1 Introduction
Although the mechanism of action of hallucinogens is incompletely understood, serotonin (5-HT) and the serotonin2A (5-HT2A) receptor are thought to play a significant role in mediating their effects (Nichols, 2004). There are two major chemical classes fitting this profile—the phenethylamines (e.g. mescaline) and the tryptamines (e.g. lysergic acid diethylamide, psilocybin). Hallucinogens have significant value as pharmacological agents. They have been used to model psychosis and to better understand human cognition and perception (Nichols, 2004). Their role in the discovery of the serotonergic system has also proven invaluable (Nichols, 2004; Passie et al., 2008). These compounds have also demonstrated clinical utility in pain, drug addiction, headache, depression, and anxiety disorders (Griffiths et al., 2006; Griffiths et al., 2008; Grob et al., 2011; Kast and Collins, 1964; Mangini, 1998; Sewell et al., 2006). While the psychedelic effects of hallucinogens may be essential for some types of therapy, these and other physiologic side effects may preclude widespread clinical use of these drugs. As opposed to other recreational drugs, however, hallucinogens are not habit-forming in humans, nor are they reinforcing in animals (Chilcoat and Shurtz, 1996; Passie et al., 2008). Deciphering the mechanisms by which hallucinogens exert their various effects will significantly benefit areas of basic and clinical science (Vollenweider and Kometer, 2010).
Drug-elicited head movement is a widely used behavioral model with which to investigate hallucinogens and there is a strong correlation between the dose of hallucinogens used to elicit mouse head twitch behavior and that used recreationally in humans (Corne and Pickering, 1967). Serotonin2A receptors have been implicated in head movements elicited by the phenethylamine hallucinogen, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; Darmani et al., 1990; Dave et al., 2002; Dave et al., 2007; Schreiber et al., 1995; Willins and Meltzer, 1997), but a role for this receptor in the action of the indoleamine hallucinogen, lysergic acid diethylamine (LSD), which also elicits head movements, is not as clear. Serotonergic antagonists such as cyproheptadine (Vetulani et al., 1980), methysergide (Yamamoto and Ueki, 1981), and bromo-LSD (Sloviter et al., 1980) block LSD-elicited rodent head movements, but these antagonists are relatively non-selective for the 5-HT2A receptor.
The report of the absence of head twitch behavior elicited by LSD in mice lacking 5-HT2A receptors suggests that the 5-HT2A receptor is necessary for LSD mediation of this behavior in this species (Gonzalez-Maeso et al., 2007). The pharmacology that characterizes the head movement response in mice may be more complex than that for other animals, however. For example, 5-HT2C receptors were not implicated in either DOI-elicited head shakes in rat (Schreiber et al., 1995) or head bobs in rabbit (Dave et al., 2002). In contrast, 5-HT2C receptors contributed significantly to DOI-elicited head twitch behavior in mice (Canal et al., 2010). More precisely, Fantegrossi and associates (2010) demonstrated that 5-HT2C receptor antagonism right shifted the descending limb of the DOI dose response curve in mice. These findings support an inhibitory role of 5-HT2C activation at high doses of DOI (Fantegrossi et al., 2010). Further investigation with non-DOI hallucinogens, such as LSD, may help further explain the contribution of 5-HT2C receptors in hallucinogen-elicited behavior.
Although 5-HT2A receptors are found in many brain regions, direct infusion of the hallucinogen, DOI, into the frontal cortex of rats (Willins and Meltzer, 1997) or rabbits (Dave et al., 2007) has been shown to elicit head shakes and head bobs, respectively. Previous studies have also demonstrated that repeated systemic administration of DOI or LSD robustly down-regulates frontocortical 5-HT2A receptors in both rats and rabbits (Aloyo et al., 2001; Smith et al., 1999). Thus, the frontocortical area is an appropriate brain region in which to investigate the role of 5-HT2A receptors in mediating the effects of hallucinogens.
The dopaminergic system is believed to play a major role in human psychosis, a condition that hallucinogens have been shown to mimic (Nichols, 2004). Hallucinogens differ in their dopaminergic pharmacology, however. For example, LSD binds dopamine receptors, but DOI does not (Burt et al., 1976; Watts et al., 1995). Investigating the role of dopaminergic receptors in the animal head movement model would not only improve our understanding of hallucinogen pharmacology, but might also offer new insight into human psychosis. Presently, dopamine1 (D1) receptor antagonists are known to block DOI-elicited head shakes in rats (Schreiber et al., 1995), but the role of D1 receptors in LSD-elicited head movement behavior has not been studied.
The present study compares and contrasts the 5-HT2A and D1 receptor actions of hallucinogens, represented by two chemical classes, the phenethylamines (DOI) and the indoleamines (LSD). The experiments include receptor binding properties and behavioral actions. The goal of these experiments is to identify the essential pharmacological components shared among hallucinogenic agents.
2 Materials and Methods
2.1 Animals
Adult male New Zealand White rabbits (Covance; Devon, PA), weighing 1.8–2.2kg upon arrival, were housed individually under a standard light-dark cycle of 12 hours in an AAALAC-approved colony maintained at 22±1°C. Rabbits were fed 2/3 cup of rabbit chow daily and had unlimited access to water. Rabbits were adapted to the colony room and the experimenter (via handling) for several days before the initiation of experiments. Experiments were carried out in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research of the National Research Council” (2003) and were approved by the Institutional Animal Care and Use Committee (IACUC) of Drexel University College of Medicine.
2.2 Drugs and Solutions
Ritanserin (FW 477.6), SCH23390-HCl (FW 324.2), prazosin-HCl (FW 419.86), (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-HCl (FW 357.6), and lysergic acid diethylamide (LSD base; FW 323.4) were purchased from Sigma-Aldrich (St. Louis, MO). Ketanserin tartrate (FW 554.5), SB206553-HCl (FW 328.8), and RS102221-HCl (FW 649.08) were purchased from Tocris Bioscience (Ellisville, MO). [3H]ketanserin and [3H]SCH23390 were purchased from Perkin-Elmer (Boston, MA). [3H]mesulergine was purchased from GE Healthcare (Arlington Heights, IL) or American Radiolabeled Chemicals (St. Louis, MO). All other reagents and supplies were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO). For in vivo injections: DOI was dissolved in physiological saline. LSD and ritanserin were dissolved in 2% tartaric acid, pH adjusted to approximately 6.5 with NaOH, and diluted with deionized water. All other drugs were dissolved in deionized water. All drugs were injected subcutaneously at a volume of 1mL/kg, except the high dose of ketanserin (10µmol/kg), which required an injection volume of 3.5mL/kg. The literature primarily reports intravenous administration of LSD (Harvey and Gormezano, 1981; Welsh et al., 1998), but in order to match mode of administration of all other drugs, LSD was also administered subcutaneously. Preliminary studies demonstrated that LSD-elicited head bob behavior was independent of route of administration (subcutaneous or intravenous; unpublished data). LSD was injected at 30nmol/kg because this was the maximally effective dose (Romano et al., 2010). DOI was injected at 300nmol/kg because the response at this dose was less variable and equal to that of the maximally effective dose of 1000nmol/kg (Dave et al., 2002). All pretreatment drugs (ketanserin, ritanserin, SB206553, SCH23390, or vehicle) were injected subcutaneously one hour prior to hallucinogen administration. Doses of pretreatment drugs were chosen based on previously used doses from both rabbit (Dave et al., 2002; Simansky et al., 1998; Welsh et al., 1998) and rat (Schreiber et al., 1995) head movement studies. For membrane binding studies, all test compounds were dissolved in ethanol and diluted into assay buffer for a final alcohol concentration of 0.025%.
2.3 Experimental Procedure
For all behavioral studies, animals were weighed, injected, and immediately replaced in their home cage, and their head bob behavior recorded for 60 minutes for later analysis. A head bob is a sequential down-up motion of the head without intervening behaviors (e.g. sniffing, chewing, hopping; Dave et al., 2002). Food and water were removed from the cage for behavioral recording. In antagonist pretreatment studies, a reduction in head bobs was considered an inhibition or rightward shift of the agonist response. Both DOI and LSD produced inverted “U” dose response curves in the rabbit (Dave et al., 2002; Romano et al., 2010). Behaviors such as lying down and staring increased with higher doses of hallucinogens (unpublished data). This downward turn of the curves likely reflects increasing systemic effects. Such behaviors were not seen when hallucinogens were preceded by antagonist administration (unpublished data). Furthermore, at the doses used, no antagonist produced head bobs or other behaviors alone (unpublished data). For chronic studies, DOI (3µmol/kg) was injected once daily for 8 days. Twenty four hours following the last DOI treatment, rabbits were challenged by administering either DOI (300nmol/kg) or LSD (30nmol/kg) and their behavioral activity recorded for one hour. Animals were then sacrificed and frontal cortex tissue harvested for later analysis of receptor density as described below. For acute studies, rabbits were administered antagonist (or vehicle) and replaced in their home cage for 60 minutes. Subsequently, DOI (300nmol/kg), LSD (30nmol/kg), or vehicle was administered, the rabbits were replaced in their home cage, and their behavior recorded for 60 minutes. Where indicated, animals were then sacrificed 10 minutes later via decapitation and frontal cortex harvested for later binding analysis. For in vitro binding studies, drug-naïve rabbits were sacrificed via guillotine decapitation, their frontal cortices harvested and frozen immediately on dry ice for later use in receptor binding studies (see below). All in vitro binding experiments were performed in dim lighting in order to protect the structural integrity of the serotonergic ligands.
