Abstract
These experiments were completed as part of an NIH “Facilities of Research Excellence in Spinal Cord Injury” contract to support independent replication of published studies that could be considered for eventual clinical testing. Recent studies have reported that selective inhibition of the P2X7 receptor improves both the functional and histopathological consequences of a contusive spinal cord injury (SCI) in rats. We repeated two published studies reporting the beneficial effects of pyridoxal-5′-phosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS) or Brilliant blue G (BBG) treatment after SCI (Wang et al., 2004 and Peng et al., 2009). Mild thoracic SCI was first produced in Experiment 1 by means of the MASCIS impactor at T10 (height 6.25 mm, weight 10 gm) followed by intraspinal administration of a P2X7 antagonist (2 μl/10mM) after injury. Treatment with PPADS or another highly selective P2X7R antagonist Brilliant Blue G (BBG) (2 μl/02mM) did not improve locomotive (BBB rating scale) over a 7 week period compared to vehicle treated rats. Also, secondary histopathological changes in terms of overall lesion and cavity volume were not significantly different between the PPADS, BBG, and vehicle treated animals. In the second experiment, the systemic administration of BBG (10 or 50 mg/kg, iv) 15 min, 24 and 72 hours after moderate (12.5 mm) SCI failed to significantly improve motor recovery or histopathological outcome over the 6 week observational period. Although we cannot conclude that there will be no long-term beneficial effects in other spinal cord injury models using selective P2X7 receptor antagonists at different doses or treatment durations, we caution researchers that this potentially exciting therapy requires further preclinical investigations before the implementation of clinical trials targeting severe SCI patients.
Keywords: inflammation, purinergic signaling, neuroprotection, spinal cord injury
Introduction
Spinal cord injury leads to both primary and secondary injury mechanisms that are both felt to participate in long-term structural and functional consequences of this devastating injury (Anderson and Hall, 1993; Popovich et al., 1997). Although much work has been accomplished regarding understanding the pathophysiology of spinal cord injury (SCI), few therapeutic interventions have been successfully translated to the clinic although methylprednisolone is a treatment option for acute SCI (Bracken et al., 1997). Thus, there is an important need to continue to investigate the pathogenesis of SCI with the long-term goal of determining novel targets of therapeutic interventions. Several new agents are currently being investigated in preclinical models of SCI that appear to show efficacy and merit special consideration as we attempt to move these preclinical data to the clinic (Baptiste & Fehlings 2007).
Following spinal cord injury, ATP is released by the traumatized tissue leading to the activation of ATP-sensitive purinergic receptors, the P2X7 receptors (P2X7R) (Bianchi et al., 1999 and North et al., 2002) which are expressed in neurons (Collo et al., 1997; Deuchars et al., 2001; Sperlágh et al 2006) and astrocytes (Duan et al., 2003; Kimelberg & Nedergaard, 2010; Neary et al 2003). As a consequence of excessive release of ATP, excessive firing of spinal cord neurons is observed followed by increases in Ca2+ that can lead to increased cell vulnerability and death (James and Butt, 2002; Cotrina and Nedergaard, 2009). Recent studies have reported that many of these physiological and destructive events can be prevented by P2X7 receptor antagonists (LeFeurre et al., 2002, 2003; Wang et al 2004; Melani et al, 2006; Peng et al. 2009). P2X7 receptors are found on various inflammatory cells and the activation of these receptors has been shown to lead to production and release of interleukins and cytokines which likely participate in the pathophysiology of SCI (Collo et al 1997; Parvathenani et al 2003; Suzuki et al., 2004; Franke et al., 2004; Davalos et al., 2005; Khakh and North, 2006). Thus, strategies that block posttraumatic inflammation, including the P2X7R antagonists could provide a novel strategy for improving outcome following SCI (Wang et al 2004; Cotrina & Nedergaard, 2009; Peng et al. 2009).
Wang and colleagues (2004) first reported the neuroprotective effects of the P2X7 receptor antagonists adenosine 5′-triphosphate-2′, 3′-dialdehyde (OxATP) and PPADS in rats after mild contusive SCI (Wang et al., 2004). In that study, both OxATP and PPADS significantly improved functional outcome as documented by the open field locomotive BBB scale as well as reducing the frequency of TUNEL-positive cells in the peritraumatic zone. The P2X7R blockers were injected directly into the cord, both rostrally and caudally from the injury epicenter. In a more recent study (Peng et al., 2009), another P2X7 receptor antagonist Brilliant Blue G (BBG) (Borzelleca et al., 1990; Jiang et al., 2000; Remy et al., 2008) was systemically administered starting 15 minutes after injury and reported to improve BBB function and reduce spinal cord histopathological damage. Taken together, these studies indicate that selectively blocking the P2X7R in the early stages after SCI may limit the detrimental effects of ATP-mediated neurotransmission including abnormal calcium signaling, posttraumatic inflammation and cell death (Cotrina & Nedergaard, 2009).
