Abstract
We previously reported that gestational dietary protein restriction in rats causes gender-related differences in development of offspring's blood pressure (BP) that is more pronounced in the males than females. Since such effects may depend on sex hormones, we investigated the role of estradiol in the development of hypertension in female offspring of protein restricted dams. Female offspring of pregnant rats fed normal (20%) or protein restricted (6%) casein diets throughout pregnancy were kept either, intact, ovariectomized or ovariectomized with estradiol supplementation. BP, estradiol and testosterone levels and vascular estrogen receptor (ER) were examined. BP was significantly higher and plasma estradiol levels were significantly lower by 34% in intact protein restricted female offspring compared to corresponding controls. Further decrease in estradiol levels by ovariectomy exacerbated hypertension in the protein restricted females with an earlier onset and more prominent elevation in BP compared to controls. Estradiol supplementation in ovariectomized protein restricted females significantly reversed ovariectomy-induced hypertension but did not normalize BP to control levels. The hypertensive protein restricted females have reduced vascular ERα expression that was unaffected by ovariectomy or estradiol replacement. In addition, the testosterone levels were significantly higher by 2.4-, 3.4-, and 2.8-fold in intact, ovariectomized and estradiol replaced protein restricted females compared to corresponding controls. Our data show that: 1) hypertension in protein restricted adult female offspring is associated with reduced plasma estradiol levels, 2) estradiol protects and limits the severity of hypertension in protein restricted females and contribute for sexual dimorphism, and 3) Estradiol replacement fails to completely reverse hypertension, which may be related to limited availability of vascular ERα receptors and/or increased circulating testosterone levels.
Keywords: blood pressure, sex steroid hormone, gender differences, fetal programming, estradiol
Introduction
Epidemiologic evidence suggests that adverse fetal environment contributes to development of adult health disorders (1,2). Animal studies have shown that nutrient, energy or oxygen restriction, or reduction in placental perfusion during pregnancy often results in fetal growth restriction and hypertension during adult life(3–5), although the mechanisms are still not completely understood. In the rat, we and others have demonstrated that protein restriction during pregnancy results in fetal growth restriction and hypertension in adult offspring(6–8). Alterations in function of vascular smooth muscle, endothelial, renin-angiotensin system and hypothalamic pituitary adrenal axis are thought to participate in both the development and the maintenance of prenatally programmed hypertension(9,10).
Studies examining the underlying mechanisms that contribute to hypertension of developmental origin should also take into account the well-established gender differences in the occurrence of cardiovascular diseases in humans at various stages of life. The incidence of cardiovascular disease and hypertension is lower in premenopausal women compared with age-matched men and postmenopausal women(11). However, after menopause, the risk of hypertension increases with age(11), suggesting that the fully functional ovaries reduce the risk for hypertension and cardiovascular disease. In animal models of programming, sex differences are also apparent, with males most often affected more severely. In the rat, moderate maternal dietary protein restriction (10% vs 20% in controls) during pregnancy results in hypertension in adult male offspring, but not in females, and it is only when the insult becomes more severe that the female develops a hypertensive phenotype(12,13). We have previously reported that severe protein restriction (6% protein diet compared to 20% in controls) during pregnancy causes hypertension in both males and female offspring but the effect is more pronounced in the males(6,8). These studies suggest that sex hormones may play an important role in modulating cardiovascular responses to an adverse fetal environment.
Sex steroids—estrogens in particular—are known to have cardiovascular protective effects(14–16). Estrogen deprivation by ovariectomy has been shown to exacerbate the existing hypertension in female rats in some experimental models of hypertension(15,17). We and others have shown previously that adult female offspring of protein-restricted dams have reduced plasma estradiol levels(6,18). Based on these findings, we hypothesize that reduced estradiol levels may contribute to moderate increases in BP in the protein-restricted females, and further reduction in estradiol levels by ovariectomy would cause elevation in BP to develop more rapidly postnatally. To establish the protective role of estrogen, it is also important to examine whether estradiol replacement reverses hypertension in the protein-restricted females. Thus, the purpose of this study is to determine whether estrogens protects against increase in BP in adult protein restricted females.
Materials and methods
Animals and experimental protocols
All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch and were in accordance with those published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996).
