Abstract
Purpose
Retinal laser photocoagulation is used to treat a variety of retinal diseases. Breakdown of the blood-aqueous barrier has been noted after retinal laser photocoagulation. The effect of vascular endothelial growth factor (VEGF) on the function of the blood-aqueous barrier after retinal laser photocoagulation remains undetermined. The current study was designed to evaluate the relationship between intraocular levels of VEGF and breakdown of the blood-aqueous barrier after retinal laser photocoagulation in rabbits.
Methods
Pigmented rabbits were treated with retinal laser photocoagulation in one eye; the other served as control. Laser flare photometry was carried out on post-treatment days 1, 3, 7, and 14. Animals were sacrificed at the time period just mentioned postlaser, the eyes were removed, and samples of vitreous and aqueous humor were collected. Intraocular VEGF levels were measured by using an immunoassay. An intravitreal injection of VEGF was administered, and the aqueous flare intensity and VEGF levels in the aqueous and vitreous humor were measured at the time periods just mentioned.
Results
A significant increase in the aqueous flare intensity after retinal laser photocoagulation was noticed on postoperative day 1, with the values returning to baseline levels on day 14. The VEGF levels in the vitreous of the lasered eyes were significantly increased on day 1 compared with the nonlasered control eyes. The VEGF levels in the aqueous humor of the lasered eyes were also significantly increased on day 1 compared with the control eyes. An intravitreal injection of VEGF induced a significant increase in the aqueous flare intensity and VEGF levels in the aqueous and vitreous humor.
Conclusions
The current results suggested that retinal laser photocoagulation can produce a breakdown of the blood-aqueous barrier. VEGF may play a role in the blood-aqueous barrier dysfunction after retinal laser photocoagulation.
Introduction
Retinal laser photocoagulation is an effective, widely accepted method for treating a variety of retinal disorders, including preretinal neovascularization in proliferative diabetic retinopathy,1 branch retinal vein occlusion,2 and rubeosis iridis complicating ischemic central retinal vein occlusion.3 The retinal lesions that develop after laser photocoagulation have been shown to alter the blood-retinal barrier.4–6 The release of chemical mediators, polymorphonuclear infiltration, and leakage of protein into the vitreous have been demonstrated to be indicative of breakdown of the blood-retinal barrier.7–9 Retinal laser photocoagulation is also noted to induce macular edema, traction retinal detachment, choriovitreal proliferation, and subretinal neovascular membrane in the posterior segment of the eye.10 Consequently, the effects of various pharmacologic agents in the reduction of the severity of the breakdown of the blood-retinal barrier after laser photocoagulation have been reported.9,11 However, the complications of laser photocoagulation in the anterior segment have not yet been fully understood. Anterior chamber depth alteration, keratopathy, lens opacity, and acute intraocular pressure elevation after panretinal photocoagulation have been shown in animals and humans.12–15
Vascular endothelial growth factor (VEGF) was originally isolated as a vascular permeability factor and later as an angiogenesis factor.16,17 The production of VEGF is enhanced by the ischemic retina and then stimulates the production of ocular neovascularization in the iris and retina.17–19 In the human eye, elevated intraocular levels of VEGF have been demonstrated to be strongly correlated with retinal ischemia-associated ocular neovasculariaztion in diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity.20–25 VEGF is known to induce the hyperpermeability of microvessels and has been demonstrated to induce breakdown of the blood-retinal barrier in animals and humans as observed in nonproliferative diabetic retinopathy.20,26,27 However, the effect of VEGF on the function of the blood-aqueous barrier after retinal laser photocoagulation remains undetermined. The recent development of laser photometry allows for quantitative, precise, and noninvasive assessment of the blood-aqueous barrier.28 We studied the relationship between VEGF and the function of the blood-aqueous barrier in this study.
We designed this study to assess the breakdown of the blood-aqueous barrier after retinal laser photocoagulation by laser photometry in rabbits. The intraocular levels of VEGF after retinal laser photocoagulation were also determined. The effect of VEGF on the blood-aqueous barrier function in the rabbit eyes was evaluated by laser photometry after an intravitreal injection of VEGF.
