Abstract
Purpose
To evaluate the 5-year follow-up of safety, efficacy, predictability, stability, and complications of anterior iris-fixated phakic intraocular lens (pIOL) implantation to correct high myopia, and patients' satisfaction after implantation.
Design
Prospective, nonrandomized, and comparative (self-controlled) trial.
Methods
A prospective clinical trial of 84 eyes of 43 patients with high myopia was conducted. Uncorrected visual acuity (UCVA), best spectacle-corrected visual acuity (BSCVA), refraction, endothelial cell count, intraocular pressure (IOP), anterior chamber depth, slit lamp biomicroscopy, and indirect ophthalmoscope were measured preoperatively and postoperatively.
Results
At the 5-year follow-up, UCVA was significantly improved, with 85.7% of eyes reaching 20/25 or better. No eyes experienced a loss in BSCVA, and 71.4% gained one or more lines of their preoperative BSCVA. There was a significant reduction in spherical errors in all patients after operation. Loss of endothelial cells was observed 3 years after operation and no more loss was observed 4 years after operation in statistical analysis. No increase in IOP was observed 5 years after operation in statistical analysis. No intraoperative complications were observed in this study. However, pigment precipitates of varying intensities on the lens optic were noted in all patients 1 day after operation, and only five eyes were observed to have the pigment residual five years after operation.
Conclusion
At the 5-year follow-up, the implantation of the anterior iris-fixated pIOL was proved to be effective, predictable and capable of reversibility to correct high myopia in phakic eyes. It was a safety addition to the laser refractive surgery. However, longer follow-up with larger numbers of patients is still necessary to evaluate long-term complications.
Keywords: iris-fixated, IOL, phakic, high myopia
Introduction
The first surgical option to correct myopia is cornea refractive, such as laser in situ keratomileusis (LASIK), photorefractive keratectomy, laser epithelial keratomileusis, epipolis LASIK, and so on. To treat high-myopia patients, the new techniques can make the cornea flap thinner and thinner; however, this increases the risk of corneal ectasia, thus decreasing visual quality.1 In recent years, phakic lens implantation in the anterior or posterior chamber has gained increasing popularity for high-myopia treatment;2 it offers a promising alternative, particularly for high-myopia correction up to −20.0 diopters (D). These intraocular operations retain the patients' clear lens, and thus the accommodation function is reserved; they maintain the original prolate shape of the cornea, as a result of which the optical qualities of the cornea are not altered.3, 4 Over the past few years, many kinds of refractive lenses have been implanted with success in phakic eyes to correct myopia, hyperopic, and astigmatism.5, 6 In 1986, Worst and Fechner modified the existing iris-fixated intraocular lens (IOL) into a negatively biconcave IOL with a convex–concave optic design. Initially, the lens was called the worst myopia-fixated IOL, later named the Artisan (Ophtec BV, Groningen, The Netherlands);7, 8, 9 now it is known as the Verisyse (Advanced Medical Optics, Inc., Santa Ana, CA, USA).
This prospective study was to evaluate the 5-year postoperative clinical and refractive results of anterior iris-fixated phakic IOL (pIOL) implantation for the correction of high myopia.
Patients and methods
In all, 84 eyes of 43 patients had surgeries at the Refractive Centre, Department of Ophthalmology, Jinling Hospital, Nanjing, China, from March 2004 to December 2005. All the patients were fully informed about the details and risks of the procedure and provided written informed consents.
Indication
Patients fulfilling the following criteria were included in the study: (1) they were aged between 18 and 40; (2) had a stable refraction for at least 1 year; (3) had myopia >−8.0 D and cannot undergo a cornea refractive operation; (4) had a clear cornea and endothelial cell count (ECC) >2200 cells/mm2; (5) had a central anterior chamber depth (ACD) >3.2 mm; (6) had an otherwise normal ophthalmologic examination and unsatisfactory correction with spectacles or contact lenses.
Contraindication
Patients with the following conditions were excluded from the study: (1) anisometropia; (2) anterior segment pathology; (3) inadequate eyelid closure; (4) ECC <1800 cells/mm2; (5) central ACD <3.0 mm; (6) abnormal iris or pupil function; (7) intraocular pressure (IOP) >21 mm Hg; (8) previous corneal or intraocular surgery; (9) any intraocular eye disease such as recurrent or chronic uveitis, cataract, glaucoma, or family history of glaucoma, retinal detachment or family history of retinal detachment, preexisting macular degeneration or macular pathology; (10) systemic diseases, chronic treatment with corticosteroids or any immunosuppressive treatment or state, and pregnancy.