The initial goal of this study was to attempt to replicate findings of the Wang et al., 2004 manuscript showing that the selective P2X7R antagonist PPADS administered directly into the spinal cord after mild contusive SCI would improve histopathological and functional outcome (Figure 5e). Because of the more recent publication (Peng et al., 2009) showing that systemic administration of Brilliant Blue G also improved outcome in an established moderate SCI model, we evaluated in a second experiment whether administration of this selective P2X7R antagonist would also improve functional and histopathological outcome (Figure 3A). To this end, attempts were made to replicate these studies by conditions described in the Wang et al., (2004) and Peng et al., (2009) manuscripts including utilizing the same injury model, injury severities and similar behavioral and histopathological outcome measures.
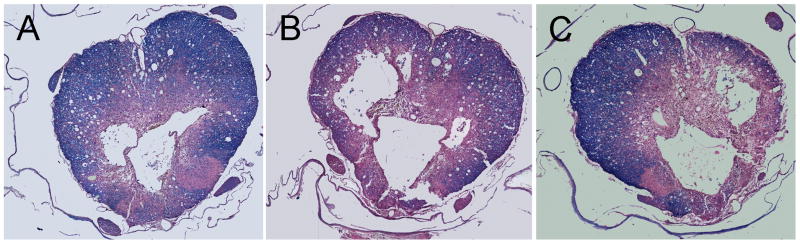
Figure 5.
Histopathological damage in Experiment 2 six weeks after moderate SCI. Note patterns of cavity and lesion formation after moderate SCI. A) 50mg/kg BBG, B) 10 mg/kg BBG, C) Vehicle treated rats.
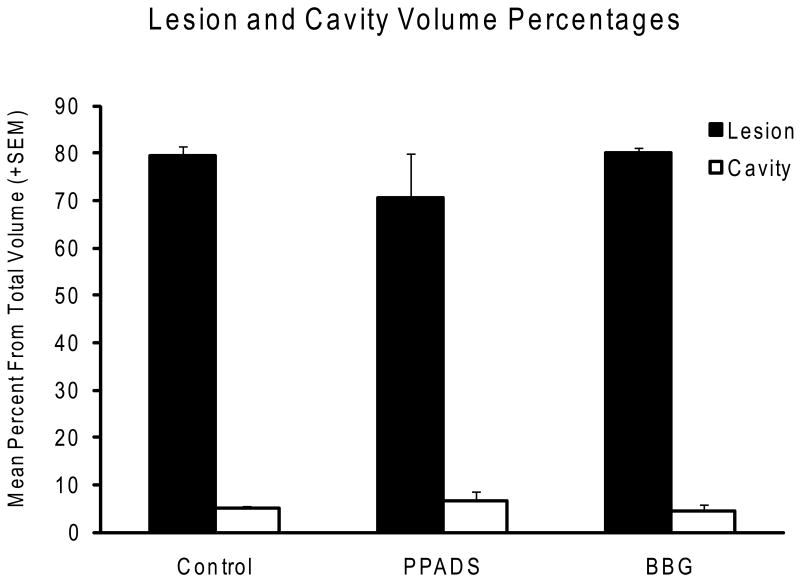
Figure 3.
Quantitative histopathological assessment of lesion and cavity volume percentages seven weeks after SCI. No significant differences in these quantitative outcome measures are demonstrated between the 3 experimental groups.
Material and methods
General Animal Procedures
Adult female Fisher rats (220-250 gms, n=29; Experiment 1) or Sprague-Dawley rats (220-250 gms, n=27; Experiment 2) were housed according to the National Institutes of Health and United States Department of Agriculture guidelines. Institutional American Animal Care and Use Committee of the University of Miami approved all animal procedures. Animals were randomly divided into various experimental groups. Anesthetic induction with Ketamine (45-75mg/kg, IP) was followed by IP injection of Xylazine (5-10 mg/kg), following verification of levels of anesthesia by assessing the corneal reflex and withdrawal reflex to stimuli for the hindlimbs. In Experiment 1 animals underwent a T10 spinal laminectomy (3.5-3.8 mm2). In Experiment 2 a spinal laminectomy was done at T12 according to Peng et al., 2009. During surgery, rats were placed on a warming pad to maintain the body temperature at 37° ± 0.5° C. These anesthetic and surgical steps followed the procedures outlined by the Wang et al. 2004 and Peng et al. 2009 manuscripts.
