Abstract
There are more microorganisms that colonize the human body than resident cells; some are commensal whereas others are pathogenic. Pathogenic microorganisms are sensed by the innate or adaptive immune system, an immune response is initiated, and the infection is often cleared. Some microorganisms have developed strategies to evade immune defenses, ensuring their long-term survival with potentially devastating consequences for the host. Approximately 18% of all cancers can be attributed to infective agents; the most common being Helicobacter pylori, Human papilloma virus (HPV) and Hepatitis B and C virus in causing stomach, cervical and liver carcinoma, respectively. This review focuses on whether HPV infection is necessary for initiating pterygia, a common benign condition and ocular-surface squamous neoplasia (OSSN), a rare disease with metastatic potential. The search engine PubMed was used to identify articles from the literature related to HPV and pterygium or conjunctival neoplasia. From 34 investigations that studied HPV in pterygia and OSSN, a prevalence rate of 18.6% (136/731) and 33.8% (144/426), respectively, was recorded. The variation in HPV prevalence (0–100%) for both disease groups may have arisen from study-design faults and the techniques used to identify the virus. Overall, the data suggest that HPV is not necessary for initiating either condition but may be a co-factor in susceptible hosts. Currently, over 60 million people worldwide have been immunized with HPV vaccines, but any effect on pterygium and OSSN development may not be known for some time as these lesions can evolve over decades or occur in older individuals.
Keywords: virus, microbes, limbus, infection, ultraviolet radiation
Introduction
When the Human Microbiome Project is complete, a comprehensive assessment of the human microbiota and its role in health and disease will be appreciated.1 The human skin2 and oral cavity3 microbiome was recently characterized, with each study identifing over 200 species-level phylotypes. Most recently, Dong et al4 disclosed a similar profile of microbes on the healthy human conjunctiva; some were characterized whereas others (31%) were novel or unclassified. Approximately 18% of all cancers can be attributed to microorganisms, the most common being Helicobacter pylori, Human Papilloma virus (HPV), Hepatitis B and C virus, Epstein-Barr virus and Human Herpes virus.5 Although these microbes are known to target specific tissues, such as the stomach, liver, and cervix, some are associated with tumors that arise on the ocular surface.6
The ocular-surface transition zone and stem-cell damage
The epithelium of the ocular surface is exposed to the environment and hence vulnerable to infection, especially when its primary defensive barriers including mucins, tears, and superficial cellular layers are compromised. Ocular-surface tumors including benign pterygia and potentially invasive ocular-surface squamous neoplasia (OSSN) have a tendency to develop from the transition zone between two functionally distinct cell types, namely the corneal and conjunctival epithelia. This propensity is not restricted to the eye and occurs in other transition zones, including esophagogastric, endo-ectocervix, and anorectal junction, where spontaneous or viral-induced transformation is common but the molecular and cellular basis is not completely understood.7 The corneo–conjunctival transition zone is known as the limbus, a narrow vascular-rich region that harbors a rare population of unipotent epithelial stem cells (SCs) whose function is to continuously replenish damaged, diseased or dead cells from the cornea,8, 9, 10 thereby maintaining corneal health and clarity, two features essential for good vision. Limbal epithelial stem cells are distinguishable morphologically as small basal cells with higher nuclear to cytoplasmic content, and phenotypically through increased expression of a number of markers including the adhesion molecule N-cadherin, integrins β1 and α6, cytokeratins (CK)-14, -15 and -19, transcription factor p63, and the membrane transporter protein, ABCG2.10, 11
It has been proposed that environmental ultraviolet radiation (UVR) is the most likely initiating agent for ocular-surface tumors and ‘peripheral light focusing' may explain the nasal limbal predilection for pterygia and possibly OSSN.12 In this model, it was demonstrated that the cornea acts as a side-on lens, focusing incident light by up to 20-fold to a distal point on the nasal limbus; the level of focusing dependent on corneal shape and depth of the anterior chamber.13, 14 The cells most likely to be affected are those residing within the limbus, as they are struck from behind by intense radiation, causing them to become activated, damaged or mutated.15 Central-corneal epithelial cells may be spared from damaging rays either due to the angle of internally reflected light or from the presence of Bowman's layer, which is thought to act as a natural UV-blocker.16
Pterygia: characteristics and pathogenic mechanisms
Although pterygia were observed some 3000 years ago,17 today the pathogenesis is still incompletely understood. Clinically, the lesion is characterized by a fleshy triangular-shaped fibrovascular pannus of inflamed conjunctival tissue that grows over the cornea. Once the growth impinges on the visual axis, causes astigmatism, reduces ocular motion or appears atypical, it is surgically excised.18 Histologically, pterygia are characterized by degenerative and proliferative changes.19 Many theories have been proposed to explain how pterygia develop including; autosomal dominant mode of inheritance, immunologically-mediated, tear film disruptions, chronic UVR exposure, and viral infection.20 After two decades of studying this disease, our group has developed a working hypothesis that proposes UVR as the principal triggering agent. We have accrued indirect evidence for this posit from modeling-, epidemiological-, and laboratory-based studies.13, 14, 19, 20, 21, 22, 23, 24, 25 However, Detorakis et al26 identified potential viral co-factors in pterygium pathogenesis and proposed a ‘two-hit' theory for its development. The first hit is a damaging reaction mediated by UVR exposure that causes genetic alteration or mutations, and the second hit is an oncogenic event mediated by viral infection in susceptible or compromised cells.26 Indeed, this is a plausible model, as viruses such as HPVs are known tumor-promoting pathogens.6
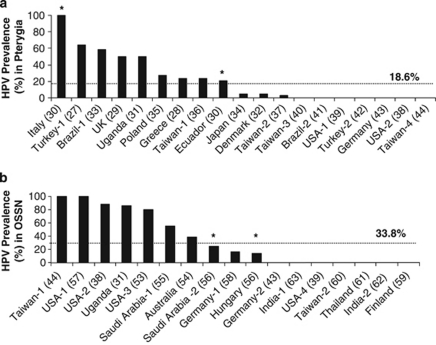
After entering key words ‘Pterygia' and ‘HPV' into PubMed and further refining the search to include only original articles written in English that assessed four or more biological samples from patients with pterygia, 18 articles were selected. Eleven investigations detected HPVs,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 whereas other studies were unable to detect the virus38, 39, 40, 41, 42, 43, 44 (Figure 1a and Table 1). From these 18 studies, the average prevalence for HPV was 18.6% (range 0–100%), corresponding to 136/731 positive specimens (Figure 1a). The wide range in prevalence between studies may reflect sampling and methodological differences. For example, racial susceptibility may have been a factor and racial mix within a study population was not always disclosed. In one study, specimens from Italian and Ecuadorean patients with pterygia were studied and HPV prevalence reported as 100% and 21% respectively, implying a geographic and/or ethnic component.30 Moreover, some studies were retrospective, some prospective, and others were both. Another reason for the variation in prevalence may have been due to differences in methodology and sensitivity between techniques. Most studies used PCR-based assays with primers for specific HPV types, sensitive enough to amplify low levels of viral DNA. Yet others were able to localize HPV proteins in infected cells using less sensitive in situ hybridization and immunohistochemical techniques. Often, selective HPVs were investigated, suggesting other genotypes that may have contributed to the disease process, were not considered. Of note, appropriate positive and negative assay and sample controls (eg, disease-free subjects) were often not included. Biospecimens (archival formalin-fixed vs fresh-frozen), quality and quantity often varied, and cross-contamination between specimens may have also contributed to variations in the assessment. Finally, only few studies confirmed their primary results with an alternative method (Table 1).
