Abstract
Aim
The aim of this study is to examine the attitudes of patients, who presented with advanced glaucoma in at least one eye, to participation in a randomised prospective trial comparing primary medical treatment with primary surgical treatment for advanced glaucoma.
Methods
Patients who had presented with advanced glaucoma (>15 dB loss mean deviation on Humphrey visual field testing) in at least one eye were asked to participate. Five focus groups comprising of 4–8 patients and consisting of 29 patients in total were undertaken. The group interviews were conducted by two experienced qualitative researchers, an ophthalmic clinician was present to clarify technical issues. The focus group discussions were taped and transcribed in full, and analysed through a process of familiarisation, open (inductive) coding, theme generation, theme refinement, and thematic mapping.
Results
Three overarching themes were identified: (1) the extent of patients' knowledge, (2) anxieties about surgery, and (3) concerns about compromised care due to trial involvement; these themes were further classified into eight sub-themes.
Conclusions
Patients' willingness to participate in randomised clinical studies is significantly connected to their level of comprehension and insight about the medical condition, its treatment, and the research process; misunderstandings about any of these aspects may act as a significant barrier to trial recruitment. Recruitment rates for future randomised trials may be enhanced by ensuring that patients have full and accurate information about the treatment alternatives, and that uncertainty exists for best patient outcomes between treatment options, and reassuring potential participants that the research process, in particular randomisation, will not compromise clinical care.
Keywords: advanced glaucoma, randomised controlled trial, patient barriers, qualitative analysis
Introduction
Between 10–33% of glaucoma patients present with advanced glaucoma,1, 2, 3, 4, 5, 6 this is a risk factor for blindness.3, 5, 7, 8, 9 Reduction in intraocular pressure is the only treatable risk factor for glaucoma and may be achieved by either medical or surgical intervention. NICE guidelines have recommended that patients who present with advanced glaucoma should be offered primary glaucoma surgery.10 A recent systematic review comparing primary medical vs surgical treatment for glaucoma11 concluded that further randomised controlled trials of current medical treatments compared with surgery are required in people with advanced glaucoma.11 A randomised, prospective clinical trial, comparing primary medical with primary surgical treatment in patients presenting with advanced glaucoma is thus warranted.
The difficulties of recruiting participants to clinical trials are well documented;12, 13, 14, 15, 16, 17, 18, 19 it is suggested that around 50% of clinical trials fail to reach their recruitment target, or require extension.12 Patient-related factors have been identified to explain this, such as a lack of understanding about research or medical condition; concerns with the research process, especially randomisation; preference for particular treatments; and a perception that research is inappropriate for serious medical conditions.16, 20, 21 In clinical trials of surgical procedures, these issues are more pronounced,22 especially where surgical interventions are being compared with medical treatment.23
Understanding the attitudes, beliefs, and insights of potential trial subjects can be helpful in recognising barriers to trial recruitment, and can be used to inform strategies to overcome these. Recruitment to a glaucoma surgery trial has previously been undertaken,24 although this research was set in a different healthcare context and was for a different patient group (those presenting with asymptomatic early glaucoma). Despite a recent study25 indicating that glaucoma patients were unconcerned about the form of their treatment, it remains necessary to clarify potential barriers to surgical trial recruitment and to establish glaucoma patients attitudes towards surgical trials in this condition.
Materials and methods
Patients attending the glaucoma clinic of one of the authors (AJK), who presented with advanced glaucoma (>15 bD loss on Humphrey visual field testing) in at least one eye, were invited to participate in the study. All those invited would potentially be eligible for a proposed future trial.
Five focus groups, consisting of between four and eight participants, were undertaken. Groups were facilitated by two researchers; an experienced ophthalmic trainee was present to help clarify clinical issues. Discussion was structured around a broad topic guide, which included willingness to undergo surgery as a primary intervention, willingness to participate in a trial comparing primary medical and surgical treatments, and information/assurances, which would encourage patients to participate in such a trial. To establish participants' prior understanding of the condition, each session started with a discussion about glaucoma and treatment options. Each focus group lasted between 60 and 90 min; all were recorded using digital recording equipment.
