Abstract
Purpose
To estimate the prevalence and associated risk factors of primary open-angle glaucoma (POAG) in a rural population in northeast China.
Methods
A population-based survey was conducted within Bin County, Harbin of northeast China. Glaucoma was diagnosed using International Society of Geographical and Epidemiological Ophthalmology criteria. All the subjects underwent a complete ophthalmic examination.
Results
A total of 4956 (86.0%) of 5762 subjects aged 40 years or older were examined. The mean intraocular pressure (IOP) of right eyes was 14.0 (95% confidence interval (CI), 13.9 to 14.1) mm Hg. The prevalence of POAG was 0.71% (35/4956, 95% CI, 0.47 to 0.93). In these POAG subjects, 17 (48.6%) had elevated IOP >21 mm Hg in either eye, 3 (8.8%) participants had been treated by laser trabeculoplasty or trabeculectomy and were known to have POAG. Vision impairment to varying degrees was present in 20 subjects (58.8%) with 1 subject blind in both eyes and 8 subjects blind in one eye. On multivariate analysis, age, family history of glaucoma, systemic hypertension, and IOP were regarded as significant independent risk factors.
Conclusions
POAG is a disease of serious consequence and of low diagnosis and treatment rates in rural northeast China. Age, family history of glaucoma, systemic hypertension, and IOP remain as significant independent risk factors for POAG.
Keywords: China, population-based study, prevalence, primary open-angle glaucoma, risk factors
Introduction
Glaucoma is the second most common cause of blindness as estimated from blindness certification,1 and can be classified into primary open-angle glaucoma (POAG), primary angle-closure glaucoma (PACG), or can be secondary to other pathology.2
It has been estimated that there will be 79.6 million people with open-angle glaucoma (OAG) and angle-closure glaucoma (ACG) in 2020, and of these, 74% will have OAG. Given the large population and future growth in China, 11.7 million of those with OAG in 2020 will be Chinese.3 Recently, surveys regarding glaucoma have been performed in Liwan4 and Beijing.5 However, the vast range of geographic, the climatic, and cultural differences present throughout China suggest that these factors may have an important role in estimating the prevalence of conditions like OAG. In particular, the diversity present and absence of data available in the northeast area of the world's most populous country imply that such a survey for this region would represent a worthwhile endeavor.
In this article, we report the prevalence of POAG, according to the definitions suggested by the International Society of Geographical and Epidemiological Ophthalmology (ISGEO),2 and the possible associated risk factors in a rural population in the North of China.
Materials and methods
Study population
The study was conducted within the town of Changan in Bin County of Harbin, an area lying in northeast China. The township encompassed 156.8 km2 and comprised 18 contiguous villages of approximately equal size. Each village was divided into 8–14 second-order villages. In total, Changan comprised 183 second-order villages. The total population of these villages was approximately 32 800 persons, according to the 2000 National Census Report of China. Of the residents, 48.88% were women, and for residents older than 40 years, women accounted for 56.23%.
Participants were selected using a random, stratified, cluster sampling process. All persons aged 40 years or older who had been a resident in the selected village for at least 6 months were eligible for inclusion. The 18 villages were numbered in clockwise order. From 18 villages, 12 were randomly enumerated by computer. Subsequently, 84 second-order villages within the 12 villages (109 second-order villages) were randomly enumerated, generating a total sample population of 5762 people.
Clinical examination
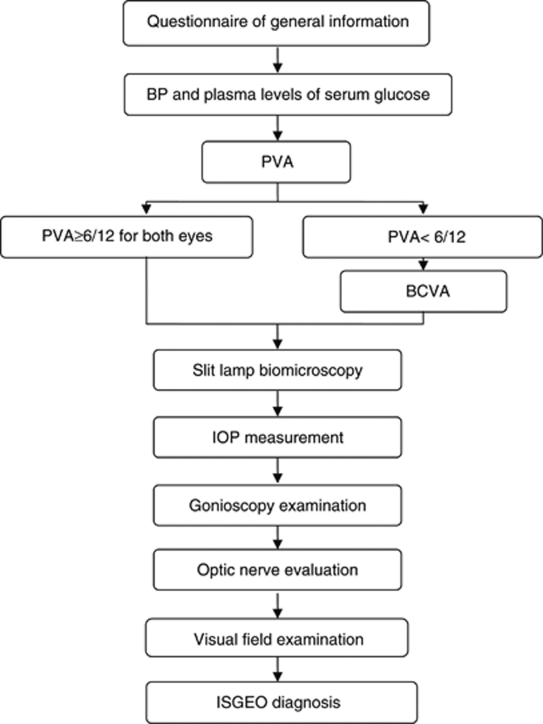
A single survey team conducted the entire study. Each team member was assigned specific tasks and received additional training in the study procedures as shown in Figure 1.