Figure 1.
Prevalence of HPV in Ocular-Surface Diseases. Data from the literature were collated on the prevalence of HPV in pterygia (a) and OSSN (b), which ranged from 0 to 100% in both disease groups. HPV was more common (1.8-fold) in OSSN than in pterygia. Asterisks (*) in panels (a and b) denote data from the same investigation but from a different ethnic source. The numbers in parenthesis along the x-axis denote respective references.
Table 1. Detection of HPV in pterygia.
| Investigators (country of origin)ref | Sample size | Prevalence (%) | HPV subtypes | Method |
|---|---|---|---|---|
| Piras et al (Italy)30 | 17 | 100 | 52, 54, candHPV90 | PCR/sequencing |
| Varinli et al (Turkey)27 | 25 | 64 | Not defined | IHC |
| Rodrigues et al (Brazil)33 | 36 | 58.3 | 1, 2 | PCR |
| Gallagher et al (UK)29 | 10 | 50 | 6, 11, 16 | PCR |
| Ateenyi-Agaba et al (Uganda)31 | 10 | 50 | 11, 37 | PCR/Southern |
| Piecyk-Sidor et al (Poland)35 | 58 (51 primary, 7 recurrent) | 27.6 | 6, 16, 18 | PCR |
| Detorakis et al (Greece)28 | 50 | 24 | 18 | PCR |
| Tsai et al (Taiwan)36 | 129 | 24 | 16, 18 | PCR/IHC |
| Piras et al (Ecuador)30 | 24 | 21 | 52, 54, candHPV90 | PCR/sequencing |
| Takamura et al (Japan)34 | 42 (40 primary, 2 recurrent) | 4.8 | Not defined | PCR/HC-II |
| Sjo et al (Denmark)32 | 90 primary | 4.4 | 6 | PCR/ISH |
| Hsiao et al (Taiwan)37 | 65 (35 primary, 30 recurrent) | 3 | 18 | PCR/ISH |
| Chen et al (Taiwan)40 | 65 | 0 | Nil detected | PCR |
| Schellini et al (Brazil)41 | 36 primary | 0 | Nil detected | PCR |
| Dushku et al (USA)39 | 13 (12 primary, 1 recurrent) | 0 | Nil detected | PCR |
| Otlu et al (Turkey)42 | 40 (30 primary, 10 recurrent) | 0 | Nil detected | PCR |
| Guthoff et al (Germany)43 | 11 | 0 | Nil detected | PCR/IHC |
| McDonnell et al (USA)38 | 6 | 0 | Nil detected | PCR |
| Kuo et al (Taiwan)44 | 4 | 0 | Nil detected | PCR |
Abbreviations: HC-II, hybrid capture; IHC, immunohistochemistry; ISH, in situ hybridization; PCR, polymerase chain reaction.
OSSN: characteristics and pathogenic mechanisms
OSSN is regarded the most common ocular-surface tumor.45 The term OSSN is used to describe a spectrum of disease that ranges from mild, moderate, and severe dysplasia, to carcinoma in situ, to invasive conjunctival epithelial squamous cell carcinoma (SCC). OSSN is regarded as a rare disease with a propensity to affect men in their sixth decade; although early onset has been noted in younger individuals who are human immunodeficiency virus (HIV) seropositive46 and in children with xeroderma pigmentosum.47 OSSN is often referred to as immune-associated (mediated by diminished immune surveillance), as the risk of developing this disease is increased by 12-fold in HIV-infected individuals48 and by 20-fold in liver-transplant patients.49
Conjunctival SCC represents the most severe form of OSSN, causing ocular morbidity and if left untreated can result in mortality.50 Metastasis to lymph nodes, enucleation, and exenteration is common.51 Newton et al52 determined that the incidence of conjunctival SCC declined by 49% with each 10-degree change in latitude, falling from >12 cases/million/year in Uganda to <0.2 cases/million/year in the UK. Additional risk factors include fair skin and ocular pigmentation, and smoking. Histologically, the disease is characterized by changes in basal, suprabasal, superficial, and full thickness epithelium. Specific cellular changes include increased hyperchromatism, pleomorphism, and loss of polarity, which in its most severe form, coincides with tumor cells breaching the basement membrane and invading into the stromal matrix.45
A strategy similar to that performed for pterygium (see above) was employed to survey the literature to identify studies that looked for HPV in OSSN. From the 42 studies identified, 16 studies were appropriate after applying the same inclusion/exclusion criteria used to assess associations between HPV and pterygium. From these investigations, 9 successfully detected HPV by various techniques,31, 38, 44, 53, 54, 55, 56, 57, 58 whereas the remaining were unsuccessfully39, 43, 59, 60, 61, 62, 63 (Figure 1b and Table 2). From these 16 studies, an average prevalence rate of 33.8% (range 0–100%) was observed, representing 144/426 HPV-positive specimens (Figure 1b). Interestingly, the prevalence for HPV was 1.8-fold greater in OSSN than for pterygia (Figures 1a and b). Overall, the data presented in the current report (Figures 1a and b) contrasts with the 80% prevalence rate of HPV in conjunctival papillomas in over 200 cases, most of which were classified as ‘low-risk' HPV types -6 and -11.64, 65 Differences in HPV prevalence rates for OSSN may be explained by the same design faults identified in studies that assessed HPV in pterygia. The most severe limitation from studies that analyzed both diseases was that HIV status was not disclosed. This is relevant particularly in regions where HIV is endemic (eg, Sub-Saharan Africa), as it has been shown that HIV infection increases the risk of developing conjunctival tumors by greater than 10-fold.48, 66 Interestingly, three investigations assessed HPV status in both disease groups. Dushku et al39 and Guthoff et al43 failed to detect HPV in their pterygium and OSSN specimens, whereas McDonnell et al38 identified HPV-16 in 88% (37/42) of their OSSN specimens but not in their pterygia (0/6). McDonnell's study suggests that HPV type may be involved in the development of one lesion over another; however their samples were not matched in number.38 Despite this, at least three independent studies observed HPV-16 in their pterygium specimens.29, 35, 36
Table 2. Detection of HPV in OSSN.
