 [2.14], P = 0.005). Although 55% of participants reported that vaginal sex with gel was no different than without gel, average ratings of participants’ sexual satisfaction with the same partner when not using the gel (9.18) were higher than when using the gel (7.05). With respect to partners’ reaction to the gel, participants reported that, overall, their main partners neither liked nor disliked the gel (rating 5.17); partner’s sexual satisfaction was rated 6.90 although in this case there was a significant difference, with participants in the SP placebo group also reporting that their partner’s sexual satisfaction with the gel was lower than partner’s satisfaction reported by participants in the VivaGel® group (5.70 [SD = 2.58] vs. 7.57 [SD = 2.34], P = 0.019). This difference parallels that in participant’s own sexual satisfaction with the gel when comparing the SP placebo to VivaGel®.
When asked about their experiences using the gel during intercourse, about half of the participants in the VivaGel® condition reported that the gel served as a lubricant and that this increased their pleasure during sex by decreasing any pain or dryness they normally might have felt with sex. Some participants stated that the use of the gel had no effect on their sexual pleasure while others complained that the gel was “messy”, “gushy”, “liquidy”, and “goopy” during sex. Several participants complained that using the gel restricted their sexual repertoire and spontaneity, e.g., the inability to receive oral sex while using the gel. One participant described how her partner reacted with the word “gross” when finding his hands full of gel after trying to manually stimulate her. Another participant described how the gel limited where she and her partner could have sex given the messiness and the clean-up that was necessary afterwards. A few participants complained that the accumulation of gel in the vagina resulted in a white discharge during sex. In addition, two participants reported having difficulty using condoms while having sex with the gel because the gel seemed to pull the condoms off the penis. One woman described how she and her partner went through three condoms in one sex act because the condom came off twice during sex while another said the only way they could avoid having the condom slip off was for her partner to hold onto the base of the condom while having sex.
A few of the participants’ partners also had positive reactions to the lubricating qualities of VivaGel®. However, several partners complained. Two partners said the gel felt “like Vaseline” and complained about messiness during sex. At least one partner was concerned about possible side effects from coming into contact with the gel, and this concern initially decreased intimacy for the participant and her partner. Another man commented that he would definitely notice if a woman was using the gel due to the white discharge associated with gel use during sex. Several women commented that their partners’ negative reactions to the gel use affected their own sexual pleasure.
Very few participants in the SP placebo condition reported liking the lubricating qualities of the gel during sex. Rather, most described vaginal sex with the gel as “sticky”, “slippery”, and “not natural.” Several participants complained of leakage during and after sex given that a lot of the product was on the condom and on their partners. A few women who did not report a difference in sex with the gel versus without it noted that some time had passed from the moment they inserted the gel to when they had sex. As in the VivaGel® condition, one participant in the SP placebo condition complained that the gel pulled the condom off her partner several times while having sex. This not only interrupted vaginal sex, but the participant would have to retrieve the condom from her vagina before being able to continue. A few partners disliked the lubricating sensation of the gel, stating that the decreased friction reduced their sexual pleasure. In addition, as in the VivaGel® condition, many participants complained of not being able to receive oral sex while using the gel, stating that it decreased spontaneity and made sex boring.
Regarding partner reaction to SP placebo use during sex, several of the participants stated that their partners complained about it. Complaints included disliking the messiness, fear of side effects from coming into contact with the gel, disliking the scent of the gel, and noticing a residue left on the condom after having sex. A participant described this residue as “white clumps.” One participant said her partner mentioned that gel use with sex was fine since they know each other well and had discussed it, but if he had been with a new or casual partner he may have thought that she had a sexually transmitted disease due to the residue left on the condom and increased vaginal secretion during sex.
