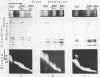
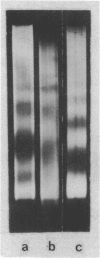
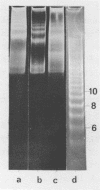
Abstract
Comparison has been made between sea urchin and starfish sperm chromatin. The only protein by which chromatins from these sources differ significantly is histone H2B. Sea urchin sperm H2B is known to contain an elongated N-terminal region enriched in Arg. Analysis of the micrococcal nuclease digests of sea urchin and starfish nuclei in one- and two-dimensional electrophoresis has shown that sperm chromatin of both animals consists of repeated units similar in general features to those of rat thymus or liver. However, DNA repeat length in chromatin of sea urchin sperm (237 bp) is higher than that of starfish sperm (224 bp), while the core DNA length does not differ and is the same as in the chromatin of rat liver or thymus. A suggestion has been made that the N-terminal region of histone H2B is associated with the linker DNA and is responsible for the increased length of sea urchin linker DNA.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt W. F., von Holt C. A histone H2B variant from the embryo of the sea urchin Parenchinus angulosus. Biochim Biophys Acta. 1978 Nov 20;537(1):177–181. doi: 10.1016/0005-2795(78)90613-x. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Moss T., Bradbury E. M. High-resolution proton-magnetic-resonance studies of chromatin core particles. Eur J Biochem. 1978 Sep 1;89(2):475–482. doi: 10.1111/j.1432-1033.1978.tb12551.x. [DOI] [PubMed] [Google Scholar]
- Cheah K. S., Osborne D. J. Analysis of nucleosomal deoxyribonucleic acid in a higher plant. Biochem J. 1977 Apr 1;163(1):141–144. doi: 10.1042/bj1630141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Iwai K., Johnson J. D., Bonner J. Pea histones H2A and H2B. Variable and conserved regions in the sequences. J Biochem. 1977 Aug;82(2):503–510. [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Keichline L. D., Wassarman P. M. Structure of chromatin in sea urchin embryos, sperm, and adult somatic cells. Biochemistry. 1979 Jan 9;18(1):214–219. doi: 10.1021/bi00568a033. [DOI] [PubMed] [Google Scholar]
- Kinkade J. M., Jr, Cole R. D. A structural comparison of different lysine-rich histones of calf thymus. J Biol Chem. 1966 Dec 25;241(24):5798–5805. [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Precise location of DNase I cutting sites in the nucleosome core determined by high resolution gel electrophoresis. Nucleic Acids Res. 1979 Jan;6(1):41–56. doi: 10.1093/nar/6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. A comparison of the structure of chicken erythrocyte and chicken liver chromatin. Cell. 1976 Dec;9(4 Pt 1):627–632. doi: 10.1016/0092-8674(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Bilek D., Chalkley R. An electrophoretic comparison of vertebrate histones. J Biol Chem. 1971 Jul 10;246(13):4206–4215. [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Nucleosome-nucleosome interaction in chromatin. FEBS Lett. 1979 Mar 1;99(1):123–128. doi: 10.1016/0014-5793(79)80263-x. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Structure of chromatin subunits: an endonuclease Serratia marcescens study. Nucleic Acids Res. 1977 Sep;4(9):3267–3279. doi: 10.1093/nar/4.9.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautière P., Tyrou D., Laine B., Mizon J., Ruffin P., Biserte G. Covalent structure of calf-thymus ALK-histone. Eur J Biochem. 1974 Feb 1;41(3):563–576. doi: 10.1111/j.1432-1033.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Whitlock J. P. Mapping DNAase l-susceptible sites in nucleosomes labeled at the 5' ends. Cell. 1976 Oct;9(2):347–353. doi: 10.1016/0092-8674(76)90124-0. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Bellard M., Compton J. L., Chambon P. The DNA repeat lengths in chromatins from sea urchin sperm and gastrule cells are markedly different. FEBS Lett. 1976 Oct 15;69(1):281–285. doi: 10.1016/0014-5793(76)80704-1. [DOI] [PubMed] [Google Scholar]
- Spiker S., Isenberg I. Cross-complexing pattern of plant histones. Biochemistry. 1977 May 3;16(9):1819–1826. doi: 10.1021/bi00628a009. [DOI] [PubMed] [Google Scholar]
- Strickland M. S., Strickland W. N., von Holt C. The histone H2B from the sperm cell of the starfish Marthasterias glacialis. Eur J Biochem. 1980 May;106(2):541–548. doi: 10.1111/j.1432-1033.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- Strickland M., Strickland W. N., Brandt W. F., Von Holt C., Wittmann-Liebold B. The complete amino-acid sequence of histone H2B(3) from sperm of the sea urchin Parechinus angulosus. Eur J Biochem. 1978 Sep 1;89(2):443–452. doi: 10.1111/j.1432-1033.1978.tb12547.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte-Durand G., Mizon J., Sautiere P., Biserte G. Histones from gonads of the star-fish Asterias rubens. Comp Biochem Physiol B. 1977;57(2):121–126. doi: 10.1016/0305-0491(77)90160-2. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Holt C., Strickland W. N., Brandt W. F., Strickland M. S. More histone structures. FEBS Lett. 1979 Apr 15;100(2):201–218. doi: 10.1016/0014-5793(79)80337-3. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Sautière P., Biserte G. Purification and characterization of glycine, arginine, lysine-rich and alanine, leucine, glycine-rich histones from sea urchin gonad. Biochim Biophys Acta. 1974 Apr 11;342(2):360–366. doi: 10.1016/0005-2795(74)90091-9. [DOI] [PubMed] [Google Scholar]