Abstract
Responding to growing concerns regarding the safety, quality, and efficacy of cancer care in the United States, the Institute of Medicine (IOM) of the National Academy of Sciences commissioned a comprehensive review of cancer care delivery in the US healthcare system in the late 1990s. The National Cancer Policy Board (NCPB), a twenty-member board with broad representation, performed this review. In its review, the NCPB focused on the state of cancer care delivery at that time, its shortcomings, and ways to measure and improve the quality of cancer care. The NCPB described an ideal cancer care system, where patients would have equitable access to coordinated, guideline-based care and novel therapies throughout the course of their disease. In 1999, the IOM published the results of this review in its influential report, Ensuring Quality Cancer Care. This report outlined ten recommendations, which, when implemented, would: 1) improve the quality of cancer care; 2) increase our understanding of quality cancer care; and, 3) reduce or eliminate access barriers to quality cancer care.
Despite the fervor generated by this report, there are lingering doubts regarding the safety and quality of cancer care in the United States today. Increased awareness of medical errors and barriers to quality care, coupled with escalating healthcare costs, has prompted national efforts to reform the healthcare system. These efforts by healthcare providers and policymakers should bridge the gap between the ideal state described in Ensuring Quality Cancer Care and the current state of cancer care in the United States.
Keywords: Oncology Service, Hospital, Quality of Health Care, Benchmarking, Guideline Adherence, Medically Uninsured, Palliative Care, Comparative Effectiveness Research
Cancer, the second leading cause of death in America,1 has a profound effect on society in terms of both human suffering and economic impact. An aging population will only increase the burden of cancer in the coming decade. While science has advanced the care of cancer patients through molecular diagnostics, epigenetics, and novel personalized treatments, elimination of this disease is a reality for some but not all Americans living today. It is imperative that healthcare providers, policymakers, and the public examine the quality of care provided to the ever-increasing number of people at risk for, living with, and surviving cancer.
Since its creation in 1970, the Institute of Medicine (IOM) of the National Academy of Sciences has demonstrated its commitment to enhancing health and improving the quality of healthcare. For example, in 1995 the IOM created the National Round Table on Health Care Quality to assess quality of care issues. The resulting workshops and commissioned papers culminated in an important consensus statement, published in 1998, in which the Round Table asserted that “quality of health care can be precisely defined and measured with a degree of scientific accuracy comparable with that of most measures used in clinical medicine” (Donaldson 1998).2 This statement highlighted widespread problems associated with the underuse, overuse, or misuse of healthcare and called for major revisions in healthcare delivery, clinician education and training, and quality improvement efforts.2 Subsequently, the IOM initiated the Quality of Health Care in America project, which led to the publication of the 2000 landmark report To Err is Human: Building a Safer Health System3 and the 2001 report Crossing the Quality Chasm.4
In parallel, Dr. Richard Klausner, then director of the National Cancer Institute (NCI), approached the leadership of the IOM and the National Academy of Sciences in late 1996 with a proposal to create a board with broad membership to address the organization and delivery of cancer care. The National Cancer Policy Board was thus established. Early in its deliberations, the board set out to articulate an ideal cancer care system from the patient’s perspective. The board then compared this ideal state with the state of cancer care delivery at that time and, in doing so, identified “a wide gulf between what could be construed as the ideal and the reality” (Hewitt 1999)5 of patients’ experience with cancer care.
Accordingly, the board developed ten recommendations for optimizing the delivery of cancer care for all Americans. These recommendations, encapsulated in the seminal report Ensuring Quality Cancer Care,5 are organized around three central questions:
What problems are evident in the quality of cancer care and what steps can be taken to improve care?
How can we improve what we know about the quality of cancer care?
What steps can be taken to overcome barriers of access to quality cancer care?
(Hewitt 1999)5
As we enter the second decade of the 21st century, it seems appropriate to examine the ten recommendations of Ensuring Quality Cancer Care with respect to the current state of cancer care delivery. This review examines each recommendation and describes the progress that has been made and what further work needs to be accomplished.
What problems are evident in the quality of cancer care and what steps can be taken to improve care?
In the report, the IOM described a substantial gap in healthcare delivery, whereby the survival and quality of life of many patients with cancer was compromised when they did not receive evidence-based care. Recommendations 1–5 of this report describe optimal cancer care, which, when fully implemented, would narrow this gap.
Recommendation 1
Ensure that patients undergoing procedures that are technically difficult to perform and have been associated with higher mortality in lower-volume settings receive care at facilities with extensive experience
The relationship between volume and favorable outcomes in cancer care, and in cancer surgery in particular, has been established across multiple oncologic diagnoses.6–8 Research in this area, together with the 1999 IOM report, has induced efforts to concentrate complex cancer care at high-volume programs. Although the success of these efforts has varied, evidence suggests that declining mortality rates for selected complex cancer operations has been achieved by redirecting certain patients to high-volume centers.9 A more dramatic, long-term shift may be achieved by increasing the capacity of and access to higher volume, higher performing cancer facilities. Currently, high-volume centers are under-resourced to care for as much as half of the complex cancer cases.9
In the interim, contingency plans, such as directing patients to high quality, lower volume providers, are needed. Throughout the nation, some lower-volume hospitals and providers are performing complex care at a high level. Several quality assessment programs aim to increase performance transparency and assist referring physicians, patients, and payors to identify high quality providers: the American College of Surgeons National Surgical Quality Improvement Program; the American Board of Medical Specialty Maintenance of Certification Evaluation of Performance in Practice requirements; the Joint Commission Ongoing Professional Practice Evaluation; and, payor-driven pay-for-performance initiatives. These programs have initiated low-cost pathways for practitioners at lower-volume hospitals to attain, verify, and maintain competence in these areas. Continued development of these programs will support provider and hospital benchmark certifications in complex cancer care. This may, in turn, guide patients with complex cancer issues to competent programs regardless of the program size.