Briefly, the rat was placed ventrally on top of the small bed of sterile gauze to elevate the surgical site, presenting an adequate exposure of the back anatomy (Pinzon et al, 2008). A 2 cm longitudinal skin incision was next centered over the T9 spinus process along the midline. Muscles and ligaments were laterally dissected and retracted followed by removal of bony elements of the posterior spine including the spinous process and laminae using micro-rongeur. In Experiment 1, the tenth thoracic (T10) spinal segment was then exposed without removing the dura mater by removing a dorsal part of the vertebra. For Experiment 2, the twelfth thoracic spinal segment was exposed without removing the dura mater by removing a dorsal part of the vertebra.
Injury Model
In the first experiment, the exposed cord was contused by a 10 gm weight dropped at a height of 6.25 mm, as previously described (Wang et al 2004). The contusion injury was induced by the weight drop device (MASCIS) developed at New York University (Gruner, 1992). The impact velocity and compression were monitored and recorded to guarantee consistency between animals. This type of injury corresponds to a relatively mild histopathological lesion in the spinal cord.
In the second experiment, a more moderate contusion was produced by the MASCIS impactor where the weight was dropped from a height of 12.5 mm as previously described (Peng et al., 2009). After injury, the muscles of all animals were sutured in layers and the skin was closed. The rats were returned to their cages with the core temperature regulated for the following 24 hours with ad libitum access to water and food.
Treatment Strategies
In the first experiment, we followed the procedures previously outlined by Wang and colleagues (2004) for the intraspinal administration of the P2X7 receptor antagonists. The P2X7 antagonist pyridoxal-5-phosphate-6-azophenyl-2, 4-disulphonic acid (PPADS, 10mM; n=8) or vehicle was injected 2.5 mm rostral and caudal to the epicenter of the injury 30 minutes after impact using a Hamilton syringe (26 gauge). For the Brilliant Blue G administration in this initial study (0.2 mM; n=10) similar injections rostral and caudal 30 minutes after impact were also conducted. Although not investigated in the Wang et al., 2004 study, we felt that this treatment would be informative since in contrast to OxATP, BBG is considered a selective P2X7R antagonist. Non-treated traumatized rats received equal amounts saline (n=11) injected rostral and caudal to the lesion epicenter. We took special precautions to prepare the proper concentrations of PPADS and BBG according to the methods outlined in the original publications.
In the second experiment, the P2X7 receptor antagonist BBG was systemically administered into the femoral vein 15 min after moderate SCI (Peng et al, 2009) followed by additional IV bolus injection for two days. After the final IV injection on day 3 post-injury, the femoral catheter was removed for behavioral testing. In these studies, a 10 (n=8) or 50 (n=8) mg/kg dose of BBG was compared to a vehicle (saline; n=6) treated or SCI only (n=5) group.
Behavioral Testing
Behavioral assessment was performed in all animals by two examiners blinded to the experimental protocols at 24 hours, 72 hours and then weekly after SCI with the open-field locomotive test (Basso et al., 1995). In each case, an examiner observed the rats in an open-field for a 4 minute period. The evaluators had no knowledge of the animal's previous score or the other observer's score during that period of assessment. It should be noted that with systemic injection of BBG, animals turn blue for a week after administration. Although this face interjects some degree of potential bias in terms of behavioral testing, the examiners had no previous knowledge of the study groups. During the next several weeks of testing, the blue color disappeared and any potential for bias due to color of the animals was not a factor.
Histopathological Analysis
At 7 weeks (first experiment) or 6 weeks (second experiment) after SCI, all animals were anesthetized and intracardially perfused with normal saline solution followed by a 4% paraformaldehyde solution (Pinzon et al., 2008). A one centimeter segment of the spinal cords were next removed, blocked and embedded in paraffin. Tissue was cut in 10 μm sections every 90 μm throughout the sample and stained with Luxol Fast Blue and counterstained with hematoxylin and eosin for myelin preservation and injury evaluation. Data from the remaining normal cord tissue, total abnormal tissue and cavities were obtained for further planimetric analysis using a Zeiss Axiovert 200 microscope and Stereo Investigator and Neurolucida Software (MicroBrightfield Bioscience, Inc.). Areas of the spinal cord, preserved myelin (Experiment 2 only), cavity and lesion were contoured every 500μm throughout the dissected piece of tissue. Lesioned cord areas were defined as damaged and/or necrotic tissue, altered size and number of neurons and their normal distribution in the gray matter, small cell infiltration and cavitation (lack of tissue). All sections were analyzed for all areas of interest, considering always the same volume of tissue from the epicenter in both rostral and caudal directions.