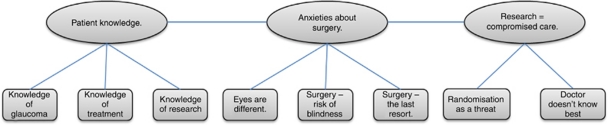
Data was managed following the conventions of Thematic Analysis.26 Data was transcribed in full and analysed through a process of familiarisation, open (inductive) coding, theme generation, theme refinement, and thematic mapping. Coding and initial theme generation were carried out by the authors separately; codes and themes were refined, redefined, and finalised through a process of mutual checking and group discussion to ensure the validity of individual interpretations. An overall thematic map (Figure 1) was generated by the authors collaboratively to both reflect the content of the focus group discussions, and also to generate insight into attitudes about surgical trial recruitment. Finally, data was extracted from the coded focus group transcripts in accordance with the final thematic map.
Figure 1.
Factors influencing willingness to participate in a trial comparing primary medical vs primary surgical treatment.
Results
A total of 29 patients agreed to participate (23 men, 6 women). Ten participants had previous experience of trabeculectomy surgery for glaucoma.
The thematic map was organised so as to address the needs of a future trial design, with a particular focus upon issues of trial recruitment. Three overarching themes were identified:
Patient knowledge.
Anxieties about surgery.
Concerns about compromised care with trial participation.
Patient knowledge
Three aspects of patient knowledge were identified from the focus group discussion:
Knowledge of glaucoma.
Knowledge of treatment.
Knowledge of research.
Knowledge of glaucoma
Patients' understanding of glaucoma varied significantly, from those who knew little about glaucoma, even several years after diagnosis, to those who held a well-researched understanding of the condition and its treatment. Knowledge of treatment goals was more uniform with a broad understanding that reducing intraocular pressure is essential. All participants were familiar with eyedrops, although knowledge of surgery was less uniform, and for some less important due to the anxiety, which it might provoke:
‘(my consultant) hasn't told me great details about the operation, and to be quite honest I don't want to, I don't want to know.… I don't need to know all the ins and outs.' P: Yeah, they didn't really explain very much [about surgery], maybe I didn't ask, maybe I didn't want to know. P: That's it, you don't want to know do you? P: Yeah, you don't want to know, yeah. P: Just carry on doctor. Yeah, just carry on doctor please.'
Knowledge of treatment
This final comment reflects a theme, which recurred throughout all aspects of the focus group, the value and trust which participants placed in expert, medical knowledge. The assumption that their doctor knows best was found to significantly influence how patients feel about the management of their glaucoma, and participants repeatedly expressed a readiness to comply with the wishes of their doctors:
‘I just do what (my consultant) says and that's it.' ‘I would sooner rely on what (my consultant) is telling me. Therefore that's the route I'm going down, I'm not interested in pros and cons, I'm relying on him to do, err. I'm just interested in, he's the expert, he's the one I'll put my faith in.'
Knowledge of research
The benefits of medical research were frequently acknowledged, ‘otherwise we wouldn't ever get anywhere, make any advances'. Yet few participants demonstrated an understanding of the research process, and some expressed concern that it is driven by intellectual curiosity rather than by clinical need:
‘I wouldn't like to lose my sight just to prove a point.'
Randomisation was an area of particular concern, with participants suggesting that observation or a survey of prior cases ought to be sufficient criteria for understanding the efficacy of a treatment:
‘I can't understand why you call it a trial, because the operation has been going on for considerable time, and so have the drops. So what is the trial?… You've already got the results from the operations you've done and the drops that have been done… let's look at the study on that, rather than just pick at random.'
In discussing recruitment to the proposed future study, participants stressed the importance of clear and complete information about both treatment and research. In all bar one focus group, it was concluded that the experiences of former patients would be valuable for potential study participants:
‘I'd like to hear from people who've had this operation [to find out] what they've gone through, and what they haven't gone through. They can tell me. Then I know what the situation is; then I can make my mind up.'