Recommendation 2
Use systematically developed guidelines based on the best available evidence for prevention, diagnosis, treatment, and palliative care
Despite recent trends toward patient-driven, personalized cancer care delivery, most cancer care is structured around accepted practice algorithms that are disease- and stage-specific. More than a thousand clinician experts, led by the National Comprehensive Cancer Network (NCCN), collaborated to develop evidence-based guidelines for more than 135 diseases or processes of cancer care. These guidelines have been adopted nationally10 and have been incorporated into a drug compendium that is used by the Centers for Medicare and Medicare Services (CMS) and other payors for reimbursement purposes.
Guidelines exist for cancer screening, diagnosis, and palliative care, thus spanning the entire cancer care cycle. The American Society of Clinical Oncology (ASCO) has incorporated many of these guidelines into its Quality Oncology Practice Initiative,11 which CMS adopted as a component of its Physician Quality Reporting System.12 Through this program, CMS awards incentive payments for eligible physicians who report on adherence to evidence-based guidelines for services furnished to Medicare beneficiaries.
Despite this progress, investigators have identified variations in adherence in selected providers and diseases and within individual institutions (including NCI-designated comprehensive cancer centers).13,14 Underlying these variations are four important factors. First, physician adoption and reporting of systematically developed guidelines is voluntary. Second, the precise relationship between the use of evidence-based guidelines and long-term outcomes has not been established. Because the guidelines are process-oriented, the relationship to intermediate outcomes is more readily ascertained. With longer time frames, other influences such as co-existent medical conditions may become the primary drivers of outcomes. Additional research is needed in this area. Third, physiological factors resulting from prior cancer treatment and comorbidities can affect adherence. Finally, patient preference sometimes prevents guideline-based treatment.
Since 1999, systematic development, adoption, and support for evidence-based guidelines in oncology practices have increased significantly. Continued adoption of electronic health records (EHRs) by individual practices will enable assessing and reporting guideline adherence without time-intensive and costly chart abstraction. Also, in the coming years, stronger mandates for systems of accountability, such as the mandatory reporting outlined in the Patient Protection and Affordable Care Act of 2010 (ACA) for selected cancer centers,15 are needed to ensure uniform adherence to evidence-based guidelines for cancer care.
Recommendation 3
Measure and monitor the quality of care using a core set of quality measures
In its third recommendation, the IOM advocated the creation of a core set of quality measures for cancer that would effectively define the output of clinical programs. This standardized reporting process would provide benchmarks to increase transparency and identify key elements of quality outcomes. The universal core measure set described by the IOM has not been defined.
Over the past decade, organizations including ASCO and NCCN have collaborated to develop cancer care measures, and the National Quality Forum has endorsed more than 150 cancer-related measures for use in public reporting.16 These measures are largely process-oriented and fail to assess the entire care cycle. An ideal core measure set would include simplified cancer outcomes metrics that are stratified by stage or extent of disease and include survival, two or three key quality of life indicators, treatment assessment, and costs of care. These measures are described below and summarized in Table 1.
Table 1. Clinical Outcomes by Disease Type.
Table 1 represents the authors’ recommendations for a core measure set stratified by stage or extent of disease
| Clinical Outcomes by Disease Site | ||||||||
|---|---|---|---|---|---|---|---|---|
| Head & Neck Cancer | Breast Cancer | Esophageal Cancer | Gynecologic Cancer | Sarcoma | Lung Cancer | Colon & Rectal Cancer | Prostate Cancer | |
| Patient Survival | One- and two-year survival rates | Five- and ten-year disease-free survival rates | Two-year survival rate | Two- and five- year survival rates | Two- and five-year survival rates | One- and two-year survival rates | Two- and five-year survival rates | Five- and ten- year survival rates |
| Patient-Defined Quality of Life | Swallowing | Satisfaction with cosmetic appearance | Swallowing | Bladder and bowel function | Amputation Rate | Pulmonary function | Bladder and bowel function | Bladder and bowel function |
| Speaking | Hormonal health | Weight | Limb function | Stoma rate | Sexual function | |||
| Long-Term Complications of Care | Second primary cancers | |||||||
| Chemotherapy toxicities | ||||||||
| Other treatment-related complications | ||||||||
| Treatment Assessment | Adherence to treatment guidelines | |||||||
| Time to complete treatment | ||||||||
| System throughput | ||||||||
| Short-term complications of care | ||||||||
| Costs of Care | Initial treatment | |||||||
| Surveillance costs | ||||||||
Survival
All cancer patients want to know their prognosis, particularly their chance of cure and long-term survival. The Surveillance, Epidemiology and End Results (SEER) program collects and reports US cancer statistics, such as incidence rates and survival.17 National registries represent the best survival data available but the data are not independently verified, and because the program lacks a central pathology review there is no consistency in pathology diagnosis.18
Patient-Defined Quality of Life
Besides survival, patients are concerned about the immediate and long-term consequences of their disease and treatment. Providers use a number of surveys to assess key indicators of quality of life for cancer patients. However, these tools are commonly used at the provider’s discretion (often as a component of a research trial) and do not always evaluate essential outcomes following treatment.