Statistical Analysis
Data are expressed as mean percentages of the lesion and cavitation areas and volumes ± SEM. Statistical analysis of data among groups was performed using two-way ANOVA for behavioral assessment, one-way ANOVA or two-way repeated measures analysis for histology followed by Tukey's test where appropriate. Statistical significance was considered at P<0.05.
Results
Experiment 1
Behavioral Assessment
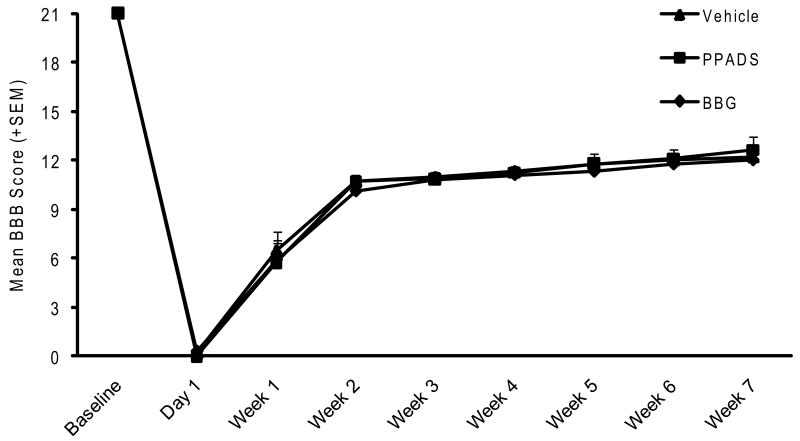
The locomotor open-field BBB animal scores of the animal groups from the first study treated with intraspinal injection of PPADS, BBG or vehicle are presented in Figure 1. In vehicle treated animals that received local injections of saline rostral and caudal to the injury site 30 minutes after mild trauma, severe motor deficits were seen at one day after injury. At one week and two weeks after injury, there was progressive recovery of locomotive function with BBB scores leveling off between 3 and 7 weeks after injury. At 7 weeks after mild injury (6.25mm), average BBB scores for the saline group were 12.18. These vehicle-treated scores are consistent with the BBB scores obtained at 28 days (Figure 5e) presented in the manuscript by Wang et al (2004). In that study, vehicle-treated rats showed BBB scores of around 12 at 28 days after SCI.
Figure 1.
Functional recovery evaluated by the BBB scale for 7 weeks after mild SCI. Vehicle, PPAGS or BBG was administered 30 minutes after SCI by direct injections rostral and caudal from the epicenter of the injury. No significant differences are seen in the pattern of locomotive recovery between the three experimental groups.
Similar to the vehicle treated animals, animals that were injected with either PPADS or BBG starting 30 minutes after trauma showed similar recovery patterns in terms of BBB scores. At 7 weeks after injury, BBB scores for PPADS animals were 12.6. For the BBG injected animals, the 7 week BBB score was 12.0. Thus, no significant difference was observed between the various treated groups at any time during the behavioral evaluation.
Histopathological Analysis
Histopathological analysis of tissue following mild contusive injury was based on area and volume values calculated by computer extrapolation from contour analysis of single sections (Figure 2). Quantitative data from the epicenter and throughout the injury spinal cord are presented in Figure 3. Lesion and cavity volumes were obtained in the saline, PPADS and BBG-treated animals. No significant difference between the percent of cavity volumes was obtained between the different groups. In addition, overall lesion volume was also calculated. Again, no significant differences were seen in overall contusion volumes at six weeks after contusive injury among the three treated groups.
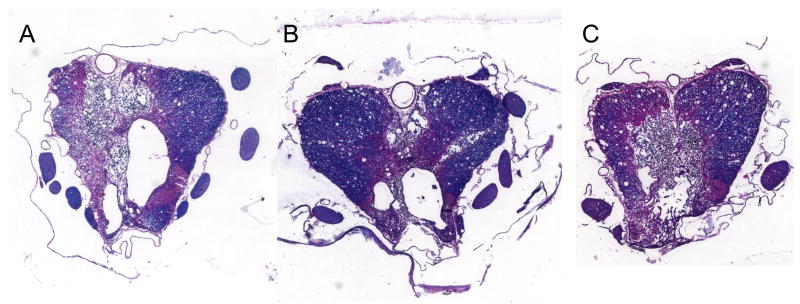
Figure 2.