Methods
Animals
Rex pigmented rabbits (2 to 3 kg) were used in this study. Animal maintenance and experimentation conformed to guidelines established by the Association for Research in Vision and Ophthalmology Statement for the use of Animals in Ophthalmic and Vision Research. During all procedures, the animals were anesthetized with intramuscular injections of a mixture of ketamine hydrochloride (3.5 mg/kg) and xylazine hydrochloride (5 mg/kg).
Retinal laser photocoagulation
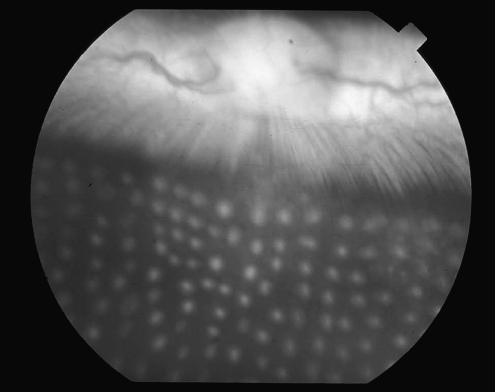
Transpupillary laser photocoagulation was carried out as described elsewhere with some modification.29 Retinal laser photocoagulation was performed on the right eyes of the rabbits by using a double-frequency yttrium aluminum garnet green laser photocoagulator (Iridex Corporation, Mountain View, CA) and a panfundus contact lens. The laser power setting (150 mW) was not altered during the scatter photocoagulation. The number of lesions per eye (400), exposure time (100 ms), and laser spot size (200 μm) were also held constant. An experienced retina specialist performed all treatments carefully to avoid hitting the iris. Lesions were scattered in the nonvascularized portion of the retina and equally distributed above and below the medullary ray. Grade Ш lesions were produced according to the classification of Tso et al.30 (Fig. 1). The left eye was not lasered and served as the control for each animal.
FIG. 1.
Fundus photography of rabbit's eye immediately after retinal laser photocoagulation.
Measurement of aqueous flare intensity after retinal laser photocoagulation
To study the effect of retinal laser photocoagulation on the aqueous flare intensity, 5 pigmented rabbits were selected and treated with scatter photocoagulation. Aqueous flare was quantified by means of photometric determination with a laser flare cell meter (FC 1000, Kowa, Tokyo, Japan).28 The aqueous flare intensity was expressed as photon counts per millisecond. If the difference between 2 sequential background signals exceeded 15%, suggesting a nonuniform background, then the measurement was repeated. The background signal donates the scattered light from the surrounding tissue in the anterior chamber. Each eye measurement consisted of 10 scans in an undilated eye. All measurements were made between the hours of 1,600 and 1,800, to avoid known diurnal variations of aqueous flare intensity in pigmented rabbits.31 The flare intensity was bilaterally measured in 5 rabbits at 1, 3, 7, and 14 days after laser photocoagulation.
Measurement of intraocular levels of VEGF after retinal laser photocoagulation
To study the effect of retinal laser photocoagulation on the intraocular levels of VEGF, twenty pigmented rabbits were observed postoperatively for a period of 1, 3, 7, or 14 days. Five rabbits were studied at each of the times just mentioned after laser photocoagulation. The animals were euthanized under anesthesia with an intracardiac injection of 5 mL sodium pentobarbital. Both eyes of each animal were then enucleated, and the intraocular contents were separated. The aqueous humor of the anterior chamber was withdrawn with a 27-gauge needle and tuberculin syringe. The anterior segment was then excised by a circumferential incision just behind the ora serrata by using corneoscleral scissors. The vitreous was then gently removed from the retina by using a pair of forceps, and the specimen was immediately placed in a −70°C freezer until analysed. When analyzed, the vitreous sample was taken from the −70°C freezer, thawed at room temperature, and then liquified by using an ultrasonicator.32
Measurements for VEGF in the vitreous and aqueous humor were performed with a sandwich enzyme-linked immunosorbent assay using a commercially available kit (Quantikine; R&D Systems, Minneapolis, MN). This kind of assay uses a monoclonal antibody specific for VEGF that is precoated onto a microtiter plate and an enzyme-linked polyclonal antibody specific for VEGF as a secondary antibody. The concentration of VEGF in each sample was calculated on the basis of the standard curve of recombinant human VEGF.