Treatment Assessment
Hundreds of tools exist to evaluate provider adherence to established standards of preventive, diagnostic, and therapeutic care. However, these tools are often limited in scope and apply to brief segments of care. Integrated care should be more rapid and efficient than fragmented care. Thus, process measures for cancer should emphasize diagnostic and staging accuracy, multidisciplinary treatment planning, and post-therapy management.
Also important is measuring treatment cycle time. Some oncologists associate shorter treatment cycles times with better long-term outcomes for certain cancers.19 More study in this area is needed to establish the precise relationship between treatment cycle time and long-term outcomes.
Costs of Care
As the US economy buckles under rising healthcare costs, there is increasing recognition that healthcare costs must be compared across providers to identify cost inefficiencies and to quantify cost increases associated with comorbidities, delayed diagnoses, and adverse events. It is unclear, however, whether costs may be used as an overall indicator of quality. Current reimbursement models utilize costs as a marker for quality, which is appropriate for hospital-acquired conditions. However, costs are a poor marker of quality for patients with late-stage diagnoses and complex co-morbidities, which require more intensive treatment.
Even getting at cost data is problematic, since there is no uniform costing methodology across providers to facilitate cost comparisons. Thus, a standardized methodology and reporting structure are needed to benchmark healthcare costs across providers throughout the cycle of care and to identify situations where healthcare costs may serve as a marker of quality.
To define and influence desired outcomes, healthcare quality measures must be standardized, reproducible, and widely available. Furthermore, these measures must span the entire treatment cycle (prevention, intervention, and survivorship) and quantify dimensions of healthcare delivery that are meaningful to a variety of audiences. The resulting meaningful measures will support comparative benchmarking of current and long-term performance.
Recommendation 4
Ensure the following elements of quality care for each individual with cancer:
that recommendations about initial cancer management, which are critical in determining long-term outcome, are made by experienced professionals;
an agreed-upon care plan that outlines goals of care;
access to the full complement of resources necessary to implement the care plan;
access to high-quality clinical trials;
policies to ensure full disclosure of information about appropriate treatment options;
a mechanism to coordinate services; and
psychosocial support services and compassionate care.
The IOM enumerated previous definitions of quality care with its fourth recommendation, which advocated care coordination, equitable access, informed decision making, and supportive care.
Care Coordination
Research suggests that outcomes are improved when patients receive cancer care from a highly functioning multidisciplinary care team, where treatment is guided by a written plan of care.20,21 Despite this recognition, healthcare remains fragmented, and patients or their caregivers bear the burden of transitioning between providers.
In a 2011 report, the IOM attributed poor care coordination to systemic issues in the US healthcare system, including lack of provider accountability for delivering patient-centered care and inadequate financial resources to incentivize providers to deliver patient-centered care.21 To date, there have been no successful national strategies to improve care coordination for cancer patients. Universal adoption of EHRs has been recommended to improve care coordination. This is a key requirement for “meaningful use” incentives outlined in the Health Information Technology for Economic and Clinical Health Act (HITECH) provision of the American Recovery and Reinvestment Act of 2009 (ARRA). The ACA also provides for shared savings to providers that deliver coordinated care through Accountable Care Organizations. The impact of these provisions has not been realized.
Equitable Access to Care
The 2010 National Healthcare Disparities Report from the Agency for Healthcare Research and Quality (AHRQ) reported continued access issues for minorities, people of low socioeconomic status, and residents in certain geographic areas. This report cited lack of insurance as an ongoing barrier to equitable access to cancer care, particularly for preventive care.22 Even insured patients experience delays in care due to primary care referrals and coverage pre-authorizations. Patient-specific psychological and socioeconomic hurdles further limit access to coordinated oncology care.23 Individuals with fewer resources tend to seek care based on geographic proximity and accessibility, often at the expense of quality.24 Thus, while the ACA seeks to redress insurance barriers to equitable access, effort is needed to eliminate the geographic, financial, and social barriers that prevent patients from seeking comprehensive cancer care.
Patient access to high-quality clinical trials is also an issue. Historically, lack of coverage for clinical trials created financial barriers to patient participation in novel and potentially beneficial interventions. This situation has improved since Medicare implemented coverage for standard costs of clinical trials in 2000 and since many states have mandated coverage of “routine care” costs for clinical trials participant.25 Investigators suggest that other barriers to participation exist, including a lack of general knowledge about clinical trials, socioeconomic status, and trial design.26,27 Hospital-based patient navigation programs have improved enrollment and retention rates in clinical trials, suggesting this as a means for improving access.21
Informed Decision Making
Patient education at the onset of treatment planning is associated with better outcomes.21 Many patients do not feel adequately informed of their treatment options and the associated prognoses21 while other patients cope with information overload. Patient-focused educational materials from reputable resources, such as the American Cancer Society, NCI, ASCO, and patient advocacy groups, may be used by clinicians to better educate patients regarding appropriate treatment options.
Supportive Care
Several studies have documented improvements in survival and multiple indicators of well-being following psychosocial support intervention.28,29 Many providers lack the resources, and in some cases the skills, to provide adequate psychosocial services to cancer patients. Where these services are offered, they are either denied for reimbursement by insurers or reimbursed at very low rates, making these services an absorbed cost for hospitals. Some experts recommend incorporating psychosocial support into oncology provider education and certification programs to improve psychosocial support for cancer patients.21
Supportive care, together with care coordination, equitable access, and informed decision making, represents ideal cancer care from the patient’s perspective.5 Additional efforts, as described above, are needed to ensure that these services are made available to all cancer patients.