Lenses
The biomaterial of the Verisyse anterior iris-fixated pIOL is a plane–concave design, made by poly methyl methacrylate, with effective ultraviolet light filtration up to approximately 400 nm. The overall length is 8.5 mm, IOL powers ranging from −3.0 to −23.5 D are available for the optic diameter of 5.0 mm, and IOL powers ranging from 3.0 to −15.5 D are available for the optic diameter of 6.0 mm. The lens calculations were based on the special calculating software, VeriCalc1.1 (Advanced Medical Optics, Inc.), and also can be received by the IOL-MASTER (Zeiss, Minneapolis, MN, USA) directly.
Operation
Thirty minutes before the operation, the miotic drop (1% pilocarpine) was given every 5 min, 3 times, then 0.4% oxybuprocaine hydrochloride topical anesthetic was given three times. A superior sclerocorneal self-sealing 5.3- to 5.5-mm incision and two paracenteses were created. A cohesive ophthalmic visco surgical device (sodium hyaluronate 1% (Healon)) was injected through the paracenteses to maintain ACD and protect endothelial cells. Further, the IOL was placed into the anterior chamber and was enclavated onto the iris, after which the visco was removed. All eyes underwent a peripheral iridotomy during the operation.
Follow-up
All the eyes were examined 1 week, 1 and 6 months, and 1, 2, 3, 4, and 5 years after surgery. The examination includes uncorrected visual acuity (UCVA), best spectacle-corrected visual acuity (BSCVA), IOP, IOL position, pupil shape, computerized corneal topography, contrast sensitivity, ECC, and indirect ophthalmoscopes. Slit lamp biomicroscopy was used to determine IOL position and to evaluate crystalline lens changes after mydriasis, as well as the shape of pupil. The IOL-MASTER (Zeiss) was used to measure the ACD. The UBM was used to examine the anterior chamber angle function.
Statistics
SPSS version 11.0. (SPSS Inc., New York, NY, USA) was used for descriptive statistical analysis. Continuous variables were described using mean, SD, median, and minimum and maximum values. Images are presented in a data graph format.
Results
In all, 84 eyes were enrolled in this prospective study between March, 2004 and December, 2005. All eyes were available for examination at 5 years. The mean preoperative sphere was −13.56±2.06 D (range, −8.05 to −22.0 D), the mean preoperative cylinder was −0.95±0.56 D (range, −1.5 to 0.00 D), and the mean preoperative spherical equivalent was −14.17±2.56 D (range, −8.75 to −22.5 D). The mean preoperative ACD was 3.4 mm (range, 3.23–3.86 mm). Mean axial length was 27.8 mm (range, 26.33–30.03 mm). Demographic data are given in Table 1.
Table 1. Summary of preoperative data for patients undergoing implantation of an anterior iris-fixated lens.
| Variable | Data |
|---|---|
| Total patients (eyes) | 43 (84eyes) |
| Average age (years) | 27.22 |
| Range of age (years) | 18–39 |
| Female (eyes) | 28 (55eyes) |
| Male (eyes) | 15 (29eyes) |
| Mean spherical equivalent±SD (D) | 14.17±2.56 |
| Range of SE (D) | −8.75 to −22.5 |
| Mean sphere±SD (D) | 13.56±2.06 |
| Range of sphere (D) | −8.05 to −22.0 |
| Mean cylinder±SD (D) | −0.95±0.56 |
| Range of cylinder (D) | −1.5 to 0.0 |
| Range of anterior chamber depth (mm) | 3.4 |
| Range of axial length (mm) | 27.8 |
| Implant power (D) | −8.5 to −22.0 |
| Range of preoperative scoptopic pupil size (mm) | 4.88–7.21 |
| Range of preoperative mesopic pupil size (mm) | 2.8–4.66 |
Abbreviation: D, diopter.