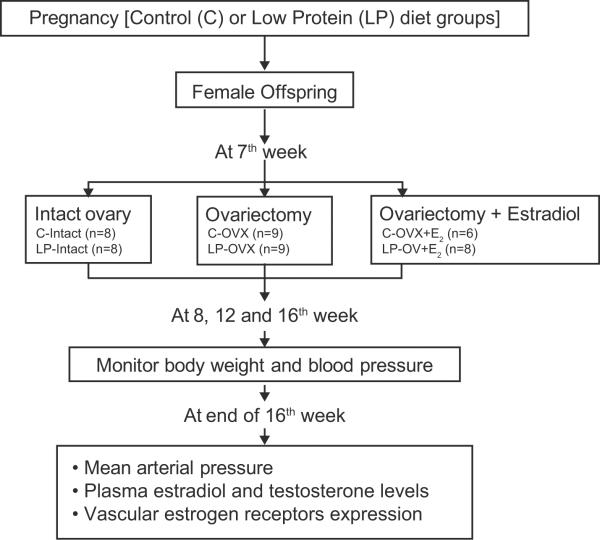
Nine- to 12-week-old virgin female Sprague-Dawley rats (Harlan Sprague Dawley, Houston, TX) were maintained at 23°C, 12:12 light-day cycles with free access to water and standard rat chow from Harlan Teklad (Madison, WI). Female rats were mated with male Sprague–Dawley rats, and conception was confirmed by the observation of sperm in vaginal smear. On day 1 of pregnancy (gestational length = 22 days), rats were randomly divided into 2 groups of 8 pregnant rats each and were fed either a control (20% casein; Harlan Teklad; Cat. No. TD.91352) or an isocaloric protein restricted (6% casein; Harlan Teklad; Cat. No. TD.90016) rat diet throughout pregnancy as in our previous studies(6,8). The pregnant rats were allowed to deliver at term, and after the delivery of pups, all lactating mothers (including protein restricted dams) were placed on standard rat chow. The pups were sexed based on anogenital distances, and litter sizes were adjusted to 10 pups per dam with equal proportion of males and females. Pups were sexed and weaned from their dams at 21 days of age, and only female offspring were used for the study. The rat offspring were fed with standard rat chow. As shown in Figure 1, prior to puberty at 7 weeks of age, offspring of both control and protein restricted dams were divided into 3 groups: sham surgery (n = 8 in each group), ovariectomy (n = 8 each in each group) and ovariectomy with estradiol replacement (control n = 6; protein restricted n = 8). In these groups of animals, changes in mean BP (noninvasive) were measured at 8, 12, and 16 weeks of age. At the end of 16 weeks of age, changes in mean BP were confirmed by measurement of mean arterial pressure (MAP) using a carotid arterial catheter. Following measurement of MAP, the animals were sacrificed by CO2 inhalation, and blood was collected by cardiac puncture for the measurement of plasma estradiol and testosterone levels. Aorta was harvested to determine the expression of estrogen receptors (ERα and ERβ). Care was taken not to take more than one sample per litter.
Figure 1.
Study design
Ovariectomy and estrogen supplementation
Rats underwent ovariectomy as described previously(19) at 7 weeks of age. It is generally considered that in rats puberty last until 54 days of age and then they are considered as adult, mature animals (20). In those rats receiving estradiol supplementation, 17-β-estradiol (5 μg/kg in 100 μL sesame oil; Sigma, St. Louis, MO) was injected subcutaneously once every 4 days immediately following ovariectomy and up to 16 weeks of age. Rats have an estrous cycle length of 4 days; hence, this dosage regimen of estradiol was chosen to closely mimic the physiological changes in 17-β-estradiol levels across the rat estrous cycle as described previously(21,22).
Noninvasive Blood Pressure measurement
Noninvasive BP was measured using a CODA computerized noninvasive BP system (Kent Scientific, Litchfield, CT) as described previously(23). Rats were acclimatized for a week to the measurement procedures prior to testing. Rats were held in a preheated restrainer with the tail exposed, and both an occlusion cuff and a volume pressure-recording cuff were placed close to the base of the tail. The cuff was then inflated and deflated automatically within 90 seconds. Blood pressure is measured during 30 consecutive, computer-automated inflation/deflation cycles of the balloon cuff (10 preliminary measurements and 20 test measurements). Unlike other tail cuff systems, CODA uses volume pressure recording to measure both systolic and diastolic BP, which is then used by the software to calculate the mean BP. Data from the preliminary measurements are discarded and data from the test measurements are averaged. Signals were recorded and analyzed using Kent Scientific software. To minimize stress-induced variations in BP, all measurements were taken by the same person in the same peaceful environment and at the same time of the day.
Mean Arterial Pressure
The MAP in various treatment groups was measured using an indwelling carotid arterial catheter in conscious free moving rats as previously described(8). Briefly, rats were anaesthetized (ketamine-45 mg/kg; xylazine-5 mg/kg; Burns Veterinary Supply, Westbury, NY) and the carotid artery was cannulated with polyethylene tubing (PE-50; Becton Dickinson, Sparks, MD). After a 1-day recovery period, the carotid cannula filled with heparinized saline (500 U mL−1, Sigma) was connected to a pressure transducer to record MAP using the DBP001 direct BP system and Workbench for Windows software (Kent Scientific). The MAP was monitored continuously for 30 minutes to determine the baseline MAP.