Recommendation 5
Ensure quality of care at the end of life, in particular, the management of cancer-related pain and timely referral to palliative and hospice care
Early referral to palliative care significantly decreases symptom burden and may also increase survival among patients with advanced lung cancer.30 Although patients with advanced cancer are diagnosed a median of 200–250 days before death, patients are referred to the palliative care team approximately forty-five days before death.31 This delay in referral, which compromises end-of-life quality for cancer patients, may relate to the misconception that palliative care reduces hope. Another barrier to early palliative care referral is the perception by oncologists that such care must occur sequentially rather than concurrently with cancer treatment. Additionally, while most cancer centers in the United States have inpatient consult teams, less than half have outpatient clinics, which provide early access to symptom control and advance care planning. Even fewer have dedicated acute palliative care units (Figure 1).32 Cancer centers that offer these services are often under-resourced to care for the high volumes of patients receiving treatment at their institutions.
Figure 1. Palliative Care Programs in US Cancer Centers.
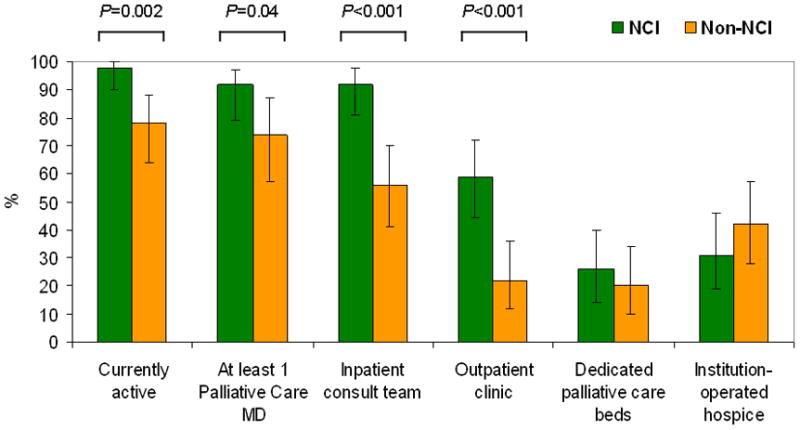
NCI-designated cancer centers were more likely to offer palliative care services through inpatient consultation teams and outpatient clinics.32
For cancer patients with a life expectancy of six months or less, hospice programs deliver excellent end-of-life care. However, it is important that patients receive optimal therapy for their physical and emotional distress before their referral to hospice. Additionally, providers must have appropriate discussions with patients and their families regarding the transition from cancer treatment to comfort care.
Several strategies have been developed to improve end-of-life care. Conceptual models are under development to help referring physicians, nurses, patients, and their families better understand the role of palliative care.33 Several studies have also identified improved quality of life and, in some cases better survival, for patients who received nurse-led palliative care.21 Some insurers have addressed end-of-life care shortages by offering palliative care and hospice services directly to patients, leading to higher utilization of hospice services and dramatic reductions in inpatient days and ICU admissions.34 Another approach is discussing advance care during treatment planning.35 As a consequence of these interventions and health reform-generated programs, supportive and psychosocial services may be better integrated into our delivery system.
How can we improve what we know about the quality of cancer care?
The IOM attributed the gaps in the quality of cancer, in part, to the lack of clinical trials to evaluate the aspects of cancer care delivery. Recommendations 6–8 of this report outline how this research may be supported.
Recommendation 6
Federal and private research sponsors such as the National Cancer Institute, the Agency for Health Care Policy and Research, and various health plans should invest in clinical trials to address questions about cancer care management
Currently, there are no adequate studies to identify why established therapies are not offered to some patients, and there is no mechanism for data collection to support this. National tumor registries, other repositories, and health plans do not collect data to address these questions. However, prospective data collection on newly diagnosed cancer cases with appropriate end points should assist investigators in identifying these issues.
With its sixth recommendation, the IOM suggested that clinical trials might be one setting where this type of prospective data collection could occur. However, in their current form, clinical trials do not identify issues of cancer care management, as they are conducted in controlled settings with pre-screened populations. A few studies have successfully incorporated a health services research approach into clinical trial design.36,37 However, cancer care management is still underrepresented in clinical trials as a whole.
Investigators have identified informed consent as a potential barrier to randomized controlled studies of care management.38 For patients with advanced disease, poor health drives high attrition rates from clinical trials. Alternative approaches, such as using telemedicine to communicate with study participants, may improve patient retention in these studies.39 By adapting clinical trials for health services research the efficacy of health care technologies and interventions may be evaluated in clinical settings. Alternatively, carefully designed observational studies may be used to answer clinical questions that cannot be addressed with a randomized control trial.40
Recommendation 7
A cancer data system is needed that can provide quality benchmarks for use by systems of care (such as hospitals, provider groups, and managed care systems)
Quality benchmarking requires the existence of measures with standardized definitions, processes, and systems for data collection, and standardized reporting formats. Despite concerted efforts in this arena, minimal progress has been made since the IOM report was first published.
