Abstract
Specific therapy of ocular infections often requires etiological diagnosis that is a combined effect of observation of characteristic clinical features and microbiological investigations. Clinical impression is central to guiding the laboratory investigation, and the aim of laboratory investigation is to confirm or rule out the clinical diagnosis. However, clinical features may vary considerably, and no one clinical feature may be pathognomonic of a particular pathogen. In addition, there may be a racial, geographical, and climatic difference in the distribution and type of causative agents associated with infections. Ophthalmologists have at their disposal in vivo and in vitro methods of diagnosis of ocular infections. The expertise of the clinician and the microbiologist along with the facilities available, determine the success with accurate diagnosis. A wide range of conventional and molecular techniques are available that not only provide rapid diagnosis for known common infections but have the potential to bring to the fore unknown organisms that may be associated with ocular infections.
Keywords: ocular infection, diagnosis, bacterial, fungal, viral, parasitic
Introduction
The external ocular surface harbors commensal organisms, such as Staphylococcus species, Corynebacterium species, and Propionibacterium species, which form the resident flora. Given a chance, any environmental organism can become a transient flora in the eye. The intraocular tissues and spaces, however, are sterile. While the conjunctiva is protected by blood supply, the cornea is avascular; therefore, the types of organisms invading these tissues may vary. The intraocular tissues are relatively immune-privileged1 and can be infected by any organism that manages to enter the inside of the eye. Trauma is an important predisposing factor for infection of the cornea and intraocular tissues. While exogenous infections are most common, eye infection may develop by spread of infection from neighbouring organs or hematogenously.2
Ocular infections may be caused by bacteria, fungi, parasites, or viruses, and each of these may produce a spectrum of disease. It is usually challenging to determine the causative agent based on clinical features because they may or may not be distinctive. Many a time, it may not even be possible to discriminate between infective or non-infective conditions. In addition, the prevalence and distribution of type of infectious agents associated with eye infections widely vary and are dependent on variety of factors. In recent times, in vivo methods, such as confocal microscopy, have come a long way in putting clinical diagnosis on firmer grounds, especially for Acanthamoeba and fungal keratitis.3 However, apart from its limited application, the equipment may not be available in all settings. Therefore, confirmation by microscopic examination and culture of the clinical samples remain the gold standard for etiological diagnosis. Currently, molecular diagnosis has added the sensitivity, specificity, and speed that have been the concerns with the conventional techniques of microscopy and culture. This review will describe the role of various methods that are currently available for the diagnosis of ocular infections.
In vivo confocal microscopy
Confocal microscopy is an attractive non-invasive technique that offers magnifications up to × 200 to × 500 with increased image contrasts and allows details of the cornea to be visualized even in hazy corneas. It allows repeated observations that aid in diagnosis, management, and follow up of cases with microbial keratitis.4 Although there are several reports published in the last decade to demonstrate the efficacy of confocal microscopy in the diagnosis of Acanthamoeba,5 fungal,6 Nocardia,7 and Microsporidia8 keratitis, it was only recently that the diagnostic accuracy was reported.3, 9 In one of the studies that took positive tissue diagnosis as the reference, confocal images were assessed on two occasions by four observers, who were masked to the tissue diagnosis and diagnostic accuracy indices were calculated.9 The authors reported highest positive likelihood ratio of 2.94 and lowest negative likelihood ratio of 0.59 with agreement values fair to moderate. Stand-alone use of confocal microscopy for the diagnosis of microbial keratitis was not recommended.9 The other study sought to determine inter and intraobserver variation in analysis and interpretation of confocal microscopy findings with conventional microbiology findings (microscopy and culture) of the corneal scrapings from patients with Acanthamoeba or fungal keratitis as the gold standard.3 The sensitivity and specificity of diagnosis by confocal microscopy were 88.3% and 91.1%, respectively. The inter- and intra-observer agreements were good, and the authors concluded that confocal microscopy provided accurate and reliable diagnosis in fungal and Acanthamoeba keratitis. Diagnosis using confocal microscopy is particularly advocated when corneal infiltrate is deep seated or patients are on treatment or microbial keratitis develops after intracorneal implants like intracorneal ring segments or refractive surgery.3
Conventional microbiological methods
Clinical samples for the diagnosis of eye infections must be collected from the site of infection. Serum samples are very rarely helpful in the diagnosis of eye infections. Generally, the sample available is very minute and requires direct processing without resorting to transport media (except for virus or Chlamydia isolation). However, use of Amies transport medium without charcoal was shown to be as good as direct patient side processing of corneal scrapings for culture of bacteria and fungi after storage for 24 h at room temperature.10 Prolonged incubation (1–2 weeks) of most media is recommended to allow growth of slow-growing/fastidious organisms. Type of clinical sample that are generally collected for the diagnosis of various eye infections are listed in Table 1 .