Plasma estradiol and testosterone levels
Blood samples were collected between 9:00 am and 10:00 am in all rats. In rats with intact ovaries, blood sample was collected on the day of estrus, as determined by vaginal cytology. In estradiol supplemented rats, blood was collected 1 hour after estradiol supplementation. Plasma was separated by centrifugation and stored at −20°C until the time of measurement. Estradiol and testosterone levels in the samples were measured using a radioimmunoassay kit (Diagnostic Systems Laboratories, Inc., Webster, TX) according to the manufacturer's instructions. The minimum detectable concentration of estradiol was of 5 pg/ml, whereas that of testosterone was of 6 pg/ml. The intra and interassay coefficients of variation for estradiol and testosterone were lower than 5%.
Western blot analysis
The aortas were homogenized in a solution containing 50 mmol/L Tris-HCl (pH 7.4), 2 mmol/L EGTA, 2 mmol/L β-mercaptoethanol, and 1 mmol/L PMSF along with complete protease inhibitors (Roche, Indianapolis, IN). Homogenates were centrifuged at 12,000 ×g for 5 minutes at 4°C, and the supernatant was used for determination of protein concentration using a BCA protein assay kit (Pierce Inc., Rockford, IL, USA). Equal amounts of protein (20 μg) were electrophoresed on 8% SDS-polyacrylamide gel and transferred onto nitrocellulose membrane. Membranes were blocked in PBS solution containing 5% dry milk for 1 hour at room temperature. After blocking, membranes were probed with either monoclonal anti-ERα (1:1000; Upstate biotechnology, NY) or polyclonal anti-ERβ (1:1000; Affinity bioreagents, Rockford, IL), with overnight incubation in a Tris buffered saline (TBS) solution containing 5% milk. Membranes were washed using TBS containing 1% milk and then incubated with horseradish peroxidase-conjugated secondary antibody (dilution 1:2,000; Cell Signaling, Danvers, MA) for 1 hour. Immunoreactive bands were visualized by enhanced chemiluminescence (Pierce biotechnology, Rockford, IL). Developed films were scanned and analyzed using Fluorchem (Alpha Innotech, San Leandro, CA). The results were normalized with β-actin (1:5000; Sigma). β-actin protein as a loading control did not differ among the groups.
Statistical analysis
Data were analyzed using GraphPad Prism for Windows (GraphPad Software, San Diego, CA). 2-way ANOVA followed by the Bonferroni post hoc tests was used for comparisons made between groups. For 2-group comparisons, Student t test was used. A value of P<0.05 was considered statistically significant.
Results
Animal data
There were no differences in feed intake between control and protein restricted dams during the gestation period. There was no significant differences in the mean litter size between control (14.4 ± 1.6) and protein restricted (13.8 ± 1.4) groups. The birth weight (female only) was significantly lower in the protein restricted offspring (5.0 ± 0.1 g) compared to controls (6.1 ± 0.1 g). This observation of low birth weight in protein restricted offspring is consistent with our previous publications(6,8).
Mean blood pressure
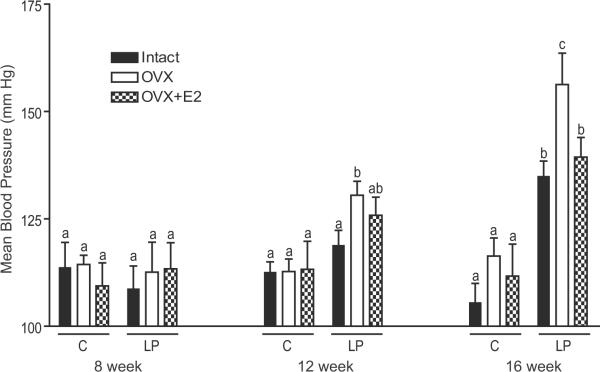
We examined mean BP at 8, 12 and 16 week of age. In the intact protein restricted offspring the increases in mean BP were significant at 16 weeks of age, but not at 8 and 12 weeks of age compared to their corresponding controls (Figure 2). Following ovariectomy in protein restricted female offspring there was an early onset of hypertension with more pronounced elevation in BP such that the BP was significantly higher at 12 and 16 weeks of age compared to the corresponding controls (Figure 2). Thus ovariectomy exacerbated mean BP in protein restricted females without any effects in controls. Estradiol replacement partially buffered the ovariectomy-induced increases in mean BP in protein restricted females. However, the mean BP was significantly higher in the estradiol-replaced ovariectomized protein restricted females compared to the corresponding controls at 16 weeks of age.
Figure 2.
Progressive changes in blood pressure in control and protein restricted female at 8, 12, and 16 weeks of age. Changes in blood pressure were measured in intact, ovariectomized (OVX), and E2 replaced (OVX +E2) offspring using a noninvasive CODA system. Bars with different letter superscripts differ significantly (P<0.05). Data points represent mean ±SEM of 6 to 9 rats in each group.