Visual acuity
In Table 2, the mean preoperative UCVA was 0.064±0.02. At 1 week postoperatively, 70.2% of the eyes reached 20/25 or better and 9.5% reached 20/15 or better. At 6 months postoperatively, 79.7% of the eyes reached 20/25 or better and 23.8% reached 20/15 or better. At 3 years postoperatively, 85.7% of the eyes reached 20/25 or better and 28.5% reached 20/15 or better. At 5 years postoperatively, 85.7% of the eyes reached 20/25 or better and 21.4% reached 20/15 or better. Results are shown in Tables 2 and 5. The mean preoperative BSCVA was 0.68±0.12 (range, 0.5–1.5), postoperatively, 71.4% of the eyes reached 20/25 or better and 7.1% reached 20/15 or better. At 5 years postoperatively, 89.2% of the eyes reached 20/25 or better and 27.4% reached 20/15 or better. Results are shown in Table 3. During the 5-year follow-up, none of the eyes experienced a loss in BSCVA, and 71.4% gained one or more lines of their preoperative BSCVA, at 5 years, 59.5% of the eyes gained one or more lines of best-corrected visual acuity, and 40.5% remained unchanged. Results are shown in Table 4.
Table 2. UCVA preoperative and 6 months, 3, and 5-years after implantation of iris-fixated pIOP.
| UCVA (eyes) | >20/20 | 20/20 | 20/25 | 20/30 | 20/40 | <20/200 |
|---|---|---|---|---|---|---|
| Pre-O(84) | 0 | 0 | 0 | 0 | 0 | 84 |
| 1-week Post-O (84) | 8 | 20 | 31 | 20 | 5 | |
| 6-month Post-O (84) | 20 | 25 | 22 | 14 | 3 | 0 |
| 3-year Post-O (84) | 24 | 27 | 21 | 12 | 0 | 0 |
| 5-year Post-O (84) | 18 | 15 | 39 | 10 | 2 | 0 |
Abbreviations: Post-O, postoperation; Pre-O, preoperation; UCVA, uncorrected visual acuity.
Table 3. BSCVA preoperative and 6 months after implantation of iris-fixated pIOP.
| BSCVA (eyes) | >20/20 | 20/20 | 20/25 | 20/30 | 20/40 | <20/40 |
|---|---|---|---|---|---|---|
| Pre-O (84) | 6 | 38 | 16 | 12 | 8 | 4 |
| 5-year Post-O (84) | 23 | 32 | 20 | 7 | 2 | 0 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; Post-O, postoperation; Pre-O, preoperation.
Table 4. Gained and lost BSCVA 6 months, 3, and 5 years after implantation of iris-fixated pIOP, postoperative compared with preoperative.
| BSCVA (eyes) | Lost | Unchanged | Gained one line | Gained two lines | Gained more lines |
|---|---|---|---|---|---|
| 6 months (84) | 0 | 51 | 18 | 13 | 2 |
| 3 years (84) | 0 | 50 | 25 | 6 | 3 |
| 5 years (84) | 0 | 34 | 14 | 28 | 8 |
| Post–pre | 0 | 24 | 32 | 12 | 16 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; Post–pre, the BSCVA at 6-month postoperative compared with preoperative.
D
The mean D preoperatively was 14.17 D, and the mean Verisyse anterior iris-fixated pIOL implanted was 14.11 D. The change in refraction (spherical equivalent) and subjective refraction remained almost stable for 1–2 years postoperatively; one to two lines of UCVA were lost during postoperative years 3–5 and the BSCVA was stable with little or no changes in the 5-year follow-up period. Results are shown in Table 5.
Table 5. UCVA, BSCVA and IOP changes preoperation and postoperative after the iris-fixated pIOL implantation.
| UCVA | BSCVA | IOP | |
|---|---|---|---|
| Preoperative | 0.064±0.02 | 0.68±0.12 | 16.86±2.99 |
| Postoperative | |||
| 1 week | 0.83±0.12 | — | 17.34±3.02 |
| 1 month | 0.88±0.14 | — | 13.23±2.88 |
| 6 month | 0.94±0.12 | 0.95+0.08 | 14.43±2.38 |
| 1 year | 0.95±0.11 | 0.96+0.08 | 14.23±3.02 |
| 2 year | 0.95±0.13 | 0.96+0.10 | 14.13±2.22 |
| 3 year | 0.96±0.11 | 0.96+0.08 | 14.26±3.01 |
| 4 year | 0.91±0.17 | 0.96+0.04 | 14.23±2.31 |
| 5 year | 0.90±0.18 | 0.95+0.08 | 14.25±1.26 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; IOP, intraocular pressure; UCVA, uncorrected visual acuity.
Intraocular pressure
No statistically significant changes were observed in IOP during the follow-up. After 5 years, the mean IOP was 14.25±1.26 mm Hg (range 9.5–18.8 mm Hg). Results are shown in Table 5.
