Abstract
Fibromyalgia (FM) has been conceptualized as a disorder of the central nervous system, characterized by augmented sensory processing and an inability to effectively modulate pain. We previously reported that physical activity (PA) is related to brain processing of pain, providing evidence for a potential mechanism of pain management. The purpose of this study was to extend our work by manipulating pain modulation and determining relationships to both PA and sustained sedentary behavior. Eleven women with FM completed accelerometer measures of PA and underwent functional magnetic resonance imaging of painful heat, administered alone and during distracting cognitive tasks. Results showed that PA was significantly (P<0.005) and positively related to brain responses during distraction from pain in regions implicated in pain modulation including the dorsolateral prefrontal cortex (DLPFC), the dorsal posterior cingulate and the periaqueducatal grey. A significant negative relationship occurred in the left anterior insula. For sedentary time, significant negative relationships were observed in areas involved in both pain modulation and the sensory-discriminative aspects of pain including the DLPFC, thalamus and superior frontal and pre and postcentral gyri. These results suggest that physical activity and sedentary behaviors are related to central nervous system regulation of pain in FM.
Keywords: fibromyalgia, pain modulation, physical activity, fMRI, sedentary behavior
Introduction
Fibromyalgia (FM) is a chronic pain disorder affecting approximately 3% of the general population (5% in women and 1.5% in men).23 There is no distinct biological marker of FM and diagnosis is based on the American College of Rheumatology criteria including pain lasting for at least 3 months in all four quadrants of the body and the axial skeleton, and the presence of pain to palpation in at least 11 of 18 specific locations on the body.47 Along with the requisite pain, patients with FM typically experience a host of additional symptoms including sleep disturbances, fatigue, cognitive difficulties and exacerbation of symptoms with physical exertion.
The mechanisms that underlie FM are unknown. At present there is no consistent evidence for peripheral tissue damage nor have any peripheral generators of pain been specifically identified.40 Data from several independent laboratories suggest that FM pain is primarily maintained by a dysregulated central nervous system (CNS).7,32 These studies have provided behavioral evidence that FM patients exhibit exaggerated temporal summation, allodynia and hyperalgesia to somatosensory stimuli paired with a lack of conditioned pain modulation and enhanced descending pain facilitation.21,22,38,39 Moreover, research employing functional magnetic resonance imaging (fMRI) has demonstrated augmented brain responses to both painful and non-painful stimuli,7,14,17 interpreted as augmented CNS processing of sensory information.
The role of physical activity in the pathophysiology of FM is not well understood. We have previously shown that: 1) FM patients are significantly less active than sedentary, healthy controls; 2) higher amounts of regular physical activity are beneficial for pain processing in FM patients; and 3) physical activity is related to brain activity during the passive receipt of a painful stimulus.27,28 Using fMRI, we demonstrated positive relationships in FM patients between physical activity and neural responses to painful stimuli in the dorsolateral prefrontal cortex (DLPFC), posterior cingulate cortex (PCC), and posterior insula – brain regions implicated in pain regulation. Negative relationships between physical activity and brain activity were observed in the postcentral gyrus and superior parietal cortex – regions implicated in the sensory-discriminative and attentional aspects of pain perception.28 FM patients who reported higher levels of physical activity demonstrated habituation to repeated painful stimuli while FM patients reporting lower levels of physical activity did not, suggesting a relationship between physical activity and pain modulation.28 Although this study provided preliminary support for a relationship between physical activity and CNS pain processing, pain modulation was not explicitly manipulated and consequently conclusions concerning the role of physical activity in pain modulation were speculative. Additionally, this study did not assess relationships between sedentary time and pain processing. Examining sedentary behavior is warranted because research has suggested that low levels of physical activity may contribute to the onset, severity and maintenance of symptoms in women with FM and because sustained sedentary time has been identified as a potential health risk.20,31 However, we are not aware of any research that has systematically determined the role of sedentary behavior in FM.
The primary purpose of the present study was to extend upon our previous brain imaging and physical activity research by manipulating pain modulation. A secondary objective was to examine the relationship between pain modulation and sedentary behaviors. We hypothesized that physical activity would be positively associated with pain modulation and that sedentary time would be negatively associated with pain modulation. These relationships would be demonstrated by differences in pain ratings and brain activity in pain-relevant regions. This research was presented in abstract form at the American Pain Society annual meeting in May, 2011.37
Materials
The institutional review board at the University of Wisconsin-Madison approved the procedures of this study and written consent was obtained from each participant. Given the gender difference in the prevalence of FM, only women were included as participants for the present investigation. In our initial work28 we included FM patients and healthy controls to explore the general relationship between physical activity and pain processing and to determine the specificity of the relationships observed in our FM patient group. For the present study we were primarily interested in testing potential pathophysiological mechanisms of CNS dysregulation in FM and determining whether physical activity was related to an FM patient’s ability to control pain during distraction. Thus, only FM patient data are reported.
Volunteers were recruited via newspaper advertisements, posted flyers, and postal and electronic mailings directed to the female population of a metropolitan area in south central Wisconsin. Confirmation of FM status was obtained from each patient’s care provider via a letter indicating that the patient met the widespread pain and tender point criteria for a diagnosis of FM.47 Participants were screened by phone for exclusionary criteria including the presence of ferrous metal in their body, pregnancy, claustrophobia, and for regular use of opioids, cardiovascular, or high-dose antidepressant medications; continuation of low-dose antidepressants was permitted. Participants taking low-dose antidepressants (4 FM patients) were instructed to maintain their current dosage throughout the course of the study. To our knowledge there has been no published research examining the effects of low-dose antidepressants on fMRI responses. However, previous analysis in our lab showed that use of these medications did not influence our fMRI data beyond reducing the power of our statistical analyses.28 The same procedures were applied to the present study. Similar to our original study, none of the individuals taking low-dose antidepressants substantially altered the pattern of the observed relationships. We did not exclude for a diagnosis of chronic fatigue syndrome in our FM patients; however none of the patients in the present study had this comorbid condition. Lastly, a trained interviewer screened all participants for exclusionary diagnoses of major depression, substance abuse, and other major Axis I psychiatric disorders with the Structured Clinical Interview for DSM-IV Disorders.10
Participants who met criteria were asked to follow several instructions prior to testing: 1) no participation in structured exercise for 48 hours, 2) no alcohol for 24 hours, 3) no pain medications for 24 hours, 4) no caffeine for 4 hours, and 5) no smoking for 2 hours. A compliance check was conducted at the beginning of each session and all participants indicated they had followed all pretesting instructions. Participants were recruited as part of a larger study investigating brain responses to pain and were paid $200 for completion of the study. Seventeen women with FM met initial inclusion criteria.
Experimental Design and Procedures
Testing took place on 2 days separated by a 7-day, physical activity-monitoring period. On the first day, participants completed a simulated MRI session to familiarize the participants with the scanning environment, conduct psychophysical assessments of pain sensitivity and allow the participants to practice the cognitive task used during functional brain imaging data collection. To characterize the sample, participants completed the Beck Depression Inventory (BDI)3 and Fibromyalgia Impact Questionnaire (FIQ).5 After the simulated session, participants were given detailed instructions for physical activity monitoring. Following the 7-day physical activity monitoring period, participants returned for a second day of testing which consisted of functional brain imaging data collection.