Mean arterial pressure
To confirm the changes in mean BP obtained by a noninvasive system, we used direct measurement of MAP using indwelling carotid arterial catheters. MAP measured at 16 weeks of age was significantly higher in protein restricted intact females compared to controls (120±2 mmHg vs 101±3 mmHg; Figure 3). Ovariectomy further increased MAP in protein restricted females (134±5 mmHg) but was without significant effect in controls (104±4 mmHg) (Figure 3). Thus, the MAP in protein restricted ovariectomized females was significantly higher compared to ovariectomized controls. Estradiol replacement in ovariectomized females significantly reduced MAP in protein restricted females (122±3 mmHg) but was without significant effect in controls (98±2 mmHg) (Figure 3). However, the mean BP in protein restricted estradiol-replaced females was significantly higher compared to their corresponding controls (Figure 3). Thus, estradiol replacement reduced ovariectomy-induced increases in mean BP but did not normalize BP to control levels.
Figure 3.
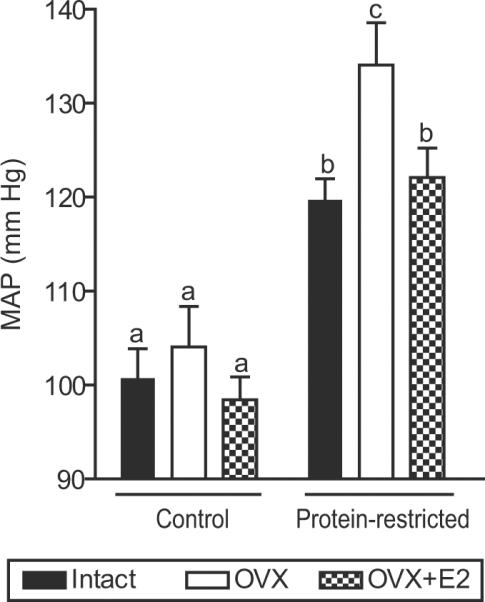
Mean arterial pressure (MAP) in adult female control and protein restricted offspring at 16 weeks of age. MAP was measured in conscious rats using indwelling carotid arterial catheter in intact, ovariectomized (OVX), and E2 replaced (OVX +E2) offspring. Data points represent mean ± SEM of 6 to 9 rats in each group. Bars with different letter superscripts differ significantly (P<0.05).
Plasma estradiol and testosterone levels
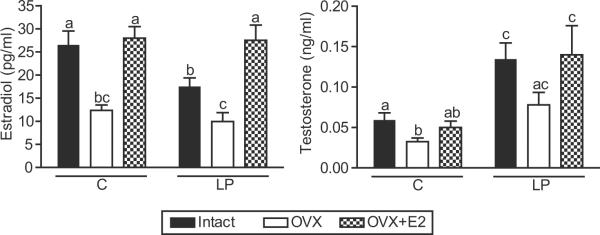
Plasma estradiol levels were significantly lower in protein restricted intact females (−34%) compared to intact controls (Figure 5). Ovariectomy significantly decreased estradiol levels in controls and protein restricted offspring compared to their respective intact offspring. There were no significant differences in estradiol levels between protein restricted ovariectomized females and their corresponding controls (Figure 5). Estradiol replacement to ovariectomized rats reinstated estradiol levels in both control and protein restricted females to levels comparable with that in controls with intact ovaries (Figure 5).
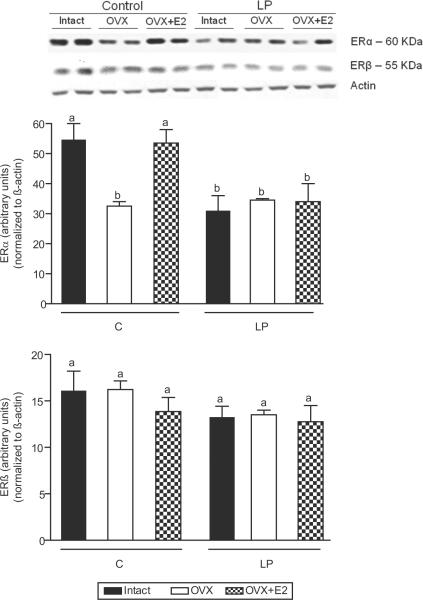
Figure 5.
Vascular protein levels of ERα (A) and ERβ (B) receptors in intact, ovariectomized (OVX), and E2 replaced (OVX +E2) control and protein restricted offspring at 16 weeks of age. The top panel shows representative blots of ERα and ERβ proteins and β-actin; the bottom panel is the summary of densitometry results of 5–6 animals in each group. Results were normalized against β-actin and expressed in arbitrary units. Data are expressed as mean ± SEM. Bars with different letter superscripts differ significantly (P<0.05).