Anxieties about surgery
Three areas of concern regarding surgery were identified from the focus group discussion:
Eyes are different.
Risk of blindness with surgery.
Surgery the option of last resort.
Eyes are different
Underpinning much of the discussion of both treatment and research was the implicit belief that eyes are somehow different to other parts of the body, more delicate, and more precious:
‘I've never missed an appointment because I think your eyes are the most precious things in your body.'
Surgery—risk of blindness
Although the risk of blindness from glaucoma surgery is very low, a preference for more conservative treatment options was directly linked to this risk:
‘Thing is, when you've got (some sight), even if you've lost a little sight, you've got something. But if the operation wasn't successful, you might end up with nothing.' ‘I think it's difficult to explain to people that maybe surgery is better, because you could lose your eyesight... in the operation. I don't know whether that's happened a lot or not. But if you're still on the drops, and your eyesight's going gradually, it might be years before you end up in the same situation that the operation [might create immediately], you know...'
A perverse consequence of this set of beliefs was that willingness to undergo surgery was perceived to be inversely related to its potential benefits. Participants felt that patients would be more willing to undergo surgery on an eye where loss of sight is already significant; in those eyes where sight is still ‘good', surgery would be deferred despite knowledge of likely further deterioration.
Surgery—the last resort
Participants drew upon their own previous medical experiences in explaining some of these opinions. Not least amongst these was the feeling that surgery was a last resort, a treatment to be utilised only when other options had been exhausted.
‘When he said we're going to have to do this operation, it seemed to me like we'd reach some point of failure, which was, yeah I felt really bad for quite a while.'
That this approach was presented as standard practice, and sanctioned by expert opinion cemented this sense of failure and deterioration:
‘If I'd come on the first time and he says, well, you've got this condition and the best thing I think is for you to have an operation. I can probably fix it, but you might need to have some drops as well for a while, or bit longer, but that's the best way. Then my attitude would have been completely different… but the way it was put to me was that, you know, we take a conservative approach here. Put you on the drops, that should control it ok, and then if not, you know, surgery is kind of like… some kind of failure. That psychologically sort of, has an impact I think. Whereas, as I say, if, if the attitude of the consultant originally would have been, I think surgery is your best option, let's do that, and it would have been different.'
Research as compromised care
Two areas of concern regarding compromised care were identified from the focus group discussion:
Randomisation as a threat to best care.
Undermining doctors treatment choices.
Randomisation as threat to best care
Randomisation was both a source of confusion and concern in each focus group.
Exploring this highlighted that participants were uncertain about the relationship between their care needs and the needs of the research study, and concerned that random allocation meant a treatment path set in concrete, which could not be changed.
‘But with randomising, as I've said before, you could be doing operations on people with glaucoma that isn't all that bad, and carrying on with drops with people who really need the operation.…' ‘I'm not being conspiracy theorist, but it sort of reflected what my concern was, in that if I went the surgery route, or the drops route, I might be going on route that's not actually good for me, but good for the study. That would be my main concern'
Convincing participants that randomisation does not compromise care, and that positive clinical outcomes are equally likely no matter which treatment arm is offered, was identified as being an important element of the information required by anyone considering participation in a future research trial:
‘If you make it that clear, that the line between the two routes is so unclear, so close that it doesn't really matter which way you go. But if there was a clear gap, obviously no one would go down that route…' ‘I need to be convinced that the likely outcome was going to be an equally good, likelihood of outcomes from either arm, you know, and I'm not sure I would be, so. I don't know, I think I'd want some persuading to leave it to randomisation…'
Undermining doctors treatment choices
Throughout the focus groups, the importance of medical knowledge was evident, and the preference for certainty in the treatment of glaucoma was stressed. Research and randomisation were perceived to undermine this and to challenge the autonomy and expertise of the doctor:
P: Well I was trying to work it out, it puzzled me, it puzzled me the why the randomisation was necessary. P: It did me too, I didn't understand that. P: And it just occurred to me that if you had all the information anyway, why do it? P: I don't think the research study should control the sort of treatment you're given, so you know. P: Well, I presume it depends on my doctor, and what, you know. They should decide whether you needed surgery?
