Abstract
Both glibenclamide and riluzole reduce necrosis and improve outcome in rat models of spinal cord injury (SCI). In SCI, gene suppression experiments show that newly upregulated sulfonylurea receptor 1 (Sur1)-regulated NCCa- ATP channels in microvascular endothelial cells are responsible for “persistent sodium currents” that cause capillary fragmentation and “progressive hemorrhagic necrosis”. Glibenclamide is a potent blocker of Sur1-regulated NCCa-ATP channels (IC50,6–48 nM). Riluzole is a pleotropic drug that blocks “persistent sodium currents” in neurons, but in SCI, its molecular mechanism of action is uncertain. We hypothesized that riluzole might block the putative pore-forming subunits of Sur1-regulated NCCa-ATP channels, Trpm4. In patch clamp experiments, riluzole blocked Sur1-regulated NCCa-ATP channels in endothelial cells and heterologously expressed Trpm4 (IC50,31 μM). Using a rat model of cervical SCI associated with high mortality, we compared the effects of glibenclamide and riluzole administered beginning at 3 h and continuing for 7 days after impact. During the acute phase, both drugs reduced capillary fragmentation and progressive hemorrhagic necrosis, and both prevented death. At 6 weeks, modified (unilateral) Basso, Beattie, Bresnahan locomotor scores were similar, but measures of complex function (grip strength, rearing, accelerating rotarod) and tissue sparing were significantly better with glibenclamide than with riluzole. We conclude that both drugs act similarly, glibenclamide on the regulatory subunit, and riluzole on the putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel. Differences in specificity, dose-limiting potency, or in spectrum of action may account for the apparent superiority of glibenclamide over riluzole in this model of severe SCI.
Keywords: Spinal cord injury, Glibenclamide, Riluzole, Sulfonylurea receptor 1 (Sur1), Transient receptor potential melastatin 4, (Trpm4), Persistent sodium current, Rat
Introduction
In spinal cord injury (SCI), de novo upregulation of sulfonylurea receptor 1 (Sur1)-regulated NCCa-ATP channels in microvascular endothelial cells has been linked to the autodestructive pathological process termed “progressive hemorrhagic necrosis” (Simard et al., 2007b, 2010). Sur1-regulated NCCa-ATP channels are comprised of a regulatory subunit, Sur1, and a pore-forming subunit, which is postulated to be transient receptor potential melastatin 4 (Trpm4) (Gerzanich et al., 2009; Simard et al., 2007a). Sur1-regulated NCCa-ATP channels are non-selective monovalent cation channels that are opened by calcium influx and by ATP depletion (Chen and Simard, 2001; Chen et al., 2003). Unchecked channel opening gives rise to a “persistent sodium current” that causes cell depolarization, oncotic cell swelling, and oncotic (necrotic) death of endothelial cells, resulting in capillary fragmentation and formation of petechial hemorrhages, the hallmarks of progressive hemorrhagic necrosis (Simard et al., 2007b, 2010).
The sulfonylurea, glibenclamide (United States adopted name: glyburide), is a potent blocker of Sur1-regulated NCCa-ATP channels (IC50,6–48 nM) (Chen et al., 2003; Simard et al., 2008). In SCI, nanomolar concentrations of glibenclamide prevent capillary fragmentation, block progressive hemorrhagic necrosis, reduce necrotic lesion volumes and improve functional outcomes.
The benzothiazole, riluzole, is a pleotropic drug that was first proposed to inhibit glutamate release (Benavides et al., 1985; Coderre et al., 2007; Martin et al., 1993; Zona et al., 2002), thus protecting neurons from excitotoxic damage (Azbill et al., 2000; Dunlop et al., 2003). At micromolar concentrations (IC50,3–10 μM), riluzole is considered to be a relatively selective blocker of “persistent sodium currents” in cardiac myocytes and CNS neurons, including spinal cord neurons, where the molecular identity of the channel(s) responsible for these currents is not known (Lamanauskas and Nistri, 2008; Lamas et al., 2009; Tazerart et al., 2007; Urbani and Belluzzi, 2000; Weiss and Saint, 2010; Xie et al., 2011).