Table 1. Type of sample and recommended procedure for sample collection in various eye infections.
| Type of infection | Type of sample | Recommended device/procedure for sample collection |
|---|---|---|
| Blepharitis | Scales/discharge from lid margin | Forceps/cotton swab |
| Conjunctivitis | Fluid/discharge from lower conjunctival sac | Calcium alginate/cotton swab |
| Dacryocystitis | Fluid/discharge from lower conjunctival sac | Calcium alginate/cotton swab |
| Keratitis | Corneal scraping | Kimura spatula, No. 15 surgical blade, bent needle |
| Uveitis | Anterior chamber fluid | Paracentesis (anterior chamber tap) with tuberculin syringe |
| Endophthalmitis | Anterior chamber fluid Vitreous aspirate Vitreous Biosy | Paracentesis (anterior chamber tap) with tuberculin syringe Tuberculin syringe Vitrectomy |
| Panophthalmitis | Vitreous biopsy Evisceration contents | Vitrectomy Evisceration |
| Deep seated stromal infiltrate in keratitis | Corneal biopsy | Lamellar biopsy |
| Non-healing keratitis requiring keratoplasty | Corneal buttons | Penetrating keratoplasty |
| Contact lens-associated keratitis | Contact lenses, lens cases, and lens solution | Aseptically removed from the eye. Aseptically collected |
| Postoperative endophthalmitis following intraocular lens implantation | Intraocular lens | Surgical removal |
| Eye injury with iris prolapse/incarceration | Iris tissue | Surgical removal |
Processing of clinical samples from the eye
Ocular samples require special handling and direct patient-side processing. In case of non-availability of the microbiology laboratory in the premises of the hospital, the required slides and media may be obtained beforehand and kept in reserve for use. The slides/media may be transported in secure boxes to the laboratory after collection of the samples. Items such as contact lens cases and contact lens solutions may be directly submitted to the laboratory for processing. The direct smear examination methods and common culture media used for isolation of bacteria and fungi are similar for majority of the samples, although the method of inoculation may vary. Sample collection for detection of parasites and viruses require special procedures. Samples such as corneal buttons/biopsies, eviscerated contents, or any other tissues, need to be simultaneously investigated by histopathology and a correlation is sought for appropriate reliable diagnosis. Table 2 provides the direct microscopy methods that may be used for detection of various organisms from ocular samples. Smears are generally not made from samples such as contact lenses, contact lens solutions, intraocular lenses, corneal biopsy/buttons, and iris tissues. These samples are directly processed for culture of bacteria, fungi, or parasites in appropriate media.
Table 2. Direct smear examination methods used for the diagnosis of eye infections.