Plasma testosterone levels were significantly higher in the protein restricted intact females (2.4-fold) compared to corresponding controls (Figure 5). Ovariectomy significantly decreased testosterone levels in controls but not in protein restricted females compared to their respective intact offspring; however, the testosterone levels were significantly higher (3.4-fold) in protein restricted ovariectomized females compared to respective controls (Figure 5). Estradiol replacement to ovariectomized rats did not significantly affect the testosterone levels in both control and protein restricted females compared to their respective ovariectomized offspring; however, the testosterone levels were significantly higher in estradiol-replaced protein restricted females (2.8-fold) compared to their corresponding controls (Figure 5).
Vascular estrogen receptors
The expression of ERα protein in the aorta was significantly lower in protein restricted intact females (−43%) compared to corresponding controls (Figure 6). Ovariectomy decreased the ERα levels in controls but was without significant effect in protein restricted females compared to their respective intact offspring (Figure 6). Thus, the vascular ERα protein in ovariectomized control and protein restricted females was at similar levels. Estradiol replacement reversed the ovariectomy-induced decrease in ERα levels in controls but was without significant effect in protein restricted females compared to their respective ovariectomized offspring (Figure 6). Thus, the ERα levels were significantly lower in estradiol-replaced protein restricted females (−34%) compared to their corresponding controls. There were no changes in ERβ levels among any control and LP treatment groups (Figure 6). Overall, it appears that protein restriction in utero programs for reduced expression of vascular ERα protein levels that may be less amenable to changes in circulating estradiol levels.
Discussion
Maternal protein restriction programs development of hypertension in the adult offspring(5,6,8,13,24). We have reported previously that the hypertension in the offspring subjected to severe protein restriction in utero is more pronounced in the males than females(6,8). This study shows that hypertensive adult protein restricted females have lower estradiol levels and ovariectomy exacerbates the hypertension, suggesting that estradiol plays a role in limiting the severity of hypertension and contributing for sexual dimorphism. Importantly this study also demonstrates that estradiol supplementation can only partially buffer BP, probably due to a limited availability of vascular ERα receptors and/or increased circulating testosterone levels.
It is now well established that adverse fetal environment have long-term influences on the adult life BP. This study shows that the postpubertal adult female offspring of protein restriction dams develop hypertension after 12 week of age. These findings are consistent with our previous reports(6,8). In contrast to our findings, Langley-Evans and colleagues reported that Wistar female offspring of modestly protein-restricted mothers developed hypertension as early as 4 weeks of age(7). The reason for this discrepancy is not clear. They used the Wistar strain, whereas we used Sprague-Dawley rats, although it seems unlikely that strain differences alone can account for the presence or absence of gender differences (25). In addition to the difference in the degree of protein restriction of Langley- Evans et al. and our present work, the other components of the diets used were not identical. However, consistent with our present findings, other investigators have reported that restriction of protein intake during pregnancy in rats lead to development of hypertension only after 75 days of age (25). It is possible that the protein restricted prepubertal females are hypertensive and this effect is stabilized with puberty as reported in the female offspring of nutrition restricted dams(4).
In addition, we show that these hypertensive adult protein restricted females have lower plasma estradiol levels compared to control females. Since estradiol is known to be vasoprotective(14–16), we suggest that suboptimal estradiol levels may allow for the moderate increases in BP. Compromised ovarian function may contribute for reduction in estradiol levels in protein restricted females as suggested by several reports: protein restriction in utero leads to delayed puberty(26), reduced ovarian size with decreased number of antral follicles and increased atretic follicles(26), impaired estrus cyclicity(8,27) and reduced fertility(27). The decrease in estradiol concentrations may be consequent to decreases in aromatase enzyme activity reported in female offspring of protein restricted dams(28–30) (this is substantiated by increase in testosterone levels observed in the gonad intact protein restricted offspring in this study). In addition, low levels of FSH and LH(27) and their receptors(28) are observed in protein restricted female offsprings. Some of these mechanisms may underlie the reductions in estradiol concentrations in protein restricted animals.
We further show that ovariectomy decreased serum estradiol to lower levels consistent with published reports of estradiol (11.2 ± 2.1 pg/ml) in ovariectomized rats(31) (which are likely attributable to non-ovarian sources) exacerbating the hypertension with an earlier onset and more prominent elevation of BP in the protein restricted females which is comparable to that observed in adult male littermates(6). This suggests that estrogen indeed protects and limits the severity of hypertension in our model and contributes for sex differences in the development of hypertension. Interestingly, the normotensive adult females that are born to pregnant rats with modest nutritional restriction have normal estradiol levels and upon ovariectomy they become hypertensive(4,27), suggesting that an optimal estradiol levels in the intact animals could have conferred cardiovascular protective effect.