Intravitreal injection
To study the effect of VEGF on the function of the blood-aqueous barrier, a sterile preparation of recombinant human VEGF 165 (Pepro Tech EC, London, United Kingdom) was diluted in Dulbecco's phosphate-buffered saline (PBS, GIBCO, Grand Island, NY) to obtain a final concentration of 100 ng/50 μL, 500 ng/50 μL, and 1,000 ng/50 μL, respectively, A single injection of 50 μL of recombinant human VEGF 165 (100, 500, and 1,000 ng, respectively) was intravitreally made in the right eyes of 5 rabbits in each group. An equal volume of sterile PBS (50 μL) was intravitreally injected into the contralateral control eyes. The aqueous flare intensity was bilaterally measured on post-treatment days 1, 3, 7, and 14 as previously detailed.
Another experiment was performed to measure the VEGF content in the aqueous and vitreous humor after an intravitreal injection of VEGF. A single injection of 50 μL of recombinant human VEGF 165 (1,000 ng) was intravitreally administered in the right eye of each of twenty pigmented rabbits. An equal volune of sterile PBS (50 μL) was intravitreally injected into the contralateral control eyes. The rabbits were observed postoperatively for a period of 1, 3, 7, or 14 days. Five rabbits were studied at each of the times just mentioned after an intravitreal injection. The animals were euthanized, and both eyes of each animal were then enucleated. The aqueous humor of the anterior chamber was withdrawn with a 27-gauge needle and tuberculin syringe. The anterior segment was then excised, and the vitreous was gently removed from the retina by using a pair of forceps. Measurements of VEGF in the aqueous and vitreous humor were performed as just described.
Statistical analysis
Statistical analysis was performed by using the Student's paired t-test and Mann–Whitney Rank Sum test. A P value of <0.05 was considered significant.
Results
Photometric determination of the effect of laser photocoagulation on the aqueous flare intensity is shown in Table 1. The flare intensity of the lasered eyes significantly increased on postoperative day 1 to 251.8±21.3 photon counts/ms (mean±standard error of the mean [SEM]) (P<0.05). Next, this value decreased to 28.5±1.1 photon counts/ms on day 3, followed by a decline on postoperative day 7. No differences were observed between lasered and nonlasered control eyes on postoperative day 14.
Table 1.
Laser Flare Intensity (Photon Counts/ms) at Different Times After Retinal Laser Photocoagulation
| Day 1 | Day 3 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Lasered eyes | 251.8±21.3a | 28.5±1.1a | 24.5±2.1a | 7.2±0.2 |
| Nonlasered control eyes | 6.7±0.3 | 6.9±0.3 | 7.0±0.2 | 6.9±0.6 |
The values represent the mean±standard error of the mean (SEM).
P<0.05 versus nonlasered eyes.
An increase in vitreous VEGF levels was observed in lasered eyes compared with nonlasered control eyes. On postoperative day 1, a marked increase to 32.0±3.2 pg/mL (mean±SEM) was noted in the levels of VEGF in the vitreous of the lasered eyes compared with the levels in the nonlasered eyes (5.7±1.3 pg/mL) (P<0.05) (Table 2). Next, this level decreased to 24.3±13.1 pg/mL on day 3, with a gradual decline through day 14. No differences were observed between lasered and nonlasered eyes on postoperative day 3 and thereafter. The vitreous levels of VEGF remained constant throughout the time course of the experiment in the nonlasered control eyes.
Table 2.