In rat models of SCI, riluzole exerts beneficial effects that are qualitatively similar to those of glibenclamide, including reduced necrotic lesion volumes and better functional outcomes (Ates et al., 2007; Schwartz and Fehlings, 2001). The specific molecular mechanism by which riluzole exerts its beneficial effects in SCI is uncertain. Involvement of glutamate antagonism is now thought to be unlikely (Lips et al., 2000; McAdoo et al., 2005). Although riluzole is known to block persistent sodium currents, and inhibition of persistent sodium currents carried by Sur1-regulated NCCa-ATP channels in microvascular endothelial cells is known to be critical for preventing capillary fragmentation and progressive hemorrhagic necrosis in SCI, the effects of riluzole have not been studied on Sur1-regulated NCCa-ATP channels in endothelial or other cells.
We hypothesized that riluzole might block the putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel, Trpm4. Here, taking advantage of the fact that Trpm4 forms functional channels even in the absence of Sur1 regulatory subunits, we carried out patch clamp experiments on heterologously expressed Trpm4, finding that riluzole blocks Trpm4 at micromolar concentrations. Subsequent experiments comparing the effects of glibenclamide and riluzole in a model of SCI revealed that optimal doses of both compounds acted similarly to inhibit capillary fragmentation and progressive hemorrhagic necrosis. However, 6 weeks after injury, measures of complex functional outcome and tissue sparing were better with glibenclamide than with riluzole. Block of the putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel, Trpm4, appears to be a key mechanism for the beneficial effect of riluzole in SCI.
Methods
Expression of Trpm4, Sur1-regulated NCCa-ATP channels and patch clamp
Expression of Trpm4-EGFPN1 in COS-7 cells (Gerzanich et al., 2009) and of Sur1-regulated NCCa-ATP channels in brain microvascular endothelial (bEnd.3) cells (Simard et al., 2009a) was carried out as described. Patch clamp electrophysiology was carried out as described (Chen and Simard, 2001; Chen et al., 2003).
Subjects and experimental series for SCI
All experimental procedures were approved by the University of Maryland Institutional Animal Care and Use Committee. In all, 50 female Long–Evans rats (~11 weeks; 250–300 g; Harlan) underwent SCI (see below for injury details) and were studied in 2 experimental series. Rats in series 1 were used for acute experiments; 9 rats (3 rats per group) were treated within 2 min of impact with a single bolus injection of vehicle, glibenclamide (10 μg/kg) or riluzole (2.5 mg/kg) IP and were euthanized 16 h after impact to determine the size of the hemorrhagic contusion, capillary fragmentation and neuron counts. Rats in series 2 were used for chronic experiments; they received vehicle (15 rats), glibenclamide (13 rats), or riluzole (13 rats) beginning 3 h after injury and continuing for 1 week (see below for treatment details). Rats in this series that were treated with glibenclamide or riluzole were maintained for 6 weeks after injury, during which they underwent serial measurements of function, and after which they were euthanized to measure lesion volumes. Two vehicle-treated rats in this series that survived were euthanized at 2 weeks, still paraplegic.