Psychophysical assessment of pain sensitivity was initially performed in a mock scanning environment. Participants were exposed to seven thermal stimuli ranging from 43–49°C in 1°C increments. Each temperature was administered twice in random sequence and separated by 1 minute. All thermal stimuli were presented via a PATHWAY Pain & Sensory Evaluation System with a 900 mm2 Peltier thermode (Medoc Advanced Medical Systems, Durham, NC) and delivered to the thenar eminence of the patient’s left hand. The baseline temperature for thermal testing procedures was maintained at 35°C and increased to the target temperature at a rate of 8°C/second. This temperature was maintained for 8 seconds before returning to baseline. Immediately following each stimulus presentation, participants were asked to rate the pain intensity and unpleasantness using the Gracely Box Scales13 viewed with a set of MRI-compatible goggles (Avotec, Inc., Stuart, FL) and presented with the use of E-Prime software (Psychology Software Tools, Pittsburg, PA). Pain intensity and unpleasantness ratings were made using a scanner-compatible button-press response unit (Current Designs, Philadelphia, PA). Linear regression of pain intensity ratings was conducted to determine the temperature each individual rated as ranging between “moderate” and “slightly intense” pain or between ‘11’ and ‘13’ on the 0–20 rating scale. This relative pain stimulus was used for all remaining pain testing procedures. Our choice of a perceptually relative pain stimulus helped to ensure that sensitivity to peripheral pain stimuli was controlled for and thus allowed for a more specific test of the relationship between physical activity and pain modulation.28
The Stroop color-word task was chosen as our distracting cognitive task. Both congruent and incongruent versions of the Stroop color-word task were employed in order to assess each participant’s ability to cognitively modulate pain. The Stroop is a sustained attention task that presents the words “red”, “green”, “yellow” and “blue” in the colors red, green, yellow and blue.41 Words are presented in either congruent (e.g. the word “red” appears in red font) or incongruent (e.g. the word “red” appears in a color of font that is not red) fashion. The incongruent version of the Stroop requires greater attention and consequently has been shown to be more distracting.25 The Stroop has previously been shown to be an effective cognitive distraction in both behavioral and neuroimaging pain studies.2,36 Participants were instructed to press the button corresponding to the color of the word and ignore the word itself. Responses were made using the scanner compatible response unit.
After the mock scanning session, participants were issued an ActiGraph GT1M accelerometer (ActiGraph, LLC, Pensacola, FL) to objectively measure physical activity during waking hours over the 7-day physical activity-monitoring period preceding the fMRI scan. Participants were given standardized instructions for wearing the monitor. Briefly, participants were told that the monitor should be worn at waist level between their hip and naval in the upright position. They were instructed that they could wear it on either the right or left side, but should be consistent throughout the week. Proper placement was demonstrated and the participant put the monitor on in front of one of the researchers in order to assure understanding of verbal instructions. Physical activity data were recorded continuously in 1-minute epochs for the 7-day period.
Participants returned 1 week later for the functional MRI session. This session was designed to determine the behavioral and neural responses during pain modulation. Scanning included five functional runs, each lasting 3 minutes and 50 seconds, presented in a pseudo-randomized, counterbalanced order. Each functional run started with a 30-second off period and had 20-second interstimulus intervals. Runs included pain alone, congruent Stroop alone, incongruent Stroop alone, pain stimuli during congruent Stroop, and pain stimuli during incongruent Stroop. During the runs involving thermal stimuli, five perceptually equivalent stimuli were administered for 20 seconds each, and pain intensity and unpleasantness ratings were collected immediately following each stimulus. For the runs involving the Stroop task, 10 word-color combinations were presented when the Stroop was presented alone and 9 words were presented in runs involving a combination of thermal stimuli and Stroop. There was one less word presented in the combined pain and Stroop run in order to avoid having the onset of the pain stimulus coincide with the presentation of a word. In order to avoid potential confounds due to expectations, participants were not told when each run-type would occur during the scan. Thus, prior to the start of each run and regardless of its type, participants were instructed to focus on the cognitive task being as fast and accurate as possible and to rate the pain stimuli whenever prompted by the rating scales.
Magnetic Resonance Imaging Acquisition
All functional and anatomical magnetic resonance images were collected on a 3-Tesla GE SIGNA MRI scanner (GE Health Systems, Waukesha, WI). A vacuum pillow was utilized to restrict head motion within the coil. High-resolution, spoiled gradient echo (SPGR) anatomical acquisitions were collected with the following parameters: 128, 1.2-mm thick, T1-weighted axial images, inversion time: 600ms, repetition time (TR): 9ms, echo time (TE): 1.7ms, field of view (FOV): 24cm, flip angle: 10°, acquisition matrix: 256 × 256). To correct inhomogeneity-induced distortions in the images, a set of 2D gradient echo fieldmaps was collected in the sagittal direction with the following parameters: 128, 4-mm thick slices, TR: 550ms, TE: 7 & 10ms, flip angle: 60°, FOV = 240mm, acquisition matrix: 256 × 256. Functional T2*-weighted blood oxygen level dependent (BOLD) images were collected using echoplanar imaging (EPI) with a whole-head transmit-receive coil. Functional MRI acquisitions were obtained with a gradient echo EPI sequence (TR: 2000ms, TE: 30ms, flip angle: 90°) and consisted of 30 4-mm thick slices collected in the sagittal direction with a 1mm gap. The acquisition matrix was 64 × 64 voxels and the FOV was 24 cm, delivering an in-plane voxel resolution of 3.75 × 3.75 × 5 mm.
Image Processing and Analysis
Analyses were conducted using Analysis of Functional Neuroimaging (AFNI) software.9 Due to saturation effects, the initial three sets of functional images were discarded from all analyses. Functional MR images were slice-time, motion and fieldmap corrected, smoothed with an 8-mm full-width, half-maximum Gaussian kernel, converted to percent signal change and registered to Montreal Neurological Institute 152 space. The criterion for inclusion of fMRI data for a given experimental run was head movement of less than 2 mm.
Physical Activity Data Processing
Criteria for inclusion of accelerometer data were at least 9 hours of valid wear time for a minimum of 3 weekdays and 1 weekend day. Accelerometer data were processed using in-house software to calculate minutes spent in sedentary, light, low-moderate, high-moderate, and vigorous levels of physical activity. Cut-off points for accelerometer counts per minute were based on previous research as follows: sedentary = 100 and below; light = 101–760; low-moderate = 761–1952; high-moderate = 1953–5724; vigorous = 5725 and above.11,26 Both average sedentary time and minutes spent during sustained sedentary behavior were calculated and used for the sedentary measurements. Sustained sedentary time was operationally defined as 100 or less counts/minute sustained for at least 60 consecutive minutes. For example, 59 consecutive minutes of sedentary time would not qualify as sustained sedentary behavior and would thus not be included in the analysis.