To clarify the importance of estradiol on BP regulation in protein restricted female offspring, estradiol replacement studies were initiated. Estradiol supplementation to protein restricted ovariectomized female offspring only partially buffered the elevated BP. Also, estradiol replacement in intact offspring of protein restricted dams given at a dose that restores plasma levels to that observed in intact control offspring(32) did not return BP back to that seen in intact control offspring (116±3.2 mm Hg compared to 100±3.1 mmHg in controls). Thus, it appears estradiol supplementation reversed only the ovariectomy-induced increase in BP but not that induced by maternal protein restriction. This finding is somewhat surprising, as it is in contrast to the findings in other rat models. In one model of genetically programmed hypertension, the mRen2.Lewis rat, it was shown that ovariectomy augments the BP of female animals and estrogen supplementation protects against this process(17). Ovariectomy also exacerbated hypertension in the Dahl salt-sensitive rat model, which was reversed by estradiol supplementation(15). The reasons for the apparent discrepancies between the above studies and the present study are not entirely clear. Both the SHR and the Dahl salt-sensitive rat are models of genetic hypertension, and thus may involve different mechanisms than fetal programming models. Presumably severe protein restriction, as in our model, could have severely impacted developing organs like the cardiovascular system such that it became less responsive to estradiol. Our results indicate that the expression of ERα (but not ERβ) receptors is reduced in the vasculature of protein restricted offspring. ERα is shown to play an important role in conveying both vasodilatory(33,34) and long-term anti-inflammatory actions of estradiol(35). Consistent with this finding of reduced ERα levels in vascular tissues, previous studies have demonstrated an attenuated vasodilatory effect of 17β-estradiol in protein restricted female offspring(36). The decreased ERα expression in protein restricted offspring might be consequent to the decreased estradiol levels since the expression of ERα in the vasculature is highly regulated by estradiol status. However, the finding that estradiol supplementation does not reverse the vascular ERα levels in protein restricted offspring suggest that maternal protein restriction may impact or program for permanent reduction in ERα levels, which might contribute to the lack of estradiol's cardiovascular protective effect. The mechanisms that contribute for the stable decrease in ERα receptor expression in the vasculature in protein restricted offspring long after the adverse exposures are gone is not known. Glucocorticoids have shown to down regulate ERα expression(37). This class of hormones appears to play a central role in mediating the programming effects of undernutrition in fetal life. Evidence of increased glucocorticoid action in brain and liver has been demonstrated in both fetal and neonatal rats exposed to low-protein diets in utero(38) and increased expression of glucocorticoid receptors persists into adult life, mediating a hypersensitivity to corticosteroids(39,40). Therefore, decrease in ERα expression in the protein restricted offspring influenced by prenatal nutritional status could in part be mediated by glucocorticoids. Loss of ERα receptor expression may also be explained by epigenetic modifications of the gene. Methylation in critical regulatory regions is believed to mark silenced genes. Offspring of dams exposed to low protein during pregnancy or after uteroplacental insufficiency often display anomalous patterns of DNA methylation in the liver of fetuses(39,41). In the vasculature of protein restricted females, one potential candidate gene for altered methylation is ERα, which is known to be differentially methylated in response to maternal care during early life(42).
Another possible explanation for increased BP in protein restricted adult female offspring may, in part, be due to endothelial dysfunction observed in adult female offspring of protein-restricted dams(8,36). Optimal functioning of the nitric oxide (NO) system is essential for estradiol's cardiovascular protective action(43). We and others have demonstrated that the endothelial function, eNOS expression, and NO release are compromised in adult female offspring of protein-restricted dams(8,36). Thus, it is possible that the compromised endothelial function together with reduced ERα levels in protein-restricted female offspring might have negated the protective effect of estradiol on BP. This is consistent with the human data which indicate a lack of protective estrogen effect in hormonal replacement therapy in postmenopausal women who have reduced expression of estrogen receptors and compromised endothelial function(44,45).
Although lower estradiol levels and reduced vascular ERα could have played a permissive role, the possibility of other direct mechanisms that contribute for hypertension to develop cannot be discounted. Studies have shown that testosterone in females may raise BP. The important factor is that the ratio of testosterone to estradiol is shown to influence BP. In earlier studies(46,47), elevated testosterone levels in ovariectomized SHR has shown to cause hypertension. In the present study, protein restricted adult female offspring have higher testosterone levels and greater testosterone-to-estradiol ratio (3.5- to 4-fold) compared to controls. Based on these findings and our recent report that hypertension could be abolished by androgen receptor blockade(6) indicates that testosterone may play a central role in causing hypertension in the females in our present model of prenatal programming by maternal diet. The reason for increased testosterone production in the protein restricted adult female offspring is not clear, however, similar increases in testosterone levels are reported in the female offspring of pregnant rats exposed to cytokines (IL-6, TNF-α and IL-1β)(48,49). Although studies suggest ovaries as a possible source for increased testosterone levels(48,49), the observation in this study that testosterone levels are also higher in protein restricted ovariectomized females suggests an extra-ovarian source, possibly adrenals or other peripheral tissues(50–52). There is growing evidence that testosterone stimulates various key components of the systemic and renal renin-angiotensin and endothelin systems while promoting oxidative stress(53,54). This may exacerbate the progression of renal disease and cardiovascular responses in females(55). Although the presence of excess testosterone during adulthood may facilitate hypertension to develop in this model, we cannot rule out testosterone playing a programming role during the neonatal period. In this regard, it is important to mention that the low birth offspring born to protein restricted mothers have increased anogenital distances compared to controls(27,56) suggesting that the fetuses are subjected to androgenic influences during intrauterine life. Further studies are needed to examine the mechanism by which testosterone contribute to the developmental programming of hypertension in females.