Finally, almost half of the participants in the HEC condition responded positively to the increased vaginal lubrication during intercourse. One respondent mentioned that the gel felt very similar to regular lubricants that she had purchased in the past. These participants said that the gel either had no effect or a positive effect on their sexual pleasure. However, several participants complained that the gel was messy and that they were bothered by product leakage during sex. They described sex with the gel as “squishy”, “unnatural”, and “gooey.” Women’s reactions to the messiness varied: one participant who disliked the leakage noted that the gel got on her clothes and bed sheets during sex, but she appreciated the lubricated sensation. Another said that the leakage during sex made her feel dirty and gross, leading her to feel bad about herself and to want to have sex less often. As in the other conditions, several women complained about not being able to receive oral sex while using the gel.
Overall, participants in the HEC condition reported that their partners did not have many complaints about using the gel with sex. A few participants reported that their partner could feel a difference in sensation: one mentioned that it felt weird, but most partners did not complain of negative effects of the gel on sexual pleasure. One participant stated that her partner liked the lubricant characteristics of the gel. Neither participants nor partners in this condition complained of any obvious vaginal discharge with sex or problems with using condoms with the gel.
Product acceptability
Table 4 (bottom) shows the ratings that participants gave on the two acceptability parameters. When participants were asked, “Overall, how much did you like the gel?” they gave an average rating of 6.21. This rating falls in the upper limit of the middle third of the scale, corresponding to neither liked nor disliked. There were no statistically significant differences in rating between the groups. After the trial was completed and participants had had the chance to use the product twice daily for up to 14 days, which may have included sexual intercourse, participants gave an average rating of 7.48 to their likelihood of using the gel in the future and a rating of 8.41 for likelihood of using the gel when condoms are not available.
Table 5 presents the proportion of participants who liked, disliked, or felt undecided about the gels, and the proportion of those expressing likelihood of future use. The table shows approximately half of the participants in all three conditions liked the gel, there being no statistically significant differences between conditions. Also, 59% of those in the VivaGel® condition, 67% of those in the HEC condition, and 76% of those in the SP placebo condition indicated likelihood of using the product (upper third of the scale). By contrast, 23% in the VivaGel® condition, 10% in the SP placebo condition and 11% in the HEC condition reported that they were unlikely to use the gel. Although participants had less enthusiasm for VivaGel®, the differences did not reach statistical significance.
Table 5.
Acceptability frequencies
| Total N = 61 |
VivaGel® N = 22 |
SP placebo N = 21 |
HEC placebo N = 18 |
VivaGel® vs. SP | VivaGel®vs. HEC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | Pa | χ2 | df | Pa | |||||
| Liking the gel | 1.67 | 2 | 0.434 | 0.10 | 2 | 0.949 | ||||
| Liked (%) | 49 | 46 | 52 | 50 | ||||||
| Neither liked nor disliked (%) | 34 | 41 | 24 | 39 | ||||||
| Disliked (%) | 16 | 14 | 24 | 11 | ||||||
| Likelihood of using in the future | 1.72 | 2 | 0.424 | 0.94 | 2 | 0.627 | ||||
| Likely (%) | 67 | 59 | 76 | 67 | ||||||
| Neither likely nor unlikely (%) | 18 | 18 | 14 | 22 | ||||||
| Unlikely (%) | 15 | 23 | 10 | 11 | ||||||
Since each contrast involves two statistical tests, P values less than 0.025 are significant
Interestingly, some participants who did not like the gel said they might use it. For example, the following participant (#61, SP placebo), rated the gel a 3 for overall like/dislike (she didn’t like it) but a 7 for intention to use it (likely to use). In the qualitative interview she explained:
It was uncomfortable. I didn’t like the fact that it would leak. I didn’t like how when I would take the applicator out, I would see yellow, greenish stuff on the applicator. […]it was just nasty, I didn’t really want to see that. And I didn’t like the fact that I’d have to use the condom every time. And I’d have to remember to do it like twice a day. […] But for instance if I didn’t know who my partner was, you know, like if it was just anybody or somebody that I just met or something like that, who I’d only been dating for a little while, and I didn’t want him to use a condom, but I wanted to be safe that he wasn’t going to transmit anything to me, I would use it [the gel]. But if I had to use it everyday, twice a day, then I would not use it.