Rat model of SCI
We used a rat model of unilateral cervical SCI (Soblosky et al., 2001) similar to that reported by us previously (Simard et al., 2007b, 2010), except that the impact force was greater. Rats were anesthetized (ketamine, 60 mg/kg plus xylazine, 7.5 mg/kg, IP) and the head was mounted in a stereotaxic apparatus (Stoelting, Wood Dale, IL). Using aseptic technique and magnification (Zeiss operating microscope with co-axial lighting), the spine was exposed dorsally from C3 to T3 via a 3-cm midline incision and subpereosteal dissection of the paraspinous muscles. The ear bars were removed and the animal was repositioned so that it was supported from the spinous process of T2 (Spinal Adaptor, Stoelting) and the snout. At C7, the spinous process, part of the right lamina, the entire left lamina and the dorsal half of the left pedicle were removed using a 1.9-mm diamond burr (#HP801-019, www.DiamondBurs.Net) and a high-speed drill, with care taken to avoid mechanical and thermal injury to the underlying dura and spinal cord. The dura was exposed by sharply removing the interlaminar ligaments rostral and caudal to the lamina of C7 and any remaining ligamentum flavum. The guide tube containing the impactor (1.55-mm tip diameter, 57 mm length, 1.01 g) (Soblosky et al., 2001) was angled 5° medially. Using the manipulator arm of the stereotaxic apparatus, the impactor was positioned on the dura mater near the left C8 nerve root. As previously (Simard et al., 2007b, 2010), the caudal edge of the impactor was placed in line with the caudal edge of the C8 nerve root at the axilla, and the medial edge of the impactor was placed 0.3 mm medial to the dorsolateral sulcus. The impactor was activated by releasing a 10 g weight from a height of 30 mm inside the guide tube. The impact caused an immediate, forceful flexion of the trunk and 70–90° flexion of the ipsilateral knee, which had been extended before impact, and resulted in a hemorrhagic contusion evident on the surface of the cord involving the ipsilateral but not the contralateral sulcus, as observed under the microscope through the translucent dura.
After injury, the surgical site was flushed with 1 ml of normal saline (NS), the musculature was replaced over the spine and the skin incision was stapled. Rats were nursed on a heating pad to maintain rectal temperature ~37 °C, and were given 10 ml of glucose-free NS subcutaneously. Food and water were placed within easy reach to ensure that the rats were able to eat and drink without assistance. Bladder function was assessed 2–3 times daily by observing for urination and, if needed, manual emptying of the bladder was carried out using the Credé maneuver.
Treatments
For glibenclamide or vehicle, treatment consisted of administering a loading dose IP plus implanting a mini-osmotic pump for continuous 1-week long subcutaneous delivery. A stock solution of glibenclamide, made as previously (Simard et al., 2007b, 2010), was prepared by placing 25 mg glibenclamide (#G2539; meets USP testing; Sigma, St. Louis, MO) into 10 ml dimethyl sulfoxide (DMSO). The solution to be loaded into the mini-osmotic pumps (Alzet 2001, 1.0 μl/h; Alzet Corp., Cupertino, CA) was made by taking 2.3 ml unbuffered NS, adding 4 μl of 10 N NaOH, then adding 200 μl stock solution, in that order to prevent precipitation of drug. The solution to be used for the loading dose was made by adding 4 μl of stock solution to 1 ml NS. For vehicle controls, solutions were made with DMSO and NS as above, but glibenclamide was omitted. After loading, the miniosmotic pumps were primed overnight in NS at 37 °C with the outlet of the pump connected to a length of PE60 tubing that extended above the level of the priming solution, to prevent H+ ions from entering the pump chamber. Prior to implanting, the pump outlet was fitted with an empty catheter (PE60 tubing), 3 μlinvolume, that resulted in a3-hour delay in start of drug infusion (Simard et al., 2009c). Loaded and primed mini-osmotic pumps were implanted subcutaneously after completing surgery to induce SCI; pumps containing drug delivered 200 ng/ h of glibenclamide for 7 days. The loading dose (10 μg/kg IP) was administered 3 h after injury, with the volume of the loading dose in microliters equal to the weight of the rat in grams.