2.4 Washed Membrane Preparation
Frontal cortex tissue was used in all binding experiments due to the importance of this brain region in eliciting head movement behavior (Dave et al., 2007; Willins and Meltzer, 1997). Except as noted, the washed membrane fraction was prepared as previously described (Aloyo and Harvey, 2000). Frozen frontal cortex was placed in 10 volumes (by weight) of ice-cold homogenization buffer (50mM Tris-HCl pH7.4 at 4°C) and homogenized with a Brinkman Polytron (10 sec at half power). The cortical homogenate was centrifuged at 40,000g for 20 min at 4°C. The resulting pellet was washed by resuspension in 50 volumes of homogenization buffer and centrifuged as described above. Where indicated, this standard tissue preparation was modified by the addition of a third wash step. Furthermore, in some experiments, the tissue preparation was warmed to 37°C for 60 min between the 2nd and 3rd wash in an attempt to remove residual ligand. The final membrane fraction was then dispersed in room temperature assay buffer (20 or 50mM Tris-HCl pH7.4 at 20°C; 5mM Mg2+ present in some assays).
2.5 Saturation Binding Analysis
Serotonin2A receptor density was quantified using the antagonist radioligand, [3H]ketanserin (SA=67Ci/mmol), in the presence of RS102221 and prazosin (30nM each) to block binding to 5-HT2C and α1 adrenergic receptors, respectively. Saturation analysis was performed using eight concentrations of [3H]ketanserin (0.02–4.0nM). The assay was initiated by the addition of washed membranes derived from 4mg of tissue prepared as described above for a final assay volume of 1mL. The mixture was incubated for 120 min at 25°C before being terminated by rapid filtration through Whatman GF/B filters (presoaked in 0.5% polyethylenimine) followed by four washes each consisting of 5mL of wash buffer (20mM Tris–HCl, pH 7.4 at 4°C). The amount of radioactivity retained on the filter was then determined by liquid scintillation counting.
Serotonin2C receptor density was quantified using eight concentrations of the antagonist radioligand, [3H]mesulergine (SA=77Ci/mmol; 0.02–4.0nM), in the presence of spiperone (30nM) to block binding to 5-HT2A and dopamine D2 receptors. [3H]mesulergine was dispensed using siliconized pipette tips and assays were carried out in glass tubes in order to prevent loss of the radioligand. The remainder of the assay was performed with the same procedure as described for 5-HT2A receptors.
Dopamine1 receptor density was quantified using eight concentrations of the antagonist radioligand, [3H]SCH23390 (SA=85Ci/mmol; 0.0035–0.45nM), in the presence of ketanserin (30nM) to block binding to 5-HT2A receptors. A 50mM Tris-HCl assay buffer containing 120mM NaCl, 5mM KCl, 2mM CaCl2, and 1mM MgCl2 was used for D1 receptor studies (Davidoff and Benes, 1998). The assay was initiated by the addition of washed membranes derived from 4mg tissue in 50mM Tris-HCl buffer (pH7.4 at 20°C), and the mixture was then incubated for 60 min at 25°C. The remainder of the assay was performed with the same procedure as described for 5-HT2A receptors except that the wash buffer was 50mM Tris–HCl, pH 7.4 at 4°C.
Analysis of saturation binding data was performed using the nonlinear curve-fitting program Ligand, which calculates the Kd (binding affinity, nM) and Bmax (receptor density, Fmol/mg tissue) values (Munson and Rodbard, 1980).
2.6 Competition Experiments
The affinity of DOI and LSD for 5-HT2A, 5-HT2C, and D1 receptors was determined by competition assays using [3H]ketanserin, [3H]mesulergine and [3H]SCH23390, respectively. Each assay tube contained DOI or LSD (0.001nM-1mM) and the labeled ligand at its approximate Kd concentration. Nonspecific binding was defined by the addition of spiperone (30 nM), RS102221 (30nM), or SCH23390 (30nM), respectively. The remainder of the assays was performed as described in the Saturation Binding Analysis section. Analysis of the competition assays was performed using the curve-fitting program GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA).
2.7 Binding Reversibility