ECC
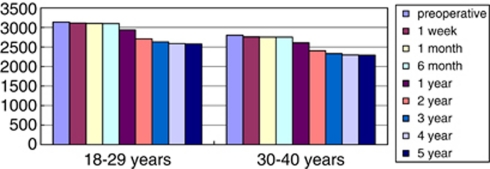
The patients were divided into two groups based on the age. In group 1, comprising patients aged between 18 and 29 years, the mean preoperative ECC was 3133.67±211 cells/mm2 (range, 2900–3423 cells/mm2). In group 2, comprising patients aged between 30 and 40 years, the mean preoperative ECC was 2800±189 cells/mm2 (range, 2200–3010 cells/mm2). At the 6-month follow-up, there was no significant ECC loss; at 1-year postoperative examination, the overall cell loss reached 5.3% at 2-year examination, the loss reached 7.8% at 3-year examination, the loss reached 2.9% at 4-year examination, the loss reached 1.5% and at 5-year examination, less loss of ECC was found. Results are shown in Figure 1.
Figure 1.
Bar graph change in ECC after implanting the iris-fixated pIOL.
Location of pIOL and shape of pupil
The location of pIOL and shape of the pupil were observed using the Slitlamp biomicroscope (Zeiss), and in no case was an pIOL optic departure from the visual centre found; the pupil turned to an oval shape in 88% of eyes on the first day after operation, but 100% recovered to a round shape with sensitivity reflection at the 6-month follow-up. Only one piece of pIOL was completely dislocated by the outside force. The post-operative eye was heavily heated by fist 4 years after the operation, the dislocated IOL caused a cornea edema. The reposition operation was performed successfully, and the UCVA was 20/20 at 1 month after surgery. This indirectly implied the good reversibility of this kind of IOL.
Computerized corneal topography and contrast sensitivity
The computerized corneal topography and contrast sensitivity were examined preoperatively, and 1 and 5 years postoperatively. The original prolate shape of the cornea did not change, and the contrast sensitivity was improved greatly.
Complication
During the 5-year follow-up, all the surgeries were uncomplicated. Spontaneous postoperative occurrence of persistent corneal edema or dystrophy and IOL dislocation was not detected. Although retinal complications have been reported to be as high as 3% in pIOL implantation,2 no retinal problems were observed after operation in this study. At 1 day after operation, 10 eyes had increased IOP (28.9 mm Hg), without additional local or systemic therapy, they all normalized at the 7-day examination and never increased in the next 5 years. All eyes had iris pigment precipitates of varying intensities on the lens optic, 87% were mostly absorbed at 6-month follow-up, and only 6% can be observed with some iris pigment remaining on the optic surface. Halos and glare were the common complaints of night-driving persons, which were usually associated with a scotopic pupil larger than the optic diameter of the lens. However, the complaint disappeared at the 2- to 5-year follow-up. None of the patients needed secondary intervention. No potentially sight-threatening complications, such as iris prolapse, iris atrophy, and touch of the anterior lenticular capsule, persistent corneal edema, pupillary block, cataract formation, retinal detachment, or endophthalmitis, were observed during the follow-up period.
Discussion
Surgical options for correcting high myopia include corneal or limbal relaxing incisions, PRK, and LASIK.2, 10, 11 Although LASIK is the leading refractive technique for correcting refractive errors, it has limited ability to correct higher myopia. It also changes the corneal curvature irreversibly and may change its optical quality by inducing additional visual aberrations.7 Refractive lens exchange as an alternative procedure is still controversial. The rate of retinal detachment ranges from 1.9 to 8.1%12, 13, 14, 15 and still increases in eyes with high myopia with this procedure. The concomitant loss of accommodation is another disadvantage, especially in younger patients.
After a 5-year follow-up, our data demonstrated that the iris-fixated pIOL implantation was a safe, effective, and a predictable option in reversible procedure for high-myopia treatment; it is an effective complementarity for refraction surgery.