Vascular Endothelial Growth Factor Levels in Aqueous and Vitreous Humor at Different Times After Retinal Laser Photocoagulation
| Day 1 | Day 3 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Aqueous humor VEGF level (pg/mL) | ||||
| Lasered eyes | 73.2±2.4a | 64.9±1.7 | 61.6±3.5 | 52.0±9.1 |
| Nonlasered control eyes | 52.2±5.8 | 50.9±4.7 | 48.4±4.2 | 49.8±6.7 |
| Vitreous VEGF level (pg/mL) | ||||
| Lasered eyes | 32.0±3.2a | 24.3±13.1 | 9.9±4.3 | 5.2±1.0 |
| Nonlasered control eyes | 5.7±1.3 | 5.5±2.8 | 5.3±0.6 | 5.3±0.9 |
The values represent the mean±SEM.
P<0.05 versus nonlasered eyes.
VEGF, vascular endothelial growth factor.
There was also an increase in the aqueous levels of VEGF in the lasered eyes compared with those in the nonlasered eyes. On postoperative day 1, an increase in the aqueous levels of VEGF to 73.2±2.4 pg/mL was noted in the lasered eyes compared with the nonlasered eyes (52.2±5.8 pg/mL) (P<0.05) (Table 2). Next, this level gradually decreased, and no differences were observed between the lasered and nonlasered eyes on postoperative day 3. The aqueous levels of VEGF remained constant throughout the time course of the study in the nonlasered control eyes.
Our results also demonstrated that the aqueous humor contained higher levels of VEGF than those in the vitreous humor in the nonlaser control eyes, with levels of about 52.2±5.8 pg/mL in the aqueous humor and about 5.7±1.3 pg/mL in the vitreous humor of the control rabbit eyes.
The mean aqueous flare intensity in the eyes significantly increased on day 1 after the intravitreal injection of VEGF 1,000 ng to 110.4±40.2 photon counts/ms (P<0.05), followed by a decrease to 25.0±1.5 photon counts/ms on day 3, and a gradual decline through day 7 (Table 3). The aqueous flare intensity decreased to baseline levels on day 14 after the injection. Eyes injected with sterile saline did not show any significant change in the aqueus flare intensity. The mean aqueous flare intensity also increased in the eyes that received the intravitreal injection of VEGF 500 ng, compared with the control eyes that received the intravitreal injection of sterile PBS. On postoperative day 1, the mean aqueous flare intensity increased in these eyes to 19.2±2.1 photon conts/ms (P<0.05) (Table 3). Next, this level gradually decreased, and the aqueous flare intensity decreased to baseline levels on day 3 after the injection. Eyes injected with sterile saline did not show any significant change in the aqueous flare intensity. However, the mean aqueous flare intensity did not show a significant change after the intravitreal injection of VEGF 100 ng, compared with the control eyes that received the intravitreal injection of sterile PBS (Table 3).
Table 3.
Effect of Intravitreal Injection of Vascular Endothelial Growth Factor on Laser Flare Intensity (Photon Counts/ms)
| Day 1 | Day 3 | Day 7 | Day 14 | |
|---|---|---|---|---|
| VEGF 1,000 ng | ||||
| Treated eyes | 110.4±40.2a | 25.0±1.5a | 10.7±0.3a | 6.9±0.3 |
| Control eyes | 7.7±0.2 | 7.0±0.3 | 7.1±0.3 | 6.4±0.2 |
| VEGF 500 ng | ||||
| Treated eyes | 19.2±2.1a | 7.5±1.2 | 7.5±1.2 | 7.5±0.4 |
| Control eyes | 6.9±0.3 | 6.7±0.3 | 7.4±0.7 | 7.1±0.3 |
| VEGF 100 ng | ||||
| Treated eyes | 11.9±2.9 | 7.9±1.3 | 8.0±3.0 | 7.3±4.5 |
| Control eyes | 7.9±2.0 | 7.4±0.4 | 7.1±0.3 | 7.8±1.3 |
The values represent the mean±SEM.
P<0.05 versus nontreated eyes.