For statistical analyses of relationships between physical activity and pain modulation, we focused primarily on the low-moderate physical activity cut-point because: 1) most of the time spent being physically active (i.e. activity greater than light intensity) in our FM sample occurred in this range, 2) this is the most likely level of physical activity that patients would be expected to engage in,27,28 3) we were interested in physical activity levels that could be used to inform future exercise training trials, and 4) our previous work indicated that the moderate and vigorous cut-points were not strongly related to pain processing in FM.28 Accelerometer-based measures of activity also allow for the objective determination of both average and sustained sedentary time, thus allowing us to examine relationships between pain modulation and sedentary behavior.
Statistical Analyses
To determine the degree of pain modulation, pain intensity and unpleasantness, ratings for the five stimuli were averaged for each functional MRI pain run. Pain modulation was defined as the difference between the ratings of the pain stimulus alone compared to ratings of the pain stimulus during the distracting cognitive tasks. Partial correlation analyses (Pearson’s r) were employed to assess the relationships between pain modulation and physical activity and sedentary behaviors. To control for disease severity, scores on the FIQ were accounted for in these analyses. Additionally, for analyses involving sedentary behavior, scores on the BDI were also controlled for because of the moderate correlation between sedentary time and BDI scores (r=0.46). Based on the directional nature of our hypotheses, a 1-tailed test was employed and the level of significance was set at 0.05. Analyses were conducted using SPSS version 19.0.
Regression analyses of whole brain data were conducted using AFNI’s 3dRegAna program9 with accelerometer (low-moderate and high-moderate physical activity and average sedentary and sustained sedentary time) data to examine the relationships between physical activity behaviors and cognitive pain modulation. Again, scores on the FIQ were statistically controlled for in all analyses and scores on the BDI were also controlled for in analyses involving sedentary behavior. Regions of interest were selected based on current research documenting brain responses to pain in patients with FM and healthy controls. Each region of interest was defined using the Harvard-Oxford cortical and subcortical structural probabilistic atlases developed at the Centre for Morphometric Analysis at Harvard University, Boston, MA (www.cma.mgh.harvard.edu). The probability threshold was set at 0.25. Regions included pre and postcentral gyri, superior parietal lobule, anterior, posterior and paracingulate cortices, brainstem, frontal medial and orbital cortices, frontal and parietal opercula, frontal pole, insula, thalamus, middle frontal gyrus, and superior temporal gyrus.
To determine the specificity of the relationship between pain modulation and physical activity, the occipital pole was employed as a control region. This region was active during the viewing of our functional pain scale and the cognitive task, though it is not typically considered a pain-relevant brain region. Thus, we considered this region to be a reasonable control.
Brain activity representative of cognitive pain modulation was generated by subtracting the BOLD responses during both the pain-alone and Stroop-alone runs from the combined pain-during-Stroop runs. This subtraction was done separately for the congruent and incongruent versions of the Stroop task. This conservative approach was intended to isolate pain modulation responses by accounting for general activity associated with pain processing (pain-alone task) and cognitive function (Stroop-alone tasks). Regression analyses of accelerometer data were conducted for both the congruent and incongruent Stroop runs in order to determine whether the level of distraction influenced our relationships. Results of the regressions for physical activity and brain responses were thresholded at P < 0.005 and clusters of activity with a volume of less than 200 mm3 were ignored to control the experiment-wise error rate. Due to the small volume of the brainstem periaqueductal grey (PAG), minimum clusters of activity were lowered to 40 mm3.
In order to further investigate the relationships between pain modulation and physical activity in women with FM, participants were categorized into higher and lower physically active and sustained sedentary time groups using a median statistical split for accelerometer data. The median split for physical activity was performed using minutes of low-moderate intensity activity. Independent-samples t-tests, controlling for FIQ scores, were performed using AFNI’s 3dttest++ program9 to assess differences in brain responses during pain modulation. BDI scores were also controlled for in analyses of sedentary time. Due to the small sample size of this comparison, these analyses should be considered exploratory. Results from t-tests were thresholded at P < 0.005 and clusters of activity with a volume of less than 200 mm3 were ignored.
Results
Of our original sample of 17 FM patients, six were excluded because they did not meet the accelerometer wear-time inclusion criteria. Thus, 11 FM patients were included for statistical analysis. Participant characteristics are presented in Table 1. Physical activity data are presented in Table 2. The participants meeting inclusion criteria averaged approximately 7 days of wear time (6.55 ± 0.69) and averaged greater than 14 hours of data per day (860 ± 92 minutes). This group of FM patients would be considered sedentary by current physical activity standards.16
Table 1.
Participant Characteristics
| Mean ± SD N = 11 |
|
|---|---|
| Age | 41.3 ± 13.2 |
| Height | 169.7 ± 4.1 |
| Weight | 74.8 ± 16.7 |
| BMI | 26.1 ± 5.1 |
| FIQ | 45.1 ± 11.1 |
| BDI | 8.8 ± 6.8 |
Table 2.
Objectively Measured Physical Activity
| Mean ± SD N = 11 |
||
|---|---|---|
| Accelerometer Data | Sedentary (mins/day) | 585 ± 92 |
| Light (mins/day) | 197 ± 47 | |
| Low-Moderate (mins/day) | 62 ± 27 | |
| High-Moderate (mins/day) | 16 ± 7 | |
| Vigorous (mins/day) | 0.2 ± 0.7 | |
| Sustained Sedentary (mins/week) | 307 ± 253* |
Measures are mean (± standard deviation) expressed as counts and minutes per day. Sedentary = ≤100 counts/minute; Light = 101–760 counts/minute; Low Moderate = 761–1952 counts/minute; High Moderate = 1953–5724 counts/minute; Vigorous = ≥ 5725
Sustained Sedentary = total minutes when participant was sedentary for at least 60 consecutive minutes per week.
The temperature used during the test day (rated as ‘13’ or “slightly intense pain”) was 47.1 ± 1.1°C. Pain intensity ratings were 11.0 ± 4.7 for the pain alone run, 9.2 ± 5.3 for the pain during congruent Stroop run and 8.0 ± 3.8 for the pain during incongruent Stroop run. Pain unpleasantness ratings were 9.7 ± 4.7 for the pain alone run, 8.0 ± 5.0 for the pain during congruent run and 7.1 ± 3.3 for the pain during incongruent run. Correlations between both minutes spent in various intensity categories of physical activity and sedentary behavior and changes in pain ratings representative of pain modulation are presented in Table 3. No data are presented for vigorous activity as only two participants had activity that reached this intensity. Significant relationships were observed between sedentary time and pain modulation for the congruent Stroop for both pain intensity (r = −0.66) and pain unpleasantness (r = −0.70) and for the incongruent Stroop for pain intensity only (r = −0.60). Significant relationships were also observed for low-moderate activity and pain modulation during the congruent Stroop for both pain intensity (r = .71) and pain unpleasantness (r = .70). Thus, the less time spent in sedentary behavior and/or the more time spent in low-moderate activity, the more participants modulated pain during the cognitive tasks.