| Type of sample | Type of organism/antigen to be detected | Staining methods for smears |
|---|---|---|
| Conjunctival swabs/scrapings | Bacteria, fungi, parasites (Microsporidia) Viral antigens | Gram, Giemsa, KOH+Calcofluor white, Kinyoun stain direct/indirect immunofluorescence or immunoperoxidase |
| Corneal scrapings | Bacteria, fungi, parasite (Acanthamoeba, Microsporidia) Viral antigens | Gram, Giemsa, KOH+Calcofluor white, Lactophenol cotton blue, Gomori methenamine silver, Ziehl-Neelsen stain, Kinyoun stain direct/indirect immunofluorescence or immunoperoxidase |
| Aqueous/vitreous fluids/biopsy | Bacteria, fungi Viral antigens | Gram, Giemsa, Calcofluor white, Gomori methenamine silver Direct/indirect immunofluorescence or immunoperoxidase |
Most samples, except fluids, for polymerase chain reaction (PCR) are placed in sterile phosphate buffered saline pH 7.2 and submitted to the laboratory, where they may be retained at 4 °C for 24 h before tested. Storage at −20 °C is recommended, if the testing is likely to be delayed. Aqueous and vitreous fluids can be directly used for DNA isolation. They can be stored at 4 or −20 °C until tested. PCR can identify the offending organisms in less than 24 h. It is considered useful in the diagnosis of bacterial (Propionibacterium acnes) as well as fungal endophthalmitis, as the sensitivity of conventional culture methods is low. Prior antibiotic therapy, small number of organisms, possible localized infection in capsular bag, and fastidious nature of the organisms are possible causes of low sensitivity. PCR with primers specific for P. acnes, has been successfully used11 on vitreous specimens negative in smear and culture but positive in PCR by eubacterial primers. DNA sequencing of the universal (eubacterial) nested PCR product allows the identification of the causative organism in a number of culture negative cases of endophthalmitis. PCR has also been found useful in the diagnosis of fungal endophthalmitis.12
Direct microscopy examination of clinical samples
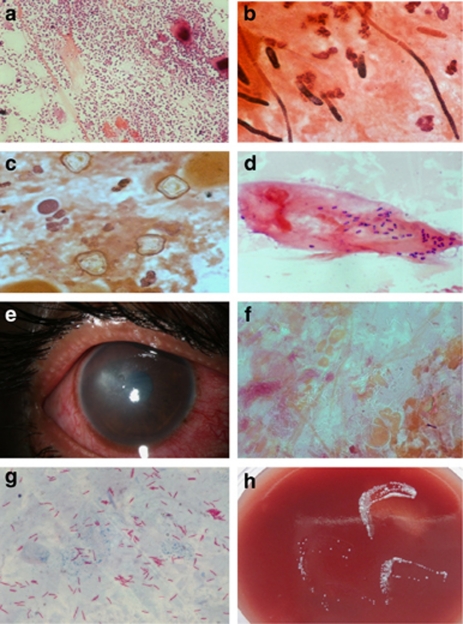
Most microbiology laboratories provide a rapid diagnosis based on initial smear examination of the clinical samples that helps initiate specific treatment early in the disease. It is a good laboratory practice to provide the initial microscopy results in the shortest possible time. Thorough microscopic examination of the smears stained appropriately can often provide etiological diagnosis that does not require further culture. Gram stain is the most commonly used stain that demonstrates the presence of bacteria, fungi, and parasites (Figures 1a–d). The sensitivity and specificity of Gram stain and potassium hydroxide with calcofluor white in the identification of bacterial and fungal elements in corneal scrapings is reported to be affected by the stage of the disease, both being higher in advanced stage.13 Observation of unstained or partially stained bacilli in corneal scrapings has been shown to provide the clue regarding Mycobacterium infection.14 Restaining of the smear with Ziehl Neelsen stain would reveal the presence of acid fast organism prompting immediate institution of therapy with 2.5% amikacin. Figure 1e shows the slit lamp picture of the cornea of a patient whose corneal scraping stained with Gram stain showed unstained bacilli that were found to be acid fast by Ziehl Neelsen stain (Figures 1f and g). A significant growth of Mycobacterium fortuitum was seen in the culture of the corneal scraping (Figure 1h). Similarly, detection of gram positive, thin, beaded, branching filaments in corneal scraping is suggestive of Actinomycetales. Presence of acid-fast filaments in Kinyoun stain (modified Ziehl Neelsen stain with 1% H2SO4) performed on the same smear would be diagnostic of Nocardia infection. Thus, a guideline for initial treatment can be formulated based on initial smear findings in the clinical sample, especially in microbial keratitis.
Figure 1.
Corneal scrapings from patients with microbial keratitis (Gram stain, x1000) showing (a) capsulated gram-positive cocci in pairs, suggestive of Streptococcus pneumoniae; (b) septate, hyaline fungal filaments; (c) double-walled, polygonal Acanthamoeba cysts; (d) intracellular gram positive, oval, well-defined microsporidia spores with characteristic paracentral or polar dark staining. (e) Shows a slit lamp photograph of a patient with stromal infiltrate of two-month duration with relatively clear surrounding cornea. Corneal scraping of the patient stained with Gram stain showed (f) unstained bacilli ( × 1000) that were found to be acid fast by (g) Ziehl Neelsen stain ( × 1000). A significant growth of Mycobacterium fortuitum was seen in culture on (h) blood agar of the corneal scraping.