Figure 1.
Participant flow chart in Bin glaucoma survey.
A trained health worker administered a standard questionnaire to collect details of demographic information, life style, general medical history, and ophthalmic history. Blood pressure, measurement of blood glucose, and detailed ophthalmic examinations were performed on all subjects.
Visual acuity (VA) was recorded using a 4-m Early Treatment Diabetic Retinopathy Study logarithm of minimum angle of resolution E chart (Precision Vision, Villa Park, IL, USA). An optometrist assessed the best-corrected VA (BCVA) by using the results of a handheld autorefraction (Topcon Corporation, Model RM-8000B, Tokyo, Japan) with necessary subjective refinement in those subjects with presenting VA of <6/12.
Slit lamp biomicroscopy was performed by two experienced glaucoma specialists to identify abnormalities of the anterior segment. Under topical anesthesia using 0.5% proparacaine, intraocular pressure (IOP) was measured with a handheld Perkins applanation tonometer (HA-2, Kowa Xinghe, Japan) using 0.5% proparacaine and fluorescein staining of the tear film. The right eye was measured first and one reliable measurement was recorded for each eye. The instrument was calibrated before use on each day of testing.
Gonioscopy was performed on all subjects with a Goldmann-type one-mirror lens (Haag Streit, Bern, Switzerland) in dim ambient illumination with a shortened slit that does not fall on the pupil. Dynamic examination was performed after the static gonioscopy of four quadrants was completed. The angle was graded using the Scheie's system, and the peripheral iris contour, degree of trabecular meshwork pigmentation, peripheral anterior synechiae, and other angle abnormalities were recorded.
Stereoscopic evaluation of the optic nerve head was performed using a +78 diopter (D) lens by the same two specialists. Standard disc images for vertical cup-disc ratio (VCDR) from 0.1 to 1.0 in 0.1 increments were used in the grading process. The margins of the cup were defined by stereoscopic view as the point of maximum inflection of the vessels crossing the neuroretinal rim.4 If the stereo view was not satisfactory, the pupil was dilated using 1% tropicamide (Mydriacyl; Alcon, Puurs, Belgium) plus 2.5% phenylephrine (Alcon), provided the participant did not have closed angles with raised IOP. The VCDR was measured and recorded. Presence of any notching, splinter hemorrhages, and peripapillary atrophy were documented. A high level of agreement (κ=0.86) was present between the two ophthalmologists regarding the VCDR (0.7 or more).
A provisional diagnosis of suspected glaucoma was made when the subject had one or more of the following conditions: IOP≥21 mm Hg in either eye; VCDR≥0.7 in either eye or VCDR asymmetry≥0.2; and focal thinning, notching, or a splinter hemorrhage.6 All subjects were advised to get a static visual field test (THG-10-BA210-013 Octopus, Interzeag AG, Bern-Köniz, Switzerland) with G1 program and TOP strategy.
Diagnostic definitions
Cases of glaucoma were defined using the ISGEO criteria.2 Glaucoma was classified in accordance with three levels of evidence.
Visual fields were judged acceptable for analysis if fixation losses <20%, false positives <33%, and false negatives <33% were present. After excluding the superior four points and the four points immediately adjacent to the blind spot, abnormal visual fields were defined when size was ≥18° × 12° and depth was ≥10 dB.7 When all visual field results were completed of the 226 subjects (452 eyes), 88.1% (398 eyes) were considered as producing reliable data.
Blindness was defined as the BCVA of <20/400 and/or visual field constriction of <10° from fixation in the better eye.
The division of cases of glaucoma into POAG and PACG was based on the gonioscopic finding of a broad angle. POAG was diagnosed if the criteria outlined above for categories 1–3 were met in one or both eyes, ≥90° of posterior (usually pigmented) trabecular meshwork was visible on static gonioscopy, and no secondary cause for glaucoma was present.2 Primary angle-closure suspect (PACS) was diagnosed based on trabecular meshwork was not seen for 270° or more on gonioscopy. Primary angle-closure (PAC) was PACS with evidence of peripheral anterior synechiae and/or elevated IOP without glaucomatous damage of the optic disc. PACG was PAC with evidence of glaucoma as defined.2 Secondary glaucoma was diagnosed if the criteria for categories 1–3 were met and a secondary cause was evident.