| Investigators (country of origin)ref | Sample size (disease) | Prevalence (%) | HPV subtypes | Method |
|---|---|---|---|---|
| Kuo et al (Taiwan)44 | 9 (Dysplasia) | 100 | 6, 11, 16, 18, 33, 37, 58, 72 | PCR |
| Scott et al (USA)57 | 10 (Dysplasia) | 100 | 16, 18 | PCR |
| McDonnell et al (USA)38 | 42 (OSSN) | 88.1 | 16 | PCR/DB |
| Ateenyi-Agaba et al (Uganda)31 | 21 (SCC) | 86 | 14, 24, 37, 38 | PCR |
| Lauer et al (USA)53 | 5 (OSSN) | 80 | 16, 18 | PCR |
| Karcioglu et al (Saudi Arabia)55 | 45 (CIS/SCC) | 55.6 | 16, 18 | PCR |
| Tabrizi et al (Australia)54 | 88 (OSSN) | 39 | 6, 11, 13, 16, 18 | PCR |
| Toth et al (Saudi Arabia)56 | 16 (SCC) | 25 | 16 | PCR |
| Auw-Haedrich et al (Germany)58 | 12 (Dysplasia) | 16.7 | 16 | PCR |
| Toth et al (Hungary)56 | 7 (SCC) | 14.3 | 18 | PCR |
| Guthoff et al (Germany)43 | 31 (OSSN) | 0 | Nil detected | PCR/IHC |
| Manderwad et al (India)63 | 48 (OSSN) | 0 | Nil detected | PCR/ISH-CARD |
| Dushku et al (USA)39 | 8 (OSSN) | 0 | Nil detected | PCR |
| Eng et al (Taiwan)60 | 20 (OSSN) | 0 | Nil detected | PCR |
| Tulvatana et al (Thailand)61 | 30 (OSSN) | 0 | Nil detected | PCR/DB |
| Sen et al (India)62 | 30 (OSSN) | 0 | Nil detected | IHC |
| Tuppurainen et al (Finland)59 | 4 (CIS/SCC) | 0 | Nil detected | PCR/ISH |
Abbreviations: CIS, carcinoma in situ; DB, dot blot; dysplasia, mild, moderate, and severe dysplasia; IHC, immunohistochemistry; ISH-CARD, in situ hybridization-catalyzed reporter deposition; OSSN, ocular surface squamous neoplasia; PCR, polymerase chain reaction.
Concurrent disease: pterygia and OSSN
Pterygia have been referred to as benign tumors due to their local invasiveness and propensity to recur but not to metastasize. Sevel and Sealy67 reported that 29% of their pterygium specimens had histological evidence of dysplasia. Since this report, other groups, including ours, have reported concurrent pre-neoplastic disease within pterygia, including OSSN at a rate of 5–10%19, 68 and primary acquired melanosis in approximately 8% of the study population.19, 69 How concurrent disease develops is not entirely clear. Moreover, it is yet to be established whether these pathological changes (often focal) represent separate disease entities or whether one arises from another as part of a continuous disease spectrum. Indeed, if HPV infection has a role in the pathogenesis of pterygia and OSSN, then viral type (ie, high vs low risk) may be a contributing factor.38 Interestingly, both high- and low-risk HPV types have been identified in the same pterygium specimen (Table 1), however a comprehensive histological review was not performed to assess for concurrent disease.29, 35
Characteristics of papilloma viruses
The papilloma virus was the first virus identified to cause cancer in mammals.70 These viruses are small non-enveloped epitheliotrophic DNA viruses able to infect stratified squamous mucosal, cutaneous, and other epithelia of birds and mammals. The viral genome comprises a circular double-stranded DNA of approximately 8000 bp that includes early-region sequences or open-reading frames designated as E1–E8 that vary in number between HPV types and are expressed shortly after infection. The genome also contains two latent regions labeled L1 and L2, which encode the capsid (outer shell) protein that has a role in viral entry. Over 200 different HPV types have been identified but not all have been characterized;71 those that have are either commensal or cause disease. HPV types are region/organ specific; for example, HPV-1 and -2 cause common warts, HPV-5 and -8 are associated with non-melanoma skin cancer (NMSC) in patients with the inherited disease epidermodysplasia verruciformis,72 and HPV-16 and -18 are responsible for over 70% of cervical cancer.71 Notably, HPV-16 is thought to be one of the most carcinogenic agents known to mankind.