In contrast, the role of smear examination in the diagnosis of infectious endophthalmitis is not very encouraging as the sensitivity is low.15 However, detection of fungal elements in vitreous sample can help early administration of antifungal intravitreal therapy.
Not many studies have reported the role of smear examination in initiating early treatment in other ocular infections. However, detection of Neisseria gonorrhoeae in Gram stain of conjunctival secretion smear is extremely useful for good therapeutic outcome. The protocol in our laboratory includes direct smear examination of all ocular samples by Gram stain and/or potassium hydroxide with calcofluor white (KOH+CFW). Special stains of Giemsa, Ziehl Neelsen, Kinyoun, and Gomori methenamine are done when indicated.
A rapid diagnosis of viral infection can be established by observing stained smears of corneal scrapings, conjunctival scrapings/swabs, or centrifuged deposits of aqueous/vitreous fluids (cytospin). This may be accomplished by using nonspecific staining techniques such as Giemsa, Papanicolaou, and Hematoxylin-eosin stain.16 These techniques help visualize multinucleated giant cells, koilocytic changes, and intranuclear/ intracytoplasmic inclusions, and various inflammatory cells that are predominantly lymphocytes.
Intranuclear inclusions are more efficiently seen in Papanicolaou stain than Giemsa-stained smears; however, Giemsa stain is good for evaluating cell types. Though these staining techniques have the advantage of being rapid and inexpensive, they are often nonspecific and offer low sensitivity in the diagnosis of viral infection. For example, these stains cannot differentiate the intranuclear inclusions of herpes simplex virus (HSV) from that of varicella zoster virus (VZV).
Specific cytology techniques used for viral diagnosis are techniques that indirectly suggest the presence of viral antigen in the clinical sample.11, 16 Detection of cell-associated viral antigen in a corneal scraping or conjunctival scraping is very useful in the diagnosis of viral keratitis. Direct and indirect immunofluorescence and indirect immunoperoxidase assays can be used in the diagnosis of HSV, VZV keratitis, and adenoviral keratoconjunctivitis. Both these tests are rapid, specific and sensitive when suitable monoclonal or purified polyclonal antibodies are used in the test system. Indirect immunoperoxidase (IP) assay has distinct advantages over indirect immunofluorescence (IF) assay. The former provides a permanent preparation for records and utilizes an ordinary light microscope, whereas the latter has the inherent problem of quenching (fading) of fluorescence and requires a sophisticated and expensive fluorescence microscope. In addition, the IP technique can be used on paraffin embedded tissue whereas the IF technique provides better results with frozen tissue sections.
Culture methods for bacteria, fungi, and parasites (Acanthamoeba)
Processing for culture involves inoculation of the sample on appropriate culture media. Prior knowledge of expected organisms helps determine the type of media to be included. The incubation conditions of the media also vary and are based on the organisms expected. Most samples are processed for isolation of bacteria and fungi. Fungi associated with eye infections are usually fast-growing saprophytic fungi that can grow on media, such as blood and chocolate agar, traditionally meant for bacteria.17 A single protocol is recommended for the culture of bacteria, fungi, and Acanthamoeba from corneal scrapings. However, in situations where only bacteria and fungi are expected (endophthalmitis) the culture for Acanthamoeba is not included. Table 3 lists the different media that are used for culture of common organisms from the ocular samples.
Table 3. Media used for culture of bacteria, fungus, Acanthamoeba from ocular samples.