We chose 12 potential risk factors for analysis. The two potential risk factors considered in the analysis were defined as follows:
Systemic hypertension: current use of systemic antihypertensive medications or a measured systolic blood pressure of 140 mm Hg or greater and/or a diastolic blood pressure of 90 mm Hg or greater.8
Diabetes mellitus: a nonfasting or postload serum glucose level of 200 mg/dl (11.1 mmol/l) or higher or the use of antidiabetic medication.9
Ethics
The study was approved by the Ethics Committee of the Second Affiliated Hospital, Harbin Medical University. Consent for participation was obtained from the head of each village before commencement of the survey, and written informed consent was obtained from all willing participants. The study was conducted in accordance with the Declaration of Helsinki.
Statistics
The database was established with Epidata3.0 (EpiData Association, Odense, Denmark). Statistical analysis was performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA) programs. Data are presented as prevalence and odds ratios (OR) with corresponding 95% confidence intervals (CIs). Age- and sex-specific prevalence of POAG and its 95% CIs were calculated. Univariate and multivariate logistic regression models were used to identify risk factors of POAG. The probable risk factors for POAG were analyzed by the univariate logistic regression. All risk factors associated with POAG on univariate analysis were modeled using multivariate logistic regression analysis. To find the best model, a forward elimination stepwise procedure was carried out in a way that the factor would be brought into the analysis if the corresponding P-value was <0.2. A value of P<0.05 was defined as statistically significant.
Results
A total of 5762 participants were considered eligible for this study (2544 men/44.2% and 3218 women/55.8%), and 4956 (2228 men and 2728 women) were examined in the clinic, therefore the response rate was 86.0%. Distribution of their age, sex, and educational level are shown in Table 1.
Table 1. Characteristics of the subjects attending for clinical examination.
| 40–49 | 50–59 | 60–69 | ≥70 | Total (%) | |
|---|---|---|---|---|---|
| Man | 612 | 688 | 539 | 389 | 2228 (44.96) |
| Woman | 819 | 933 | 594 | 382 | 2728 (55.04) |
| Educational level | |||||
| University education | 3 | — | — | — | 3 (0.06) |
| College education | 3 | 7 | 6 | 1 | 17 (0.34) |
| High schooling | 69 | 55 | 14 | 2 | 140 (2.82) |
| Middle schooling | 409 | 225 | 88 | 11 | 733 (14.79) |
| Primary Schooling | 708 | 748 | 590 | 81 | 2127 (42.92) |
| Illiterate | 239 | 586 | 435 | 676 | 1936 (39.06) |
| Total for age (%) | 1431 (28.87) | 1621 (32.71) | 1133 (22.86) | 771 (15.56) | 4956 (100.00) |
Tables 2 and 3 provide data on the IOP in right eyes and VCDR parameters for the population without glaucoma. Of the 4956 participants, IOP values were missing from 13 right eyes and 1 left eye. VCDR values were missing from 165 right and 153 left eyes. These data were absent as a result of media opacities, trauma, and inflammation or refusal to be examined (Table 4). In total, the optic discs of 4854 subjects were measured in at least in one eye. IOP declined with age in normal participants.
Table 2. IOP in normal participants (right eyes)a.
| Age | Number | Median (mm Hg) | Mean (mm Hg) (95% CI) | 97.5th Centile | 99.5th Centile |
|---|---|---|---|---|---|
| 40∼ | 1429 | 15 | 14.8 (14.7–15.0) | 21 | 25 |
| 50∼ | 1601 | 14 | 14.3 (14.2–14.5) | 21 | 25 |
| 60∼ | 1098 | 13 | 13.4 (13.2–13.5) | 20 | 25 |
| ≥70 | 719 | 12 | 12.5 (12.3–12.7) | 20 | 25 |
| Total | 4847 | 14 | 14.0 (13.9–14.1) | 21 | 25 |
Abbreviation: IOP, intraocular pressure.
Defined as those with available vertical CDR data and excluding eyes with definitive glaucomatous field defect.
Table 3. VCDR and VCDR asymmetry in normal participants.
| Number | Median | Mean (95% CI) | 97.5th Centile | 99.5th Centile | |
|---|---|---|---|---|---|
| Right VCDR | 4751 | 0.30 | 0.313 (0.311–0.315) | 0.5 | 0.6 |
| Left VCDR | 4732 | 0.30 | 0.312 (0.310–0.314) | 0.5 | 0.6 |
| VCDR asymmetrya | 4712 | 0.00 | 0.012 (0.011–0.013) | 0.1 | 0.3 |
Abbreviation: VCDR, vertical cup-disc ratio.
Absolute value of the difference in VCDR in two eyes.
Table 4. The reason for missing data of IOP and VCDR (n=subjects).
| IOP | VCDR values | |
|---|---|---|
| Media opacities | 0 | 98 |
| Trauma | 9 | 21 |
| Inflammation | 1 | 42 |
| Refusal to be examined | 3 | 9 |
The distributions of IOP and VCDR parameters for glaucoma cases are shown in Table 5. VCDR values were missing from 1 right eye.