Table 3.
Correlations between accelerometer data and change in pain ratings, controlling for disease severity
| Intensity | Unpleasantness | |||
|---|---|---|---|---|
| PA - CS | PA - IS | PA - CS | PA - IS | |
| Sedentary | −0.66*† | −0.60*† | −0.70*† | −0.54† |
| Sustained Sedentary | −0.46† | −0.15† | −0.50† | −0.11† |
| Light | 0.24‡ | −0.13‡ | 0.22‡ | −0.19‡ |
| Low-Moderate | 0.72*‡ | 0.53‡ | 0.72* ‡ | 0.45‡ |
| High-Moderate | 0.02‡ | 0.13‡ | −0.03 ‡ | 0.10‡ |
NOTE: All results are Pearson’s r
Significant at P = 0.05 by 1-tailed test.
Controlling for FIQ
Controlling for both FIQ and BDI
PA = Pain alone, CS = Congruent Stroop plus pain, IS = Incongruent Stroop plus pain
Functional MRI Analyses
Table 4 presents brain responses during pain modulation that were significantly correlated with accelerometer data. For the congruent Stroop, low-moderate physical activity was significantly and negatively related to brain responses in the ipsilateral anterior insula and positively related to responses in the dorsal region of the PCC. For the incongruent Stroop, brain activity in the contralateral DLPFC was significantly and positively related to low-moderate physical activity. For high-moderate activity, significant negative relationships were observed in the contralateral postcentral gyrus and the ventral region of the PCC.
Table 4.
Clusters showing significant relationships between low-moderate and high moderate physical activity and sustained sedentary time with brain responses during pain modulation, controlling for FIQ scores in all analyses and BDI scores in sedentary analyses (N=11)
| Direction | Peak X,Y,X | Volume (mm3) | Peak R2 | F-Statistic | |
|---|---|---|---|---|---|
| Low-Moderate | |||||
| Congruent Stroop | |||||
| L Anterior Insula | − | 30, −8, −14 | 376 | 0.78 | 22.81 |
| Dorsal Posterior Cingulate | + | −10, 20, 44 | 232 | 0.75 | 16.42 |
| Incongruent Stroop | |||||
| R DLPFC | + | −30, −52, 26 | 472 | 0.86 | 8.93 |
| PAG | + | 2, 36, −16 | 40 | 0.72 | 19.88 |
| High-Moderate | |||||
| Congruent Stroop | |||||
| R Postcentral Gyrus | − | −40, 22, 52 | 632 | 0.76 | 18.54 |
| Ventral Posterior Cingulate | − | 6, 46, 2 | 472 | 0.77 | 21.54 |
| Sustained Sedentary | |||||
| Congruent Stroop | |||||
| Thalamus | − | 0, 8, 8 | 4000 | 0.82 | 29.33 |
| R Postcentral Gyrus | − | −52, 32, 54 | 2064 | 0.87 | 28.82 |
| R DLPFC | − | −28, −50, 10 | 776 | 0.78 | 16.67 |
| L Superior Frontal Gyrus | − | 14, −52, 42 | 584 | 0.78 | 22.03 |
| R Precentral Gyrus | − | −50, −10, 28 | 544 | 0.69 | 17.81 |
| L DLPFC | − | 36, −40, 36 | 360 | 0.78 | 16.87 |
| L Precentral gyrus | − | 4, 16, 74 | 272 | 0.61 | 18.34 |
| L Postcentral Gyrus | − | 66, 20, 30 | 232 | 0.77 | 18.47 |
| Incongruent Stroop | |||||
| L Middle Frontal Gyrus | − | 46, −2, 56 | 744 | 0.85 | 23.01 |
| Average Sedentary | |||||
| Congruent Stroop | |||||
| Dorsal Posterior Cingulate | − | −10, 24, 42 | 872 | 0.82 | 16.87 |
| R DLPFC | − | −28, −50, 14 | 232 | 0.84 | 23.71 |
| R Postcentral Gyrus | − | −30, 44, 62 | 200 | 0.82 | 21.70 |
Note: All values significant at voxel-wise threshold of P = 0.005 and cluster threshold at 200 mm3
For sustained sedentary time, significant negative relationships were observed for brain activity in the thalamus, superior frontral gyrus, bilateral DLPFC, bilateral pre-central gyrus, and bilateral postcentral gyrus for the congruent Stroop. No significant relationships were observed between sustained sedentary time and brain activity for the incongruent Stroop task. For average sedentary time, significant negative relationships were observed in the dorsal region of the PCC, the contralateral DLPFC and the contralateral postcentral gyrus. Light activity was not significantly related to pain modulation. These data are presented in Figures 1–3. No significant relationships were observed between measures of physical activity or sedentary time and brain responses in the experimental control region, the occipital pole (range of r = 0.10 to 0.26).
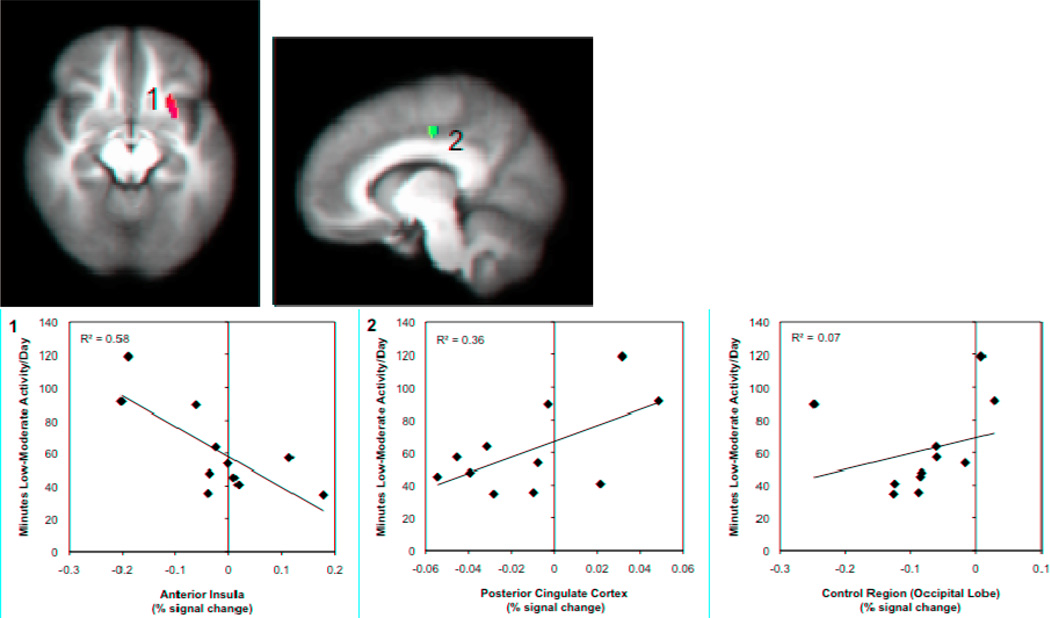
Figure 1. Relationships between low-moderate activity and brain responses to pain modulation during the congruent Stroop task.