Actively consenting to participate in a research study might similarly be perceived as a mechanism for redirecting control of treatment away from the doctor, something which many focus group participants seemed uncomfortable with:
‘The way I see it, I think you making things difficult for yourself, because we are patients and we have trust in our doctors. We believe in them, and we know what they do for us is right, so I would say there's no point in asking whether you'll prefer A or B.…' P: Tell him straight away, this is a case of, err, operation, and we have no choice.…
Discussion
This study confirms many factors previously identified as influencing recruitment to surgical clinical trials, and adds to the limited work previously undertaken in glaucoma surgery.24 It demonstrates a number of common patient misunderstandings which may potentially jeopardise recruitment to any future trial in this field.
There was a lack of consistent knowledge about glaucoma and uncertainty about the preventative nature of its treatment. However, in the context of patients presenting with glaucoma for the first time, this lack of historical understanding is less important. This information would be provided before entry into any trial as part of a patient-oriented information package to ensure patient understanding of the natural history and outcome of the disease, the treatment options, and potential implications of treatment options for patients.
Although understanding of the research process was poor, a general consensus that research was helpful and essential was demonstrated. However, it was clear that patients require more information about why the research is being undertaken, and more about the clinical questions that are being addressed. In addition, it is important to highlight the rigorous process undertaken to have a research project approved, including scrutiny of the evidence to justify the research, and assessment of the research techniques by independent experts, both clinical and non-clinical. It should be emphasised that patient safety is at the forefront when these assessments are being made.
A reported willingness to undergo surgery where sight is already significantly deteriorated may benefit recruitment to a study recruiting patients with advanced glaucoma, who are likely to be symptomatic with their disease,27, 28 and the observation that eyes are special rather than being an obstacle to research may be used to support research, as it becomes more justifiable and essential to identify the treatment most likely to preserve this valuable resource.
It is important to acknowledge that surgery is associated with risks although the risk of blindness in modern glaucoma surgery is small;29, 30 similarly, medical treatments are not without potential complications and side effects, and may affect surgical success at a later stage.31, 32, 33 Accurate information regarding the true risks of vision loss and potential side effects of all potential interventions must form part of any information pack for potential trial patients.
It is unlikely that perceptions about surgery being the treatment of last resort would apply to newly diagnosed patients; recent NICE guidelines10 now recommend that surgery should be offered as a primary treatment for advanced glaucoma, although it is acknowledged that the evidence base supporting this is poor; however, this approach is consistent with the opinion of the majority of UK consultants.34
Concerns that randomisation might undermine the expertise of the doctor, and may lead to unnecessary or even ineffective treatment, was paramount. That chance should have such a significant role in governing medical treatment was considered to be inappropriate by many participants. This is a major barrier for recruitment to surgical trials in this field. Although such concerns about clinical trials have been identified elsewhere, they are seemingly heightened here due to the belief that eyes are different, and sight too precious to gamble with.
Despite a lack of clear evidence about what might constitute an effective strategy for increasing trial recruitment,5 the importance of educating and reassuring patients about the nature and process of clinical research might be inferred from this study.
In the context of glaucoma treatment, participants need to be reassured that both medical and surgical treatments are commonly used, and that both have a good degree of success. That surgery is a mature intervention, rather than an untried technique, should be stressed. Data on the risks and side effects associated with both treatments should be presented to support informed decision-making by potential participants. It should be highlighted in all recruitment and trial materials that, although short-term differences between treatment outcomes are marginal, there is real uncertainty about which treatment offers best disease control over the patients' lifetime.
Strengths and limitations
The exploration of patients' attitudes through a qualitative research process allows in-depth evaluation of the genuine concerns and opinions of patients who would be potential participants in an advanced glaucoma trial, thus generating a patient's perspective on aspects that are important to them, and identifying key barriers to recruitment. Anticipation of such barriers allows development of strategies to answer patients' concerns and material to address potential concerns, thus maximising the chances of recruitment by minimising the patients' uncertainty regarding the justification and process of the proposed research.