Table 5. IOP, VCDR and VCDR asymmetry in glaucoma cases.
|
IOP |
VCDR |
VCDR asymmetrya | |||
|---|---|---|---|---|---|
| Right | Left | Right | Left | ||
| Number | 35 | 35 | 34 | 35 | 34 |
| Median (mm Hg) | 20 | 20 | 0.60 | 0.60 | 0.00 |
| Mean (95% CI) (mm Hg) | 20.17 (18.17–22.18) | 19.17 (17.24–21.10) | 0.64 (0.59–0.68) | 0.58 (0.52–0.64) | 0.10 (0.05–0.14) |
Absolute value of the difference in VCDR in two eyes.
Table 6 summarizes the prevalence, age, and sex distribution of POAG.
Table 6. Prevalence, distribution of age and sex for POAG.
| Age | Case detection rates |
|||||
|---|---|---|---|---|---|---|
|
Men |
Women |
Total |
||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | |
| 40∼ | 0 | — | 0 | — | 0 | — |
| 50∼ | 2 (0.29) | 0.03∼1.03 | 3 (0.32) | 0.06∼0.94 | 5 (0.31) | 0.04∼0.57 |
| 60∼ | 6 (1.11) | 0.22∼1.97 | 7 (1.18) | 0.31∼2.03 | 13 (1.15) | 0.52∼1.75 |
| ≥70 | 8 (2.06) | 0.64∼3.41 | 9 (2.36) | 0.82∼3.82 | 17 (2.20) | 1.15∼3.19 |
| Total | 16 (0.72) | 0.36∼1.06 | 19 (0.70) | 0.38∼1.00 | 35 (0.71) | 0.47∼0.93 |
The prevalence of POAG in any category within at least one eye was 0.71% (95% CI, 0.47 to 0.93). This prevalence increased with age in both women and men, and was similar in male (0.72%) and female (0.70%) POAG patients. Suspected glaucoma on the basis of optic disc exam alone was reported for 203 subjects (203/4854, 4.2%), 187 subjects (187/4854, 3.9%) were found to have peripapillary atrophy, and 8 subjects (8/35, 22.9%) were diagnosed POAG. Splinter hemorrhages were observed in 27 subjects (27/4854, 0.6%) and 4 subjects (4/35, 11.4%) were POAG. Diagnosis is as based on category 1 comprised 26 subjects (74.3%), 4 subjects (11.4%) based on category 2, and 5 subjects (14.3%) based on category 3. In all, 17 (48.6%) participants had elevated IOP of >21 mm Hg in either eye, and only 3 (8.6%) participants had been treated by laser trabeculoplasty or trabeculectomy. Varying degrees of vision impairment was observed in 21 (60.0%) of the subjects. The prevalence of bilateral blindness in the patients with glaucoma was 2.9% (1/35) and unilateral blindness was 22.9% (8/35). Table 7 shows the prevalence of POAG in selected population-based studies as resulting from the ISGEO criteria.
Table 7. Prevalence of POAG in selected population-based studies used ISGEO criteria.
| Author | Location | Setting | Age (years) | Prevalence, % |
|---|---|---|---|---|
| He et al4 | Liwan, Guangzhou | Urban | ≥50 | 1.8 |
| Shen et al42 | Singapore Malay | Urban | ≥40 | 2.5 |
| Casson et al12 | Meiktila, Myanmar | Rural | ≥40 | 2.0 |
| Raychaudhuri et al11 | Calcutta, West Bengal | Rural | ≥50 | 3.0 |
| Vijaya et al6 | Chennai, South India | Rural | ≥40 | 1.62 |
| Rahman et al13 | Dhaka, Bangladesh | Rural | ≥35 | 2.5 |
| Bourne et al7 | Rom Klao, Thailand | Urban | ≥50 | 2.3 |
| Rotchford et al43 | Temba, South Africa | Urban | ≥40 | 2.9 |
| Rotchford et al44 | Zulu, South Africa | Rural | ≥40 | 2.7 |
| Present study | Bin, China | Rural | ≥40 | 0.71 |
Abbreviation: ISGEO, International Society of Geographical and Epidemiologic Ophthalmology.
Univariate and multivariate logistic regression analyses of risk factors for POAG are shown in Tables 8 and 9. Age, educational level, family history of glaucoma, diabetes, systemic hypertension, and IOP were significant independent risk factors as derived from the univariate analysis. After multivariate analysis, age, family history of glaucoma, systemic hypertension, and IOP were identified as significant independent risk factors.