Mode of HPV infection and DNA integration
Precisely how HPV infects epithelial cells is not fully understood but it is thought that the virus gains access to a selective cell population through a micro-abrasion or other trauma.73 In the cervix, the virus targets basal-epithelial progenitor cells, a strategy it uses to ensure stable infection and indefinite passage of genetic information to the host. In terms of ocular-surface disease, it is possible that HPV targets epithelial SCs that have been traumatized or damaged from UVR exposure,8, 15, 74 a model that would certainly support Detorakis's two-hit hypothesis.26 Another reason for targeting epithelial SCs may be that these cells harbor unique viral-entry receptors that are not expressed on differentiated superficial cells. It has been shown that many viruses including Rota,75 Foot and Mouth Disease,76 Coxsackie,77 West Nile,78 and Herpes Simplex79, 80 utilize RGD (Arg-Gly-Asp)-dependent integrin receptors, most commonly αvβ3. There is recent evidence that HPV-entry receptors include α6 intergin81 and laminin 5.82 The virus adheres to the plasma membrane through engagement of the caspid L1 protein with either receptor and is subsequently absorbed through vesicles. Interestingly, α6 intergin is a potential corneal83 and cutaneous84 epithelial SCs marker, hence a receptor exists on these cells for HPV entry. Upon internalization, HPV L2 disrupts the vesicle membrane, the capsid ruptures spilling its contents into the cytosol. The viral genome is transported to the nucleus, integrates into the host's genome, and is transcribed into functionally active proteins responsible for not only maintaining the viral genome but also negatively influencing a number of host tumor-suppressor genes, including p53 and retinoblastoma protein (pRb), thereby promoting immortalization. Once expressed, E6 and E7 are the predominant proteins responsible for suppressing p53 and pRb function.85
Role of HPV in p53 tumor-suppressor inactivation
Abnormal proliferation is a characteristic feature of the pterygium epithelium as indicated by increased expression of cell-cycle-related proteins, such as cyclin D1, Ki-67 and the proliferating cell-nuclear antigen.86, 87 This process is further amplified through elevated anti-apoptotic proteins survivin88 and BCL-2.89 The tumor-suppressor protein p53 is a widely studied factor in pterygia; normally present in low or undetectable levels within a cell, this protein functions to induce cell-cycle arrest, DNA repair or apoptosis.90 In pterygia, p53 levels are increased39, 87, 91 and several studies have reported abnormal p53 associated with HPV,33, 36 suggesting a role for viral oncoproteins in suppressing p53 activity. This contrasts with Dushku et al,39 who detected increased p53 in pterygium without evidence of HPV infection, leading the authors to postulate that enhanced p53 was due to UV-induced mutagenesis.
Anti-apoptotic and cell-proliferative markers are also elevated in OSSN,92 some studies reporting even higher levels than in pterygia.93 In one study that sourced cases and controls from Uganda, a region where the prevalence of HIV infection and sun exposure is high, genetic changes in p53 (consistent with a molecular signature of UV-induced mutagenesis) were identified in 52% of cases and 14% of controls, and DNA from epidermodysplasia verruciformis HPV-38 was detected in 10 out of 11 cases with p53 mutations.94 This is in contrast to Toth's study56 which detected p53 in 78% of ocular-surface tumors, 22% of which were HPV positive, again implying no association between HPV and p53. Despite these mixed data on the effect of HPV on p53 function; HPV-18 E6 is known to bind p53 and induce its degradation through the ubiquitin-proteolysis pathway.85 This in turn compromises the cell's ability to effect growth arrest upon UV-induced DNA damage.95 Overall these data imply that HPV is not required for p53 dysfunction in OSSN. However, in a susceptible host, HPV may act as a contributing pathogenic factor.
Host response to HPV and immune evasion
The response to HPV infection can be slow. If professional antigen-presenting cells such as dendritic cells or macrophages encounter virus, then the innate immune system is activated to clear the infection. If the virus manages to evade being phagocytosed and infects its target epithelial cell, antigen presentation by the epithelium, although possible,96 may be inefficient or delayed. An immune reaction is initiated when cytotoxic T-cell sense viral peptides presented on the host cell's plasma membrane through major histocompatibility complexes (MHC). HPV may also evade immune surveillance by either suppressing MHC expression97 or depleting professional antigen-presenting cells.98 Recently, HPV-16 E6 and E7 proteins were shown to abolish Toll-like receptor 9 expression.99 These data support the notion that reducing the immune response may be a critical step towards carcinogenic events mediated by HPVs.
HPV activity and UVR
The observation that HPV was found in an involved and uninvolved eye with OSSN 8 years after excision and eradication of the lesion, suggests the virus is able to remain latent.38 The mechanism of HPV latency is not well understood, however, UVR may have a role in re-activation in cutaneous and ocular-surface-associated cancers. Purdie et al100 demonstrated that HPV-77 has a consensus binding site for p53 and its promoter activity is enhanced by UVR. Furthermore, HPV-77 localizes to sun-exposed skin and could be involved in the development of NMSC. In addition, Akjul et al101 demonstrated that the non-coding promoter region of HPV-5 and HPV-8 (also associated with NMSC) was strongly attenuated following UVB exposure. UVR also stimulates promoter activity of the epidermodysplasia verruciformis-related HPV type -5, -20, -23, and -38 in a skin keratinocyte cell line.102 UVR-mediated re-activation of viral latency has been observed in Herpes Simplex virus103 and HIV.104
Conclusions
The current study reports the prevalence of HPV in ophthalmic pterygia and OSSN as 18% and 33%, respectively. This contrasts with stronger associations (∼80%) for conjunctival papilloma. Overall, these data suggest that the involvement of HPV in pterygia and OSSN is inconclusive. Moreover, the International Agency for Research on Cancer (IARC) regarded the evidence as weak105 and in 2011 the IARC's report did not mention HPV as a carcinogenic mediator of ocular-surface disease.106 As seen from the evidence presented in Figure 1 and Tables 1 and 2, the field is divided and conclusions made have often been based on sub-optimal study designs and techniques used to identify HPVs in biological specimens. The ocular field is in desperate need of a ‘gold-standard' detection and tissue-collection protocol for assessing HPV infection. A multiplex approach to identify all known HPV within a single specimen and larger case-control studies are warranted.