Separate aliquots of washed frontocortical membranes were mixed with drug plus 5mM MgSO4 and incubated at 25°C for 60 min. Drug concentrations were chosen at roughly 10 times their respective Kd values—4nM for LSD, ketanserin, and ritanserin, 100nM for DOI (Aloyo and Harvey, 2000; Leysen et al., 1985). The mixture was then centrifuged at 40,000g for 20 min at 4°C to remove unbound drug. The final membrane fraction was then dispersed in room temperature assay buffer (20mM Tris-HCl and 5mM Mg2+ pH7.4 at 20°C). Each assay contained washed membranes derived from 4mg of tissue, [3H]ketanserin at 80% 5-HT2A receptor saturation (0.8nM), and RS10222 (30nM) and prazosin (30nM) to block binding to 5-HT2C and α1 adrenergic receptors, respectively. Separate tubes also contained spiperone (30nM) in order to define non-specific binding. Assay samples were incubated for 2 hours at 25° followed by rapid filtration through Whatman GF/B filters (presoaked in 0.5% polyethylenimine) and then four washes each consisting of 5mL of wash buffer (20mM Tris–HCl, pH7.4 at 4°C). The amount of radioactivity retained on the filter was then determined by liquid scintillation counting.
2.8 Statistical Analysis
Data were reported as group mean ± SEM. Some results are presented as a fraction of the control group value. The data were analyzed via t-test, analysis of variance (ANOVA), or 2-way ANOVA. Post hoc Dunnett or Bonferroni tests were carried out when applicable. Significance for all statistical comparisons was set at p<0.05 using a two-tailed test.
3 Results
3.1 Effects of systemic drug injection
3.1.1 Manipulation through chronic in vivo treatment
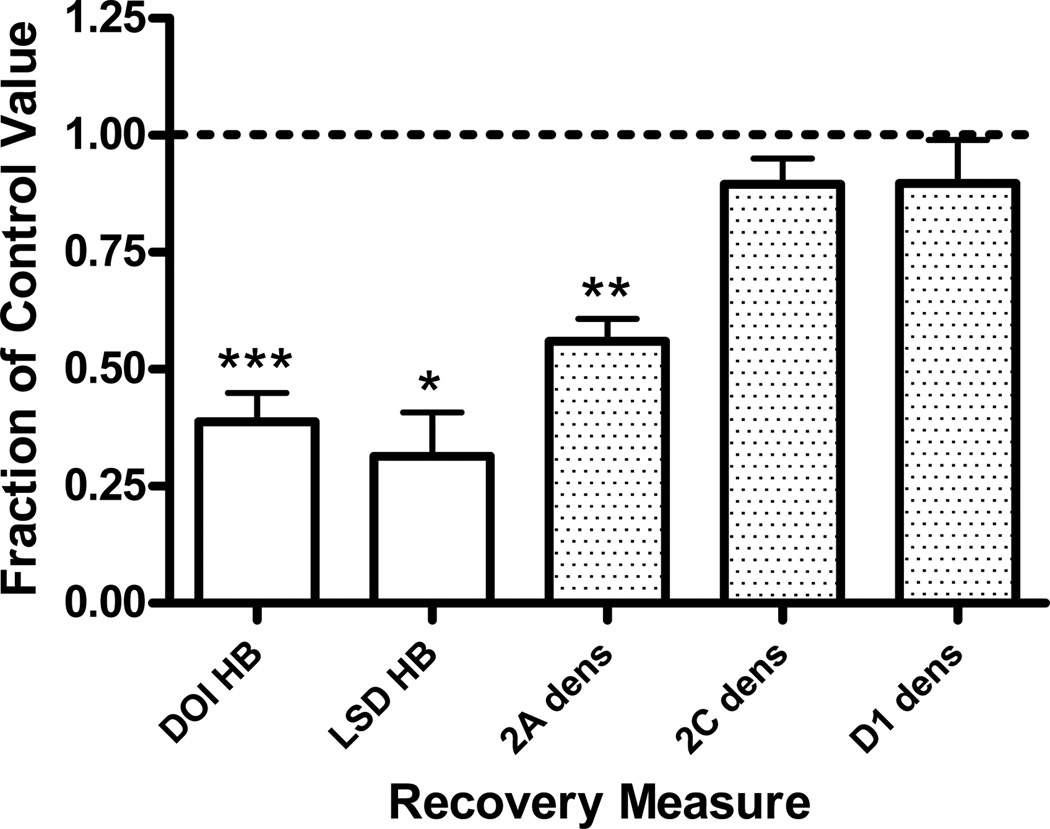
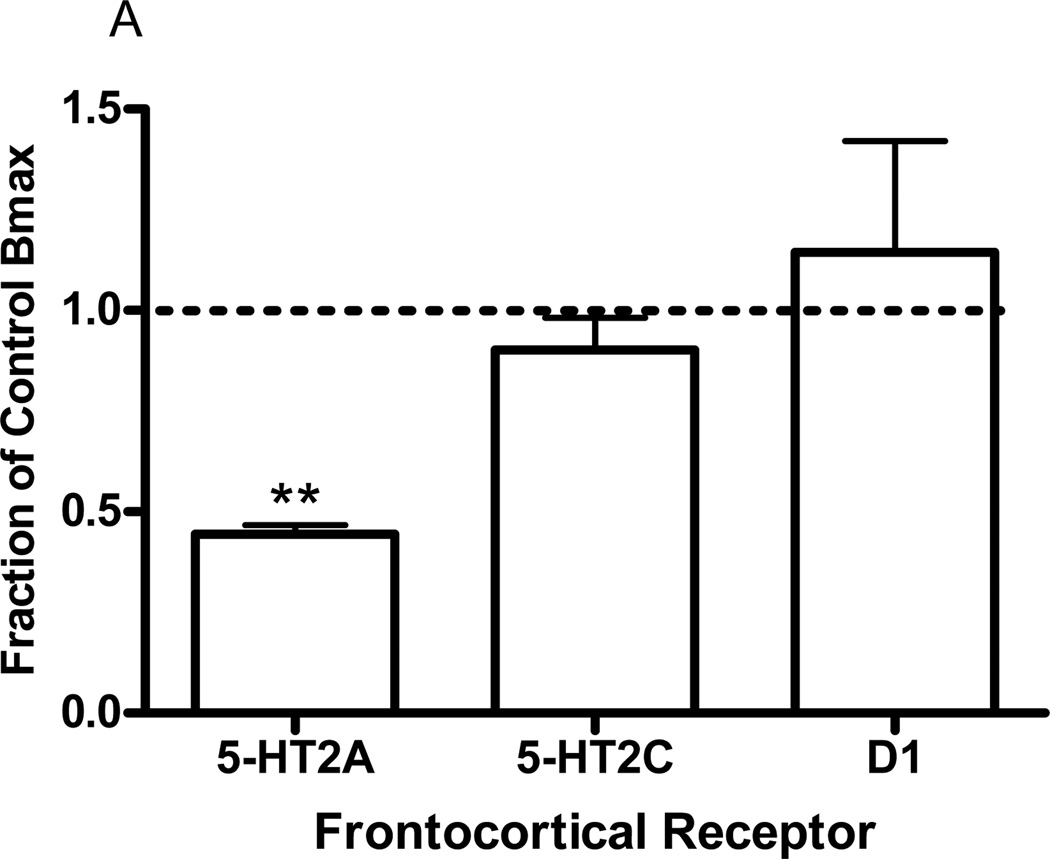
Eight daily injections of DOI (3µmol/kg) selectively reduced frontocortical 5-HT2A receptor density from 12.36±0.8 (control, saline treatment) to 6.88±0.5Fmol/mg tissue (DOI treatment; p<0.005, t-test). Frontocortical 5-HT2C receptor density was unchanged: control value, 5.23±0.2; DOI treatment, 4.68±0.4Fmol/mg tissue. Similarly, frontocortical D1 receptor density was unchanged: control value, 4.84±0.5; DOI treatment, 4.30±0.01Fmol/mg tissue. In addition, both DOI- and LSD-elicited head bobs were also significantly decreased from 41.8±3 to 16.3±4 (p<0.001, t-test) and from 32.3±7 to 8.8±3 (p<0.01, t-test), respectively. Data are represented graphically as a fraction of the control value (Fig 1).
Fig. 1.
Effects of chronic in vivo DOI administration on head bob and receptor density measures. Twenty-four hours after 8 daily injections of DOI (3µmol/kg) DOI- and LSD-elicited head bobs as well as frontocortical 5-HT2A, 5-HT2C, and D1 receptor densities were measured. Data are represented as the mean fraction of control ± SEM. For each measure investigated, the head bob response or receptor density of the animal that received chronic DOI treatment is divided by the head bob response or receptor density of the paired animal that received chronic vehicle treatment (control). These calculations are made for each animal pair. *p<0.01, **p<0.005, ***p<0.001, t-test, significantly different from vehicle treated group, n=3 to 4
3.1.2 Antagonist modulation of drug-elicited head bob behavior
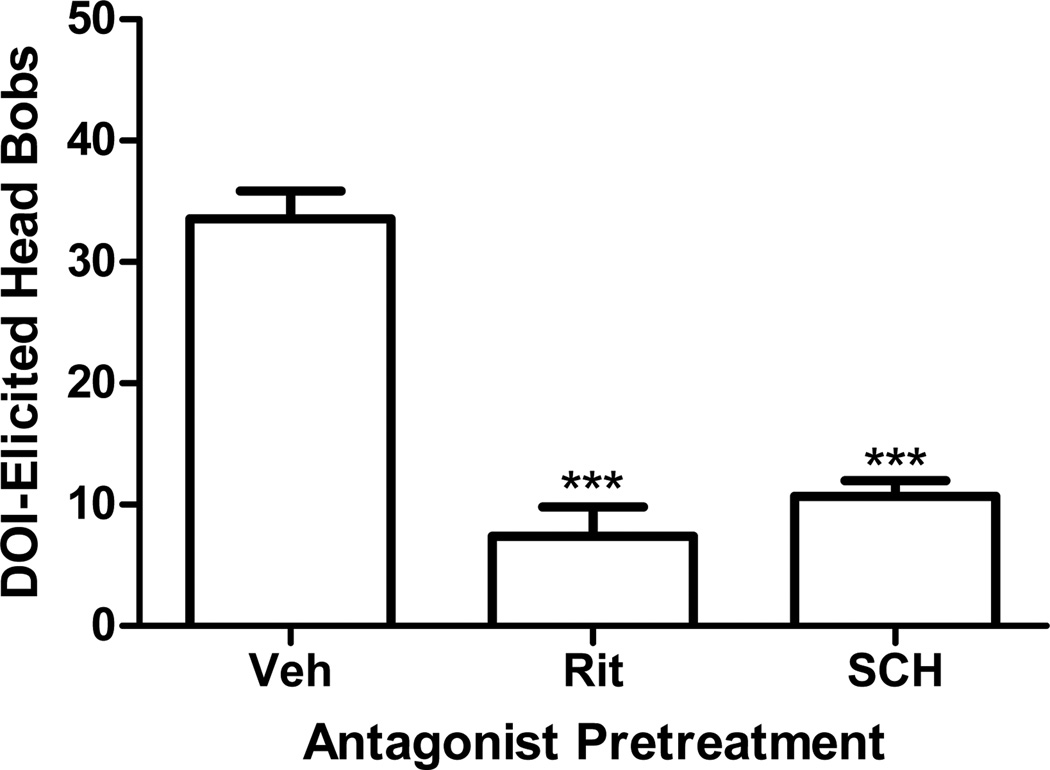
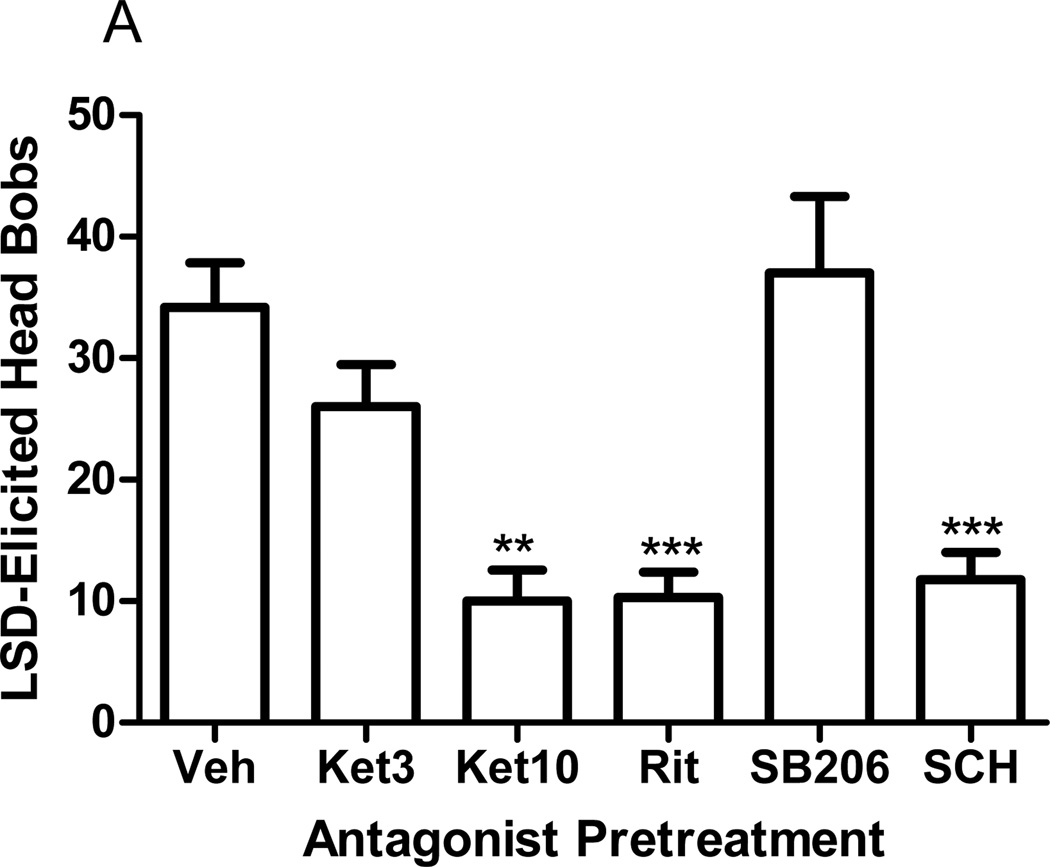
Pretreatment of rabbits with the 5-HT2A/2C receptor antagonist, ritanserin (0.67µmol/kg, p<0.0001, post hoc Dunnett test), or the D1 receptor antagonist, SCH23390 (0.05µmol/kg, p<0.0001, post hoc Dunnett test), significantly reduced DOI elicited head bobs (F=36.9, p<0.0001, ANOVA, Fig 2). Similarly, ritanserin (p<0.0001, post hoc Dunnett test) or SCH23390 (p<0.0001, post hoc Dunnett test) significantly reduced LSD-elicited head bobs (F=9.22, p<0.0001, ANOVA, Fig 3a). Pretreatment with the 5-HT2A/2C antagonist, ketanserin (3µmol/kg), did not affect LSD-elicited head bobs (p>0.5, post hoc Dunnett test; Fig 3a). A higher dose of ketanserin (10µmol/kg) was required to significantly inhibit the LSD-elicited response (p<0.001, post hoc Dunnett test, Fig 3a). Pretreatment with the 5-HT2B/2C antagonist, SB206553 (1µmol/kg), failed to alter LSD-elicited head bobs (Fig 3a).