Table 8. Univariate logistic regression analysis of risk factors for POAG.
| Variable | ß | SE | Wald x2 | P | Adjusted OR (95% CI) |
|---|---|---|---|---|---|
| Sex | 0.03 | 0.34 | 0.01 | 0.924 | 1.03 (0.53∼2.01) |
| Age | 1.10 | 0.20 | 30.17 | <0.001 | 2.99 (2.02 ∼ 4.42) |
| Educational level | 1.24 | 0.33 | 13.94 | <0.001 | 3.47 (1.81∼6.66) |
| Family history | 3.83 | 0.36 | 113.26 | <0.001 | 45.97 (22.71∼93.02) |
| Diabetes | 1.84 | 0.46 | 16.29 | <0.001 | 6.30 (2.58∼15.40) |
| Systemic hypertension | 1.01 | 0.34 | 8.90 | 0.003 | 2.75 (1.42∼5.36) |
| Migraine | 0.89 | 0.60 | 2.19 | 0.139 | 0.41 (0.13∼1.34) |
| Blood type | |||||
| A and B | 0.18 | 0.39 | 0.21 | 0.644 | 1.20 (0.56∼2.56) |
| A and AB | 0.21 | 0.61 | 0.12 | 0.733 | 0.81 (0.25∼2.67) |
| A and O | 0.47 | 0.36 | 1.73 | 0.188 | 0.63 (0.31∼1.26) |
| Smoking | 0.24 | 0.17 | 1.94 | 0.164 | 1.27 (0.91∼1.79) |
| Alcohol | 0.12 | 0.15 | 0.60 | 0.439 | 0.89 (0.66∼1.20) |
| Intraocular pressure | 3.00 | 0.34 | 76.43 | <0.001 | 20.13 (10.27∼39.45) |
Table 9. Multivariate logistic regression analysis of risk factors for POAG.
| Variable | ß | SE | Wald x2 | P | Adjusted OR (95% CI) |
|---|---|---|---|---|---|
| Age | 0.72 | 0.23 | 9.60 | 0.002 | 2.06 (1.31∼3.26) |
| Family history | 2.68 | 0.45 | 35.62 | <0.001 | 14.58 (6.05∼35.15) |
| Systemic hypertension | 0.90 | 0.38 | 5.58 | 0.018 | 2.45 (1.17∼5.16) |
| Intraocular pressure | 2.00 | 0.40 | 24.79 | <0.001 | 7.39 (3.36∼16.24) |
Discussion
This study, to our knowledge, is the first population-based study from northeast China to classify OAG based on the ISGEO criteria2 and therefore can be compared with other studies that use the same criteria.
Of the 5762 people aged 40 years or older in this study, the participant rate was 86.01%. This response rate is reasonable for population-based surveys and similar to the studies of the Urban South Indian Study (80.2%),10 West Bengal Study (83.1%),11 and Mandalay Division (83.7%),12 but higher than that of Dhaka (66%).13 The high response rate suggests the data obtained are representative of the population.
The prevalence of POAG in our survey was 0.71% (95% CI, 0.47–0.93). When compared with that of studies conducted in other countries, the prevalence in northeast China was substantially lower than that of Bangladesh (2.1%),13 Australia (3.0%),14 Japan (3.6%),15 and Singaporean Chinese (3.2%),16 but slightly higher than Mongolia (0.5%).17 Although no clear explanation exists for these differences, genetic, environmental, and/or methods used for detecting and defining POAG would seem to represent likely sources for these variations.
When considering regions inside China, however, the prevalence in our survey remained lower than Beijing (1.76%, 2004)5 and urban Guangzhou (2.1%, 2006),4 but higher than the Shunyi district of Beijing (0.29%, 2002).18 One reason for this variation observed within China may be the differences in the ages of the study participants. In the Guangzhou survey, subjects were aged 50 years or older. As the prevalence of POAG shows an increasing trend with age, the younger age profile of our population could have resulted in an underestimation of its prevalence. In addition, Guangzhou is located 3430 km from our study area where climatic and ethnic conditions are very different. The inhabitants of northeast China belong to the Han nationality, Mongolian race and are of the north-Asia type, while in south China, the Malay type are mostly a transition group with origins from Far Eastern to South Asian. After analyzing the relationship between POAG prevalence and temperature, Weale19 hypothesized that high temperatures can accelerate the onset, and increase the prevalence of POAG. While conclusions generated from this study can be subject to ecologic fallacies, the prevalence of POAG in our study is in line with the concept that lower rates are associated with colder climates. As for the two surveys in Beijing, the glaucoma criteria adopted was different from ours and the mean IOP in normal participants in the 2004 Beijing investigation (∼15–16 mm Hg) was higher than ours (14.0 mm Hg), which can lead to the differences reported in POAG prevalence.