There was an increase in the aqueous levels of VEGF in the eyes that received the intravitreal injection of VEGF 1,000 ng compared with those in the control eyes that received the intravitreal injection of sterile PBS. On postoperative day 1, an increase in the aqueous levels of VEGF to 2471.6±40.0 pg/mL was noted in the eyes that received the intravitreal injection of VEGF compared with the control eyes that received the intravitreal injection of sterile saline (57.4±10.4 pg/mL) (P<0.05) (Table 4). Next, this level decreased to 2239.8±299.4 pg/mL on day 3, with a gradual decline through day 14. No differences were observed between the eyes that received the intravitreal injection of VEGF and the eyes that received the intravitreal injection of sterile saline on postoperative day 7 and thereafter. Eyes injected with sterile saline did not show any sigmificant change in the aqueous levels of VEGF.
Table 4.
Vascular Endothelial Growth Factor Levels in Aqueous and Vitreous Humor at Different Times After Intravitreal Injection of Vascular Endothelial Growth Factor 1,000 ng
| Day 1 | Day 3 | Day 7 | Day 14 | |
|---|---|---|---|---|
| Aqueous humor VEGF level (pg/mL) | ||||
| Treated eyes | 2471.6±40.0a | 2239.8±299.4a | 56.9±8.2 | 54.6±6.2 |
| Control eyes | 57.4±10.4 | 56.0±9.3 | 53.6±10.7 | 53.9±7.3 |
| Vitreous VEGF level (pg/mL) | ||||
| Treated eyes | 2635.1±47.2a | 2576.8±58.6a | 66.1±6.9a | 5.5±1.6 |
| Control eyes | 5.9±1.9 | 5.7±1.3 | 5.5±2.8 | 5.3±0.6 |
The values represent the mean±SEM.
P<0.05 versus nontreated eyes.
There was also an increase in the vitreous levels of VEGF in the eyes that received the intravitreal injection of VEGF 1,000 ng compared with those in the control eyes that received the intravitreal injection of sterile PBS. On postoperative day 1, an increase in the vitreous levels of VEGF to 2635.1±47.2 pg/mL was noted in the eyes that received the intravitreal injection of VEGF compared with the control eyes that received the intravitreal injection of sterile saline (5.9±1.9 pg/mL) (P<0.05) (Table 4). Next, this level decreased to 2576.8±58.6 pg/mL on day 3, with a gradual decline through day 7. No differences were observed between the eyes that received the intravitreal injection of VEGF and the eyes that received the intravitreal injection of sterile saline on postoperative day 14. Eyes injected with sterile saline did not show any significant change in the vitreous levels of VEGF.
Discussion
In this study, we evaluated the blood-aqueous barrier function after retinal laser photocoagulation by using laser flare photometry. The peak aqueous flare intensity value of the lasered eyes occured on postoperative day 1, then gradually decreased, and became insignificant on day 14. This increase in the aqueous flare intensity confirms that breakdown of the blood-aqueous barrier can be induced by retinal laser photocoagulation. Moriarty et al.33 have used laser flare photometry to evaluate the breakdown of the blood-aqueous barrier after panretinal photocoagulation in patients with proliferative diabetic retinopathy. It has been suggested that panretinal photocoagulation can induce uveal effusion and anterior chamber shallowing, which may lead to increased ciliary body venous pressure. The reduced ciliary body venous drainage can lead to subsequent leakage of protein into the aqueous.12,15,33 However, Inoue et al.34 have evaluated the disruption of the blood-aqueous barrier after retinal laser photocoagulation in pigmented rabbits by laser flare photometry. Since anterior chamber changes were not observed in this study, it is postulated that breakdown of the blood-retinal barrier after retinal laser photocoagulation may allow for the release of protein and exogenous inflammatory substances, such as prostaglandins and cytokines, into the vitreous. The released proteins may diffuse anteriorly into the anterior chamber, or exert a direct effect on the vascular permeability of the anterior segment to produce an increase in the aqueous flare values.35