Fig. 2.
Effect of antagonist pretreatment on DOI-elicited head bobs. Rabbits were pretreated with ritanserin (0.67µmol/kg) or SCH23390 (0.05µmol/kg) before administration of DOI (300nmol/kg). DOI-elicited head bobs are shown as the mean ± SEM. ***p<0.0001, post-hoc Dunnett test, significantly different from vehicle pretreated group, n=5 to 14
Fig. 3.
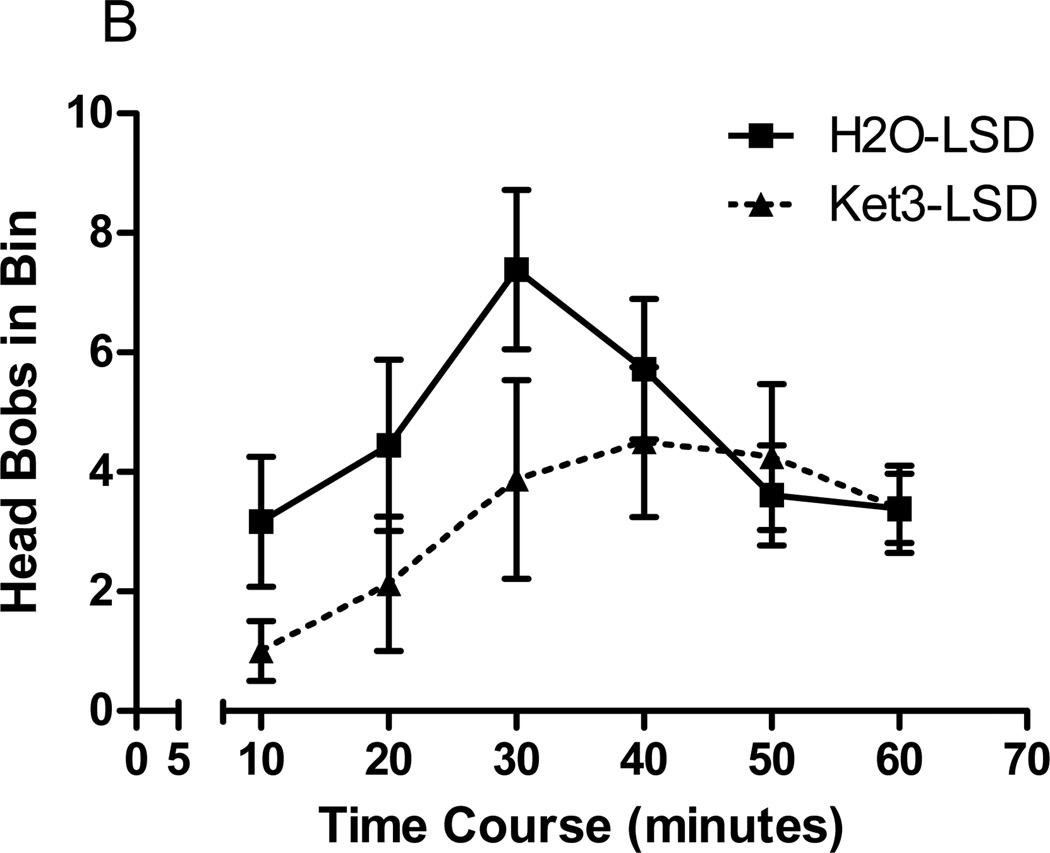
Effect of antagonist pretreatment on LSD-elicited head bobs. a Rabbits were pretreated with ketanserin (3 or 10µmol/kg), ritanserin (0.67µmol/kg), SB206553 (1µmol/kg), or SCH23390 (0.05µmol/kg) before administration of LSD (30nmol/kg). LSD-elicited head bobs are shown as the mean ± SEM. b Analysis of the behavioral time course of LSD-elicited head bobs following pretreatment with vehicle (H2O) or ketanserin (3µmol/kg). LSD-elicited head bobs are shown in 10 minute bins. The first 5 minutes is not included in the analysis due to the large stress response immediately after injection. **p<0.001, ***p<0.0001, post-hoc Dunnett test, significantly different from vehicle pretreated group, n=4 to 18.
Time course analysis of the effect of ketanserin pretreatment (3µmol/kg) on LSD (30nmol/kg)-elicited head bobs revealed an effect of time (F=2.43, p<0.039, 2-way ANOVA), but not of treatment (F=1.88, p>0.184, 2-way ANOVA; Fig 3b). There was a clear tendency for ketanserin to suppress LSD-elicited head bobs early in the time course, however. The LSD response then returned in the second half of the time course (Fig 3b).
3.2 Ligand-receptor interactions
3.2.1 Frontocortical receptor binding affinity
Both DOI and LSD bound frontocortical 5-HT2A receptors as measured by competition with [3H]ketanserin; affinity values (Ki) were 12.5±0.74nM (n=3) and 0.46±0.045nM (n=4), respectively. The affinity for frontocortical 5-HT2C receptors as measured by [3H]mesulergine competition was 73.5±14nM (n=4) and 7.5±1.5nM (n=4) for DOI and LSD, respectively. Dopamine1 receptor binding affinity in frontal cortex as measured by [3H]SCH23390 competition was 16980±3610nM (n=4) and 161.2±32.8nM (n=3) for DOI and LSD, respectively.
3.2.2 In vitro binding reversibility at frontocortical 5-HT2A receptors
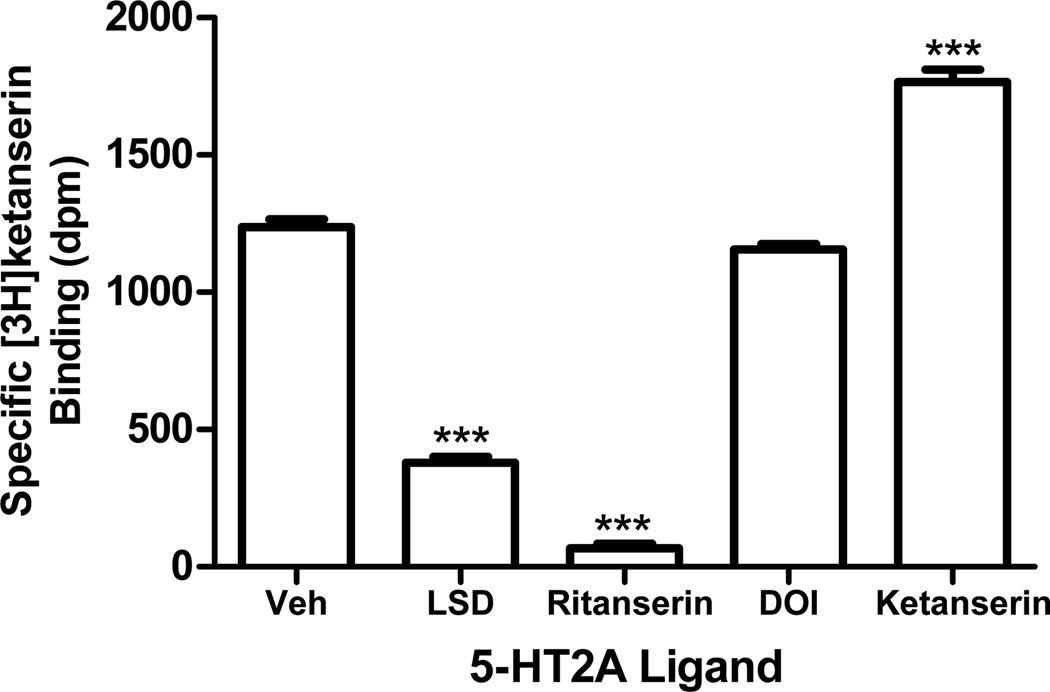
Drug pre-incubation significantly affected the ability of [3H]ketanserin to bind rabbit frontocortical membranes (F=590.5, p<0.0001, ANOVA; Fig 4). Pre-incubation of membranes with LSD (p<0.0001, post-hoc Dunnett test) and ritanserin (p<0.0001, post hoc Dunnett test) inhibited [3H]ketanserin binding (Fig 4). In contrast, pre-incubation with DOI or ketanserin did not inhibit [3H]ketanserin binding in frontocortical rabbit membranes (Fig 4). Interestingly, ketanserin pre-incubation led to a 43% increase in [3H]ketanserin binding (1238±36 to 1766±51 disintegrations per minute; Fig 4; p<0.0001, post hoc Dunnett test).