In our study, some subjects who had media opacities and were not examined for disc assessment could also have had glaucoma. Additionally, we used strict criteria for defining POAG, which could have excluded a large group of possible POAG cases from the analyses. This approach potentially reduced the number of cases but led to fewer false positives. Therefore, for these reasons the overall estimate for POAG prevalence in our study may be underestimated.
Conversely, we classified glaucoma as POAG in the case of media opacity and IOP exceeding the 99.5th percentile or evidence with the history of previous glaucoma filtering surgery. In fact, subjects meetings this criteria were not necessarily POAG. Therefore, as based on this definition, our survey may have over-estimated POAG prevalence to some extent.
In our study, the prevalence of PACG, PAC and PACS were 1.57% (95% CI, 1.469–1.671), 1.33% (95% CI, 1.236–1.424), and 4.68% (95% CI, 4.541–4.819), respectively.20 The ratio of the prevalence of PACG to POAG was in excess of 2 : 1. This high ratio in our study suggested that, some of the cases may have both mechanisms but be diagnoses as angle closure, thus reducing those who have only OAG.
Applanation tomometer as used in this study is a relatively accurate method for IOP measurement. In our study, we only present results from right eyes, and the mean IOP in our normal subjects was 14.0 (95% Cl, 13.9–14.1) mm Hg. This is broadly consistent with mean IOP in other population-based studies that had used the same criterion or same method for measuring IOP such as 14.1 mm Hg in Japan,21 13.5 mm Hg in China,18 and 12.7 mm Hg in Mongolia.17 These values are much lower than those reported in white populations.14, 22 Similarly, Lin et al23 have concluded that the mean IOP values in elderly Chinese population were lower than in Cavcasians.
Most European and American studies have reported a positive association between age and IOP.24, 25, 26 While in our study this relationship was not significant.27 In contrast, Chinese,18 Korean, and Japanese studies,21, 28, 29, 30, 31 as well as a recent Australian study,32 have reported a negative association. Like that of these latter studies, which indicate a negative relationship, our data showed that the mean IOP in non-glaucomatous eyes declined with age.
Results from several studies have shown that POAG prevalence of increases with age.4, 5, 6, 18 Our findings show a similar trend in that POAG was more prevalent among older subjects.
Some studies have shown a higher prevalence of POAG in men,33 but others have shown no gender difference,34 and the Blue Mountains Eye Study35 reported a higher prevalence of glaucoma in women. In our study, we found no difference in prevalence between male and female subjects.
As based on the univariate and multivariate logistic regression analysis in our survey, we report that age, family history of glaucoma, systemic hypertension, and IOP remained as significant independent risk factors for POAG.
Old age had often been shown to be associated with increased risk of POAG, which is consistent with our findings.
Family history was found to be strong risk factor for POAG in this study as revealed from data derived both from history and ophthalmic examination. Among persons with less education, knowledge of glaucoma is low, so family history may be underestimated. The importance of this risk factor has also been highlighted in other studies.
IOP was another strong risk factor for POAG in our study. This finding is in agreement with virtually every study performed. However, only 17 (48.6%) participants with glaucoma in our survey had elevated IOP >21 mm Hg in either eye. Our results reinforce the opinion that POAG diagnosis cannot be based only on the level of IOP, but higher IOP represents an important risk factor for POAG. Accordingly, normal or low tension glaucoma should not be ignored.
The relationship between as the vascular system, in particular systemic hypertension, and POAG remains controversial. Conclusions resulting from data obtained in several population-based studies have failed to arrive at any consensus regarding the relationship between BP and OAG.36, 37, 38, 39, 40 Our results suggest that systemic hypertension might be a possible risk factor for the development of POAG.
According to the BCVA, glaucoma was the second leading cause of visual impairment and blindness in our study.41 The causes of glaucoma blindness in total 13 participants were PACG (11/13), POAG (1/13), and secondary to other pathology (1/13).41 Poor medical treatment consciousness and poor medical treatment level in rural area, which resulted in that the subjects did not receive timely treatment or did not further consult with a doctor after treatment may explain in part our finding that high prevalence of PACG blindness. Among all 35 POAG patients in our study, the proportion of high tension glaucoma was 48.6%. Only three subjects (8.6%) were known to have POAG and had been treated, whereas for the remainder (91.4%) the disease was diagnosed during the course of study. This low rate of diagnosis and low treatment rate are a matter of concern from a public health point of view. Some factors that can contribute to these unsatisfactory rates include the lack of facilities for comprehensive ophthalmic examination for the population, the widespread use of inappropriate eye examination techniques, and/or lower socioeconomic status and disease awareness. In such a situation, a silent disease like glaucoma especially POAG can go unnoticed.