Brain regions showing significant associations between accelerometer measures of low-moderate physical activity and brain responses during pain modulation (congruent Stroop task), controlling for FIQ scores. A significant negative correlation was found in the left anterior insula (1) and a significant positive correlation was founding in the dorsal regions of the posterior cingulate cortex (2). Images are shown with a voxelwise threshold set at P = 0.005 and cluster-size thresholding at 200 mm3. Functional timeseries data (percent signal change) for each individual were extracted and are shown plotted against average minutes of low-moderate physical activity per day with the corresponding R2 values. Percent signal change data values are based on the subtraction of the BOLD responses during both the pain-alone and Stroop-alone runs from the combined pain-during-Stroop runs.
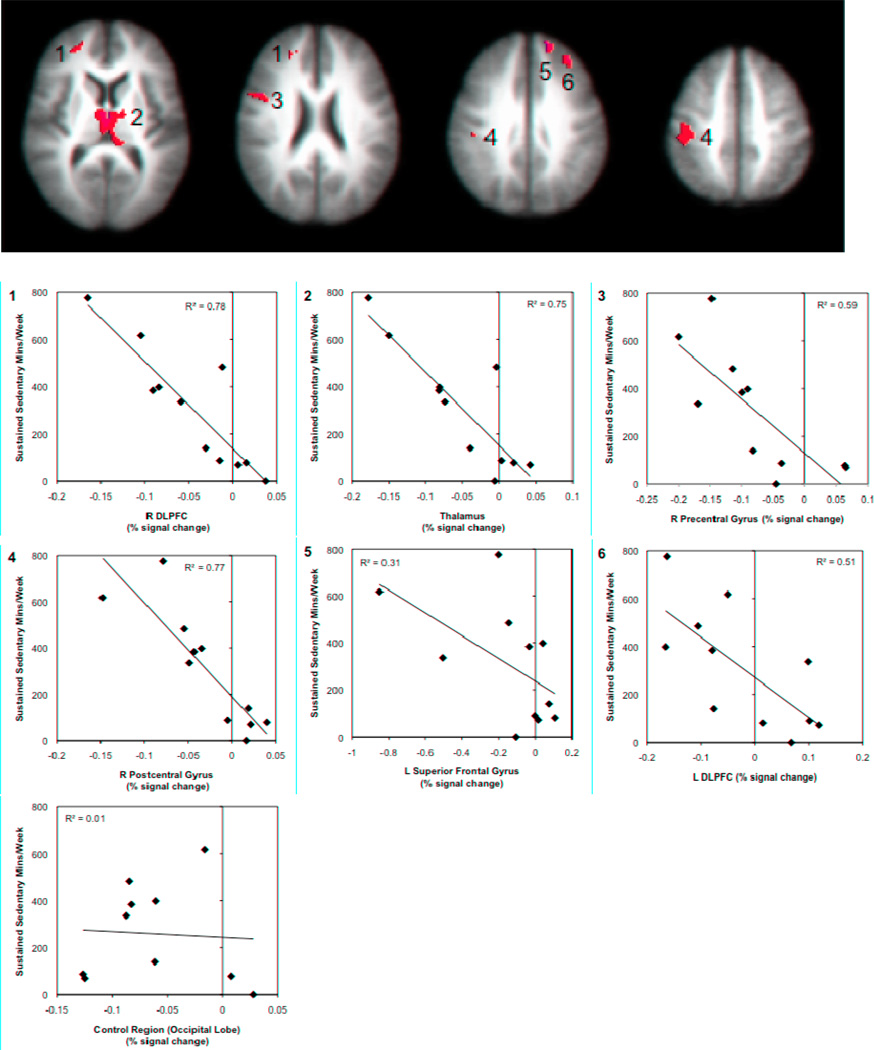
Figure 3. Relationships between sustained sedentary time and brain responses to pain modulation during the congruent Stroop task.
Brain regions showing significant associations between accelerometer measures of sustained sedentary time and brain responses during pain modulation (congruent Stroop task), controlling for FIQ and BID scores. Negative correlations were found in the bilateral DLPFC (1 and 6), thalamus (2), right pre and postcentral gyri (3, 4) and right superior frontal gyrus (5); left pre and postcentral gyri are not pictured. Images are shown with a voxelwise threshold set at P = 0.005 and cluster-size thresholding at 200 mm3. Functional timeseries data (percent signal change) for each individual were extracted and are shown plotted against minutes of sustained sedentary time per week with the corresponding R2 values. Percent signal change data values are based on the subtraction of the BOLD responses during both the pain-alone and Stroop-alone runs from the combined pain-during-Stroop runs.
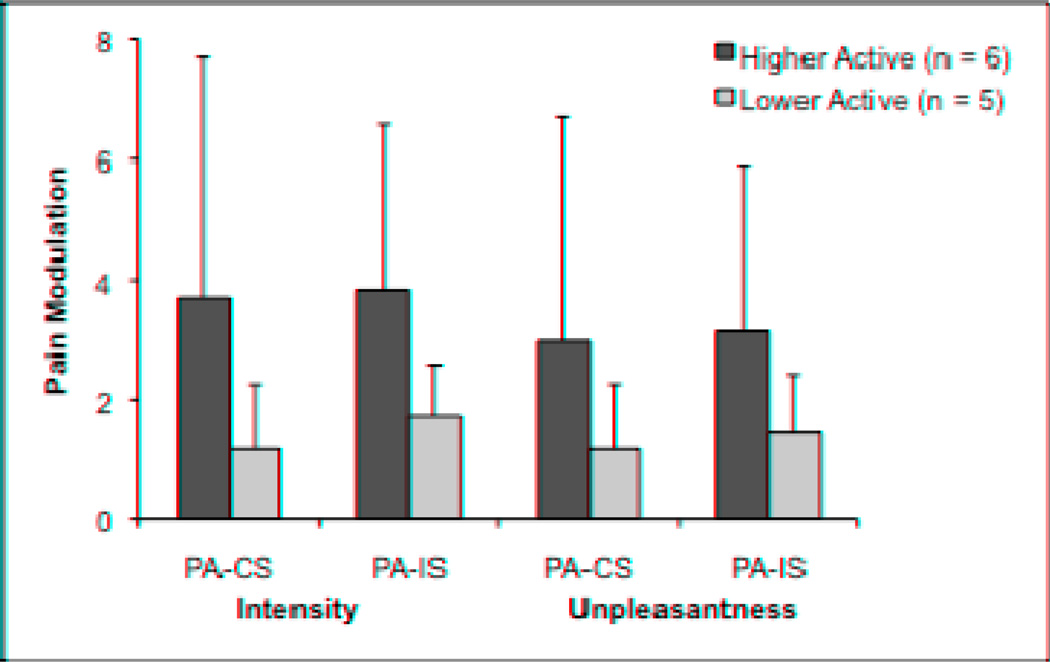
Pain Modulation Responses Between Higher and Lower Active FM Patients
Splitting participants by low-moderate physical activity resulted in six higher active and five lower active participants. Two participants in each subgroup were taking low-dose antidepressants and scores on the BDI and FIQ did not differ between groups (P > 0.05). Pain modulation ratings for each activity group are presented in Figure 4. Effect sizes (Cohen’s d) between the higher and lower active group means were small to moderate and ranged from 0.31 to 0.49 for ratings of intensity and unpleasantness. The individuals in the higher physical activity group had significantly (P < 0.005) less activity in the contralateral precentral gyrus [t(9) = −6.16] during pain modulation for the congruent Stroop. Higher active FM patients also showed significantly (P < 0.005) more activity in the ipsilateral precentral gyrus [t(9) = 5.82], ipsilateral anterior insula [t(9) = 10.41, contralateral DLPFC [t(9) = 5.61], and the dorsal region of the PCC [t(9) = 7.19] compared to lower active FM patients for the incongruent Stroop.