Fig. 4.
Binding reversibility of 5-HT2A receptor ligands in rabbit frontal cortex. Frontocortical embranes were pre-incubated with LSD (4nM), ritanserin (4nM), DOI (100nM), or ketanserin (4nM). Following the pre-incubation, the membranes were washed and [3H]ketnaserin binding was measured (see Methods 2.7). Specific binding is shown as mean disintegrations per minute (dpm) ± SEM. ***p<0.0001, post-hoc Dunnett test, significantly different from vehicle pre-incubated membranes, n=4
3.2.3 Ex vivo binding at frontocortical 5-HT2A receptors
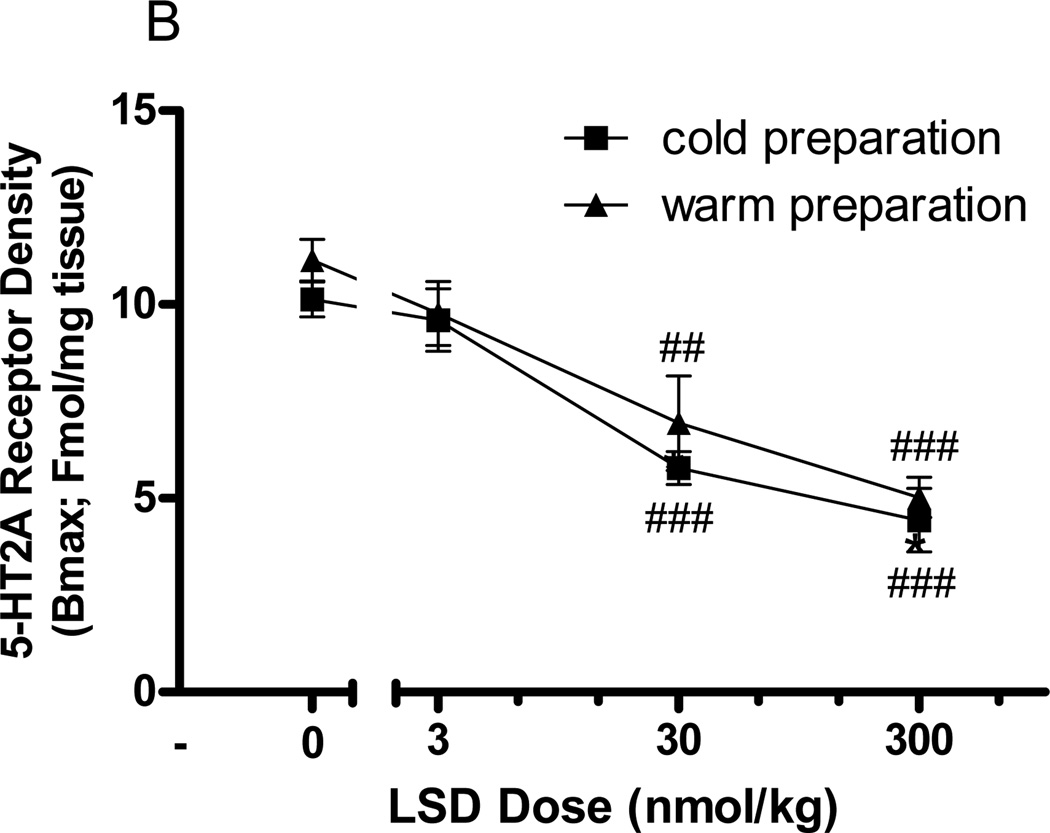
Acute administration of DOI (300nmol/kg) did not change the maximum binding (Bmax) of [3H]ketanserin, [3H]mesulergine, or [3H]SCH23390 to the frontocortical membranes, indicating no change in frontocortical 5-HT2A, 5-HT2C, or D1 receptor binding, respectively (data not shown). As with DOI, acute administration of LSD (30nmol/kg SC) had no effect on frontocortical [3H]mesulergine (3.84±0.5 to 3.53±0.9Fmol/mg tissue) or [3H]SCH23390 (3.47±0.7 to 3.66±0.2Fmol/mg tissue) binding. In contrast to DOI, however, acute LSD administration (30nmol/kg SC) significantly reduced the maximum binding of [3H]ketanserin in frontocortical membranes from 11.29±0.9 to 5.21±0.6Fmol/mg tissue (p<0.001, t-test). Data are represented graphically as a fraction of the control value (Fig 5a). This reduction in [3H]ketanserin binding after acute in vivo LSD administration was dose-dependent (F=27.9, p<0.0001, 2-way ANOVA; Fig 5b). The doses of 30 and 300nmol/kg LSD significantly reduced maximum binding of [3H]ketanserin in frontocortical membranes (p<0.001 and p<0.0001, post-hoc Bonferroni tests), but the dose of 3nmol/kg did not (Fig 5b). As residual drug would artificially decrease the Bmax for [3H]ketanserin binding, membranes were warmed prior to [3H]ketanserin incubation in order to remove any potentially residual drug. The LSD-elicited reduction in frontocortical membrane [3H]ketanserin binding did not differ between cold and warm membrane preparations (F=2.0, p>0.18, 2-way ANOVA, Fig 5b). Neither acute DOI nor acute LSD injection altered receptor binding affinity (Kd) for any of the radioligands tested (data not shown).
Fig. 5.
Effect of acute in vivo LSD administration on frontocortical [3H]ketanserin binding. a Seventy minutes after in vivo LSD (30nmol/kg SC) or vehicle (control) injection, the frontocortical density (Bmax) of 5-HT2A, 5-HT2C, and D1 receptors were measured using [3H]ketanserin, [3H]mesulergine, and [3H]SCH23390, respectively. Data are represented as the mean fraction of control ± SEM. For each receptor, the receptor density of the animal that received acute LSD treatment is divided by the receptor density of the paired animal that received acute vehicle treatment. These calculations are made for each animal pair. b Seventy minutes after in vivo LSD injection (0, 3, 30, or 300nmol/kg IV), the density of frontocortical 5-HT2A receptors was measured using [3H]ketanserin. Receptor density is reported as Fmol/mg tissue and shown as mean ± SEM. Frontocortical membranes were prepared with either a cold (square) or warm (triangle) pre-incubation. **p<0.001, t-test, significantly different from vehicle treated group, n=4; ##p<0.001, ###p<0.0001, post-hoc Bonferroni test, significantly different from vehicle treated group, n=4
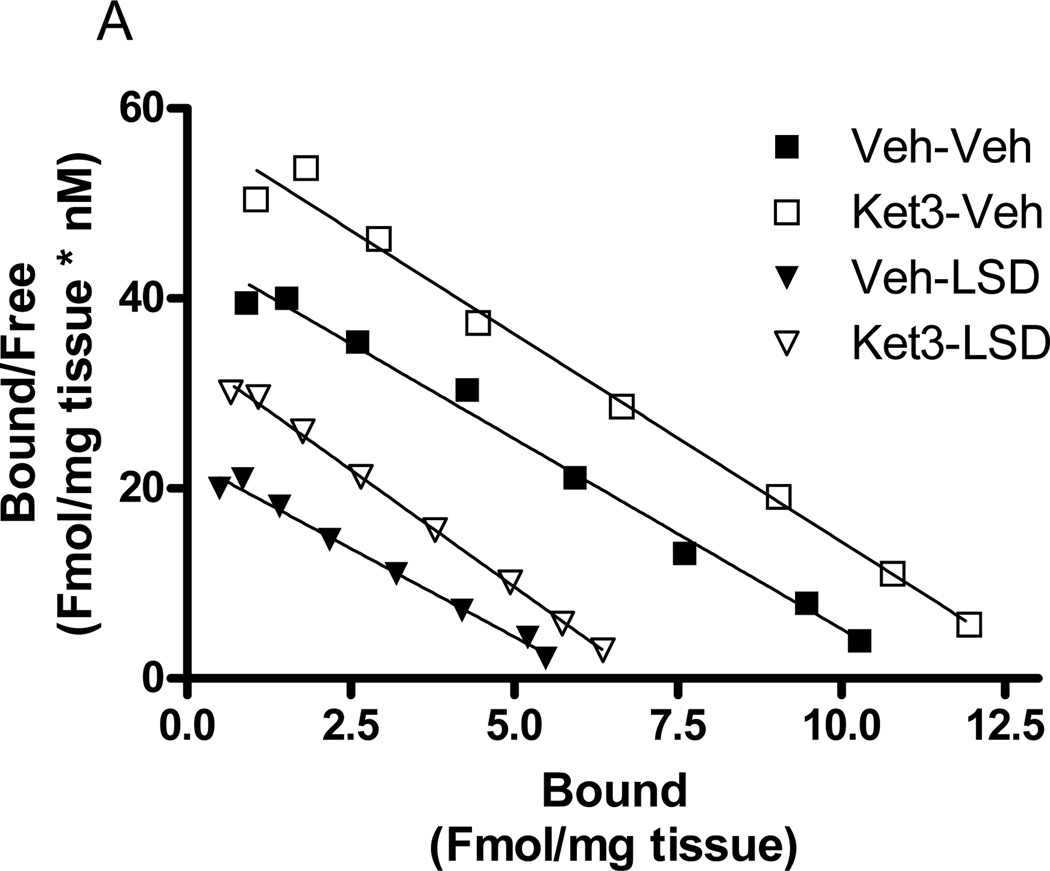
Pre-treatment of rabbits with ketanserin (3µmol/kg) did not affect the LSD-induced decrease in frontocortical [3H]ketanserin binding. Interestingly, acute injection of ketanserin (3µmol/kg) led to an increase in the maximum binding (Bmax) of [3H]ketanserin to rabbit frontocortical membranes (F=13.1, p<0.004, 2-way ANOVA). In animals that received the LSD vehicle, the control value, 10.83±0.8Fmol/mg tissue, increased to 13.46±0.2Fmol/mg tissue with in vivo ketanserin pre-treatment (p<0.001, post hoc Bonferroni test). In animals that received LSD (30nmol/kg), the control value, 6.05±0.2Fmol/mg tissue, increased to 8.04±1Fmol/mg tissue with in vivo ketanserin pre-treatment (p<0.001, post hoc Bonferroni test). ([3H]Ketanserin binding affinity (Kd) remained unchanged in all treatment groups. A representative Scatchard plot of these effects is shown in Figure 6a.