In the qualitative interviews, participants elaborated on the circumstances that would affect their likelihood of using the gel in the future. In the VivaGel® condition, those reporting likelihood of future use had expressed fewer problems with the gel than those unlikely to use it and also said they appreciated having both an HIV prevention method and a lubricant in one product. Most of those who said they would be unlikely to use the gel made reference to their long-term relationships in which they trusted their partners. Hence, perception of risk was a key point in terms of likelihood of gel use. One participant noted that the product would be more likely to be used by single women who had multiple partners or by those who were in a steady relationship but knew their partners were unfaithful.
Participants also stated that their likelihood of using the gel would depend on when and how often they would be expected to use it. Most participants stated that having to use the gel twice a day (as required by the protocol although participants were not told that this would be the frequency required for future use if the gel were efficacious) was too often and that it would be better to use it before sex (as the product is intended to be used). Participants varied in their perceptions of the ideal moment to use the gel before sex, ranging from right before to 1 h before sexual intercourse. However, some participants noted that it was useful to get into a daily routine gel use, albeit no more than once a day. A few participants mentioned that the gel could get in the way of spontaneity if they had to stop and insert it or avoid receiving oral sex after inserting the gel.
Several participants in the SP placebo condition stated they would be likely to use the product in the future, but many of them were not enthusiastic about it. Reasons given for wanting to use the gel included believing it would prevent HIV (although they had been told no product was proven to offer HIV protection in humans), that it would allow women to take control of their own health, and that it would serve as a lubricant. As in the VivaGel® condition, many women said they would not use it with their current, monogamous partners but they would likely use it if they had new or casual partners. Nevertheless, one participant noted that it would be easier to use the gel with a steady partner because given the established communication with that partner, it would be easier to bring up. In addition, issues such as messiness, the interruption of sex, the impossibility of using the product covertly due to its obvious visual and tactile presence in the vagina, and the restrictions product use imposed on the sexual repertoire (e.g., interfering with oral sex), were mentioned by participants as reasons for not wanting to use the gel. Two participants were adamant in their dislike of the product, stating that it ruined their sexual experiences.
Finally, some participants in the HEC condition were quite enthusiastic about the product. Many said they would be likely to use the product but only with a new or casual partner, as was expressed in the other conditions. However, a few participants mentioned that they would only use the product with people they knew and trusted because with occasional partners they would prefer to use condoms. One said that she would use the gel with her boyfriend because even though she trusted him, “Who knows?”
Those who rated themselves as unlikely to use the gel in the future gave several reasons: being monogamous, trust in their partners, that they previously tested HIV negative, or in some cases that the gel was too messy and uncomfortable to use during sex. Most participants believed they could not use the gel covertly because its messiness would be noticeable to their sexual partners as well as the inability to receive oral sex. Others mentioned that timing and amount of gel use would be important. Most felt that it would be better if it could be used right before sex and if a smaller volume of gel could be used.
Regression Analyses
As described above, three linear regression models were tested that specified likelihood of use as the dependent variable and controlled for product type (VivaGel®, SP placebo, or HEC). In the first regression including background variables (age, education, income, employment status, country of residence, lifetime number of partners [ranked], frequency of vaginal sex in past 3 months [ranked], number of vaginal products used, and frequency of lubricant use for vaginal sex in past 3 months), only country of residence was statistically significant. Participants residing in Puerto Rico were more likely to intend to use the product in the future (b = 3.16, se = 0.98, P = 0.003). In the second regression, which included three summary scores representing the proximal determinants (vehicle, application, and use), higher use ratings were associated with future intentions (b = 0.53, se = 0.26, P = 0.044). Finally, a third regression included country of residence and the use summary score as independent variables, and only country of residence remained significant (b = 1.86, se = 0.90, P = 0.045). Space limitations preclude the discussion of differences in acceptability between Puerto Rican and US women; qualitative and quantitative data are presented in a separate publication (R. Giguere, MPH et al., unpublished data, April 2011).