Histopathological damage at 7 weeks after mild SCI is shown at the epicenter of the insult. Note patterns of cavity and lesion formation in gray and white matter structures of A) vehicle, B) PPADS and C) BBG treated rats. Sections are stained by hematoxylin and eosin and luxol fast blue.
Experiment 2
Behavioral Analysis
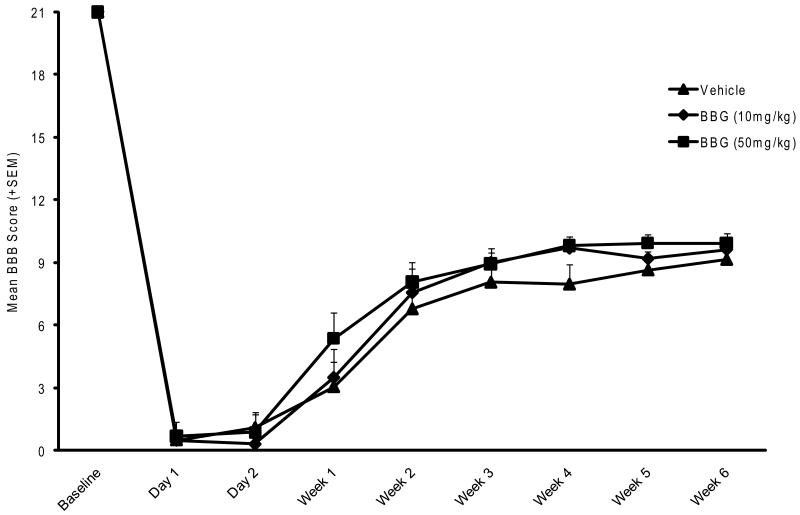
In this second study, the effects of two doses of BBG (10mg/kg or 50 mg/kg) given over a 3 day posttraumatic period were compared to vehicle treated rats after moderate SCI. Prior to analysis a comparison was made between vehicle treated SCI and SCI alone groups with no significant differences found. Therefore, these two groups were combined and indicated within the graph as the vehicle treated group. These BBB values were similar to the Peng et al paper (Figure 3A). The systemic administration of BBG resulted in color changes in the rat that slowly subsided over the course of one week. Thus, this change in color did not complicate the blind assessment of BBB over the course of six weeks. As shown in Figure 4, all rats demonstrate severe locomotor deficits at 24 and 48 hours after moderate SCI. Between 2 and 14 days after injury, animal scores improved and then gradually plateaued better 21 and 42 days. Some trends were observed with the 50mg/kg treated groups showing some accelerated recovery in motor function compared to the vehicle and 10 mg/kg BBG treated groups. However, by 42 days, no significant differences were seen between the 50mg BBG (9.9), 10 mg BBG (9.6) and the vehicle (9.1) treated SCI rats.
Figure 4.
Functional recovery in Experiment 2 by the BBB scale for six weeks after moderate SCI. BBG (10 or 50 mg/kg) or vehicle was administered systemically 15 min after trauma. No significant differences are seen in the pattern of locomotive recovery between the three experimental groups.
Histopathological Analysis
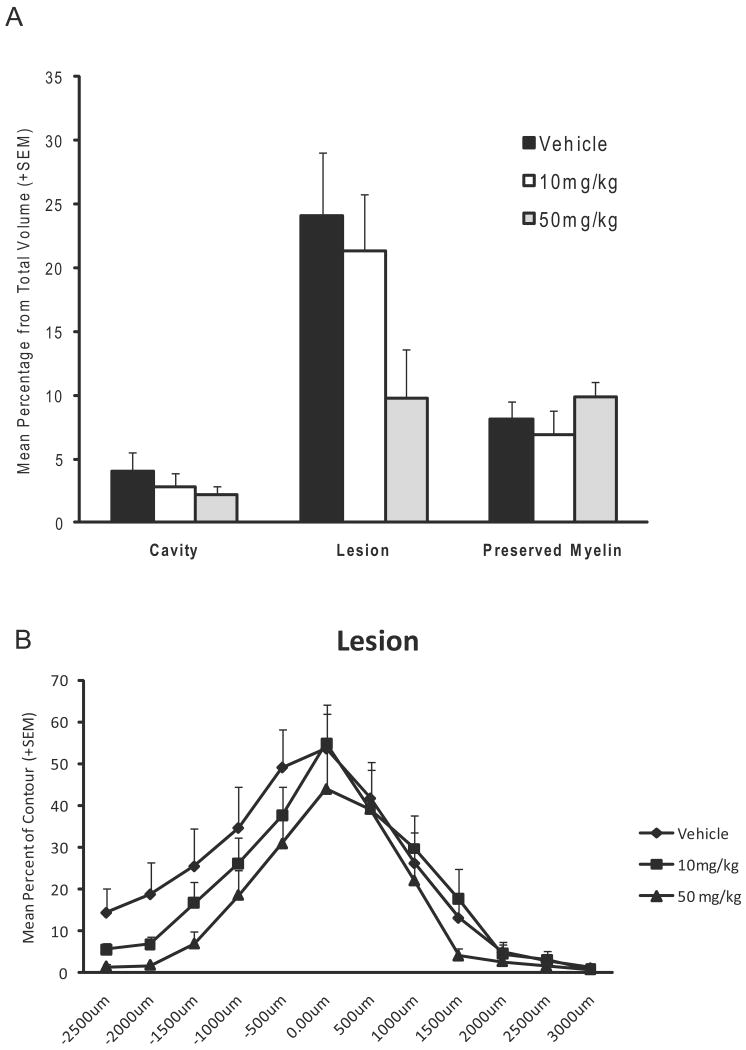
At 6 weeks after moderate SCI, spinal cords were processed and analyzed as previously described under Experiment 1. A well demarcated contusion was observed in all animals (Figure 5). Cavitation, lesion and preserved myelin were obtained in the 3 groups as percent of tissue from total volume between 50mg/kg BBG, 10mg/kg BBG and vehicle treated animals (Figure 6). Again, vehicle treated and SCI animals were compared across all histopathological outcomes and no significant differences were present. Therefore, these two groups were combined for further analysis. There was a strong trend for the 50mg/kg BBG animals to show a smaller lesion compared to vehicle animals but this was not significant (Figure 6A). Interestingly, when lesion area was analyzed across the rostral/caudal extent of the spinal cord, a strong trend was again seen in areas rostral to the epicenter (Figure 6B). Preserved myelin showed a non-significant pattern of preservation with the 50mg/kg group demonstrating more preserved myelin compared to the other groups.
Figure 6.