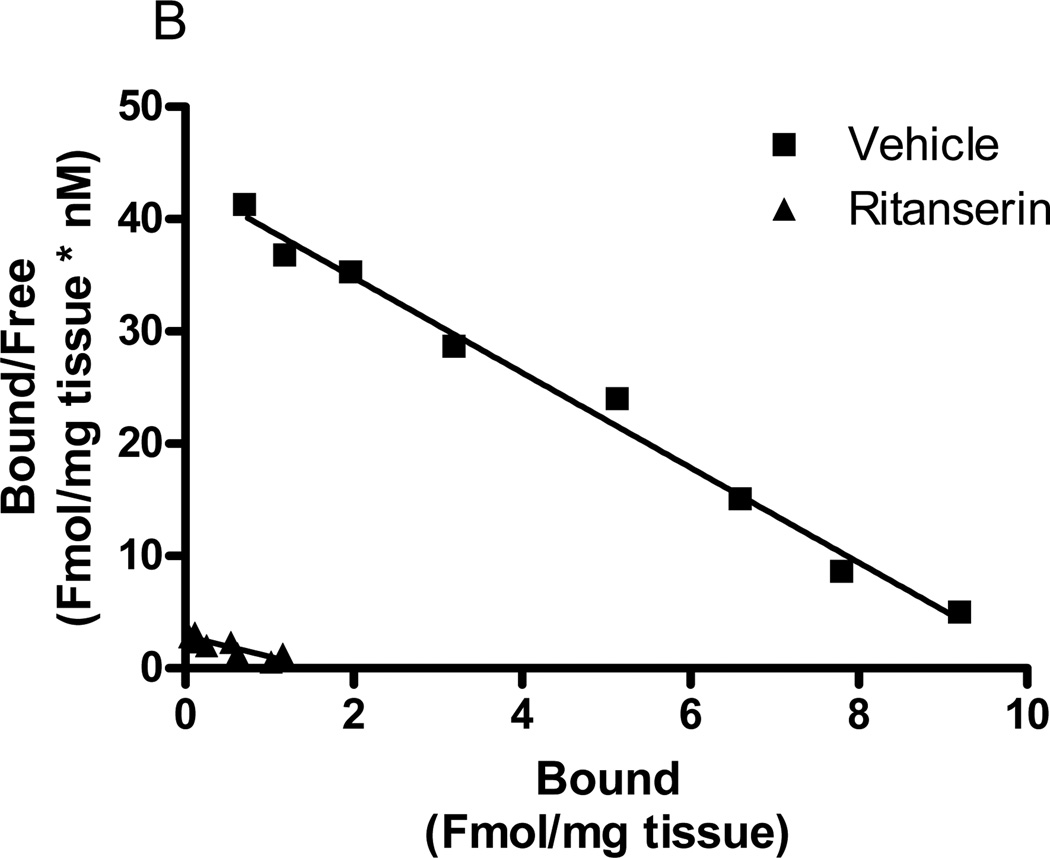
Fig. 6.
Effects of acute in vivo ketanserin or ritanserin on frontocortical [3H]ketanserin binding. a Rabbits were injected with ketanserin (3µmol/kg) or vehicle (H2O) followed 60 minutes later by LSD (30nmol/kg) or vehicle (control). Seventy minutes later, rabbits were sacrificed and frontal cortex tissue harvested for analysis of [3H]ketanserin binding. A Scatchard plot shows binding in representative animals from Veh-Veh (filled square), Ket-Veh (open square), Veh-LSD (filled triangle), and Ket-LSD (open triangle) groups. b Other rabbits were acutely injected with ritanserin (0.67µmol/kg), sacrificed 70 minutes later, and frontal cortex tissues harvested for analysis of [3H]ketanserin binding. A Scatchard plot shows binding in a representative vehicle (square) and ritanserin (triangle) animal. All membranes were warmed for 60 minutes at 37°C (see Methods 2.4). n=3 to 5
Seventy minutes after acute administration of ritanserin (0.67µmol/kg), there was a significant reduction in the Bmax for [3H]ketanserin binding in rabbit frontocortical membranes (9.40±1.2 to 1.91±0.5Fmol/mg tissue; p<0.0005, t-test). A representative Scatchard plot of the effect is shown in Figure 6b. Acute ritanserin injection significantly decreased frontocortical [3H]ketanserin binding affinity (0.21±0.01 to 0.5±0.1nM; p<0.01, t-test), but this apparent Kd shift is probably due to calculation errors resulting from the very low Bmax values (1.9±0.5Fmol/mg tissue).
4 Discussion
4.1 Serotonin2A-mediated effects of DOI and LSD
The present work demonstrates that the indoleamine class of hallucinogens, exemplified by LSD, elicited head bobs in rabbits through 5-HT2A receptor activation. This conclusion is consistent with previous reports establishing that the phenethylamine hallucinogen, DOI, elicits head movements in rodents via 5-HT2A receptors (Fantegrossi et al., 2010; Gonzalez-Maeso et al., 2007; Schreiber et al., 1995; Willins and Meltzer, 1997). Likewise, we have previously demonstrated that DOI elicits head movements in the rabbit by activation of this same receptor (Dave et al., 2002; Dave et al., 2007). The current study provides two lines of evidence to support the conclusion that DOI and LSD elicit rabbit head bobs by activation of 5-HT2A receptors. Firstly, down regulation of frontocortical 5-HT2A receptors resulted in decreased head movement behavior elicited by both hallucinogens. Secondly, ritanserin inhibited head bobs elicited by both DOI and LSD. Although ritanserin is also an antagonist at 5-HT2C receptors, the inability of SB206553, which is 750 times more selective for rabbit frontocortical 5-HT2C receptors over 5-HT2A receptors, to affect either DOI- or LSD-elicited head bobs highlights the 5-HT2A receptor in the effects of ritanserin (Dave et al., 2002).
The present study also explored the receptor binding characteristics of DOI and LSD at the rabbit frontocortical 5-HT2A receptor. Pre-incubation of rabbit frontocortical membranes with LSD, but not DOI, resulted in a significant reduction in [3H]ketanserin binding. To test the hypothesis that residual, unbound LSD in the membrane preparation accounted for the observed change in [3H]ketanserin binding, the tissue was warmed to facilitate drug removal. Warming the tissue during preparation, however, failed to alter this binding parameter. Acute in vivo administration of LSD, but not DOI, also significantly reduced [3H]ketanserin binding ex vivo. Radioligand binding to 5-HT2C and dopamine D1 receptors was unaffected by this in vivo manipulation. The selective and dose-dependent effects of LSD suggest that the drug remains pseudo-irreversibly bound to frontocortical 5-HT2A receptors for at least 60 minutes after in vitro incubation or 70 minutes after in vivo administration. In contrast, DOI binds the rabbit frontocortical 5-HT2A receptor in a reversible manner.