Figure 4.
Pain modulation as a function of level of low-moderate physical activity in FM patients. Pain modulation reflects the difference in pain intensity and unpleasantness ratings between the pain alone run and the combined pain and cognitive task runs.
PA = Pain alone, CS = Congruent Stroop plus pain, IS = Incongruent Stroop plus pain
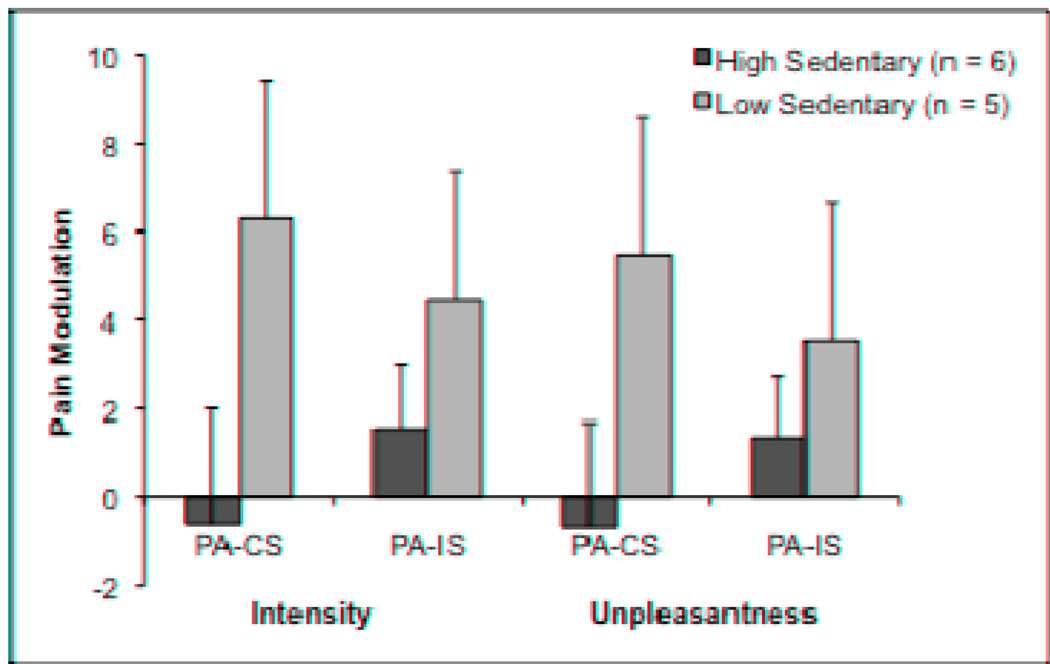
Pain Modulation Responses Between High and Low Sedentary FM Patients
Splitting participants by sustained sedentary time resulted in six high sedentary and five low sedentary participants; these groups were not comprised of the same individuals as the higher and lower physical activity groups. In the high sedentary group, participants averaged 499 ± 168 minutes of sustained sedentary behavior during the week of physical activity monitoring, while those in the low sedentary group averaged 75 ± 50 minutes. Two participants in each subgroup were taking low-dose antidepressants and scores on the BDI and FIQ did not differ between groups (P > 0.05). Pain modulation ratings for each sedentary time group are presented in Figure 5. Effect sizes between group means were small to large and ranged from 0.43 to 1.03 for intensity and unpleasantness ratings. High sedentary FM patients showed significantly (P < 0.005) less activity in the bilateral DLPFC [t(9) = −5.82], contralateral postcentral gyrus [t(9) = −5.61], ACC [t(9) = −5.55], and contralateral precentral gyrus [t(9) = −5.21] compared to the low sedentary FM patients during the congruent Stroop.
Figure 5.
Pain modulation as a function of level of sustained sedentary time in FM patients. Pain modulation reflects the difference in pain intensity and unpleasantness ratings between the pain alone run and the combined pain and cognitive task runs.
PA = Pain alone, CS = Congruent Stroop plus pain, IS = Incongruent Stroop plus pain
Discussion
Our data demonstrate significant relationships between brain responses during pain modulation and both physical activity and sedentary behaviors for women diagnosed with FM. These results extend upon our earlier data by manipulating pain modulation and considering the previously unexplored influence of a sedentary lifestyle. In general, physical activity was positively related to brain activity during pain modulation while sedentary time showed negative relationships. Together, these data suggest that physical activity behaviors are important for CNS regulation of pain in FM.
For this study, we chose to include two separate measures of sedentary behavior, average minutes of sedentary time and minutes of sustained sedentary time. While these variables are related, they differ in one important way – sustained sedentary time refers to prolonged periods of sedentary behavior that are greater than an hour in length while average sedentary time includes all sedentary time, regardless of duration. Recent research has suggested that prolonged periods of sedentary time may be more detrimental to one’s health than shorter periods of sedentary time that are broken up by higher intensity activity.20, 31 As FM patients tend to be highly sedentary,20,27 we were particularly interested in determining the effects of prolonged sedentary behavior on pain modulation.
Our results demonstrate that for behavioral assessments of pain modulation, average sedentary time and sustained sedentary time provide relatively equivalent information in both direction and magnitude of the relationships. However, our functional neuroimaging data demonstrate that sustained sedentary behavior is associated with a broader network of brain regions involved in pain modulation suggesting that pain modulation may be more influenced by sustained sedentary behavior than average sedentary time.
Pain Modulation Brain Regions
Patients with FM demonstrated significant relationships between objective measurements of physical activity and sedentary time and brain responses indicative of cognitive pain modulation. These included the prefrontal cortex (PFC), insula, cingulate cortex, thalamus, and PAG. All of these regions have been reported to respond to painful stimuli and to play a role in pain modulation in healthy controls.2,7,33,45
The prefrontal cortices are considered as critical regions for endogenous pain control.4 Specifically, the DLPFC has been shown to be involved in the top-down modulation of pain possibly by inhibiting connectivity between the thalamus and midbrain.24 We have previously reported that physical activity was positively related to activity in the lateral regions of the PFC during pain processing in FM patients, suggestive of top-down pain control.28 In the current study, we observed negative relationships between sustained sedentary time and brain activity during pain modulation bilaterally in the DLPFC. Moreover, significantly less activity was observed in the contralateral DLPFC for the high sedentary FM group and this same region was significantly more active in the higher active group during pain modulation. These results provide additional support for the potential benefits of physical activity for pain regulation in FM driven in part by activity in the DLPFC.