Quantitative histopathological assessment of lesion, cavity and preserved myelin volume and area percentages six weeks after moderate SCI from Experiment 2. A) Volume assessment of histopathological changes showed no significant differences in the qualitative outcome measures. There was a strong trend for the 50mg/kg group to have smaller lesions compared to the other two groups. B) An assessment of the lesion area across the rostral/caudal extent of the spinal cord clearly shows lesion sparing in the rostral part of the spinal cord for the 50mg/kg group.
Discussion
Recently, several studies have emphasized the beneficial effects of blocking P2X7 receptor activation in models of SCI (Wang et al., 2004; Peng et al., 2009; Cotrina & Nedergaard, 2009). Wang and colleagues (2004) first reported that administering non-selective and selective blockers of the P2X7 receptor including OxATP or PPADS in rats acutely after impact injury significantly improved behavioral performance in terms of motor function as well as diminished cell death in the peritraumatic zone. Because of the importance of ATP-gated channels in controlling calcium signaling in neurons and astrocyte (James and Butt, 2002; Khakh and North, 2006; Neary et al 2003; Wang et al 2004), this would appear to be an important target for therapeutic interventions targeting SCI.
In the study by Wang and colleagues (2004), spinal cord neurons were shown to express P2X7 purine receptors and exposure to ATP led to high frequency spiking, irreversible increases in cytosolic calcium and cell death (abnormal TUNEL staining). Blockage of these calcium-mediated effects by administering P2X7 receptor blockers early after contusive SCI also improved functional outcome (Wang et al., 2004). In a more recent but related study, the systemic administration of the P2X7 receptor blocker Brilliant Blue G was reported to significantly improve motor recovery and reduce histological damage following contusive SCI. In that study, BBG systemically administered 15 minutes after moderate SCI improved BBB scores, reduced anatomical damage and limited the degree of focal activation of astrocytes and microglia as well as neutrophil infiltration. Taken together, these studies indicate that the administration of selective P2X7 receptor antagonists following SCI improve outcome by targeting ATP-mediated signaling events and inflammatory cascades.
Based on these interesting results, we attempted to replicate some of the more clinically relevant post-injury treatment findings of these two studies, specifically targeting long-term behavioral and histopathological outcome measures after SCI. To this end, we first chose to follow the treatment procedures outlined in the Wang et al., (2004) study to determine whether direct injections of the selected P2X7 receptor blocker PPADS into the injured spinal cord would improve functional recovery and limit gray and white matter damage. We chose PPADS specifically and not OxATP because it is in a more selective blocker of P2X7 receptors. As summarized in this study, we observed no significant improvement in BBB recovery patterns with PPADS administration compared to vehicle treated animals.