| Type of sample | Culture media | Expected organisms |
|---|---|---|
| Lid margin scales | Sheep blood agar Brain heart infusion broth Sabouraud dextrose agara | Bacteria Fungi |
| Conjunctival swab | Sheep blood agar Sheep blood chocolate agar Brain heart infusion broth Sabouraud dextrose agara | Bacteria Fungi |
| Corneal scrapings | Sheep blood agar (aerobic, anaerobic) Sheep blood chocolate agar Brain heart infusion broth Sabouraud dextrose agara Thioglycollate broth Non-nutrient agar with E.coli | Bacteria (aerobic, anaerobic) Fungi Acanthamoeba |
| Aqueous/vitreous | Sheep blood agar (aerobic, anaerobic) Sheep blood chocolate agar Brain heart infusion broth Sabouraud dextrose agara Thioglycollate brotha | Bacteria (aerobic, anaerobic) Fungi |
| Corneal biopsy/ buttons | Sheep blood agar (aerobic, anaerobic) Sheep blood chocolate agar Brain heart infusion broth Sabouraud dextrose agara Non-nutrient agar with E.coli | Bacteria (aerobic, anaerobic) Fungi Acanthamoeba |
| Contact lenses | Sheep blood chocolate agar (aerobic, anaerobic) Sabouraud dextrose agara Non-nutrient agar with E.coli | Bacteria (aerobic, anaerobic) Fungi Acanthamoeba |
| Contacat lens solutions | Sheep blood chocolate agar (aerobic, anaerobic) Sabouraud dextrose agara Non-nutrient agar with E.coli | Bacteria Fungi Acanthamoeba |
| Intraocular lens/iris tissue | Sheep blood agar | Bacteria Fungi |
With antibiotics (gentamicin or chloramphenicol) but without cycloheximide. Potato dextrose agar may be used in addition to Sabouraud dextrose agar for better sporulation.
The number of media may be reduced as per the availability of the samples. All media are incubated at 37 °C except Sabouraud dextrose agar, which requires 25–27 °C (BOD incubator). Chocolate agar is incubated in 3–5% CO2 in a candle jar or CO2 incubator, and blood agar for anaerobic culture requires anaerobic chamber or anaerobic jar with gas pack. All other media are incubated aerobically.
All media are examined for growth daily and are incubated for 1–2 weeks before discarding. Bacteria such as Nocardia species, atypical mycobacteria, and Acanthamoeba grow slowly and require prolonged incubation. Although most fungi associated with eye infections are saprophytes and grow within a week, they may require incubation for 2–4 weeks for proper sporulation and identification.
Although bacterial and fungal colonies are examined with unaided eyes, the observation of Acanthamoeba growth requires use of microscope. Non-nutrient agar plates (with lid on) are placed under × 4 objective lens of the microscope, and the presence of trophozoites is looked for in the vicinity of the inoculation mark on the surface of the medium. One may be able to see the characteristic track marks made by the migration of the trophozoites on the Escherichia coli lawn. Acanthamoeba forms no colonies. Bacterial growth in liquid media appears as turbidity that requires to be subcultured and Gram stained for identification. The growth of bacteria or fungus in culture is considered significant if the growth is confluent (more than 10 colonies) on the site of inoculation on solid media, or the organism was seen in the smears, or if the same organism was grown in more than one medium.
Antibiotic susceptibility testing
Susceptibility tests help to determine the most effective drug that can be used for treatment. Susceptibility of bacterial isolates to antibiotics is well standardized by clinical laboratory standards institute and guidelines are available for disc diffusion assay (CLSI M02-A10, 2009), as well as for broth dilution and agar dilution methods (CLSI M07-A8, 2009) for determination of minimum inhibitory concentration (MIC) of antibacterial agents. Susceptibility tests for bacteria using disc diffusion method are well standardized, and availability of commercial antibiotic discs makes it a commonplace practice by all microbiology laboratories. The antibiotic discs, however, contain obtainable serum level of the drug and not the level obtainable in the tear film or cornea or intraocular space by usual topical or intraocular therapy. Therefore, organisms reported as resistant may be susceptible in ophthalmic situation. Whereas disc diffusion method labels an organism as susceptible, resistant, or intermediately susceptible, broth dilution procedures can determine minimum lethal concentration or minimum bactericidal concentration apart from MIC. This is clinically important, especially in endophthalmitis, as the effective peak concentration should be 2–4 times higher than the MIC. A simple method of MIC determination has become available in the form of E-test, which is a commercially available quantitative antimicrobial susceptibility test. It combines the simplicity and flexibility of disc diffusion test with the ability to determine MICs of up to five antibiotics at one go. Routine testing of bacterial isolates for MIC of antibiotics is a commonly used procedure in many laboratories despite the high cost.