Retinal laser photocoagulation has been demonstrated as causing retinal damage, which could induce the release of inflammatory mediators, cytokines, and various proteolytic enzymes into the vitreous, thus exacerbating breakdown of the blood-retinal barrier.36,37 Prostaglandin E2 has been shown as playing a major role in postoperative inflammation. It has been shown that exposure to retinal laser photocoagulation resulted in an elevation of the amount of prostaglandin E2.9 An intravitreal injection of prostaglandin E2, interleukin-1, and interleukin-6 has been demonstrated as inducing a significant increase in aqueous flare values.34 It was suggested that these inflammatory mediators, including prostaglandin E2, interleukin-1, and interleukin-6, may play an important role and together participate in the breakdown of the blood-aqueous barrier after retinal laser photocoagulation.34
Our study further demonstrated that exposure to retinal laser photocoagulation could induce an elevation in the levels of VEGF in both the vitreous and aqueous humor. There was a similar time-dependent responce in the elevated levels of aqueous flare intensity and intraocular concentrations of VEGF throughout the time course of the experiment, although the VEGF levels were significantly elevated only on day 1 after retinal laser photocoagulation in both the vitreous and aqueous humor. In this study, we did not examine the aqueous flare intensity and measured the aqueous and vitreous levels of VEGF in the same rabbit eyes after retinal laser photocoagulation to avoid the possible effect of laser photometry on the intraocular levels of VEGF. Meanwhile, our study further demonstrated that an intravitreal injection of VEGF could induce a significant increase in the aqueous flare values and VEGF levels in the aqueous and vitreous humor. There was a similar time-dependent response in the elevated levels of aqueous flare intensity and intraocular levels of VEGF through the time course of the experiment, although the VEGF levels in the aqueous humor were significantly elevated only on days 1 and 3 after the intravitreal injection of VEGF 1,000 ng. Meanwhile, the VEGF levels in the vitreous were significantly elevated on days 1, 3, and 7 after the intravitreal injection of VEGF. In conclusion, our study demonstrated that VEGF may play a role and participate in the breakdown of the blood-aqueous barrier after retinal laser photocoagulation.
Our study further demonstrated that the aqueous humor contained higher levels of VEGF than those in the vitreous in the normal rabbit eyes. The VEGF levels were increased after retinal laser photocoagulation in both the anterior and posterior compartments of the eye. The vitreous had a slightly higher concentration of elevated VEGF than the aqueous humor, that is, about 26 versus 21 pg/mL, respectively, when comparing the lasered and nonlasered eyes on postoperative day 1. However, previous studies have demonstrated that the vitreous had higher concentrations of both the basic fibroblast growth factor and transforming growth factor-beta 2 than the aqueous humor in normal rabbit eyes. The vitreous had a higher concentration of the elevated basic fibroblast growth factor and transforming growth factor-beta 2 than the aqueous humor after retinal laser phohtcoagulation.29,38 This result suggests that diffusion of the basic fibroblast growth factor and transforming growth factor-beta 2 from the posterior compartment toward the anterior compartment of the eye may occur. The vitreous, which is in closer proximity to the laser-damaged retina, has a higher concentration of both liberated basic fibroblast growth factor and transforming growth factor-beta 2, whereas the more distant aqueous humor has a lower, yet still elevated, concentration of basic fibroblast growth factor and transforming growth factor-beta 2. A similar trend was observed with increased VEGF levels in both vitreous and aqueous humor after retinal laser photocoagulation in the rabbit retina in our study. Meanwhile, our study further demonstrated that an intravitreal injection of VEGF could increase the aqueous level of VEGF. The importance of the difference in the distribution of concentration of VEGF, compared with basic fibroblast growth factor and transforming growth factor-beta 2, in the aqueous and vitreous humor in normal rabbit eyes needs further clarification in future.
Acknowledgments
This work was supported by a grant from the National Science Council of Taiwan (NSC 95-2314-B-002-162).
Autor Disclosure Statement
No competing financial interests exist for any author.