HPV genotypes most represented in pterygia and OSSN include types 6, 11, 16, and 18 (Tables 1 and 2). Infection with these genotypes is preventable by the quadrivalent vaccine Gardacil (Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA). Hence, if effective, the vaccine may reduce the incidence or may potentially eradicate ocular disease attributable to HPV infection. The global immunization program for HPV began in 2007 and currently 60 million individuals (mainly young women) have been vaccinated. Unfortunately, immediate benefits (if any) for patients with eye disease will not be apparent for years as pterygia can require decades to develop, and OSSN is generally a disease of elderly men. Alternative treatments that do not involve surgery or a vaccine are being trialed. Immunotherapy with interferon-alpha 2b has been used in patients with HPV-16/18-positive conjunctival neoplasia. This naturally occurring cytokine has anti-viral, anti-neoplastic, and anti-proliferative actions, which involve immune-enhancing properties. However, local and systemic discomforts, as well as recurrences have been noted.107
Precisely how HPV infects the ocular surface is unknown, however infection from mother to newborn during birth has been speculated.108 Moreover, digital self-inoculation is possible as HPV DNA was noted on the fingers of males and females positive for genital HPV.109 Although none of the studies highlighted in this review screened for HPV-related genital disease, good personal hygiene is a reasonable starting point to minimize the risk of ocular infection.
Acknowledgments
N Di Girolamo is supported by a Career Development Award (no. 455358) from the National Health and Medial Research Council of Australia.
The author declares no conflict of interest.
References
- NIH Human Microbiome Project . http://nihroadmap.nih.gov/hmp .
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E, Keijser BJF, Huse SM, Crielaard W. Defining the healthy ‘core microbiome' of oral microbial communities. BMC Microbiol. 2009;9:259–271. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller D, et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52:5408–5413. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Verma V, Shen D, Sieving PC, Chan C-C. The role of infectious agents in the etiology of ocular adnexal neoplasia. Surv Ophthalmol. 2008;53:312–331. doi: 10.1016/j.survophthal.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNairn AJ, Guasch G. Epithelial transition zones: merging microenvironments, niches, and cellular transformation. Eur J Dermatol. 2011;21 (Suppl 2:21–28. doi: 10.1684/ejd.2011.1267. [DOI] [PubMed] [Google Scholar]
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng S-Z, Dong G, Sun T-T, Lavker RM. Existance of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Li W, Hayashida Y, Chen Y-T, Tseng SCG. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria TJ, Di Girolamo N. Tissue-regenerating, vision-restoring corneal epithelial stem cells. Stem Cell Rev Rep. 2011;7:256–268. doi: 10.1007/s12015-010-9199-1. [DOI] [PubMed] [Google Scholar]
- Coroneo MT. Albedo concentration in the anterior eye: A phenomenon that locates some solar diseases. Ophthalmic Surg. 1990;21:60–66. [PubMed] [Google Scholar]
- Coroneo MT. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg. 1991;22:705–711. [PubMed] [Google Scholar]
- Maloof AJ, Ho A, Coroneo MT. Influence of corneal shape on limbal light focusing. Invest Ophthalmol Vis Sci. 1994;35:2592–2598. [PubMed] [Google Scholar]
- Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- Kolozsvari L, Nogradi A, Hopp B, Bor Z. UV absorbance of the human cornea in the 240- to 400-nm range. Invest Ophthalmol Vis Sci. 2002;43:2165–2168. [PubMed] [Google Scholar]
- Bidyadhar NK. Susruta and his ophthalmic operations. Arch Ophthalmol. 1939;22:550–574. [Google Scholar]
- Figueira EC, Coroneo MT, Francis IC. Preventing conjunctival autograft inversion in pterygium surgery. Br J Ophthalmol. 2007;91:83–84. doi: 10.1136/bjo.2006.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui J, Coroneo MT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygia: a stem cell disorder with pre-neoplastic features. Am J Pathol. 2011;178:817–827. doi: 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, metalloproteinases, and ultraviolet light. Prog Ret Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, McCluskey PJ, Lloyd A, Coroneo M, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–679. [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin/MMP-7 in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–1968. [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Wakefield D. UVB-mediated induction of Interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–3437. [PubMed] [Google Scholar]
- Nolan TM, Di Girolamo N, Coroneo MT, Wakefield D. Proliferative effects of heparin-binding epidermal growth-like growth factor on pterygium epithelial cells and fibroblasts. Invest Ophthalmol Vis Sci. 2004;45:110–113. doi: 10.1167/iovs.03-0046. [DOI] [PubMed] [Google Scholar]
- Chui J, Di Girolamo N, Coroneo MT, Wakefield D. The role of substance P in the pathogenesis of pterygia. Invest Ophthalmol Vis Sci. 2007;48:4482–4489. doi: 10.1167/iovs.07-0123. [DOI] [PubMed] [Google Scholar]
- Detorakis ET, Drakonaki EE, Spandidos DA. Molecular genetic alterations and viral presence in ophthalmic pterygium. Int J Mol Med. 2000;6:35–41. [PubMed] [Google Scholar]
- Varinli S, Varinli I, Koksal-Erkisi M, Doran F. Human papillomavirus in pterygium. Central Afr J Med. 1994;40:24–26. [PubMed] [Google Scholar]
- Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001;20:164–167. doi: 10.1097/00003226-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Giannoudis A, Herrington CS, Hiscott P. Human papillomavirus in pterygium. Br J Ophthalmol. 2001;85:782–784. doi: 10.1136/bjo.85.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Moore PS, Ugalde J, Perra MT, Scarpa A, Sirigu P. Detection of human papillomavirus DNA in pterygia from different geographic regions. Br J Ophthalmol. 2003;87:864–866. doi: 10.1136/bjo.87.7.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateenyi-Agaba C, Weiderpass E, Smet A, Dong W, Dai M, Kahwa B, et al. Epidermodysplasia verruciformis human papillomavirus types and carcinoma of the conjunctiva: a pilot study. Br J Cancer. 2004;90:1777–1779. doi: 10.1038/sj.bjc.6601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjo NC, von Buchwald C, Ulrik Prause J, Norrild B, Vinding T, Heegaard S. Human papillomavirus and pterygium. Is the virus a risk factor. Br J Ophthalmol. 2007;91:1016–1018. doi: 10.1136/bjo.2006.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriues FW, Arruda JT, Silva RE, Moura KKVO. TP53 gene expression, codon 72 polymorphism and human papillomavirus DNA associated with pterygium. Genet Mol Res. 2008;7:1251–1258. doi: 10.4238/vol7-4gmr528. [DOI] [PubMed] [Google Scholar]
- Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Detection of human papillomavirus in pterygium and conjunctival papilloma by hybrid capture II and PCR assays. Eye. 2008;22:1442–1445. doi: 10.1038/eye.2008.176. [DOI] [PubMed] [Google Scholar]
- Piecyk-Sidor M, Polz-Dacewicz M, Zagorski Z, Zarnowski T. Occurrence of human papillomavirus in pterygia. Acta Ophthalmol. 2009;87:890–895. doi: 10.1111/j.1755-3768.2008.01372.x. [DOI] [PubMed] [Google Scholar]
- Tsai Y-Y, Chang C-C, Chiang C-C, Yeh K-T, Chen P-L, Chang C-H, et al. HPV infection and p53 inactivation in pterygium. Mol Vis. 2009;15:1092–1097. [PMC free article] [PubMed] [Google Scholar]
- Hsiao CH, Lee BH, Ngan KW, Chuang WY, Yeung L, Yeh LK, et al. Presence of human papillomavirus in pterygium in Taiwan. Cornea. 2010;29:123–127. doi: 10.1097/ICO.0b013e3181afdb06. [DOI] [PubMed] [Google Scholar]
- McDonnell JM, McDonnell PJ, Sun YY. Human papillomavirus DNA in tissues and ocular surface swabs of patients with conjunctival epithelial neoplasia. Invest Ophthalmol Vis Sci. 1992;33:184–189. [PubMed] [Google Scholar]
- Dushku N, Hatcher SL, Albert DM, Reid TW. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch Ophthalmol. 1999;117:1593–1599. doi: 10.1001/archopht.117.12.1593. [DOI] [PubMed] [Google Scholar]
- Chen K-H, Hsu W-M, Cheng C-C, Li Y-S. Lack of human papillomavirus in pterygium of Chinese patients from Taiwan. Br J Ophthalmol. 2003;87:1046–1048. doi: 10.1136/bjo.87.8.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellini SA, Hoyama E, Shiratori CA, Sakamota RH, Candeias JMG. Lack of papillomavirus (HPV) in pterygia of a Brazilian sample. Arq Bras Oftalmol. 2006;69:519–521. doi: 10.1590/s0004-27492006000400012. [DOI] [PubMed] [Google Scholar]
- Otlu B, Emre S, Turkcuoglu P, Doganay S, Durmaz R. Investigation of human papillomavirus and Epstein-Barr virus DNAs in pterygium tissue. Eur J Ophthalmol. 2009;19:175–179. doi: 10.1177/112067210901900201. [DOI] [PubMed] [Google Scholar]
- Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–671. doi: 10.1080/02713680903007162. [DOI] [PubMed] [Google Scholar]
- Kuo K-T, Chang H-C, Hsiao C-H, Lin M-C. Increased Ki-67 proliferation index and absence of P16INK4 in CIN-HPV related pathogenic pathways different from cervical squamous intraepithelial lesion. Br J Ophthalmol. 2006;90:894–899. doi: 10.1136/bjo.2005.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults; a clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- Chisi SK, Kollmann MK, Karimurio J. Conjunctival squamous cell carcinoma in patients with human immunodeficiency virus infection seen at two hospitals in Kenya. East Afr Med J. 2006;83:267–270. doi: 10.4314/eamj.v83i5.9432. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Hassan RY, Pindiga UH. Xeroderma pigmentosum in three consecutive siblings of a Nigerian family: observations on oculocutaneous manifestations in black African children. Br J Ophthalmol. 2001;85:110–111. doi: 10.1136/bjo.85.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guech-Ongey M, Engels EA, Goedert JJ, Biggar RJ, Mbulaiteye SM. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer. 2008;122:2590–2593. doi: 10.1002/ijc.23384. [DOI] [PubMed] [Google Scholar]
- Vajdic CM, van Leeuwen MT, McDonald SP, McCredie MRE, Law M, Chapman JR, et al. Increased incidence of squamous cell carcinoma of eye after kidney transplantation. J Natl Cancer Inst. 2007;99:1340–1342. doi: 10.1093/jnci/djm085. [DOI] [PubMed] [Google Scholar]
- Ogun GO, Ogun OA, Bekibele CO, Akang EE. Intraepithelial and invasive squamous neoplasms of the conjunctiva in Ibadan, Nigeria: a clinicopathological study of 46 cases. Int Ophthalmol. 2009;29:401–409. doi: 10.1007/s10792-008-9257-8. [DOI] [PubMed] [Google Scholar]
- McKelvie PA, Daniell M, McNab A, Loughnan M, Santamaria JD. Squamous cell carcinoma of the conjunctiva: a series of 26 cases. Br J Ophthalmol. 2002;86:168–173. doi: 10.1136/bjo.86.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R, Ferlay J, Reeves G, Beral V, Parkin DM. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. Lancet. 1996;347:1450–1451. doi: 10.1016/s0140-6736(96)91685-2. [DOI] [PubMed] [Google Scholar]
- Lauer SA, Malter JS, Meier JR. Human papillomavirus type 18 in conjunctival intraepithelial neoplasia. Am J Ophthalmol. 1990;110:23–27. doi: 10.1016/s0002-9394(14)76932-6. [DOI] [PubMed] [Google Scholar]