Susceptibility testing against antifungal drugs is performed for yeasts and filamentous fungi by broth or agar dilution methods, where MIC is determined, and by disc diffusion method. Disc diffusion method similar to bacterial susceptibility testing is available for yeasts (CLSI M44-A, 2009) and some of the non-dermatophyte filamentous fungi (CLSI M51-A, 2010). Drugs commonly tested include 5FC (flucytosine), ketoconazole, miconazole, fluconazole, itraconazole, and Amphotericin B. Only a limited number of antifungal susceptibility testing of ocular isolates has been reported.18, 19, 20 E-test of several antifungals is also available for yeast and filamentous fungi. However, unlike bacterial isolates, testing of fungal isolates for susceptibility to antifungal drugs is yet to become a routine practice.
Culture of ocular samples for viruses
The sample for viral diagnosis always needs to be collected in an appropriate transport medium (except the smears) and sent to the laboratory. Methods of transport would vary according to the type of sample. Hank's balanced salt solution or 2 sucrose phosphate broth may be used.11, 16
Samples received in a virology laboratory may be processed using a variety of techniques. The choice of technique would depend on the type of sample and the specific virus that is being looked for. Most of the procedures can be performed in a moderately equipped laboratory. Of all available laboratory techniques for diagnosis of viral infections, only a few can be adopted in a particular laboratory. The choice is made on the basis of the advantages, disadvantages, and cost effectiveness of the techniques and their overall utility. Established cell lines such as HeLa, Vero, HEp 2, MRC-5 etc. have been used for isolation of HSV from corneal scrapings and other ocular samples. An immortalized human corneal epithelial cell line has been reported to be very sensitive for isolation of HSV.21
Growth of virus in the cell lines can be confirmed either by characteristic cellular changes or cytopathic effect (CPE) or by IF or IP techniques, which detect viral antigens in the infected cell lines. Appearance of CPE may take several days but antigens can be detected even before CPE occurs, thereby rendering the latter a more rapid method. Viruses may be cultured in cell lines maintained in tubes (tube culture) or on cover slips in vials (shell vial).22 In recent times, viral molecular diagnostic methods for demonstration of viral DNA in clinical samples have taken over the virus isolation. Molecular methods are ideal for viral diagnosis as virus isolation is time consuming, technically demanding and requires special and expensive virology laboratory set up.
Molecular methods in the diagnosis of ocular infections
By virtue of being extremely sensitive and specific, molecular techniques, especially PCR, is presently the most sought-after test for viral diagnosis and detection of organisms that are difficult to culture such as Microsporidia, Propionibacterium acnes, Toxoplasma gondii etc. or that take long time to grow, such as Mycobacterium tuberculosis. PCR is a rapid, reliable and sensitive tool for the diagnosis of bacterial and fungal endophthalmitis.23 PCR-based techniques with several of its modifications have been widely used for the diagnosis of viral infections of the eye. Conjunctivitis or keratoconjunctivitis caused by adenoviruses,24 herpes simplex virus,25 Chlamydia,26 or microsporidia27 are usually confirmed by PCR-based techniques. Uniplex, as well as multiplex, PCRs are used for the diagnosis of viral retinitis.28
The utility of PCR techniques for improving diagnosis of fungal infections of the eye has been demonstrated by several investigators.29, 30, 31 Panfungal PCR using ITS primers was shown to be very sensitive for the diagnosis of fungal endophthalmitis.12 A recent study has compared three different panfungal primers for the diagnosis of fungal keratitis and has concluded that the sensitivity of PCR using ITS primers is higher than 18 S rDNA and 28S rDNA primers.32 DNA technology in the form of DNA chip has been developed by Xcyton Diagnostics Bangalore, India, and provides a platform to apply multiplex PCR and hybridization for simultaneous diagnosis of several ocular infections.33 Real-time PCR has added a great advantage in being quantitative and its application in diagnosis and follow up of several ocular viral infections has been reported.34, 35 Sequencing of genomic fragments often helps identify organisms that are difficult to identify by conventional methods. New organisms have been associated with eye diseases in recent studies. These versatile techniques have opened our eyes to the fact that much remains to be learnt as far as ocular infections are concerned.
Conclusions
The repertoire of investigations for the diagnosis of ocular infections depends on the facilities and expertise available. A judicious combination of clinical acumen and laboratory tests would help make an etiological diagnosis and initiate specific treatment without losing time.