Considering the safety, there was less intraocular tissue damage, accommodation and corneal curvature were preserved, and no major complications were found. The Verisyse iris-fixated pIOL is a plane–concave design, the thin optic part can protect the endothelial cells and self-lens; this may minimize the cataract postoperatively. Development of glaucoma and progressive endothelial cell loss are major concerns.7 An anterior pIOL usually can induce glaucoma by compromising the anterior chamber angle, but the iris-fixated anterior IOL was fixated on the iris, avoiding the trabecular meshwork damage. Preoperative iridotomy or an intraoperative iridectomy can avoid the pupil block, and thus, in this study, all eyes underwent a peripheral iridotomy during the operation. Transitory increase in IOP after operation for several days was observed, some due to the Healon leftover, some due to the intense local steroidal eye drops, but no persistent increase in IOP was observed during the 5-year follow-up. Progressive endothelial cell loss has been a major concern with anterior chamber IOLs. In this study, we did not detect significant endothelial cell loss during the 6-month follow-up, but a mean endothelial loss of 5.3% at 1-year, 7.8% at 2-year, 2.9% at 3-year, 1.5% at 4-year, and less loss at 5-year follow-up was detected; the hexagonality and coefficient variation in cell size were close to the preoperative levels at the 3-year follow-up. These morphological changes recovered to reach nearly preoperative levels, suggesting that endothelial damage occurred primarily during the operation procedure and the damage stopped after the procedure. The cause of pigment dispersion may be mechanical, forces and the rigid haptics may lead to iris pigment abrasion during pupillary movement. Pupillary block can also occur in patients with anterior chamber IOLs, either by direct blocking by the optic or by the adhesions between vitreous and posterior iris, but none was observed in this study. Nonprogressive lens vacuoles had not been observed.1 Pupil ovalization can also be a complication of anterior chamber IOLs; in this study, all pupils returned to round after 6 months.
Considering the efficiency, the UCVA after operation is important. Mean BSCVA was 0.68±0.12 preoperatively and improved to 0.95±0.08 postoperatively. Spherical error was significantly reduced. Underestimate effects caused by the spectrum were eliminated in all cases. Compared with equal diameter laser ablation, this IOL is closer to the pupil, resulting in a better coverage, which achieves less night-associated photic phenomena.1 LASIK and PRK also have been associated with alteration of the corneal shape in myopic patients.16, 17 This can increase coma and spherical-order aberrations and decrease contrast sensitivity. Implantation of iris-fixated IOLs has been shown to maintain optical performance,4 also with the potential to improve contrast sensitivity.18
Considering predictability, 93.2% of the eyes were within the ±1.0 D of the desired correction. Postoperative optical performance was also significantly enhanced. In all the eyes, postoperative UCVA was improved, with 85.7% of the eyes achieving 20/25 or better. In all, 59.5% of the eyes gained one or two lines of BSCVA after operation at the 5-year follow-up.
Iris-fixated IOLs have been associated with various other complications, such as cataract, glaucoma, chronic subclinical inflammation, and pupil ovalization.2 Surgically related complications, including transient corneal edema, transient IOP elevation, or hyphema, can be minimized by accurate surgical training and by mastering the special iris-fixated implantation technique.10 Eyes with a shallow anterior chamber, extensive eye rubbing, or IOL dislocation, will induce significant endothelial cell loss.2 Therefore, strict preoperative exclusion criteria are mandatory; moreover, high-precision measurements will contribute to excellent postoperative outcomes and minimize potential complications.
In this 5-year follow-up after implantation of an anterior iris-fixated lens, good refractive results with excellent predictability and efficacy were found. None of the patients experienced permanent IOP increase, corneal decompensation, synechia, iris atrophy, chronic inflammation, or pupil ovalization. All patients felt satisfied with the visual acuity. Progressive endothelial cell loss could not be detected after 3 years. Although there are some risks associated with pIOLs, they still remain the most promising alternative to keratorefractive options.19 In this procedure, suture is not required, and many complications can be avoided; thus, pIOLs are widely used in aphakic eye at present. More patients and a longer follow-up period are needed to determine further benefits pertaining to this treatment modality.

The authors declare no conflict of interest.