Discussion
This Phase 1 randomized controlled trial sought to assess the acceptability of VivaGel® among young, ethnically diverse, sexually active women. Of the participants assigned to the VivaGel® condition, fewer than half indicated liking it, and 41% said they were undecided. Fifty-nine percent of participants who used VivaGel® indicated they were likely to use it in the future, 23% said they were unlikely to use it, and 18% were undecided. Although participants in the VivaGel® condition appeared less enthusiastic about the product than those in the placebo conditions, the differences did not reach statistical significance.
The proportion of women indicating they were likely to use VivaGel® (as well as the placebo gels) may be an overestimate. We specifically asked, “If a gel were available that provided some protection against HIV, and it looked like the one you have used in this study, how likely would you be to use it every time you have vaginal intercourse?” Thus, we put the risk of HIV in the foreground. The qualitative data showed that women often thought that if they ran the chance of getting HIV, then they would lean towards using the gel especially if they were not using condoms. Yet, most women believed they were not at risk, mainly because they were in steady relationships. If using the gel results in some discomfort (e.g., leakage, soiling of underwear and linen, need to use panty liners, decreased sexual pleasure, restriction from practices like oral sex, partner’s dissatisfaction), it is likely that the perceived risk of contracting HIV may be downplayed (e.g., “I am not at such high risk that I need to sacrifice so much to protect myself”). Alternatively, given the dosing regimen required by the protocol, the number of women indicating that they were likely to use the gels may be an underestimate of those who would use it if the gel were to be used only prior to sexual intercourse.
Of participants receiving VivaGel®, 41% were either non-committal concerning likelihood of future use of the product or said they would definitely not use it, compared with 33% receiving HEC and 24% receiving SP placebo. Attention needs to be paid to this because in a large trial, lack of adherence to product use, missing data and loss to follow up can have a serious impact on the possibility of determining the HIV prevention efficacy of a product [15]. If a Phase 1 trial already shows this level of lack of enthusiasm, efforts should be made to improve the product’s acceptability before embarking on larger trials.
This study highlighted that the issues leading to dissatisfaction with the study products are not restricted to VivaGel®. The HEC placebo was developed as a safe and ineffective placebo product against which different microbicide candidates could be tested. Our participants had as many complaints and problems with the HEC placebo as with VivaGel® or the SP placebo. Many of the complaints replicated those recorded in prior studies [6, 7, 9]. Interestingly, the complaints were not one-sided. For example, some participants thought the gel they used was too thick, but others who used the same product thought it was too thin. There may be variations in women’s physiology that cause them to react differently to the characteristics of the gel (e.g., its osmolarity). Just as with hair gels, with which some people prefer a “firm hold” whereas others prefer a “wet look,” maybe microbicide candidates will need to be formulated in two versions (thick and thin) to allow study participants the choice of which one to use in a trial. Also, a woman may experience the need for different gel consistencies according to her partner: in some cases, a smooth, lubricated penetration may be favored whereas in others more friction may result in more satisfaction.
Introduction of variations in the gel characteristics before an agent has been shown to be HIV preventive may constitute an extra hurdle in the process of product development. Yet, considering the extraordinary cost of Phase III trials and how seriously results can be affected by lack of adherence to product use [15], any steps that can be taken to increase product acceptability and consistent product use during the trial may be crucial to demonstrating microbicide efficacy.
Our trial design had several advantages: Participants had the opportunity to use the actual microbicide during vaginal sex with their partners, and they used it more than once, thus gaining familiarity with it. Therefore, participants could respond to acceptability questions from their real-life experience. Although this Phase 1 trial required participants to use the gel twice daily, some products are being considered for coitally independent administration, which would require consistent application; other products including VivaGel® will require pre-coital administration. In other words, the conditions of this Phase 1 trial may not be too far from the expected use of a microbicidal gel in the future, and the lessons learned may apply to other products and larger trials.