One weakness of this study was that although it interviewed patients who presented with advanced glaucoma, many of them had been followed up and treated for several years, and thus possessed insights, which would not be available to patients presenting with advanced glaucoma for the first time. It is unclear whether the attitudes presented here, and a general willingness to participate in a clinical trial, would be replicated with those newly diagnosed. However, importantly, there did not appear to be a general rejection of the proposed trial, which could bring into doubt its feasibility. Furthermore, participants with experience of both glaucoma and its treatment (medical and surgical) are well placed to comment upon the information needs of possible trial participants regarding the study, the proposed treatment options, and potential complications and outcomes. Therefore, consulting with those with prior experience offer benefits in the development of patient information resources for the clinical trial.
Although more males were interviewed in our study, this was not an intended bias and simply represents those patients who presented with advanced glaucoma, attending clinic during the recruitment phase of the study.
Conclusions
Qualitative analysis is an appropriate technique for extracting patients concerns regarding participation in a prospective, randomised trial. Comprehensive explanation of the need for research and that uncertainty in treatment options exist is essential. Reassuring potential participants that the research process, randomisation particularly, will not compromise clinical care is critical.
Understanding the concerns of patients and prediction of the questions potential participants are likely to ask allows the development of patient-centred material to aid them in making an informed decision about whether to agree to participation in a future trial.
Acknowledgments
This research was supported by a grant from the International Glaucoma Association.
The authors declare no conflict of interest.
References
- Elkington AR LJ, MacKean J, Sargent P. A collaborative hospital glaucoma survey. Res Clin Forums. 1982;4:31–40. [Google Scholar]
- Coffey M, Reidy A, Wormald R, Xian WX, Wright L, Courtney P. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993;77 (1:17–21. doi: 10.1136/bjo.77.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105 (11:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- Sheldrick JH, Ng C, Austin DJ, Rosenthal AR. An analysis of referral routes and diagnostic accuracy in cases of suspected glaucoma. Ophthalmic Epidemiol. 1994;1 (1:31–39. doi: 10.3109/09286589409071443. [DOI] [PubMed] [Google Scholar]
- Grant WM, Burke JF., Jr Why do some people go blind from glaucoma. Ophthalmology. 1982;89 (9:991–998. doi: 10.1016/s0161-6420(82)34675-8. [DOI] [PubMed] [Google Scholar]
- Ng WS, Agarwal PK, Sidiki S, McKay L, Townend J, Azuara-Blanco A. The effect of socio-economic deprivation on severity of glaucoma at presentation. Br J Ophthalmol. 2010;94 (1:85–87. doi: 10.1136/bjo.2008.153312. [DOI] [PubMed] [Google Scholar]
- Odberg T.Visual field prognosis in advanced glaucoma Acta Ophthalmol 198765(suppl27–29.3033979 [Google Scholar]
- Mikelberg FS, Schulzer M, Drance SM, Lau W. The rate of progression of scotomas in glaucoma. Am J Ophthalmol. 1986;101 (1:1–6. doi: 10.1016/0002-9394(86)90457-5. [DOI] [PubMed] [Google Scholar]
- Wilson R, Walker AM, Dueker DK, Crick RP. Risk factors for rate of progression of glaucomatous visual field loss: a computer-based analysis. Arch Ophthalmol. 1982;100 (5:737–741. doi: 10.1001/archopht.1982.01030030741002. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) Glaucoma; Diagnosis and management of chronic open angle glaucoma and ocular hypertension CG85In: (NICE) NIfHaCE, (ed.).National Institute for Health and Clinical Excellence (NICE): London; 2009 [Google Scholar]
- Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005;2:CD004399. doi: 10.1002/14651858.CD004399.pub2. [DOI] [PubMed] [Google Scholar]