Another important region involved in pain perception and modulation is the insula, an area related to pain unpleasantness, intensity encoding, opioid-related pain modulation, anxiety, depression and aversive body states.12,34,35,43 Activity in this region is augmented in FM patients compared to controls in response to both warm and painful heat stimuli7 and insula responses have been consistently shown to discriminate FM from control groups.8 Our lab recently reported a positive relationship between physical activity and responses in the contralateral posterior insula, a region with a somatotopic representation1 and involvement in pain regulation,44 in FM patients during the passive processing of a painful heat stimulus.28 In the current study, the ipsilateral anterior insula was negatively related to physical activity in FM patients suggesting that physical activity might affect the general emotional evaluation of afferent sensory signals during active pain modulation. The physiological aspects of being physically active (i.e. repetitive interpretations of interoceptive signals) may influence the way that pain is evaluated during a distracting cognitive task and may be a potential target brain region for physical activity interventions that are designed to treat chronic widespread pain.
The cingulate cortex is comprised of several different regions, plays an integrative role in pain processing and has been proposed to process feelings of unpleasantness and emotional memories as they relate to painful stimuli.46 The anterior portion of the cingulate is related to executive control and processing emotion. In the current study, the more sedentary FM patients showed significantly less activity in this region than the less sedentary FM patients during pain modulation. In addition, the posterior portion of the cingulate cortex is related to the evaluation of internal states.29 The PCC has been subdivided into two regions with distinct functions.46 The dorsal PCC (dPCC) has been associated with visuospatial orientation towards a noxious stimulus and the ventral PCC (vPCC) has been implicated in formation and retrieval of self-referential memories associated with pain.46 Previously, we reported that responses in the ipsilateral PCC were positively related to physical activity during passive pain processing.28 The current data show a significant positive relationship between physical activity and activity in the dPCC and a significant negative relationship between physical activity and activity in the vPCC during pain modulation. Moreover, data demonstrate a significant negative relationship between average sedentary time and activity in the dPCC. The results suggest that being active may help to maintain or improve a patient’s ability to evaluate and regulate sensory information during a concurrent distracting task while being sedentary may challenge this ability.
The thalamus is a sensory integration region and is involved in several aspects of pain such as general arousal and attention.36,43 Resting regional cerebral blood flow to the thalamus has been reported to be lower in neuropathic pain patients15 and pain relief is associated with increased blood flow to the thalamus.19 In our study, FM patients exhibited a strong negative relationship in the bilateral thalamus for sustained sedentary time supporting the potential detrimental effects of sedentary time for pain modulation.
The PAG is an integral component of the descending pain-regulation circuitry. Tracey and colleagues42 reported increased PAG activity during distraction from pain that was predictive of decreased pain ratings. The current data show a positive relationship between physical activity and PAG activity, but only during the more attention demanding incongruent Stroop. Thus, during high levels of distraction, women with FM who were more physically active engaged this region to a greater degree and this was moderately associated (d = 0.49) with reductions in pain ratings. Importantly, in our previous study we did not observe significant relationships between physical activity and the PAG during the passive receipt of a painful stimulus. These results show that physical activity is related to PAG activity, but only during explicit modulation of painful stimuli.
Pain Sensory-Discriminative Brain Regions
We also observed significant relationships for brain regions classically involved in the sensory-discriminative dimensions of pain. These included the pre and postcentral gyri. The postcentral gyrus (primary somatosensory cortex) has been implicated in the spatial summation, temporal, anticipation, intensity, and discriminative aspects of pain processing.1,2,35,36,45 Previously we reported a negative relationship between physical activity and brain responses to pain in the ispilateral postcentral gyrus, suggesting that being physically active was associated with reduced pain sensitivity.28 In the current study, a negative relationship between sustained sedentary time and brain activity in the bilateral postcentral gyrus during pain modulation was found.
Activity of the precentral gyrus (primary motor cortex) might reflect sensory pain response to repeated pain stimuli, motor inhibition of the stimulated hand or facial muscle tension.7,33 Responses of the bilateral precentral gyrus (primary somatosensory cortex) were negatively related to sustained sedentary time in FM patients. The similar pattern of relationships between these two regions (pre and postcentral gyri) suggests that being sedentary may interfere with the sensory-discriminative components of pain processing during a competing cognitive task.
Interestingly, a study assessing functional connectivity during cognitive pain modulation using the Stroop task identified a number of similar regions (ACC, PAG and posterior thalamus) to form a network of cognitive modulation in healthy participants.45 The authors concluded that during pain modulation, the cingulo-frontal area was responsible for inhibitory control of sensory processing in order to perform attention and cognitive tasks.44 Thus for the current study, the negative relationships between frontal cortical (DLPFC) and sensory (pre and postcentral gyri) regions in FM patients and sustained sedentary time would suggest that the communication with the sensory processing regions are impaired and therefore, the experience of pain might be augmented.
Limitations and Considerations
The primary limitation of the current study is the small sample size. This was due to a large portion of our sample not meeting inclusion criteria for accelerometer data. We chose to be stringent in our accelerometer data inclusion criteria in order to perform more conservative analyses. Two participants were close to meeting the criteria but did not and when their data were included in the brain analyses, the same patterns of activity were observed but the strength of those relationships was greater. Furthermore, we reported the results of high-low splits with small group sizes. Though these were only exploratory, the observed significant relationships in brain regions involved with both sensory discrimination and modulation of pain support the potential role of physical activity and sedentary behavior in the experience of pain. In addition, this sample of FM patients was free of chronic fatigue syndrome and major depressive disorder, common comorbid diagnoses, and therefore applying these data to the larger heterogeneous population of FM patients should be done cautiously.
Because of this small sample size, we were unable to employ the current best-practice methods for controlling for multiple comparisons in our fMRI analyses. Thus, it is possible that some of our reported regions are spurious. However, our primary results showed relationships between physical activity behaviors and brain responses to pain modulation in a priori designated regions and in the hypothesized directions. Further, these results corresponded with our behavioral data. Consequently, our results may provide important information on which to base future studies in this area.
Other limitations of the study include the physical activity level of the sample. Our participants were largely inactive and it is unclear how our observed relationships would generalize to a more active FM sample. There was also no manipulation of physical activity, thereby precluding statements concerning causal mechanisms. The novel findings of this study were the relationships with sustained sedentary time. Future research should focus on mechanistic studies of exercise interventions in FM. These studies could test how physical activity and sedentary behaviors influence brain regions involved in pain modulation in terms of brain volume, structure and function.
Conclusions
Though FM is often characterized as a central nervous system disorder, the causative mechanisms are most likely multifaceted and include psychosocial factors, physiological factors and the complex integration of neural circuits. Our results suggest that FM patients who can maintain even a low level of physical activity and/or avoid periods of sustained sedentary time are more able to modulate pain during distraction. How these results translate to patient symptoms (i.e. the illness of FM) is currently unknown, however exercise training is recognized as a consistently beneficial treatment for FM.6 Larger prospective studies that manipulate physical activity and sedentary behaviors and determine central nervous system responses to pain and pain modulation are needed to test the generalizability and specificity of our observed relationships. These future studies could help us to determine the mechanism by which physical activity is beneficial for patients with chronic musculoskeletal pain.