Large healthcare systems in certain geographic areas have implemented EHRs that capture relevant data fields. However, these systems focus on primary care.41 Oncology-specific data elements that capture current disease status, functional status, and adherence to national treatment standards are not standard in such systems,42 although vendors are beginning to market oncology-specific EHRs.43,44 For most providers, this reporting is dependent upon time- and resource-intensive abstraction of medical charts in the absence of EHR adoption by individual practices.
To date, it has not been economically feasible to develop a scalable cancer-specific data system that integrates the increasingly sophisticated, tumor-specific biology with more general clinical aspects. Stronger mandates, such as those outlined in the ACA for selected cancer centers, are needed to advance the development of this cancer-specific data system.
Recommendation 8
Public and private sponsors of cancer care research should support national studies of recently diagnosed individuals with cancer, using information sources with sufficient detail to assess patterns of cancer care and factors associated with the receipt of good care. Research sponsors should also support training of cancer care providers interested in health services research
In recent decades, there has been significant progress in altering the progression of a number of malignancies through screening, appropriate local therapies, and incorporation of systemic therapies to reduce the risk of recurrence and death. However, these advances are not universally offered to appropriate candidates for these approaches, and little is known about why this occurs.
The ARRA, which directed $1.1 billion to comparative effectiveness research (CER), accelerated activity in this area.45 In June 2011, the Government Accountability Office reported that the funds had been assigned to new CER programs.46 To guide funding agencies in the use of ARRA-generated monies, the IOM prepared a report outlining national CER priorities. Cancer was addressed in six primary CER topics.47,48
This activity reignited interest in CER throughout the cancer community. The National Institutes of Health directed funding to previously approved, but unfunded CER, Challenge Grants and Grand Opportunity award programs, and other ongoing programs as administrative supplements. The NCI and AHRQ awarded more than eighty grants to CER studies evaluating cancer prevention, screening, and treatment. The NCI also funded network programs designed to organize smaller programs into national programs of cancer control by merging clinical research and community-based programs. Funding was directed to link the SEER national database with other databases to enumerate cancer outcome issues specific to Medicare patients.49 Furthermore, the Secretary of Health and Human Services directed funding to improve clinical registries and data networks and recently announced the creation of a Comparative Effectiveness Research Inventory to merge CER databases.50
This first round of major funding of CER for cancer care represents the first major national funding initiative that corresponds to the IOM’s recommendation. The impact of this funding has yet to be realized. It is unclear whether this funding effort will continue in the current legislative and economic climate. However, the Patient-Centered Outcomes Research Institute created by the ACA has been charged with directing and funding ongoing CER. There is little evidence that health plans have made similar investments in this type of research.
To address the shortage of investigators trained in health services research, the NCI issued eight career development awards aimed at training investigators in CER. Training more investigators in CER, identifying which outcomes are important to patients, and determining how to build better patient/provider partnerships will set the future course for CER in cancer care.
What steps can be taken to overcome barriers of access to quality cancer care?
While advances in medical science have improved short- and long-term outcomes for insured cancer patients, certain populations with cancer have not experienced similar progress. Recommendations 9 and 10 of this report examine the barriers these individuals face in accessing quality cancer care.
Recommendation 9
Services for the un- and underinsured should be enhanced to ensure entry to, and equitable treatment within, the cancer care system
Access and coverage issues for the un- and underinsured have worsened since the publication of this recommendation. The uninsured population stresses the healthcare system, and the rising costs of cancer care coupled with access issues magnify the problem. 2010 cancer care expenditures were estimated at $125 billion; these costs are projected to rise to $207 billion in 2020.51 Beyond the financial implications to patients and their families, lack of insurance is associated with poorer outcomes and lower survival rates.52
AHRQ’s 2009 and 2010 National Healthcare Disparities Reports cited ongoing disparities in access to care. Racial and ethnic minorities and individuals with lower incomes and education levels were found to have disproportionate uninsured rates. These reports also identified poorer quality of care, including lower utilization rates for breast and colon cancer screening, and increased barriers to care, leading to advanced-stage breast and colon cancer diagnoses for minority populations when compared to non-Hispanic Whites.22,53
Several agencies have attempted to address access issues through the development of programs, such as the National Breast and Cervical Cancer Early Detection Program. These programs provide funding to states for the screening for and early detection of cancer in underserved populations. Several states, such as Massachusetts, have implemented insurance reforms with reductions in rates of uninsured. The federal prescription drug program, Medicare Part D, was enacted in 2006 to subsidize the costs of prescription drugs for Medicare beneficiaries. This program is particularly important for cancer patients because the cost of pharmaceutical therapies will continue to rise as treatment options become more advanced. In 2010, legislators attempted to broaden the scope of coverage through the ACA. If the ACA survives the current legal and legislative challenges, its access provisions may improve coverage in several years.
Recommendation 10
Studies are needed to find out why specific segments of the population (e.g., members of certain racial or ethnic groups, older patients) do not receive appropriate cancer care. These studies should measure provider and individual knowledge, attitudes, and beliefs, as well as other potential barriers to access to care
Since this recommendation was published, studies have examined racial, cultural, age-related, and gender-related disparities to identify why, when, and how patients seek care. One study found that providers are inadequately prepared to address and communicate the complexities of cancer care to diverse patient populations.54 Despite compelling data describing the nature and impact of disparities in cancer care, more than ten years after the IOM report, there has been limited progress.