An alternative hypothesis is that the acute administration of LSD to rabbits results in the down-regulation of 5-HT2A receptors. Indeed, Ferry and colleagues (1993) demonstrated rapid reduction of 5-HT2A receptor density in cultured P11 cells after LSD incubation. This group showed the same effect and time course for DOI-induced 5-HT2A receptor reduction in P11 cells (Ferry et al., 1993). These findings are in contrast to the current study, which showed no change in [3H]ketanserin binding after either in vitro incubation or in vivo injection with DOI. Similar to the findings in cells, DOI significantly decreased 5-HT2 receptor binding in rat aortic smooth muscle, but required at least four hours to do so (Rinaldi-Carmona et al., 1994). Thus, it is unlikely that the one hour following drug administration used in the current study would be sufficient to down-regulate 5-HT2A receptor density. Instead, the reduction in [3H]ketanserin binding observed in the current study likely represents the presence of pseudo-irreversibly bound LSD. This conclusion is supported by previous studies that used similar methods to show that LSD binds the rat and blowfly 5-HT2A receptor in an irreversible or pseudo-irreversible manner (Berridge and Prince, 1974; Burris and Sanders-Bush, 1992).
Consistent with our previous results demonstrating rapid dissociation of [3H]ketanserin from rabbit 5-HT2A receptors (Aloyo et al., 2001), the present studies found that ketanserin bound reversibly to rabbit cortical membranes. Interestingly, frontocortical [3H]ketanserin binding was increased after in vitro incubation or acute in vivo administration of ketanserin. This effect suggests that ketanserin may possess unique interactions with the 5-HT2A receptor or its associated proteins. Further research will be required to test this hypothesis.
In behavioral studies, a relatively low dose of ketanserin (1µmol/kg) maximally inhibited DOI-elicited head bobs in rabbits (Dave et al., 2002). In contrast, the current study showed that a ten-fold higher dose of ketanserin (10µmol/kg) was required to inhibit the LSD-elicited behavior. On a molar basis, the high dose of ketanserin was more than 300 times the dose of LSD. The present work shows that pretreatment with a lower dose of ketanserin (3µmol/kg) was also able to reduce LSD-elicited head bobs, but only in the early period of agonist exposure (0–30 minutes). The behavioral effect of LSD then returned in the latter portion of the time course (30–60 minutes). Thus, when averaged over the entire 60 minute observation period, the data show that ketanserin (3µmol/kg) did not alter LSD elicited head bobs. Based on our demonstration that LSD binds in a pseudo-irreversible manner whereas ketanserin binds reversibly, a likely explanation is that LSD displaced ketanserin from the 5-HT2A receptor and thereby elicited sufficient head bob behavior to compensate for the initial ketanserin-induced inhibition.
In keeping with previous work in rodents (Leysen et al., 1985), the current studies demonstrated that ritanserin differed from ketanserin in that it bound to frontocortical 5-HT2A receptors in a pseudo-irreversible manner, a property demonstrated both in vitro and ex vivo. In contrast to ketanserin, ritanserin blocked head bobs elicited by either DOI or LSD at a relatively low dose (0.67µmol/kg). The pseudo-irreversible binding of ritanserin likely prevented its displacement by LSD, allowing it to inhibit LSD-elicited effects at low doses. This property of ritanserin supports its preferred use over ketanserin in studying the 5-HT2A-mediated effects of the pseudo-irreversibly binding LSD. Ritanserin is also preferable to more selective 5-HT2A antagonists such as M-100,907 or sarpogrelate, which have both been described as reversible antagonists (Ito et al., 1998; Kanamori et al., 1994). If LSD displaced ketanserin in the current experiments, it would likely displace these reversibly binding antagonists as well.
4.2 The role of 5-HT2B/2C receptors in hallucinogen-elicited behavior
The present work demonstrates that LSD did not elicit head bobs through 5-HT2B/2C receptor activation. The inability of SB206553 to alter LSD-elicited head bobs is, to our knowledge, the first reported investigation into the role of 5-HT2B/2C receptors in LSD-elicited head movement behavior. This finding also extends the work by Dave and colleagues (2002) showing that 5-HT2B/2C receptors were not involved with DOI-elicited head bobs in rabbits. Other reports have also shown that 5-HT2C receptors are not involved in either DOI-elicited rat head twitches (Schreiber et al., 1995) or the DOI stimulus cue in rats (Smith et al., 1999). The lack of a role for 5-HT2C receptors in hallucinogen-mediated head movements in both rabbits and rats contrasts with the findings in mice, which identify a role for 5-HT2C receptors in both DOI-elicited head twitches (Canal et al., 2010) and the DOI stimulus cue (Smith et al., 2003). Additional studies should examine the possibility that a species factor accounts for the differential roles of 5-HT2C receptors in rat, rabbit, and mouse.
Dave and associates (2002) showed that 5-HT2B/2C receptors were in fact involved in another rabbit behavior, the body shake. DOI-elicited body shakes were blocked by SB206553 (1µmol/kg), but not by ketanserin (1 and 3µmol/kg; Dave et al., 2002). The authors concluded that head bobs and body shakes were mediated by 5-HT2A and 5-HT2C receptors, respectively (Dave et al., 2002). Given the inability of this same behaviorally active dose of SB206553 (1µmol/kg) to modify LSD-elicited head bobs (either inhibit or potentiate) we concluded that 5-HT2B/2C receptors were not involved in this behavior. As Fantegrossi and associates (2010) clearly demonstrated, the role of 5-HT2C receptors in mouse head twitches is conditional on the dose of DOI administered. Further studies using doses of DOI and LSD on the descending arm of the dose response curve are warranted to test the hypothesis that 5-HT2C antagonism may potentiate rabbit head bobs under these conditions.
4.3 The role of D1 receptors in hallucinogen-elicited behavior
The dopaminergic system is not a major focus of hallucinogen study, although dopamine is strongly tied to human psychotic disease, a condition mimicked by hallucinogens (Nichols, 2004). Dopamine1 receptors have been reported to mediate DOI-elicited rat head shakes (Schreiber et al., 1995), but the role of this or any dopaminergic receptor in LSD-elicited head movement behavior was not previously investigated. The current studies found that both DOI and LSD required the activation of D1 receptors to elicit head bobs, strongly supporting the hypothesis that D1 receptors may play a part in the hallucinogenic mechanism of action. The antagonist used to reach this conclusion was SCH23390, a ligand used previously to demonstrate the role of D1 receptors in DOI-elicited head movement behavior (Schreiber et al., 1995). SCH23390 is known to bind 5-HT2A receptors as well as D1 receptors, suggesting that SCH23390 may be inhibiting head bobs via antagonist action at 5-HT2A receptors. Although the relative affinity for these two receptors varies among animal species, SCH23390 exhibits approximately 100 fold selectivity for D1 receptors as compared to 5-HT2A receptors (Ekelund et al., 2007; Neumeyer et al., 2003; Schreiber et al., 1995). Thus, it is unlikely that SCH23390 is inhibiting head movement behavior via 5-HT2A receptors.
Previously it was shown that LSD binds dopamine receptors whereas DOI and other hallucinogens do not (Burt et al., 1976; Watts et al., 1995). The present work showed that LSD had modest binding affinity for D1 receptors in rabbit frontal cortex, whereas DOI had negligible affinity. Thus, LSD may act directly on D1 receptors to elicit head bobs in rabbits, whereas the dopaminergic component of the DOI-elicited behavior is likely an indirect effect. One possibility for an indirect mechanism is the release of dopamine in frontal cortex mediated via 5-HT2A receptor activation (Gobert and Millan, 1999; Ichikawa et al., 2001). Importantly, both ritanserin and SCH23390 independently and equally diminished head bob behavior elicited by the hallucinogens studied. Thus, both 5-HT2A and D1 receptors appear to play key roles in producing head bobs in rabbits. To confirm the role of D1 receptors in DOI- and LSD- elicited head bobs, other more selective D1 receptor antagonist should be tested (see Schreiber et al., 1995).
5 Conclusion
In keeping with the original goals of this study, the current report delineates some of the pharmacology behind hallucinogen-mediated behavior. Importantly, these studies showed that both 5-HT2A and D1 receptors were crucial for DOI- and LSD-elicited head bobs in rabbits. This conclusion suggests a central role for these receptors in the effects of hallucinogens in humans. The pseudo-irreversible binding of LSD at frontocortical 5-HT2A receptors along with its affinity for D1 receptors may also explain some of the unique characteristics of this particular hallucinogen. The various findings discussed in this report are supported by the known effects of hallucinogens in humans, which are also similar, yet distinct (Shulgin and Shulgin, 1991; Shulgin and Shulgin, 1997). Ultimately, the ability of these compounds to have such profound and lasting effects on the human psyche, even after limited exposure (Griffiths et al., 2006; Griffiths et al., 2008; Grob et al., 2011), implicates complex interactions, such as genetic effects or perhaps even changes in neurocircuitry (Passie et al., 2008; Vollenweider and Kometer, 2010). These and other post-receptor effects are driving the latest studies (Nichols and Sanders-Bush, 2004; Schmid and Bohn, 2010). The benefits of continued investigation of hallucinogenic compounds are clear. The contributions of this distinctive group of compounds to science and medicine have only begun to be realized.
Highlights.
-
>
The pharmacology of DOI and LSD were compared in vivo and in vitro using rabbits.