Another important outcome measure that is commonly used to assess treatment strategies following SCI injury is the amount of tissue sparing that is seen chronically. Following the 7 week observational period, animals were perfusion-fixed for quantitative histopathological damage. We compared several aspects of tissue damage including lesion and cavity volumes. Compared to the non-treated saline group, no significant differences in histopathological damage were seen. Degrees of cord sparing as well as cavity area did not appear to differ in the treated and non-treated groups. Thus, our replication study did not show evidence for the local administration of a P2X7 receptor blocker improving long-term functional or histopathological outcome following mild SCI in rats.
More recently, an interesting study was presented showing that another selective P2X7 receptor antagonist improved outcome in a model of SCI (Peng et al., 2009). In that study, the P2X7 receptor antagonist Brilliant Blue G (Borzelleca et al. 1990) was administered systemically starting 15 minutes after moderate (12.5 mm) weight drop injury. In our initial experiment, we therefore attempted to determine whether or not injections of this selective P2X7R antagonist directly into the spinal cord would result in altered outcome following mild SCI. Similar to the results with PPADS, we did not show a significant improvement in BBB outcome or a reduction in overall histopathological damage with this intraspinal treatment. It would be interesting in future experiments to conduct a dose response study to determine if a more optimal dose of BBG would alter chronic outcome in this model of SCI.
In Experiment 2, we attempted to replicate the encouraging data published by Peng and colleagues (2009) that reported that the systemic administration of two different doses of BBG improved chronic outcome. In that study, treatment with BBG (10 or 50 mg/kg) improved BBB locomotor function and reduced tissue loss at 6 weeks post-trauma. At day 42, the BBB scores were 11.7 and 11.9 in rats receiving 10 or 50 mg/kg BBG respectively with vehicle treatment rats showing 9.4. In the present study, the BBB scores were 9.6 and 9.9 in rats receiving 10 or 50mg/kg BBG with control treatment rats showing 9.1. Thus, we were not able to replicate these behavioral results. In addition, total lesion size and degrees of atrophy did not show significant difference between the experimental groups. An interesting pattern of treatment related changes in lesion areas rostral to the lesion epicenter was seen with BBG treatment. In future studies, it might be important to investigate larger numbers of animals per group so that potential trends can be identified and statistical differences noted. In our experiment some animals had to be discarded because of autophagy of the hindlimb due to the femoral catheter placement.
It is evident that some procedural variables were different in our experiments compared to the Wang et al., and Peng et al., publications. First, we used a different strain of rat in the first replication study. In the second replication study however, Sprague-Dawley rats were used as according to Peng et al., 2009. Although both strains of rats are commonly used in SCI studies, the Fischer rat is routinely used in experimental SCI studies. This is due to the consistency of the spinal cord lesion that is produced in Fischer rats as well as increased ability to produce long-term survival in these animals. In addition, in the first experiment a different level of spinal cord injury was performed. In our study we chose to evaluate T10 because this is the level of injury that we routinely utilize to test therapeutic interventions. We do not feel that this is a major diversion from the original manuscript since similar patterns of acute behavioral severity and spontaneous recovery were observed in vehicle-treated rats between the two studies with our contusive injury in terms of open locomotive behavioral assessment. Thus, injury severity and functional outcomes in the vehicle treated animals appear to be similar in the two studies.
Another variable that differed between the two studies was the method of histopathologically evaluating the treated and non-treated groups. In our studies we utilized paraffin histology in contrast to cryosectioning. This method of processing tissue for histopathological analysis is commonly used in many laboratories that evaluate the morphological consequences of SCI. We also used hematoxylin/eosin and luxol fast blue to assess lesion volume and cavity in contrast to Kluver-Barrera procedure for myelin staining. Both staining procedures are accepted methods of identifying white matter sparing and most likely did not interfere with our ability to accurately assess histopathological outcome. In the Wang and colleagues study (2004), chronic contusion volume was not quantitated. Instead, the frequency of TUNEL-positive cells in peritraumatic zone at 24 hrs after SCI was reported. Although, numbers of damaged cells at an early posttraumatic period is an informative outcome measure especially when considering mechanisms of action, we felt that it would be more instructive to assess overall lesion and cavity volumes because these chronic outcome measures are commonly emphasized in treatment studies. Also, the degree of white matter injury or sparing is commonly felt to correlate closely with the behavioral findings assessed with the BBB open-motor scale (Basso et al 1995). In our first replication study, no significant differences in lesion or cavity volumes were documented with the treatment strategies. Thus, although we did not evaluate patterns of dying cells, our study showed no evidence with this treatment paradigm reduced overall pathology after mild SCI.