- Tabrizi SN, McCurrach FE, Drewe RH, Borg AJ, Garland SM, Taylor HR. Human papilomavirus in corneal and conjunctival carcinoma. Aust NZ J Ophthalmol. 1997;25:211–215. doi: 10.1111/j.1442-9071.1997.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Karcioglu ZA, Issa TM. Human papilloma virus in neoplastic and non-neoplastic conditions of the external eye. Br J Ophthalmol. 1997;81:595–598. doi: 10.1136/bjo.81.7.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth J, Karcioglu ZA, Moshfeghi AA, Issa TM, Al-Ma'ani JR, Patel KV. The relationship between human papillomavirus and p53 gene in conjunctival squamous cell carcinoma. Cornea. 2000;19:159–162. doi: 10.1097/00003226-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–547. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- AuW-Haedrich C, Martin G, Spelsberg H, Sundmacher R, Freudenberg N, Maier P, et al. Expression of p16 in conjunctival intraepithelial neoplasia does not correlate with HPV-infection. Open J Ophthalmol. 2008;2:48–56. doi: 10.2174/1874364100802010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppurainen K, Raninen A, Kosunen O, Kankkunen JP, Kellokoski J, Syrjanen S, et al. Squamous cell carcinoma of the conjunctiva: failure to demonstrate HPV DNA by in situ hybridization and polymerase chain reaction. Acta Ophthalmol. 1992;70:248–254. doi: 10.1111/j.1755-3768.1992.tb04132.x. [DOI] [PubMed] [Google Scholar]
- Eng H-L, Lin T-M, Chen S-Y, Wu S-M, Chen W-J. Failure to human papillomavirus DNA in malignant epithelial neoplasms of the conjunctiva by polymerase chain reaction. Am J Clin Pathol. 2002;117:429–436. doi: 10.1309/RVUP-QMU3-5X6W-3CQ1. [DOI] [PubMed] [Google Scholar]
- Tulvatana W, Bhattarakosol P, Sansopha L, Sipiyarak W, Kowitdamrong E, Paisuntornsug T, et al. Risk factors for conjunctival squamous cell neoplasia: a matched case-control study. Br J Ophthalmol. 2003;87:396–398. doi: 10.1136/bjo.87.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Sharma A, Panda A. Immunohistochemical localization of human papilloma virus in conjunctival neoplasias: a retrospective study. Ind J Ophthalmol. 2007;55:361–363. doi: 10.4103/0301-4738.33822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderwad GP, Kannabiran C, Honavar SG, Vemuganti GK. Lack of association of high-risk human papillomavirus in ocular surface squamous neoplasia in India. Arch Pathol Lab Med. 2009;133:1246–1250. doi: 10.5858/133.8.1246. [DOI] [PubMed] [Google Scholar]
- Sjo NC, von Buchwald C, Cassonnet P, Norrild B, Prause JU, Vinding T, et al. Human papillomavirus in normal conjunctival tissue and in conjunctival papilloma: types and frequencies in a large series. Br J Ophthalmol. 2007;91:1014–1015. doi: 10.1136/bjo.2006.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DS, Powell N, Fiander AN. Will vaccination against human papillomavirus prevent eye disease? A review of the evidence. Br J Ophthalmol. 2008;92:460–465. doi: 10.1136/bjo.2007.135038. [DOI] [PubMed] [Google Scholar]
- Newton R, Ziegler J, Ateenyi-Agaba C, Bousarghin L, Casabonne D, Beral V, et al. The epidemiology of conjunctival squamous cell carcinoma in Uganda. Br J Cancer. 2002;87:301–308. doi: 10.1038/sj.bjc.6600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevel D, Sealy R. Pterygia and carcinoma of the conjunctiva. Trans Ophthalmol Soc UK. 1969;88:567–578. [PubMed] [Google Scholar]
- Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127:31–32. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- Perra MT, Colombari R, Maxia C, Zucca I, Piras F, Corbu A, et al. Finding of conjunctival melanocytic pigmented lesions within pterygium. Histopathology. 2006;48:387–393. doi: 10.1111/j.1365-2559.2006.02346.x. [DOI] [PubMed] [Google Scholar]
- Rous P, Beard JW. Carcinomatous changes in virus-induced papilloma of the skin of rabbits. Proc Soc Exp Biol Med. 1934;32:578–580. [Google Scholar]
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117 (Suppl 1:S5–S10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Majewski S, Jablonska S. Epidermodysplasia verruciforms as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312–1318. [PubMed] [Google Scholar]
- Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nature Rev Immunol. 2004;4:46–55. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- Tseng SCG, Chen JJY, Huang AJW, Kruse FE, Maskin SL, Tsai RJF. Classification of conjunctival surgeries for corneal diseases based on stem cell concept. Ophthalmic Clin Nth Am. 1990;3:595–609. [Google Scholar]
- Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. Integrin αvβ3 mediates Rotavirus cell entry. Proc Natl Acad Sci USA. 2000;97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff S, Mason PW, Baxt B. High-efficiency utilization of the bovine integrin αvβ3 as a receptor of Foot-and-Mouth Disease virus is dependent on the bovine β3 subunit. J Virol. 2000;74:7298–7306. doi: 10.1128/jvi.74.16.7298-7306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, et al. Human Coxackie-adenovirus receptor is colocalized with integrins αvβ3 and αvβ5 on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy. Circulation. 2001;104:275–280. doi: 10.1161/01.cir.104.3.275. [DOI] [PubMed] [Google Scholar]
- Chu JJ-h, Ng M-L. Interaction of West Nile virus with αvβ3 integrin mediates virus entry into cells. J Biol Chem. 2004;279:54533–54541. doi: 10.1074/jbc.M410208200. [DOI] [PubMed] [Google Scholar]