Perspective.
Our results support a promising benefit of physical activity and highlight the potentially deleterious effects of sustained sedentary behavior for pain regulation in FM. Studies aimed at increasing physical activity or reducing sedentary behavior and determining the impact of these on pain regulation are warranted.
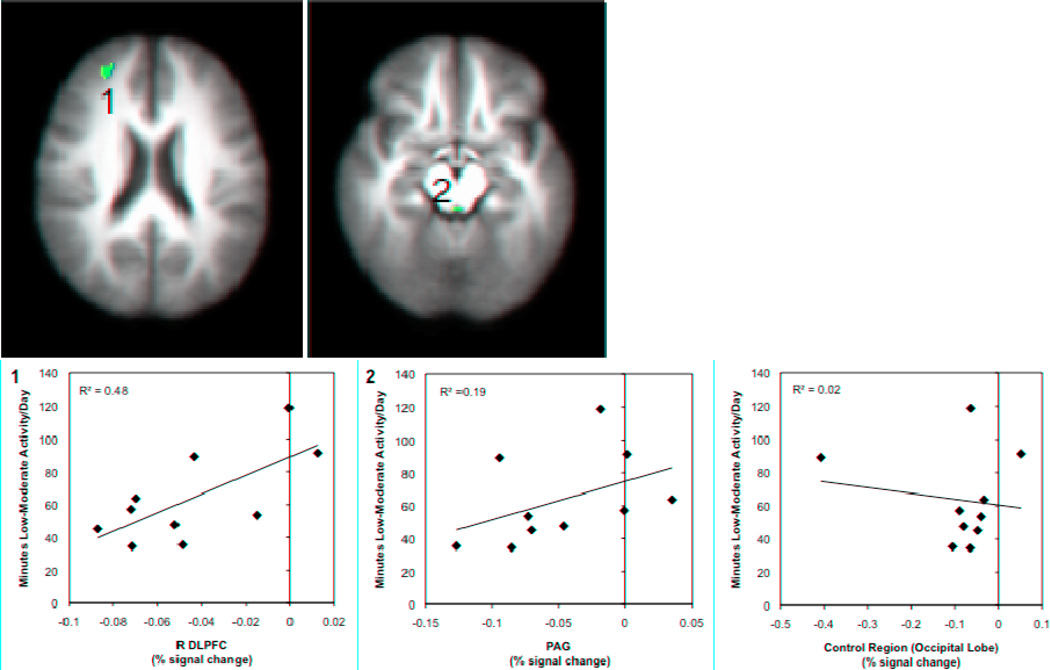
Figure 2. Relationships between low-moderate activity and brain responses to pain modulation during the incongruent Stroop task.
Brain regions showing significant associations between accelerometer measures of low-moderate physical activity and brain responses during pain modulation (incongruent Stroop task), controlling for FIQ scores. Significant positive correlations were found between accelerometer measures of low-moderate physical activity and brain activity in the R DLPFC (1) and the PAG (2). Images are shown with a voxelwise threshold set at P = 0.005. For the DLPFC, cluster-size thresholding was set at 200 mm3 and for the PAG cluster-size thresholding was set at 40 mm3. Functional timeseries data (percent signal change) were extracted and are shown plotted against average minutes of low-moderate physical activity per day with the corresponding R2 value. Percent signal change data values are based on the subtraction of the BOLD responses during both the pain-alone and Stroop-alone runs from the combined pain-during-Stroop runs.
Acknowledgements
The authors would like to acknowledge the significant contribution of Nathan J. Vack in physical activity data processing and analysis. With respect to the use of the Harvard-Oxford Cortical and Subcortical Structural Atlases, we are very grateful for the training data for FIRST, particularly to David Kennedy at the CMA, and also to: Christian Haselgrove, Centre for Morphometric Analysis, Harvard; Bruce Fischl, Martinos Center for Biomedical Imaging, MGH; Janis Breeze and Jean Frazier, Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance; Larry Seidman and Jill Goldstein, Department of Psychiatry of Harvard Medical School; Barry Kosofsky, Weill Cornell Medical Center.
This research was supported by National Institutes of Health grant ROI 5R01AR050969, the National Institute of Arthritis and Musculoskeletal and Skin Disorders (NIAMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There were no conflicts of interest during this study.
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 4.Bingel U, Schoell E, Buchel C. Imaging pain modulation in health and disease. Curr Opin Neurol. 2007;20:424–431. doi: 10.1097/WCO.0b013e328259c34d. [DOI] [PubMed] [Google Scholar]
- 5.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 6.Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KAR. Exercise for fibromyalgia: A systematic review. J Rheumatol. 2008;35:1130–1144. [PubMed] [Google Scholar]
- 7.Cook DB, Lange G, Ciccone DS, Lui WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 8.Cook DB, Stegner AJ, McLoughlin MJ. Imaging pain of fibromyalgia. Curr Pain Headache Rep. 2007;11:190–200. doi: 10.1007/s11916-007-0190-8. [DOI] [PubMed] [Google Scholar]
- 9.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput and Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 10.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 11.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 13.Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 14.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 15.Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- 16.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 17.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in FM reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Karmath HO, Baier B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Struct Funct. 2010;214:411–417. doi: 10.1007/s00429-010-0250-4. [DOI] [PubMed] [Google Scholar]
- 19.Kishima H, Saitoh Y, Oshino S, Hosomi K, Ali M, Maruo T, Hirata M, Goto T, Yanagisawa T, Sumitani M, Osaki Y, Hatazawa J, Yoshimine T. Modulation of neuronal activity after spinal chord stimulation for neuropathic pain; H(2)150 PET study. Neuroimage. 2010;49:2564–2569. doi: 10.1016/j.neuroimage.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 21.Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375–383. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- 22.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psych Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 26.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37:S512–S522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 27.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc. 2011:905–912. doi: 10.1249/MSS.0b013e3181fca1ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain. 2011;12:640–651. doi: 10.1016/j.jpain.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medford N, Critchley HD. Conjoint activity of the anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersel DL, Dror V, Cheung R. Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res. 2011;89:29–34. doi: 10.1002/jnr.22512. [DOI] [PubMed] [Google Scholar]
- 33.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 34.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 35.Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122:1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- 36.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 37.Shields M, Ellingson L, Stegner A, Cook DB. How does physical activity relate to cognitive pain modulation in women? J Pain. 2011;12:S39. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 40.Staud R, Robinson ME, Weyl EE, Price DD. Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J Pain. 2010;11:1376–1383. doi: 10.1016/j.jpain.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psych. 1935;18:643–662. [Google Scholar]
- 42.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Tran TD, Wang H, Tandon A, Hernandez-Garcia L, Case KL. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010;150:93–102. doi: 10.1016/j.pain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain- an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McGain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]