In the second experiment, more chronic histopathological outcome measures were assessed in both our replication study and the Peng et al., manuscript. In both studies, volumes of tissue injury were quantitated at 6 weeks after moderate SCI. Peng and colleagues reported a loss of tissue which was calculated by the sum of volumes of tissue injury and atrophy which was reduced in BBG treated animals compared to vehicle controls. Interestingly in that study, degrees of atrophy assessed by measuring the area between the reduced actual border of the spinal cord and a straight line connecting the rostral and caudal sides in the longitudinal section, and not lesion volume alone was reduced significantly with BBG administration. In our study, lesion volumes and patterns of cavity formation calculated in coronal sections were not attenuated by BBG administration. Thus, although strategies for lesion volume assessment were not exactly the same in both studies, we could not demonstrate a long-term beneficial effect of systemic administration of BBG on either lesion volume or cavity formation. It should be noted that a reduction in overall lesion volume with 50mg/kg BBG was seen. In this regard, a pattern of reduced lesion area was noted with the BBG treatment rostral to the lesion epicenter. This response may signal a regionally specific treatment effect on P2X7 receptor activation that should be pursued in future studies. Also, it is possible that an alternative dosing schedule of BBG may be efficacious in significantly attenuating these outcome measures.
Evidence is emerging regarding the importance of P2X7 receptor inhibition on some of the pathophysiological consequences of CNS injury (Le Feuvre et al., 2003; Cotrina & Nedergaard, 2009). Various studies have reported P2X7 activation in several experimental conditions and provided data on ATP-mediating signaling following ischemic and traumatic insults (James and Butt, 2002; Le Feuvre et al., 2002, 2003; Franke et al., 2004; Davalos et al., 2005; Khakh & Norton, 2006; Melani et al., 2006). Indeed, recent studies after experimental SCI emphasize the specific actions of ATP and abnormal P2X7 receptor activation and report that this cell signaling pathway appears to be critical in several pathological responses to trauma (Cotrina & Nedergaard, 2009). Based on the present findings, we would suggest that additional SCI studies showing robust effects on clinically relevant outcome measures using more delayed treatment strategies be demonstrated before the implementation of clinical trials in acutely injured SCI patients.
Research Highlights.
Replication study of previously published spinal cord injury data
Inhibition of P2X7 receptor on outcomes after contusive spinal cord injury
Discussion of difficulties in replicating published work
Additional preclinical studies needed to translate this therapy into patients
Acknowledgments
We would like to thank Ileana Oropesa, Denise Koivisto, Rosa Abril, and Monica Stagg for animal and behavioral analysis; Paulo Diaz for performing the contusion injuries. We would also like to thank Ronald Zambrano for assisting in the quantitation of the contusion volumes, Vermilla Rusakowa for tissue processing and Jessie Truettner for helping to prepare the pharmacological agents for a demonstration. We thank Mr. Jeremy Lytle for expert editorial assistance and word processing. This work was supported by funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Facilities of Research Excellence and Spinal Cord Injury (FORE-SCI) under contract #NO1-NS-3-2352, and The Miami Project to Cure Paralysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann Emerg Med. 1993;22:987–992. doi: 10.1016/s0196-0644(05)82739-8. [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–33. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bianchi DM, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Borzelleca JF, Depukat K, Hallagan JB. Lifetime toxicity/carcinogenicity studies of FD & C Blue No. 1 (brilliant blue FCF) in rats and mice. Food Chem Toxicol. 1990;28:221–234. doi: 10.1016/0278-6915(90)90034-k. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63:686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signaling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–60. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–32. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–53. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol. 2002;447:261–269. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- Le Feuvre RA, Brough D, Touzani O, Rothwell NJ. Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J Cereb Blood Flow Metab. 2003;23:381–384. doi: 10.1097/01.WCB.0000048519.34839.97. [DOI] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volontè C, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;32:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon A, Marcillo A, Pabon D, Bramlett HM, Bunge MB, Dietrich WD. A reassessment of erythropoietin as a neuroprotective agent following rat spinal cord compression or contusion injury. Exp Neurol. 2008;213:129–136. doi: 10.1016/j.expneurol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Remy M, Thaler S, Schumann RG, May CA, Fiedorowicz M, Schuettauf F, Grüterich M, Priglinger SG, Nentwich MM, Kampik A, Haritoglou C. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol. 2008;92:1142–1147. doi: 10.1136/bjo.2008.138164. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]