Several programs established through the NCI’s Center to Reduce Cancer Health Disparities are in place to address these issues. For example, the Comprehensive Partnerships to Reduce Cancer Health Disparities program is designed to increase the participation of institutions targeting communities with cancer health disparities in cancer research and research training. The initiative also aims to increase the involvement and effectiveness of NCI-designated cancer centers in developing effective research, education, and outreach programs, to encourage diversity among competitive researchers and to reduce cancer health disparities.55 Additionally, the AHRQ Health Care Innovations Exchange program was launched in 2008 to accelerate healthcare delivery improvements and reduce nationwide healthcare disparities through early adoption of evidence-based innovations.56
Important progress has been made in understanding the factors contributing to health disparities. Significant work is now needed to develop and implement interventions for those population segments that do not receive appropriate cancer care.57
Conclusion
The 1999 IOM report presented a well-researched and timely description of the state of US cancer care delivery. Its ten recommendations described what constitutes quality cancer care; how we can improve our knowledge of quality cancer care; and, how to overcome the barriers of access to quality cancer care. The ferment created by the report reignited interest in patient safety, preventing medical errors, and improving the overall quality of care, leading to significant healthcare improvements through the efforts of the healthcare industry and the government. Much of the progress has been stimulated and funded by two recent pieces of legislation, the ARRA and the ACA.
As we enter the second decade of the 21st century, it is clear that some cancer patients are not receiving ideal care despite these efforts. Progress-to-date, current gaps, and potential management strategies are summarized in Table 2. The greatest challenges center on how to define and implement nationwide solutions, especially universal transparent metrics, access to care, and end-of-life care. More work is needed, especially in the areas of cancer care access and treatment of minority populations, to ensure that all Americans have access to the finest quality care throughout the cycle of care. Increased awareness of the current deficiencies of our cancer care delivery system and action by providers and policy experts should further accelerate improvement.
Table 2. Summary of Progress-to-Date, Current Gaps, and Management Strategies by Recommendation.
Table 2 represents the authors’ view of progress-to-date, current gaps, and potential management strategies for the ten recommendations outlined in Ensuring Quality Cancer Care.5
| Summary of Progress-to-Date, Current Gaps, and Management Strategies by Recommendation |
|---|
|
Recommendation 1 Ensure that patients undergoing procedures that are technically difficult to perform and have been associated with higher mortality in lower-volume settings receive care at facilities with extensive experience. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 2 Use systematically developed guidelines based on the best available evidence for prevention, diagnosis, treatment, and palliative care. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 3 Measure and monitor the quality of care using a core set of quality measures. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 4 Ensure the following elements of quality care for each individual with cancer:
|
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 5 Ensure quality of care at the end of life, in particular, the management of cancer-related pain and timely referral to palliative and hospice care. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 6 Federal and private research sponsors such as the National Cancer Institute, the Agency for Health Care Policy and Research, and various health plans should invest in clinical trials to address questions about cancer care management. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 7 A cancer data system is needed that can provide quality benchmarks for use by systems of care (such as hospitals, provider groups, and managed care systems). |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 8 Public and private sponsors of cancer care research should support national studies of recently diagnosed individuals with cancer, using information sources with sufficient detail to assess patterns of cancer care and factors associated with the receipt of good care. Research sponsors should also support training of cancer care providers interested in health services research. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 9 Services for the un- and underinsured should be enhanced to ensure entry to, and equitable treatment within, the cancer care system. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
|
Recommendation 10 Studies are needed to find out why specific segments of the population (e.g., members of certain racial or ethnic groups, older patients) do not receive appropriate cancer care. |
Progress-to-Date
|
Current Gaps
|
Management Strategies
|
Acknowledgments
This work is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. The authors have no financial disclosures to make.
Contributor Information
Tracy Spinks, Institute for Cancer Care Excellence, The University of Texas MD Anderson Cancer Center.
Heidi W. Albright, Institute for Cancer Care Excellence, The University of Texas MD Anderson Cancer Center.
Thomas W. Feeley, Anesthesiology & Critical Care, The University of Texas MD Anderson Cancer Center.
Ron Walters, Medical Operations & Informatics, The University of Texas MD Anderson Cancer Center.
Thomas W. Burke, The University of Texas MD Anderson Cancer Center.
Thomas Aloia, Surgical Oncology, The University of Texas MD Anderson Cancer Center.
Eduardo Bruera, Palliative Care and Rehabilitation Medicine, The University of Texas MD Anderson Cancer Center.
Aman Buzdar, Breast Medical Oncology, The University of Texas MD Anderson Cancer Center.
Lewis Foxhall, Health Policy, The University of Texas MD Anderson Cancer Center.
David Hui, Palliative Care and Rehabilitation Medicine, The University of Texas MD Anderson Cancer Center.
Barbara Summers, The University of Texas MD Anderson Cancer Center.
Alma Rodriguez, Lymphoma, The University of Texas MD Anderson Cancer Center.
Raymond DuBois, The University of Texas MD Anderson Cancer Center.
Kenneth I. Shine, The University of Texas System.
References
- 1.American Cancer Society. [Accessed June 10, 2011];Cancer Facts and Figures. 2010 http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2010-rev.