- Garrigues HJ, Rubinchikova YE, DiPersio CM, Rose TM. Integrin αvβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpsvirus and functions as an RGD-dependent entry receptor. J Virol. 2008;82:1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni T, Gatta V, Campadelli-Fiume G. αvβ3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc Natl Acad Sci USA. 2010;107:22260–22265. doi: 10.1073/pnas.1014923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C-S, Kim K-D, Park S-N, Cheong S-W. α6 integrin is the main receptor of human papillomavirus type 16 VLP. Biochem Biophys Res Commun. 2001;2283:668–673. doi: 10.1006/bbrc.2001.4838. [DOI] [PubMed] [Google Scholar]
- Culp TD, Budgeon LR, Marinkovich MP, Meneguzzi G, Christensen ND. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruss by binding virions and transferring them to adjacent cells. J Virol. 2006;80:8940–8950. doi: 10.1128/JVI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Saito T, Oshima T, Okano T, Tano Y, et al. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin α6 and CD71. Biochem Biophy Res Comm. 2008;367:256–263. doi: 10.1016/j.bbrc.2007.12.077. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Viestra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kanazawa S, Kitaoka T, Dake Y, Ohira A, Quertani AM, et al. Immunohistochemical study of p53, p21 and PCNA in pterygium. Acta Histochem. 2001;103:159–165. doi: 10.1078/0065-1281-00584. [DOI] [PubMed] [Google Scholar]
- Chowers I, Pe'er J, Zamir E, Livni N, Ilsar M, Frucht-Pery J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 2001;108:985–988. doi: 10.1016/s0161-6420(00)00651-5. [DOI] [PubMed] [Google Scholar]
- Maxia C, Perra MT, Demurtas P, Minerba L, Murtas D, Piras F, et al. Expression of survivin protein in pterygium and relationship with oxidative DNA damage. J Cell Mol Med. 2008;12:2372–2380. doi: 10.1111/j.1582-4934.2008.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000;84:212–216. doi: 10.1136/bjo.84.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–7655. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997;123:404–405. doi: 10.1016/s0002-9394(14)70141-2. [DOI] [PubMed] [Google Scholar]
- Mahomed A, Chetty R. Human immunodeficiency virus infection, Bcl-2, p53 protein, and Ki-67 analysis in ocular surface squamous neoplasia. Arch Ophthalmol. 2002;120:554–558. doi: 10.1001/archopht.120.5.554. [DOI] [PubMed] [Google Scholar]
- Ohara M, Sotozono C, Tsuchihashi Y, Kinoshita S. Ki-67 labeling index as a marker of malignancy in ocular surface neoplasms. Jpn J Ophthalmol. 2004;48:524–529. doi: 10.1007/s10384-004-0129-0. [DOI] [PubMed] [Google Scholar]
- Ateenyi-Agaba C, Dai M, Le Calvez F, Katongole-Mbidde E, Smet A, Tommasino M, et al. TP53 mutations in squamous-cell carcinomas of the conjunctiva: evidence qfor UV-induced mutagenesis. Mutagenesis. 2004;19:399–401. doi: 10.1093/mutage/geh048. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Storey A. Repair of UV-induced thymine dimers is compromised in cells expressing the E6 protein from human papillomavirus types 5 and 18. Br J Cancer. 2004;90:2203–2209. doi: 10.1038/sj.bjc.6601829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy GT, Hooper DC, Easty DL. Antigen presentation of herpes simplex virus by corneal epithelium- an in vitro and in vivo study. Br J Ophthalmol. 1993;77:440–444. doi: 10.1136/bjo.77.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi GH, Brown DR, Fife KH, Campo MS. Down-regulation of MHC class I is a property common to papillomavirus E5 protein. Virus Res. 2006;120:208–211. doi: 10.1016/j.virusres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-caherin. J Virol. 2003;77:8376–8385. doi: 10.1128/JVI.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- Purdie KJ, Pennington J, Proby CM, Khalaf S, de Villiers E-M, Leigh IM, et al. The promoter of a novel human papillomavirus (HPV77) associated with skin cancer displays UV responsiveness, which is mediated through a consensus p53 binding sequence. EMBO J. 1999;18:5359–5369. doi: 10.1093/emboj/18.19.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul B, Lemme W, Garcia-Escudero R, Story A, Pfister HJ. UV-B irradiation stimulates the promoter activity of the high-risk, cutaneous human papillomavirus 5 and 8 in primary keratinocytes. Arch Virol. 2005;150:145–151. doi: 10.1007/s00705-004-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Villiers E-M, Ruhland A, Sekaric P. Human papillomavirus in non-melanoma skin cancer. Cancer Biol. 1999;9:413–422. doi: 10.1006/scbi.1999.0145. [DOI] [PubMed] [Google Scholar]
- Spruance SL. Pathogenesis of Herpes Simplex Labialis: experimental induction of lesions with UV light. J Clin Microbiol. 1985;22:366–368. doi: 10.1128/jcm.22.3.366-368.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka BZ, Beer JZ. Activation of human immunodeficiency virus by ultraviolet radiation. Photochem Photobiol. 1990;52:1153–1162. doi: 10.1111/j.1751-1097.1990.tb08454.x. [DOI] [PubMed] [Google Scholar]
- IARC IARC Monographs on the evaluation of carcinogenic risks to humansHuman papillomaviruses, 2007. Available at http://monographs.iarc.fr/ ENG/Monographs/vol90/index.php .
- IARC IARC Monographs on the Evaluation of Carcinogenic Risks to HumansA Review of Human Carcinogens: Biological Agents, 2011. Available at http://monographs.iarc.fr/ENG/ Monographs/vol100B/index.php .
- Chen H-C, Chang S-W, Huang S-F. Adjunctive treatment with interferon a-2b may decrease the risk of papilloma-associated conjunctival intraepithelial neoplasm recurrence. Cornea. 2004;23:726–729. doi: 10.1097/01.ico.0000126320.36014.d1. [DOI] [PubMed] [Google Scholar]
- Egbert JE, Kersten RC. Female genital tract papillomavirus in conjunctival papillomas of infancy. Am J Ophthalmol. 1997;123:551–552. doi: 10.1016/s0002-9394(14)70184-9. [DOI] [PubMed] [Google Scholar]
- Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect. 1999;75:317–319. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]