References
- 1.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings. DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 2.Branch Vein Occlusion Study Group. Argon laser photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. Arch. Ophthalmol. 1986;104:34–41. doi: 10.1001/archopht.1986.01050130044017. [DOI] [PubMed] [Google Scholar]
- 3.Kohner E. Laatikinen L. Oughton J. The management of central retinal vein occlusion. Ophthalmology. 1983;90:484–487. doi: 10.1016/s0161-6420(83)34527-9. [DOI] [PubMed] [Google Scholar]
- 4.Zweig K. Cunha-Vaz J. Peyman G. Stein M. Raichand M. Effect of argon laser photocoagulation on fluorescein transport across the blood-retinal barrier. Exp. Eye Res. 1981;32:323–329. doi: 10.1016/0014-4835(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 5.Jaccoma E.H. Conway B.P. Campochiaro P.A. Cryotherapy causes extensive breakdown of the blood-retinal barrier: a comparison with argon laser photocoagulation. Arch. Ophthalmol. 1985;103:1728–1730. doi: 10.1001/archopht.1985.01050110124039. [DOI] [PubMed] [Google Scholar]
- 6.Arrindell E.L. Wu J.C. Wolf M.D., et al. High-resolution magnetic resonance imaging evaluation of blood-retinal barrier integrity following transscleral diode laser treatment. Arch. Ophthalmol. 1995;113:96–102. doi: 10.1001/archopht.1995.01100010098027. [DOI] [PubMed] [Google Scholar]
- 7.Marshall J. Hamilton A.M. Bird A.C. Histopathology of ruby and argon laser lesions in monkey and human retina. Br. J. Ophthalmol. 1975;59:610–630. doi: 10.1136/bjo.59.11.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lincoff H. Kreissig I. Jakobiec F. Iwamoto T. Remodeling of the cryosurgical adhesion. Arch. Ophthalmol. 1981;99:1845–1849. doi: 10.1001/archopht.1981.03930020719019. [DOI] [PubMed] [Google Scholar]
- 9.Naveh N. Weissman C. Corticosteroid treatment of laser retinal damage affects prostaglandin E2 response. Invest. Ophthalmol. Vis. Sci. 1990;31:9–13. [PubMed] [Google Scholar]
- 10.Little H.L. Complications of argon laser retinal photocoagulation: a five year study. Int. Ophthalmol. Clin. 1976;16:145–159. [PubMed] [Google Scholar]
- 11.Wilson C.A. Berkowitz B.A. Sato Y., et al. Treatment with intravitreal steroid reduced blood-retinal barrier breakdown due to retinal photocoagulation. Arch. Ophthalmol. 1992;110:1155–1159. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]
- 12.Mensher J.H. Anterior chamber depth alteration after retinal photocoagulation. Arch. Ophthalmol. 1977;95:113–116. doi: 10.1001/archopht.1977.04450010113011. [DOI] [PubMed] [Google Scholar]
- 13.Hirst L.W. Robin A.L. Sheroman S. Greem W.R. D'Anna S. Corneal endothelial changes after argon laser iridotomy and panretinal photocoagulation. Am. J. Ophthalmol. 1982;93:473–481. doi: 10.1016/0002-9394(82)90137-4. [DOI] [PubMed] [Google Scholar]
- 14.Lakhanpal V. Schocket S.S. Richards R.D. Nirankari V.S. Photocoagulation-induced lens opacity. Arch. Ophthalmol. 1982;100:1068–1070. doi: 10.1001/archopht.1982.01030040046003. [DOI] [PubMed] [Google Scholar]
- 15.Blondeau P. Pavan P.R. Phelps C.D. Acute pressure elevation following panretinal photocoagulation. Arch. Ophthalmol. 1981;99:1239–1241. doi: 10.1001/archopht.1981.03930020113011. [DOI] [PubMed] [Google Scholar]
- 16.Keck P.J. Hauser S.D. Krivi G., et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak H.F. Brown L.F. Detmar M. Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 18.Minchenko A. Bauer T. Salceda S. Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab. Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- 19.Pe'er J. Shweiki D. Itin A., et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab. Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 20.Aiello L.P. Avery R.L. Arrigg P.G., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal diaorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 21.Adamis A.P. Miller J. Bernal M., et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 22.Pe'er J. Folberg R. Itin A., et al. Vascualr endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105:412–416. doi: 10.1016/S0161-6420(98)93020-2. [DOI] [PubMed] [Google Scholar]