- Treweek S, Pitkethly M, Kjeldstrom M, Taskila T, Johansen M, Sullivan F, et al. Strategies to improve recruitment to randomised clinical trials (Review) Cochrane Database Syst Rev 2010. Issue4No. MR000013. [DOI] [PubMed] [Google Scholar]
- Rendell J, Merritt R, Geddes J.Incentives and disincentives to participation by clinicians in randomised controlled trials Cochrane Database Syst Rev 2007. Issue 2: No. MR000021. [DOI] [PMC free article] [PubMed]
- Campbell M, Snowdon C, Francis D, Elbourne D, McDonald A, Knight R, et al. Recruitment to randomised trials: strategies for trial enrolment and participation study. Health Technol Assess. 2007;11:48. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]
- McDonald A, Knight R, Campbell M, Entwistle V, Grant A, Cook J, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid C, Hodges Z, Fayter D, Stirk L, Eastwood A. Increasing participation of cancer patients in randomised controlled trials: a systematic review. Trials. 2006;7:16. doi: 10.1186/1745-6215-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J, Torgerson D. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol. 2006;6:34. doi: 10.1186/1471-2288-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffer S, Torgerson D. Recruitment difficulties in randomised controlled trials. Controlled Clinical Trials. 2003;24:3. [Google Scholar]
- Spaar A, Frey M, Turk A, Karrer W, Puhan M. Recruitment barriers in a randomised controlled trial form the physicians' perspective – a postal survey. BMC Med Res Methodol. 2009;9:14. doi: 10.1186/1471-2288-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- Prescott R, Counsell C, Gillespie W, Grant A, Russell I, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3:20. [PubMed] [Google Scholar]
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324:1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Ramsay C, Norrie J. Recruitment to publicly funded trials – are surgical trials really different. Contemp Clin Trials. 2008;29:631. doi: 10.1016/j.cct.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch DC LP, CIGTS Study Group The Collaborative Initial Glaucoma Treatment Study (CIGTS): reasons for refusing to participate in a surgery vs. medicine trial. Controlled Clin Trials. 1998;19 (3:S56–S57. [Google Scholar]
- Bhargava JS, Bhan-Bhargava A, Foss AJ, King AJ.Views of glaucoma patients on provision of follow-up carean assessment of patient preferences by conjoint analysis. Br J Ophthalmol 200892(121601–1605. [DOI] [PubMed] [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3 (2:77–101. [Google Scholar]
- Freeman EE, Munoz B, West SK, Jampel HD, Friedman DS. Glaucoma and quality of life: the Salisbury Eye Evaluation. Ophthalmology. 2008;115 (2:233–238. doi: 10.1016/j.ophtha.2007.04.050. [DOI] [PubMed] [Google Scholar]
- Gupta V, Srinivasan G, Mei SS, Gazzard G, Sihota R, Kapoor KS. Utility values among glaucoma patients: an impact on the quality of life. Br J Ophthalmol. 2005;89 (10:1241–1244. doi: 10.1136/bjo.2005.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Mehta K, Shaikh NM, Tsai JC, Moster MR, Budenz DL, et al. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology. 2000;107 (12:2305–2309. doi: 10.1016/s0161-6420(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Wong TT, Khaw PT, Aung T, Foster PJ, Htoon HM, Oen FT, et al. The Singapore 5-Fluorouracil Trabeculectomy Study: effects on intraocular pressure control and disease progression at 3 years. Ophthalmology. 2009;116 (2:175–184. doi: 10.1016/j.ophtha.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Broadway D, Hitchings R. Conjunctival damage induced by long-term topical anti-glaucoma therapy. Acta Ophthalmol Scand. 1996;74 (1:97. [PubMed] [Google Scholar]
- Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993;77 (9:590–596. doi: 10.1136/bjo.77.9.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112 (11:1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- Stead R, King AJ, Azuara-Blanco A.Attitudes of consultant ophthalmologists in the UK to initial management of glaucoma patients presenting with severe visual field loss: a national survey Clin Experiment Ophthalmol 2011. e-pub ahead of print 14 June 2011; doi: 10.1111/j.1442-9071.2011.02574.x [DOI] [PubMed]