It is essential to emphasize the following cautionary remarks regarding the aforesaid interpretations. Although it appears that the defects of the sex hormone axis contributes to the observed elevation in arterial pressure, the alterations in sex steroid levels may also be secondary to arterial pressure alterations. Further analysis of longitudinal changes in sex steroid levels and their correlation with BP is essential to establish a cause-effect relationship. In conclusion, these observations suggest that estradiol plays a major role in limiting the severity of hypertension and in maintaining the normal differences in BP between male and female rats that are developmentally programmed. Failure of estradiol supplementation to reduce BP in these animals may be due to permanent reduction in the availability of estrogen receptors and/or increased testosterone levels. The fact that testosterone levels are higher in protein restricted adult females together with our previous report of hypertension being abolished with androgen receptor blockade suggests that testosterone may play an important role in maintaining the elevated BP in this model of maternal protein restriction.
Figure 4.
Hormone levels in control and protein restricted offspring at 16 weeks of age. Estradiol (A) and testosterone (B) were measured by radioimmunoassay in intact, ovariectomized (OVX), and E2 replaced (OVX + E2) offspring. Data points represent mean ± SEM of 6 to 9 rats in each group. Bars with different letter superscripts differ significantly (P<0.05).
Acknowledgements
The present study was supported in part by grants HL58144, HL102866 and HL72650 from the National Institute of Health (grant to (ChandraYallampalli) K. Sathishkumar designed the study, performed the surgeries and mean arterial pressure measurements, analyzed the data and wrote the manuscript, Rebekah Elkins and Uma Yallampalli performed non invasive blood pressure measurements, hormone quantification, Western blot analysis and organized the collection of tissues, and Chandra Yallampalli conceived the study and participated in experimental design, analysis and discussion of the results, and review of the manuscript. All authors read and approved the final manuscript. There were no conflicts of interest. Thanks also to the staff of the publication division, department of obstetrics and gynecology, University of Texas Medical Branch, Galveston, Texas for assistance with technical editing and preparation of figures.
Reference List
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Shiell AW, Barker ME, et al. Growth in utero and blood pressure levels in the next generation. J Hypertens. 2000;18:843–846. doi: 10.1097/00004872-200018070-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hemmings DG, Williams SJ, et al. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–H682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ojeda NB, Grigore D, Robertson, et al. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 6.Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- 8.Sathishkumar K, Elkins R, Yallampalli U, et al. Protein Restriction during Pregnancy Induces Hypertension and Impairs Endothelium-Dependent Vascular Function in Adult Female Offspring. J Vasc Res. 2008;46:229–239. doi: 10.1159/000166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 10.Moritz KM, Cuhen-McEwen LA. Kidney development and fetal programming. Early Life Origins of Health and Disease. 2006;573:130–144. [Google Scholar]
- 11.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 12.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 13.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 15.Hinojosa-Laborde C, Craig T, Zheng W, et al. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 16.Oparil S. Corcocran Memorial Lecture. Hormones and vasoprotection. Hypertension. 1999:170–176. doi: 10.1161/01.hyp.33.1.170. [DOI] [PubMed] [Google Scholar]
- 17.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2). Lewis rat. AJP - Regulatory, Integrative and Comparative Physiology. 2006;291:R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 18.Franco MC, Arruda RM, Dantas AP, et al. Intrauterine undernutrition: expression and activity of the endothelial nitric oxide synthase in male and female adult offspring. Cardiovasc Res. 2002;56:145–153. doi: 10.1016/s0008-6363(02)00508-4. [DOI] [PubMed] [Google Scholar]
- 19.Ross GR, Chauhan M, Gangula PR, et al. Female sex steroids increase adrenomedullin-induced vasodilation by increasing the expression of adrenomedullin2 receptor components in rat mesenteric artery. Endocrinology. 2006;147:389–396. doi: 10.1210/en.2005-0664. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56:619–625. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- 21.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 22.Wells CC, Riazi S, Mankhey RW, et al. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005;2:227–237. doi: 10.1016/s1550-8579(05)80052-x. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Rateri D, Hong L, et al. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009 doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods LL, Ingelfinger JR, Nyengaard JR, et al. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Musha Y, Itoh S, Hanson MA, et al. Does estrogen affect the development of abnormal vascular function in offspring of rats fed a low-protein diet in pregnancy? Pediatr Res. 2006;59:784–789. doi: 10.1203/01.pdr.0000219126.78372.c8. [DOI] [PubMed] [Google Scholar]