- 23.Lashkari K. Hirose T. Yazdany J., et al. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am. J. Pathol. 2000;156:1337–1344. doi: 10.1016/S0002-9440(10)65004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd S.R. Zachary I. Chakravarthy U., et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch. Ophthalmol. 2002;120:1644–1650. doi: 10.1001/archopht.120.12.1644. [DOI] [PubMed] [Google Scholar]
- 25.Duh E.J. Yang H.S. Haller J.A., et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am. J. Ophthalmol. 2004;137:668–674. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Murata T. Nakagawa K. Khalil A., et al. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab. Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- 27.Pe'er J. Folberg R. Itin A., et al. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 1996;80:241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawa M. Tsurimaki Y. Tsuru T. Shimizu H. New quantitative method to determine protein concentration and cell number in aqueous in vivo. Jpn. J. Ophthalmol. 1988;32:132–142. [PubMed] [Google Scholar]
- 29.Chen M.S. Chang C.C. Lin S.Y., et al. Basic fibroblast growth factor levels increase following retinal laser photocoagulation in the Rex pigminted rabbit retina. Taiwan J. Ophthalmol. 2007;46:104–110. [Google Scholar]
- 30.Tso M.O.M. Wallow I.H.L. Elgin S. Experimental photocoagulation of the human retina. I: correlations of physical, clinical and pathologic data. Arch. Ophthalmol. 1977;95:1035–1040. doi: 10.1001/archopht.1977.04450060121012. [DOI] [PubMed] [Google Scholar]
- 31.Sawa M. Clinical application of laser flare-cell meter. Jpn. J. Ophthalmol. 1990;34:346–363. [PubMed] [Google Scholar]
- 32.Boulton M.E. Lane C. Singh A., et al. Effects of vitreous from photocoagulated eyes on retianl microvascular cells in culture: a preliminary report. Curr. Eye Res. 1988;7:465–470. doi: 10.3109/02713688809031799. [DOI] [PubMed] [Google Scholar]
- 33.Moriarty A.P. Spalton D.J. Shilling J.S. Efytche T.J. Bulsara M. Breakdown of the blood-aqueous barrier after argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1996;103:833–838. doi: 10.1016/s0161-6420(96)30607-6. [DOI] [PubMed] [Google Scholar]
- 34.Inoue M. Tsukahara Y. Shirabe H. Yamamoto M. Disruption of the blood-aqueous barrier following retinal laser photocoagulation and cryopexy in pigmented rabbits. Ophthalmic. Res. 2001;33:37–41. doi: 10.1159/000055639. [DOI] [PubMed] [Google Scholar]
- 35.Inoue M. Azumi A. Shirabe H. Tsukahara Y. Yamamoto M. Laser flare inetnsity in diabetics: correlation with retinopathy and aqueous protein concentration. Br. J. Ophthalmol. 1994;78:694–697. doi: 10.1136/bjo.78.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray M.A. Gordon D. Prostaglandin production by macrophages and the effect of anti-inflammatory drugs. Br. J. Pharm. 1978;63:635–642. doi: 10.1111/j.1476-5381.1978.tb17276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twining S. Potts D. Burke J. Acid protease of vitreous macrophages. Curr. Eye Res. 1984;3:1055–1062. doi: 10.3109/02713688409011752. [DOI] [PubMed] [Google Scholar]
- 38.Ie D. Gordon L.W. Glaser B.M. Pena R.A. Transforming growth factor-beta 2 levels increase following retinal laser photocoagulation. Curr. Eye Res. 1994;13:743–746. doi: 10.3109/02713689409047009. [DOI] [PubMed] [Google Scholar]