Many changes have occurred in the field of glaucoma in recent years. These include modifications in the definition of the disease itself, new instruments for glaucoma diagnosis and monitoring, safer and more efficient medications, and laser treatments. These advances will positively influence the design of future glaucoma screening programs and should now permit such a program of diagnosis and treatment to be both feasible and cost effective. Collaboration among diverse specialists, including community health-care experts, glaucoma specialists, optometrists, and health economists will be integral in organizing and implementing an effective glaucoma screening strategy. Information and prevention campaigns to raise glaucoma awareness in high-risk populations could also have a positive impact on visual health and aid in identifying areas in need for future investigation.
In conclusion, POAG is a disease of low diagnosis and treatment rates in rural northeast China. The overall prevalence of POAG was 0.71% in this population. Age, family history of glaucoma, systemic hypertension, and IOP remained as significant independent risk factors for POAG in rural northeast China. The increase in prevalence with age is a cause of concern, as this segment of the Chinese population is expected to increase dramatically over the next few decades. These rates of prevalence must improve significantly, to reduce ocular morbidity, and minimize blindness because of glaucoma.

Acknowledgments
We are grateful to all volunteers for their participation and the government of Bin County for support, organization and help in this investigation. This study was supported by Special Fund for Major Research Projects (2008-02) and PhD Research Fund (BS2010-16) of the Second Hospital of HMU, China.
The authors declare no conflict of interest.
References
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He MG, Foster PJ, Ge J, Huang W, Zheng Y, Friedman DS, et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan district, Guangzhou. Invest Ophthalmol Vis Sc. 2006;47:2782–2788. doi: 10.1167/iovs.06-0051. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen JH, Li JJ, Luo L, Yang H, Zhang RX, et al. The prevalence and its screening methods of primary open angle glaucoma in defined population based study of rural and urban in Beijing. Chin J Ophthalmol. 2004;40:726–732. [PubMed] [Google Scholar]
- Vijaya L, George R, Paul PG, Baskaran M, Arvind H, Raju P, et al. Prevalence of open-angle glaucoma in a rural south Indian population. Invest Ophthalmol Vis Sci. 2005;46:4461–4467. doi: 10.1167/iovs.04-1529. [DOI] [PubMed] [Google Scholar]
- Bourne RR, Sukudom P, Foster PJ, Tantisevi V, Jitapunkul S, Lee PS, et al. Prevalence of glaucoma in Thailand: a population based survey in Rom Klao District. Br J Ophthalmol. 2003;87:1069–1074. doi: 10.1136/bjo.87.9.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya L, George R, Arvind H, Baskaran M, Paul PG, Ramesh SV, et al. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol. 2006;124 (3:403–409. doi: 10.1001/archopht.124.3.403. [DOI] [PubMed] [Google Scholar]
- Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam Study. Arch Ophthalmol. 2007;125 (6:805–812. doi: 10.1001/archopht.125.6.805. [DOI] [PubMed] [Google Scholar]
- Vijaya L, George R, Arvind H, Baskaran M, Ve Ramesh S, Raju P, et al. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population: the Chennai Glaucoma Study. Ophthalmology. 2008;115:648–654. doi: 10.1016/j.ophtha.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri A, Lahiri SK, Bandyopadhyay M, Foster PJ, Reeves BC, Johnson GJ. A population based survey of the prevalence and types of glaucoma in rural West Bengal: the West Bengal Glaucoma Study. Br J Ophthalmol. 2005;89:1559–1564. doi: 10.1136/bjo.2005.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson RJ, Newland HS, Muecke J, McGovern S, Abraham L, Shein WK, et al. Prevalence of glaucoma in rural Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2007;91:710–714. doi: 10.1136/bjo.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Rahman N, Foster PJ, Haque Z, Zaman AU, Dineen B, et al. The prevalence of glaucoma in Bangladesh: a population based survey in Dhaka. Br J Ophthalmol. 2004;88:1493–1497. doi: 10.1136/bjo.2004.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Oen FT, Machin D, Ng TP, Devereux JG, Johnson GJ, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118:1105–1111. doi: 10.1001/archopht.118.8.1105. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996;114:1235–1241. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sui R, Jia L, Ellwein LB. Prevalence of glaucoma and normal intraocular pressure among adults aged 50 years or above in Shunyi county of Beijing. Zhonghua Yan Ke Za Zhi. 2002;38:335–339. [PubMed] [Google Scholar]