References
- Maloney RK, Nguyen LH, John ME. Artisan phakic intraocular lens for myopia: short-term results of a prospective, multicenter study. Ophthalmology. 2002;109:1631–1641. doi: 10.1016/s0161-6420(02)01170-3. [DOI] [PubMed] [Google Scholar]
- Dick HB, Tehrani M. Phakic intraocular lenses. Current status and limitations (in German) Ophthalmologe. 2004;101:232–245. doi: 10.1007/s00347-004-0990-8. [DOI] [PubMed] [Google Scholar]
- Sarver EJ, Sanders DR, Vukich JA. Image quality in myopic eyes corrected with laser in situ keratomileusis and phakic intraocular lens. J Refract Surg. 2003;19:397–404. doi: 10.3928/1081-597X-20030701-04. [DOI] [PubMed] [Google Scholar]
- Brunette I, Bueno JM, Harissi-Dagher M, Parent M, Podtetenev M, Hamam H. Optical quality of the eye with the Artisan phakic lens for the correction of high myopia. Optom Vis Sci. 2003;80:167–174. doi: 10.1097/00006324-200302000-00013. [DOI] [PubMed] [Google Scholar]
- Landesz M, van Rij G, Luyten G. Irisclaw phakic intraocular lens for high myopia. J Refract Surg. 2001;17:634–640. doi: 10.3928/1081-597X-20011101-01. [DOI] [PubMed] [Google Scholar]
- Landesz M, Worst JG, van Rij G. Long-term results of correction of high myopia with an iris claw phakic intraocular lens. J Refract Surg. 2000;16:310–316. doi: 10.3928/1081-597X-20000501-03. [DOI] [PubMed] [Google Scholar]
- Dick HB, Alió J, Bianchetti M, Budo C, Christiaans BJ, El-Danasoury MA, et al. Toric phakic intraocular lens; European multicenter study. Ophthalmology. 2003;110:150–162. doi: 10.1016/s0161-6420(02)01447-1. [DOI] [PubMed] [Google Scholar]
- Landesz M, van Rij G, Luyten G. Iris-claw phakic intraocular lens for high myopia. J Refract Surg. 2001;17:634–640. doi: 10.3928/1081-597X-20011101-01. [DOI] [PubMed] [Google Scholar]
- Landesz M, Worst JGF, Van Rij G. Long-term results of correction of high myopia with an iris claw phakic intraocular lens. J Refract Surg. 2000;16:310–316. doi: 10.3928/1081-597X-20000501-03. [DOI] [PubMed] [Google Scholar]
- Budo C, Hessloehl JC, Izak M, Luyten GP, Menezo JL, Sener BA, et al. Multicenter study of the Artisan phakic intraocular lens. J Cataract Refract Surg. 2000;26:1163–1117. doi: 10.1016/s0886-3350(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Güell JL, Vázquez M, Malecaze F, Manero F, Gris O, Velasco F, et al. Artisan toric phakic intraocular lens for the correction of high astigmatism. Am J Ophthalmol. 2003;136:442–447. doi: 10.1016/s0002-9394(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Colin J, Robinet A, Cochener B.Retinal detachment after clear lens extraction for high myopia; seven-year follow-up Ophthalmology 19991062281–2284.discussion by M Stirpe, 2285. [DOI] [PubMed] [Google Scholar]
- Colin J, Robinet A. Retinal detachment after clear lens extraction in 41 eyes with high axial myopia (letter) Retina. 1997;17:78–79. [PubMed] [Google Scholar]
- Barraquer C, Cavelier C, Mejia LF. Incidence of retinal detachment following clear-lens extraction in myopic patients; retrospective analysis. Arch Ophthalmol. 1994;112:336–339. doi: 10.1001/archopht.1994.01090150066025. [DOI] [PubMed] [Google Scholar]
- Alió JL, de la Hoz F, Pérez-Santonja JJ, Ruiz-Moreno JM, Quesada JA. Phakic anterior chamber lenses for the correction of myopia; a 7-year cumulative analysis of complications in 263 cases. Ophthalmology. 1999;106:458–466. doi: 10.1016/S0161-6420(99)90103-3. [DOI] [PubMed] [Google Scholar]
- Marcos S, Barbero S, Llorente L, Merayo-Lloves J. Optical response to LASIK surgery for myopia from total and corneal aberration measurements. Invest Ophthalmol Vis Sci. 2001;42:3349–3356. [PubMed] [Google Scholar]
- Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery:LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001;42:1396–1403. [PubMed] [Google Scholar]
- Dick HB, Tehrani M, Aliyeva S. Contrast sensitivity after implantation of toric iris-claw lenses in phakic eyes. J Cataract Refract Surg. 2004;30:2284–2289. doi: 10.1016/j.jcrs.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Menezo JL, Avino JA, Cisneros A, Rodriguez-Salvador V, Martinez-Costa R. Iris claw phakic intraocular lens for high myopia. J Refract Surg. 1997;13:545–555. doi: 10.3928/1081-597X-19970901-11. [DOI] [PubMed] [Google Scholar]