- 2.Donaldson MS, editor. Statement on Quality of Care. Washington, DC: Institute of Medicine, National Academy of Sciences; 1998. [Accessed June 23, 2011]. http://www.nap.edu/catalog/9439.html. [Google Scholar]
- 3.Kohn KT, Corrigan JM, Donaldson MS, editors. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000. [Accessed June 6, 2011]. http://www.nap.edu/catalog.php?record_id=9728. [PubMed] [Google Scholar]
- 4.Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 5.Hewitt M, Simone JV, editors. Ensuring Quality Cancer Care. Washington, DC: Institute of Medicine National Research Council, National Academy Press; 1999. [PubMed] [Google Scholar]
- 6.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital Volume and Operative Mortality in Cancer Surgery: A National Study. Arch Surg. 2003;138(7):721–725. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon Volume and Operative Mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 8.Ho V, Heslin MJ, Yun H, et al. Trends in Hospital and Surgeon Volume and Operative Mortality for Cancer Surgery. Ann Surg Oncol. 2006;13:851–858. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Finks JF, Osborne NH, Birkmeyer JD. Trends in Hospital Volume and Operative Mortality for High-Risk Surgery. N Engl J Med. 2011;364:322. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. [Accessed June 6, 2010];National Comprehensive Cancer Network Annual Report. 2010 http://www.nccn.org/about/pdf/annual_report.pdf.
- 11.American Society of Clinical Oncology. [Accessed June 6, 2011];The Quality Oncology Practice Initiative: Program Overview. http://qopi.asco.org/program.
- 12.Physician Quality Reporting System formerly known as the Physician Quality Reporting Initiative: Overview. U.S. Department of Health and Human Services; [Accessed June 6, 2011]. http://www.cms.gov/PQRS/ [Google Scholar]
- 13.Foster JA, Abdolrasulnia M, Doroodchi H, et al. Practice patterns and guideline adherence of medical oncologists in managing patients with early breast cancer. J Natl Compr Canc Netw. 2009;7(7):697–706. doi: 10.6004/jnccn.2009.0049. [DOI] [PubMed] [Google Scholar]
- 14.Romanus D, Weiser MR, Skibber JM, et al. Concordance with NCCN Colorectal Cancer Guidelines and ASCO/NCCN Quality Measures: an NCCN institutional analysis. J Natl Compr Canc Netw. 2009;7(8):895–904. doi: 10.6004/jnccn.2009.0059. [DOI] [PubMed] [Google Scholar]
- 15.Patient Protection and Affordable Care Act. Vol Pub. L. No. 111–148, 124 Stat. 119. Mar. 23, 2010.
- 16. [Accessed June 6, 2011];National Quality Forum-Endorsed Standards [Internet] http://www.qualityforum.org/Measures_List.aspx.
- 17.National Cancer Institute. [Accessed June 6, 2011];Overview of the SEER Program [Internet] http://seer.cancer.gov/about/overview.html.
- 18.Kumar S, Shah JP, Bryant CS, et al. Second neoplasms in survivors of endometrial cancer: Impact of radiation therapy. Gynecol Oncol. 2009;113:233–239. doi: 10.1016/j.ygyno.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24(2):115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C, Munro AJ, Glynne-Jones R, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Washington, DC: National Academies Press; 2011. [Accessed August 1, 2011]. Patient-Centered Cancer Treatment Planning: Improving the Quality of Oncology Care: Workshop Summary. http://www.nap.edu/catalog.php?record_id=13155. [Google Scholar]
- 22.Agency for Healthcare Research and Quality. National Healthcare Disparities Report, 2010. Rockville, MD: 2011. [Accessed August 1, 2011]. http://www.ahrq.gov/qual/nhdr10/nhdr10.pdf. [Google Scholar]
- 23.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable Access to Cancer Services: A Review of Barriers to Quality Care. Cancer. 1999;86(11):2378–2390. [PubMed] [Google Scholar]
- 24.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF., Jr Patient Preferences for Location of Care: Implications for Regionalization. Med Care. 1999;37:204–209. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 25.National Conference of State Legislatures. [Accessed June 6, 2011];Clinical Trials: What are States Doing [Internet]? http://www.ncsl.org/default.aspx?tabid=14331.
- 26.Gross CP, Murthy V, Li Y, Kaluzny AD, Krumholz HM. Cancer Trial Enrollment After State-Mandated Reimbursement. J Natl Cancer Inst. 2004;96(14):1063–1069. doi: 10.1093/jnci/djh193. [DOI] [PubMed] [Google Scholar]
- 27.Gross CP, Wong N, Dubin JA, Mayne ST, Krumholz HM. Enrollment of Older Persons in Cancer Trials After the Medicare Reimbursement Policy Change. Arch Intern Med. 2005;165(13):1514–1520. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet Oct. 1989;2(8668):888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 29.Stanton AL. Psychosocial Concerns and Interventions for Cancer Survivors. J Clin Oncol. 2006;24(32):5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 30.Temel JS, Greer JA, Muzikansky A, et al. Early Palliative Care for Patients with Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 31.Osta BE, Palmer JL, Paraskevopoulos T, et al. Interval Between First Palliative Care Consult and Death in Patients Diagnosed with Advanced Cancer at a Comprehensive Cancer Center. J Palliat Med. 2008;11(1):51–57. doi: 10.1089/jpm.2007.0103. [DOI] [PubMed] [Google Scholar]