-
>
LSD elicited head bobs through activation of 5-HT2A, but not 5-HT2B/2C, receptors.
-
>
Both DOI and LSD elicited head bobs through activation of D1 receptors.
-
>
Both DOI and LSD bound 5-HT2A receptors, but only LSD bound D1 receptors.
-
>
LSD and ritanserin pseudo-irreversibly bound frontocortical 5-HT2A receptors.
-
>
DOI and ketanserin reversibly bound frontocortical 5-HT2A receptors.
Acknowledgments
Funding:
This work was supported by NIMH grant MH16841-40 (JAH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For all authors there are no conflicts of interested involving these data.
References
- 1.Aloyo VJ, Dave KD, Rahman T, Harvey JA. Selective and divergent regulation of cortical 5-HT(2A) receptors in rabbit. J Pharmacol Exp Ther. 2001;299(3):1066–1072. [PubMed] [Google Scholar]
- 2.Aloyo VJ, Harvey JA. Antagonist binding at 5-HT(2A) and 5-HT(2C) receptors in the rabbit: high correlation with the profile for the human receptors. Eur J Pharmacol. 2000;406(2):163–169. doi: 10.1016/s0014-2999(00)00645-2. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Prince WT. The nature of the binding between LSD and a 5-HT receptor: a possible explanation for hallucinogenic activity. Br J Pharmacol. 1974;51(2):269–278. doi: 10.1111/j.1476-5381.1974.tb09657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris KD, Sanders-Bush E. Unsurmountable antagonism of brain 5-hydroxytryptamine2 receptors by (+)-lysergic acid diethylamide and bromo-lysergic acid diethylamide. Mol Pharmacol. 1992;42(5):826–830. [PubMed] [Google Scholar]
- 5.Burt DR, Creese I, Snyder SH. Binding interactions of lysergic acid diethylamide and related agents with dopamine receptors in the brain. Mol Pharmacol. 1976;12(4):631–638. [PubMed] [Google Scholar]
- 6.Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology. 2010;209(2):163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilcoat HD, Schutz CG. Age-specific patterns of hallucinogen use in the US population: an analysis using generalized additive models. Drug Alcohol Depend. 1996;43(3):143–153. doi: 10.1016/s0376-8716(96)01297-5. [DOI] [PubMed] [Google Scholar]
- 8.Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11(1):65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- 9.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36(4):901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 10.Dave KD, Harvey JA, Aloyo VJ. A novel behavioral model that discriminates between 5-HT2A and 5-HT2C receptor activation. Pharmacol Biochem Behav. 2002;72(1–2):371–378. doi: 10.1016/s0091-3057(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 11.Dave KD, Harvey JA, Aloyo VJ. The time-course for up- and down-regulation of the cortical 5-hydroxytryptamine (5-HT)2A receptor density predicts 5-HT2A receptor-mediated behavior in the rabbit. J Pharmacol Exp Ther. 2007;323(1):327–335. doi: 10.1124/jpet.107.121707. [DOI] [PubMed] [Google Scholar]
- 12.Davidoff SA, Benes FM. High-resolution scatchard analysis shows D1 receptor binding on pyramidal and nonpyramidal neurons. Synapse. 1998;28:83–90. doi: 10.1002/(SICI)1098-2396(199801)28:1<83::AID-SYN10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, et al. In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9(3):117–125. doi: 10.1007/s11307-007-0077-4. [DOI] [PubMed] [Google Scholar]
- 14.Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335(3):728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferry RC, Unsworth CD, Molinoff PB. Effects of agonists, partial agonists, and antagonists on the regulation of 5-hydroxytryptamine2 receptors in P11 cells. Mol Pharmacol. 1993;43(5):726–733. [PubMed] [Google Scholar]
- 16.Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38(2):315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 17.González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths RR, Richards WA, Johnson MW, McCann UD, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22(6):621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187(3):268–292. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 20.Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 21.Harvey JA, Gormezano I. Effects of haloperidol and pimozide on classical conditioning of the rabbit nictitating membrane response. J Pharmacol Exp Ther. 1981;218(3):712–719. [PubMed] [Google Scholar]
- 22.Ichikawa J, Dai J, Meltzer HY. DOI, a 5-HT2A/2C receptor agonist, attenuates clozapine-induced cortical dopamine release. Brain Res. 2001;907(1–2):151–155. doi: 10.1016/s0006-8993(01)02596-3. [DOI] [PubMed] [Google Scholar]
- 23.Ito H, Nyberg S, Halldin C, Lundkvist C, Farde L. PET imaging of central 5-HT2A receptors with carbon-11-MDL 100,907. J Nucl Med. 1998;39(1):208–214. [PubMed] [Google Scholar]
- 24.Kanamori A, Matoba K, Yajima Y. Effects of sarpogrelate on serotonin-induced increases in cytosolic Ca2+ in cultured rat mesangial cells. Life Sci. 1994;55(18):PL365–PL370. doi: 10.1016/0024-3205(94)00762-4. [DOI] [PubMed] [Google Scholar]
- 25.Kast EC, Collins VJ. Lysergic acid diethylamide as an analgesic agent. Anesth Analg. 1964;45:285–291. [PubMed] [Google Scholar]
- 26.Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27(6):600–611. [PubMed] [Google Scholar]
- 27.Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs. 1998;30(4):381–418. doi: 10.1080/02791072.1998.10399714. [DOI] [PubMed] [Google Scholar]
- 28.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 29.Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474(2–3):137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- 30.Nichols CD, Sanders-Bush E. Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase-1, C/EBP-beta and ILAD-1, a novel gene with homology to arrestins. J Neurochem. 2004;90(3):576–584. doi: 10.1111/j.1471-4159.2004.02515.x. [DOI] [PubMed] [Google Scholar]
- 31.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther. 2008:295–314. doi: 10.1111/j.1755-5949.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi-Carmona M, Prabonnaud V, Bouaboula M, Poinot-Chazel C, Casellas P, Le Fur G, Herbert JM. Regulation of 5-hydroxytryptamine2 (5-HT2) receptor expression in cultured rat aortic smooth muscle cells by SR 46349B, a selective 5-HT2 receptor antagonist. J Biol Chem. 1994;269(1):396–401. [PubMed] [Google Scholar]
- 34.Romano AG, Quinn JL, Li L, Dave KD, Schindler EA, Aloyo VJ, Harvey JA. Intrahippocampal LSD accelerates learning and desensitizes the 5-HT2A receptor in the rabbit. Psychopharmacology. 2010;212:441–448. doi: 10.1007/s00213-010-2004-7. [DOI] [PubMed] [Google Scholar]
- 35.Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30(40):13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273(1):101–112. [PubMed] [Google Scholar]
- 37.Sewell RA, Halpern JH, Pope HG., Jr Response of cluster headache to psilocybin and LSD. Neurology. 2006;66(12):1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- 38.Shulgin AT, Shulgin A. Phenethylamines I Have Known And Loved: A Chemical Love Story. Berkley: Transform Press; 1991. [Google Scholar]
- 39.Shulgin AT, Shulgin A. Tryptamines I Have Known And Loved: The Continuation. Berkley: Transform Press; 1997. [Google Scholar]
- 40.Simansky KJ, Baker G, Kachelries WJ, Hood H, Romano AG, Harvey JA. Prenatal exposure to cocaine reduces dopaminergic D1-mediated motor function but spares the enhancement of learning by amphetamine in rabbits. Annals Ann N Y Acad Sci. 1998;846:375–378. [PubMed] [Google Scholar]
- 41.Sloviter RS, Drust EG, Damiano BP, Connor JD. A common mechanism for lysergic acid, indolealkylamine and phenethylamine hallucinogens: serotonergic medication of behavioral effects in rats. J Pharmacol Exp Ther. 1980;214(2):231–238. [PubMed] [Google Scholar]
- 42.Smith RL, Barrett RJ, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology. 2003;166(1):61–68. doi: 10.1007/s00213-002-1252-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology. 1999;144(3):248–254. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- 44.Vetulani J, Bednarczyk B, Reichenberg K, Rokosz A. Head twitches induced by LSD and quipazine: similarities and differences. Neuropharmacology. 1980;19(2):155–158. doi: 10.1016/0028-3908(80)90131-8. [DOI] [PubMed] [Google Scholar]
- 45.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11(9):642–645. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 46.Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology. 1995;118(4):401–409. doi: 10.1007/BF02245940. [DOI] [PubMed] [Google Scholar]
- 47.Welsh SE, Kachelries WJ, Romano AG, Simansky KJ, Harvey JA. Effects of LSD, ritanserin, 8-OH-DPAT, and lisuride on classical conditioning in the rabbit. Pharmacol Biochem Behav. 1998;59(2):469–475. doi: 10.1016/s0091-3057(97)00436-x. [DOI] [PubMed] [Google Scholar]
- 48.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282(2):699–706. [PubMed] [Google Scholar]
- 49.Yamamoto T, Ueki S. The role of central serotonergic mechanisms on head-twitch and backward locomotion induced by hallucinogenic drugs. Pharmacol Biochem Behav. 1981;14(1):89–95. doi: 10.1016/0091-3057(81)90108-8. [DOI] [PubMed] [Google Scholar]