Researchers have become progressively aware that many women have anal sex [16, 17]. In this study of young, mainly college educated women, half of participants reported having had anal sex at least once in their lives with their first anal sex experience occurring on average a couple of years after their first vaginal sex. Furthermore, 16.3% of the participants reported having had anal sex within the 90 days prior to baseline. Given that rectal intercourse is riskier than vaginal intercourse for HIV transmission [18–20], development of microbicidal products that will confer both vaginal and rectal protection is of paramount importance. The simpler the method (e.g., one product protecting both compartments), the higher the likelihood of acceptability.
Some questions remain unanswered. For example, it is unclear why many participants reported experiencing more leakage 30 min after insertion than immediately. Is this attributable to the rheological characteristics of the gel, body temperature, mix with vaginal fluids, or other factors? Can it be prevented? It is important to understand not only how protective a microbicide gel may be, but also how it performs once inserted in the body and what happens to it over time. Acceptability of the gel may be affected by these factors.
Our study has several limitations. The small n in each of the study groups limits the power to detect statistically significant differences among the groups. Furthermore, a Phase 1 trial of short duration, twice-daily product use, and close monitoring is quite different from the real life circumstances in which women could end up using the product. Therefore, the results cannot be generalized. However, in such conditions, complaints and negative ratings that overpower social desirability (the wish to be a good participant and please investigators) are important warnings that need to be heeded. Another limitation of our data is that participants were repeatedly instructed that any adverse event (e.g., rash, discomfort, pain) experienced during the trial should be reported directly to the clinical staff as soon as possible, and that the phone reporting system, email, and video teleconferences did not take the place of direct reporting. Participants who followed these instructions may not have also reported to the behavioral team some problematic experiences they may have had with the gels. Nevertheless, despite such limitations, the combination of quantitative and qualitative methods of inquiry used in this study generated a deep understanding of women’s experiences using gels in the context of vaginal sex, and also pointed to the need for improvement of gel formulations to seek better acceptability of future microbicide candidates.
Acknowledgments
This research was sponsored by the US National Institutes of Health (NIH) and co-sponsored by Starpharma Pty Ltd. The studies were designed and implemented by the Microbicide Trials Network (MTN-004) and the Adolescent Trials Network (ATN-062). The MTN (U01AI068633) has been funded by NIAID, NICHD, and NIMH. MTN-004 and ATN-062 were also funded through NICHD awards to the Adolescent Trials Network (U01HD040533 and U01 HD040474) co-funded by NIMH and NIDA. The study products were provided free of charge by Starpharma Pty Ltd. The Statistical Center was supported by NIAID (U01AI068615). Additional support came from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator: Anke A. Ehrhardt, Ph.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors want to thank Ana Ventuneac, Ph.D., Pamina Gorbach, Ph.D., and the MTN 004 and ATN 062 study teams, including staff from the study sites, the coordinating center, and the statistical center for their contributions to this study. They also want to thank the participants who generously offered their time and candidly discussed intimate experiences.
Footnotes
The study was conducted on behalf of the MTN 004 and ATN 062 Protocol Teams
Contributor Information
Alex Carballo-Diéguez, Email: ac72@columbia.edu, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, 1051 Riverside Drive, Unit 15, New York, NY 10032, USA.
Rebecca Giguere, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, 1051 Riverside Drive, Unit 15, New York, NY 10032, USA.
Curtis Dolezal, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, 1051 Riverside Drive, Unit 15, New York, NY 10032, USA.
Beatrice A. Chen, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA, USA Center for Family Planning Research, Magee-Womens Research Institute, Pittsburgh, PA, USA.
Jessica Kahn, Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Greg Zimet, Division of Medicine, Indiana University, Indianapolis, IN, USA.
Marina Mabragaña, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, 1051 Riverside Drive, Unit 15, New York, NY 10032, USA.