- Weale RA. Ethnicity and glaucoma: higher environmental temperatures may accelerate the onset and increase the prevalence, of primary open-angle glaucoma. Med Hypotheses. 2007;69:432–437. doi: 10.1016/j.mehy.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Qu W, Li Y, Song W, Zhou X, Sui H, Yuan HP. Prevalence and risk factors for angle-closure disease in a rural Northeast China population: a population based survey in Bin district, Harbin. Acta Ophthalmol. 2011;89:e515–e520. doi: 10.1111/j.1755-3768.2011.02146.x. [DOI] [PubMed] [Google Scholar]
- Fukuoka S, Aihara M, Iwase A, Araie M. Intraocular pressure in an ophthalmologically normal Japanese population. Acta Ophthalmol. 2008;86:434–439. doi: 10.1111/j.1600-0420.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- Lin HY, Hsu WM, Chou P, Liu CJ, Chou JC, Tsai SY. Intraocular pressure measured with a noncontact tonometer in an elderly Chinese population: the Shihpai Eye Study. Arch Ophthalmol. 2005;123:381–386. doi: 10.1001/archopht.123.3.381. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, Bernardi P, De Franco I, Perfetti S, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105:209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- Leske MC, Connell AM, Wu SY, Hyman L, Schachat AP. Distribution of intraocular pressure. The Barbados Eye Study. Arch Ophthalmol. 1997;115:1051–1057. doi: 10.1001/archopht.1997.01100160221012. [DOI] [PubMed] [Google Scholar]
- Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: the Rotterdam study. Ophthalmology. 1994;101:1851–1855. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- Shiose Y, Kitazawa Y, Tsukahara S, Akamatsu T, Mizokami K, Futa R, et al. Epidemiology of glaucoma in Japan—a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35:133–155. [PubMed] [Google Scholar]
- Lee JS, Lee SH, Oum BS, Choi HY, Lee SH, Oum BS. Relationship between intraocular pressure and systemic health parameters in a Korean population. Clin Experiment Ophthalmol. 2002;30:237–241. doi: 10.1046/j.1442-9071.2002.00527.x. [DOI] [PubMed] [Google Scholar]
- Shiose Y, Kawase Y. A new approach to stratified normal intraocular pressure in a general population. Am J Ophthalmol. 1986;101:714–721. doi: 10.1016/0002-9394(86)90776-2. [DOI] [PubMed] [Google Scholar]
- Shiose Y. The aging effect on intraocular pressure in an apparently normal population. Arch Ophthalmol. 1984;102:883–887. doi: 10.1001/archopht.1984.01040030703023. [DOI] [PubMed] [Google Scholar]
- Weih LM, Mukesh BN, McCarty CA, Taylor HR. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119:875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- Antón A, Andrada MT, Mujica V, Calle MA, Portela J, Mayo A. Prevalence of primary open-angle glaucoma in a Spanish population, the Segovia Study. J Glaucoma. 2004;13:371–376. doi: 10.1097/01.ijg.0000133385.74502.29. [DOI] [PubMed] [Google Scholar]
- Varma R, Ying-Lai M, Francis BA, Nguyen BB, Deneen J, Wilson MR, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos-The Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29:1151–1158. [PubMed] [Google Scholar]
- Leske MC, Podgor MJ. Intraocular pressure, cardiovascular risk variables, and visual field defects. Am J Epidemiol. 1983;118:280–287. doi: 10.1093/oxfordjournals.aje.a113634. [DOI] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma: the Barbados eye study. Arch Ophthalmol. 1995;113:918–924. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954–959. doi: 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the Blue mountains eye study. J Glaucoma. 2004;13:319–326. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- Wilson MR, Hertzmark E, Walker AM, Childs-Shaw K, Epstein DL. A case-control study of risk factors in open-angle glaucoma. Arch Ophthalmol. 1987;105:1066–1071. doi: 10.1001/archopht.1987.01060080068030. [DOI] [PubMed] [Google Scholar]
- Song W, Sun X, Shao Z, Zhou X, Kang Y, Sui H, et al. Prevalence and causes of visual impairment in a rural North-east China adult population: a population-based survey in Bin County, Harbin. A case-control study of risk factors in open-angle glaucoma. Acta Ophthalmol. 2009;88:669–674. doi: 10.1111/j.1755-3768.2009.01768.x. [DOI] [PubMed] [Google Scholar]
- Shen SY, Wong TY, Foster PJ, Loo JL, Rosman M, Loon SC, et al. The prevalence and types of glaucoma in malay people: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2008;49 (9:3846–3851. doi: 10.1167/iovs.08-1759. [DOI] [PubMed] [Google Scholar]
- Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology. 2003;110 (2:376–382. doi: 10.1016/S0161-6420(02)01568-3. [DOI] [PubMed] [Google Scholar]
- Rotchford AP, Johnson GJ. Glaucoma in Zulus: a population-based cross-sectional survey in a rural district in South Africa. Arch Ophthalmol. 2002;120 (4:471–478. doi: 10.1001/archopht.120.4.471. [DOI] [PubMed] [Google Scholar]