- 32.Hui D, Elsayem A, De la Cruz M, et al. Availability and Integration of Palliative Care at US Cancer Centers. JAMA. 2010;303(11):1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruera E, Hui D. Integrating Supportive and Palliative Care in the Trajectory of Cancer: Establishing Goals and Models of Care. J Clin Oncol. 2010;28(25):4013–4017. doi: 10.1200/JCO.2010.29.5618. [DOI] [PubMed] [Google Scholar]
- 34.Spettell CM, Rawlins WS, Krakauer R, et al. A comprehensive case management program to improve palliative care. J Palliat Med. 2009;12(9):827–832. doi: 10.1089/jpm.2009.0089. [DOI] [PubMed] [Google Scholar]
- 35.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leader A, Daskalakis C, Braddock CH, et al. Measuring Informed Decision Making about Prostate Cancer Screening in Primary Care. Med Decis Making. 2011 doi: 10.1177/0272989X11410064. [DOI] [PubMed] [Google Scholar]
- 37.White VM, Macvean ML, Grogan S, et al. Can a tailored telephone intervention delivered by volunteers reduce the supportive care needs, anxiety and depression of people with colorectal cancer? A randomised controlled trial. Psychooncology. 2011 doi: 10.1002/pon.2019. [DOI] [PubMed] [Google Scholar]
- 38.Poustchi H, Farrell G, Strasser S, et al. Feasibility of conducting a randomised control trial for liver cancer screening: Is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011 doi: 10.1002/hep.24581. [DOI] [PubMed] [Google Scholar]
- 39.Applebaum AJ, Lichtenthal WG, Pessin HA, et al. Factors associated with attrition from a randomized controlled trial of meaning-centered group psychotherapy for patients with advanced cancer. Psychooncology. 2011 doi: 10.1002/pon.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkow RP, Ko CY. Evidence-based medicine in surgery: the importance of both experimental and observational study designs. JAMA. 2011;306(4):436–437. doi: 10.1001/jama.2011.1059. [DOI] [PubMed] [Google Scholar]
- 41.Simon J. [Accessed June 6, 2011];The State of Health Information Technology in California [Internet] http://www.chcf.org/publications/2011/05/health-information-technology-california.
- 42.PWC Health Research Institute. [Accessed June 6, 2011];Putting patients into “meaningful use”. http://www.pwc.com/us/en/health-industries/publications/putting-patients-into-meaningful-use.jhtml.
- 43.Varian Medical Systems. [Accessed August 3, 2011];Press Releases: Varian’s ARIA® for Medical Oncology Receives ONC-ATCB EMR Certification by Drummond Group. 2011 May 4; http://varian.mediaroom.com/index.php?s=43&item=855.
- 44.Elekta AB. [Accessed August 3, 2011];Press Release: National Comprehensive Cancer Network Guidelines Available in Elekta’s MOSAIQ EMR. 2011 Jun 08; http://www.elekta.com/healthcare_international_press_release_20071275.php.
- 45.Department of Health and Human Services. [Accessed May 5, 2011];Text of the Recovery Act Related to Comparative Effectiveness Funding, American Recovery Reinvestment Act of 2009. http://www.hhs.gov/recovery/programs/cer/recoveryacttext.html.
- 46.U.S. Government Accountability Office. [Accessed June 27, 2011];HHS Research Awards: Use of Recovery Act and Patient Protection and Affordable Care Act Funds for Comparative Effectiveness Research. 2011 Jun 14; Publication No. GAO-11-712R. http://www.gao.gov/products/GAO-11-712R.
- 47.Initial National Priorities for Comparative Effectiveness Research. Committee on Comparative Effectiveness Research Prioritization, Institute of Medicine. Washington, DC: National Academies Press; 2009. [Accessed July 1, 2011]. http://www.nap.edu/catalog.php?record_id=12648#toc. [Google Scholar]
- 48.Inglehart JK. Prioritizing Comparative-Effectiveness Research – Institute of Medicine Recommendations. N Engl J Med. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 49.National Cancer Institute. Comparative Effectiveness Research (CER) [Accessed May 5, 2011];CER Supported by National Cancer Institute: Projects Supported by ARRA Funding [Internet] http://cancercontrol.cancer.gov/cer/cer_supported_nci.html.
- 50.Proposed Project: Comparative Effectiveness Research Inventory (Public Comment) [Accessed May 5, 2011];Federal Register 2011-310. 2011 Jan 11;:1616–1617. Available from URL: http://www.gpo.gov/fdsys/pkg/FR-2011-01-11/html/2011-310.htm.
- 51.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward E, Halpern M, Schrag N, et al. Association of Insurance with Cancer Care Utilization and Outcomes. CA Cancer J Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 53.Agency for Healthcare Research and Quality. National Healthcare Disparities Report, 2009. Rockville, MD: 2010. [Accessed August 1, 2011]. http://www.ahrq.gov/qual/nhdr09/nhdr09.pdf. [Google Scholar]
- 54.Agency for Healthcare Research and Quality. National Healthcare Disparities Report, 2008. Rockville, MD: 2009. [Accessed June 28, 2011]. http://www.ahrq.gov/qual/nhdr08/nhdr08.pdf. [Google Scholar]
- 55.National Cancer Institute. Center to Reduce Cancer Health Disparities Research. CRCHD Research; [Accessed June 6, 2011]. http://crchd.cancer.gov/research/prchd-overview.html. [Google Scholar]
- 56.Agency for Healthcare Research and Quality. [Accessed August 3, 2011];About the AHRQ Health Care Innovations Exchange [Internet] http://www.innovations.ahrq.gov/about.aspx.
- 57.Presidents Cancer Panel. [Accessed July 1, 2011];President’s Cancer Panel: 2009–2010 Annual Report. 2011 Mar; http://deainfo.nci.nih.gov/ADVISORY/pcp/pcp.htm.