The author declares no conflict of interest.
Footnotes
The paper was presented at the Cambridge Ophthalmological Symposium, 7–9 September 2011, St John's College Cambridge.
References
- Pararajasegaram G.Mechanisms of uveitis. Chapter 159In: Yanoff M, Duker JS (eds)Ophthalmology2nd Vol. 2nd ed. Reed Elsevier India Pvt. Ltd: New Delhi; 20041111 [Google Scholar]
- Sharma S.Ocular Microbiology1st ed. Aravind Eye Hospital and Postgraduate Institute of Ophthalmology: Madurai; 1988 [Google Scholar]
- Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and Acanthamoeba keratitis. Ophthalmology. 2011;118:29–35. doi: 10.1016/j.ophtha.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Kaufman SC, Musch DC, Belin MW, Cohen EJ, Meisler DM, Reinhart WJ, et al. Ophthalmic technology assessment committee cornea panel. Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:396–406. doi: 10.1016/j.ophtha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD. Ophthalmology. 2006;113:538–547. doi: 10.1016/j.ophtha.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Avunduk AM, Beuerman RW, Varnell ED, Kaufman HE. Confocal microscopy of Aspergillus fumigatus keratitis. Br J Ophthalmol. 2003;87:409–410. doi: 10.1136/bjo.87.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaddavalli PK, Garg P, Sharma S, Thomas R, Rao GN. Confocal microscopy for Nocardia keratitis. Ophthalmology. 2006;113:1645–1650. doi: 10.1016/j.ophtha.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Sagoo MS, Mehta JS, Hau S, Irion LD, Curry A, Bonshek RE, et al. Microsporidial stromal keratitis: in vivo confocal findings. Cornea. 2007;26:870–873. doi: 10.1097/ICO.0b013e31806c7a3c. [DOI] [PubMed] [Google Scholar]
- Hau SC, Dart JKG, Vesaluoma M, Parmar DN, Claerhout I, Bibi K, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94:982–987. doi: 10.1136/bjo.2009.175083. [DOI] [PubMed] [Google Scholar]
- McLeod SD, Kumar A, Cevallos V, Srinivasan M, Whitcher JP. Reliability of transport medium in the laboratory evaluation of corneal ulcers. Am J Ophthalmol. 2005;140:1027–1031. doi: 10.1016/j.ajo.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Therese KL, Madhavan HN.Microbiological procedures for diagnosis of ocular infections . www.ijmm.org/documents/ocular.pdf . Accessed 26 December 2006.
- Bagyalakshmi R, Therese KL, Madhavan HN. Application of semi-nested polymerase chain reaction targeting internal transcribed spacer region or rapid detection of panfungal genome directly from ocular specimens. Indian J Ophthalmol. 2005;55:261–265. doi: 10.4103/0301-4738.33037. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis. A survey of eight years of laboratory experience. Cornea. 2002;21:643–647. doi: 10.1097/00003226-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Garg P, Bansal AK, Sharma S, Vemuganti GK. Bilateral infectious keratitis following laser in situ keratomileusis: A case report and review of the literature. Ophthalmology. 2001;108:121–125. doi: 10.1016/s0161-6420(00)00435-8. [DOI] [PubMed] [Google Scholar]
- Sharma S, Jalali S, Adiraju MV, Gopinathan U, Das T. Sensitivity and predictability of vitreouscytology, biopsy and membrane filter culture in endophthalmitis. Retina. 1996;16:525–529. doi: 10.1097/00006982-199616060-00010. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sreedharan A.Diagnostic procedures in infectious keratitisIn: Nema HV, Nema N (eds).Diagnostic Procedures in Ophthalmology2nd ed. Jaypee Brothers Medical Publishers: New Delhi; 2009316–332. [Google Scholar]
- Das S, Sharma S, Kar S, Sahu SK, Samal B, Mallick A. Is inclusion of Sabouraud dextrose agar essential for the laboratory diagnosis of fungal keratitis. Indian J Ophthalmol. 2010;58:281–286. doi: 10.4103/0301-4738.64122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in south India. Cornea. 2002;21:555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Shapiro BL, Lalitha P, Loh AR, Fothergill AW, Prajna NV, Srinivasan M, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94:384–385. doi: 10.1136/bjo.2009.158675. [DOI] [PubMed] [Google Scholar]