- 26.Leonhardt M, Lesage J, Croix D, et al. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- 27.Guzman C, Cabrera R, Cardenas M, et al. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Silva FT, de Bittencourt BF, Sampaio FJ, et al. Maternal malnutrition during lactation affects folliculogenesis, gonadotropins, and leptin receptors in adult rats. Nutrition. 2010;26:1000–1007. doi: 10.1016/j.nut.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh AJ, Erickson GF. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids. 1978;32:639–648. doi: 10.1016/0039-128x(78)90074-0. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeven G, Cailleau J. Prolonged exposure to androgens suppresses follicle-stimulating hormone-induced aromatase activity in rat Sertoli cell cultures. Mol Cell Endocrinol. 1988;57:61–67. doi: 10.1016/0303-7207(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 31.Ricchiuti V, Lian CG, Oestreicher EM, et al. Estradiol increases angiotensin II type 1 receptor in hearts of ovariectomized rats. J Endocrinol. 2009;200:75–84. doi: 10.1677/JOE-08-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol RZ, Okuda H, Stanczyk FZ, et al. Normative reproductive indices for male and female adult Sprague-Dawley rats. Contraception. 1999;59:203–207. doi: 10.1016/s0010-7824(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 33.Bolego C, Cignarella A, Sanvito P, et al. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203–1208. doi: 10.1124/jpet.104.082867. [DOI] [PubMed] [Google Scholar]
- 34.Pinna C, Cignarella A, Sanvito P, et al. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension. 2008;51:1210–1217. doi: 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- 35.Bolego C, Vegeto E, Pinna C, et al. Selective agonists of estrogen receptor isoforms: new perspectives for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2192–2199. doi: 10.1161/01.ATV.0000242186.93243.25. [DOI] [PubMed] [Google Scholar]
- 36.Torrens C, Brawley L, Barker AC, et al. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton MJ, Nelson LD, Taylor AH, et al. The modulation of aromatase and estrogen receptor alpha in cultured human dermal papilla cells by dexamethasone: a novel mechanism for selective action of estrogen via estrogen receptor beta? J Invest Dermatol. 2006;126:2010–2018. doi: 10.1038/sj.jid.5700344. [DOI] [PubMed] [Google Scholar]
- 38.Langley-Evans SC, Nwagwu M. Impaired growth and increased glucocorticoid-sensitive enzyme activities in tissues of rat fetuses exposed to maternal low protein diets. Life Sci. 1998;63:605–615. doi: 10.1016/s0024-3205(98)00311-7. [DOI] [PubMed] [Google Scholar]
- 39.Bertram C, Trowern AR, Copin N, et al. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 40.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 41.MacLennan NK, James SJ, Melnyk S, et al. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics. 2004;18:43–50. doi: 10.1152/physiolgenomics.00042.2004. [DOI] [PubMed] [Google Scholar]
- 42.Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiner CP, Lizasoain I, Baylis SA, et al. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 45.Prelevic GM, Kwong P, Byrne DJ, et al. A cross-sectional study of the effects of hormon replacement therapy on the cardiovascular disease risk profile in healthy postmenopausal women. Fertil Steril. 2002;77:945–951. doi: 10.1016/s0015-0282(02)03078-9. [DOI] [PubMed] [Google Scholar]
- 46.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. [DOI] [PubMed] [Google Scholar]
- 47.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 48.Dahlgren J, Nilsson C, Jennische E, et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–E334. doi: 10.1152/ajpendo.2001.281.2.E326. [DOI] [PubMed] [Google Scholar]
- 49.Gotz F, Dorner G, Malz U, et al. Short- and long-term effects of perinatal interleukin-1 beta-application in rats. Neuroendocrinology. 1993;58:344–351. doi: 10.1159/000126560. [DOI] [PubMed] [Google Scholar]
- 50.Flores A, Rodriguez JO, Palafox MT, et al. The acute asymmetric effects of hemiovariectomy on testosterone secretion vary along the estrous cycle. The participation of the cholinergic system. Reprod Biol Endocrinol. 2006;4:11. doi: 10.1186/1477-7827-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labrie F, Luu-The V, Labrie C, et al. Intracrinology and the skin. Horm Res. 2000;54:218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 52.Quinkler M, Sinha B, Tomlinson JW, et al. Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 53.Iliescu R, Cucchiarelli VE, Yanes LL, et al. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol. 2007;292:R731–R735. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- 54.Yanes LL, Romero DG, Cucchiarelli VE, et al. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–R233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 55.Elliot SJ, Berho M, Korach K, et al. Gender-specific effects of endogenous testosterone: female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–472. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- 56.Zambrano E, Rodriguez-Gonzalez GL, Guzman C, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]