Cheng-Shiun Leu, HIV Center for Clinical and Behavioral Studies, New York State Psychiatric Institute and Columbia University, 1051 Riverside Drive, Unit 15, New York, NY 10032, USA.
Ian McGowan, Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA, USA; Center for Family Planning Research, Magee-Womens Research Institute, Pittsburgh, PA, USA.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantell JE, Myer L, Carballo-Diéguez A, et al. Microbicide acceptability research: current approaches and future directions. Soc Sci Med. 2005;60(2):319–330. doi: 10.1016/j.socscimed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Coly A, Gorbach PM. Microbicide acceptability research: recent findings and evolution across phases of product development. Curr Opin HIV AIDS. 2008;3(5):581–586. doi: 10.1097/COH.0b013e32830aba00. [DOI] [PubMed] [Google Scholar]
- 4.Severy LJ, Tolley E, Woodsong C, Guest G. A framework for examining the sustained acceptability of microbicides. AIDS Behav. 2005;9(1):121–131. doi: 10.1007/s10461-005-1687-y. [DOI] [PubMed] [Google Scholar]
- 5.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–283. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley ME, Morrow KM, Fullem A, et al. Acceptability of a novel vaginal microbicide during a safety trial among low-risk women. Fam Plan Perspect. 2000;32(4):184–188. [PubMed] [Google Scholar]
- 7.Rosen RK, Morrow KM, Carballo-Diéguez A, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health. 2008;17(3):383–392. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead SJ, Kilmarx PH, Blanchard K, et al. Acceptability of Carraguard vaginal gel use among Thai couples. AIDS. 2006;20(17):2141–2148. doi: 10.1097/QAD.0b013e32801086c9. [DOI] [PubMed] [Google Scholar]
- 9.Joglekar N, Joshi S, Kakde M, et al. Acceptability of PRO2000 vaginal gel among HIV un-infected women in Pune, India. AIDS Care. 2007;19(6):817–821. doi: 10.1080/09540120601133576. [DOI] [PubMed] [Google Scholar]
- 10.Morrow K, Rosen R, Richter L, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J Womens Health. 2003;12(7):655–666. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- 11.Mason TH, Foster SE, Finlinson HA, et al. Perspectives related to the potential use of vaginal microbicides among drug-involved women: focus groups in three cities in the United States and Puerto Rico. AIDS Behav. 2003;7(4):339–351. doi: 10.1023/b:aibe.0000004726.61630.96. [DOI] [PubMed] [Google Scholar]
- 12.Bentley ME, Fullem AM, Tolley EE, et al. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94(7):1159–1164. doi: 10.2105/ajph.94.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004) AIDS. 2011;25(8):1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SPSS Inc. SPSS for Windows, Rel. 17.0.0.2008. Chicago, IL: [Google Scholar]
- 15.Mâsse BR, Boily MC, Dimitrov D, Desai K. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerg Themes Epidemiol. 2009;6:5. doi: 10.1186/1742-7622-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenness SM, Begier EM, Neaigus A, Murrill CS, Wendel T, Hagan H. Unprotected anal intercourse and sexually transmitted diseases in high-risk heterosexual women. Am J Public Health. 2011;101(4):745–750. doi: 10.2105/AJPH.2009.181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard E, Carballo-Diéguez A, Ventuneac A, Exner T, Mayer K. Women’s experiences with anal sex: motivations and implications for STD prevention. Perspect Sex Reprod Health. 2009;41(3):142–149. doi: 10.1363/4114209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol. 1998;148(1):88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 19.Vernazza PL, Eron JJ, Fiscus SA, Cohen MS. Sexual transmission of HIV: infectiousness and prevention. AIDS. 1999;13(2):155–166. doi: 10.1097/00002030-199902040-00003. [DOI] [PubMed] [Google Scholar]
- 20.Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS. 1996;10(Suppl A):S75–S82. doi: 10.1097/00002030-199601001-00011. [DOI] [PubMed] [Google Scholar]