- Lalitha P, Vijaykumar R, Prajna NV, Fothergill AW. In vitro natamycin susceptibility of ocular isolates of Fusarium and Aspergillus species: Comparison of commercially formulated natamycin eye drops to pharmaceutical-grade powder. J Clin Microbiol. 2008;46:3477–3478. doi: 10.1128/JCM.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki KA, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- Johnson FB, Luker G, Chow C. Comparison of shell vial culture and the suspension-infection method for the rapid detection of herpes simplex viruses. Diagn Microbiol Infect Dis. 1993;16:61–66. doi: 10.1016/0732-8893(93)90131-p. [DOI] [PubMed] [Google Scholar]
- Bagyalakshmi R, Madhavan HN, Therese KL. Development and application of multiplex polymerase chain reaction for the etiological diagnosis of infectious endophthalmitis. J Postgrad Med. 2006;52:179–182. [PubMed] [Google Scholar]
- Dalapathy S, Therese KL, Roy S, Madhavan HN. Development and use of nested polymerase chain reaction (PCR) for the detection of adenovirus from conjunctivitis specimens. J Clin Virol. 1998;11:77–84. doi: 10.1016/s0928-0197(98)00021-x. [DOI] [PubMed] [Google Scholar]
- Madhavan HN, Priya K, Anand AR. Detection of herpes simplex virus (HSV) genome using polymerase chain reaction (PCR) in clinical samples comparison of PCR with standard laboratory methods for the detection of HSV. J Clin Virol. 1999;14:145–151. doi: 10.1016/s1386-6532(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Fan J, Zhang WH, Wu YY, Jing XY, Claas EC. Detection of infections of the eye with Chlamydiatrachomatis by the polymerase chain reaction. Int Ophthalmol. 1993;17:327–330. doi: 10.1007/BF00915738. [DOI] [PubMed] [Google Scholar]
- Joseph J, Sharma S, Murthy SI, Krishna PV, Garg P, Nutheti R, et al. Microsporidial keratitis in India: 16S rRNA gene-based assay for diagnosis and species identification of microsporidia in clinical samples. Invest Ophthalmol Vis Sci. 2006;47:4468–4473. doi: 10.1167/iovs.06-0376. [DOI] [PubMed] [Google Scholar]
- Priya K, Madhavan HN, Malathi J. Use of uniplex polymerase chain reaction and evaluation of multiplex PCR in the rapid diagnosis of viral retinitis. Indian J Med Res. 2003;117:205–210. [PubMed] [Google Scholar]
- Embong Z, Wan Hitam WH, Yean CY, Rashid NH, Kamarudin B, Abidin SK, et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008;8:7. doi: 10.1186/1471-2415-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarai B, Gupta A, Ray P, Shivaprakash MR, Chakrabarti A. Polymerase chain reaction for early diagnosis of post operative fungal endophthalmitis. Indian J Med Res. 2006;123:671–678. [PubMed] [Google Scholar]
- Anand A, Madhavan H, Neelam V, Lily T. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology. 2001;108:326–330. doi: 10.1016/s0161-6420(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Balne PK, Reddy AK, Manjulatha K, Durga C, Gorli SR, Garg P.Evaluation of three polymerase chain reaction assays for the detection of fungi in patients with mycotic keratitis Br J Ophthalmol 2011(in press). [DOI] [PubMed]
- Basu S, Sharma S, Kar S, Das T. DNA chip-assisted diagnosis of a previously unknown etiology of intermediate uveitis-Toxoplasma gondii. Indian J Ophthalmol. 2010;58:535–537. doi: 10.4103/0301-4738.71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Deai T, Hibino T, Higaki S, Hayashi K, Shimomura Y. Quantitative analysis of herpes simplex virus genome in tears from patients with herpetic keratitis. Cornea. 2003;22 (7 Suppl:S55–S60. doi: 10.1097/00003226-200310001-00008. [DOI] [PubMed] [Google Scholar]
- Kandori M, Inoue T, Takamatsu F, Kojima Y, Hori Y, Maeda N. Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology. 2010;117:216–222. doi: 10.1016/j.ophtha.2009.06.059. [DOI] [PubMed] [Google Scholar]