For riluzole, we first explored the possibility of constant infusion using a mini-osmotic pump. However, its poor solubility in water combined with the high concentrations needed, thwarted attempts to develop a formulation without undue amounts of organic solvents that could be used with a mini-osmotic pump. Studies on acute treatment with riluzole in contusive SCI in rats in which tissue necrosis was measured used doses of 5–8 mg/kg IP as a single injection at the time of injury (Ates et al., 2007; Schwartz and Fehlings, 2001). We reasoned that twice daily dosing maintained for several days, as used in humans [0.7 mg/kg PO twice daily (Siniscalchi, 2004)], would yield better serum levels than a single injection, thus affording greater likelihood of demonstrating efficacy. We performed a pilot study to determine the optimal dose. We found that 5–8 mg/kg IP repeated twice daily left the animals in a somnolent, comatose or moribund state (Kitzman, 2009; Mantz et al., 1992). A dose of 2.5 mg/kg IP every 12 h was found to be beneficial in terms of outcome after SCI (see Results), but did not produce undue sedation (Wahl and Stutzmann, 1999).
Treatment allocation and outcome assessment
Treatment allocation was determined by an investigator not other-wise involved in the experiments. Serial mBBB scores were assessed by two independent investigators, with the worse of the two scores being used. Objective outcomes, including rearing times, grip strength, and time on accelerating rotarod were determined by a single investigator. Lesion volumes were determined by yet another investigator not otherwise involved in the experiments.
Contusion size
At 16 h after injury, the rat was anesthetized with sodium pentobarbital (100 mg/kg), a thoracotomy was performed, and the rat was transcardially perfused with NS (50 ml) followed by 10% neutral buffered formalin (50 ml) at a pressure of 100 cm H2O. The spinal cord was harvested, sectioned longitudinally in the plane one third of the way up from ventral to dorsal (midway through the ventral horns), and the cut faces were imaged at high resolution using a flat-bed scanner. Contusion areas were measured using Photoshop (Adobe).
Capillary length and neuron counts
Longitudinal cryosections (10 μm) through the middle of the ventral horns were immunolabeled for NeuN and analyzed for viable neurons, or were immunolabeled for laminin and analyzed for capillary length, as described (Simard et al., 2009b). For neuron counts, regions of interest (ROI) in the ventral horns contralateral to the impact were identified. For each rat, 5 ROIs of 250× 1000 μm were processed, with the middle one centered opposite the epicenter of injury. Individual “objects” within each ROI, defined as areas with contiguous specific labeling 3× above background, were counted. Unbiased measurements of capillary length were obtained using specialized commercial software (NIS-Elements, Advanced Research v3.06; Nikon Instruments). ROI in the penumbra adjacent to the necrotic void were identified. For each rat, an ROI of 350×450 μm was processed. Individual “objects” within each ROI were defined as areas with contiguous specific labeling 3× above background. The maximum length of each object was determined for objects >5 μm. Based on the rod model, the length of the central axis of a thin rod is given by: L=[P+(P2–16×A)0.5]/4, where P and A are the perimeter and area of the object, respectively. Frequency count histograms of object lengths (bin width, 2 μm) were constructed by combining data from 3 rats per group (vehicle, riluzole and glibenclamide).
Functional tests
Basso, Beattie and Bresnahan (BBB) scores were determined as described (Basso et al., 1995), except that modifications were introduced to allow more accurate assessment of the functional asymmetry associated with hemicord injury. The scoring sheet shown in Fig. 1 of the original paper (Basso et al., 1995) provides for scoring of the right and left hindlimbs separately, with the final score for the animal being based on the two scores. Here, because we were studying hemicord injuries, we maintained the two scores separately, which we refer to as modified BBB (mBBB) scores. The method for scoring up to and including scores of 11 is identical for mBBB and BBB. For mBBB scoring of coordination, forelimb–hindlimb coordination was operationally defined as a one-to-one correspondence between forelimb and ipsilateral hindlimb steps on each side; “alternation in hindlimb stepping”, which is taken into account in BBB scoring, was ignored for mBBB scoring. For scores above 19, both right and left sides were credited for “tail consistently up” and for “trunk stability”.
Fig. 1.
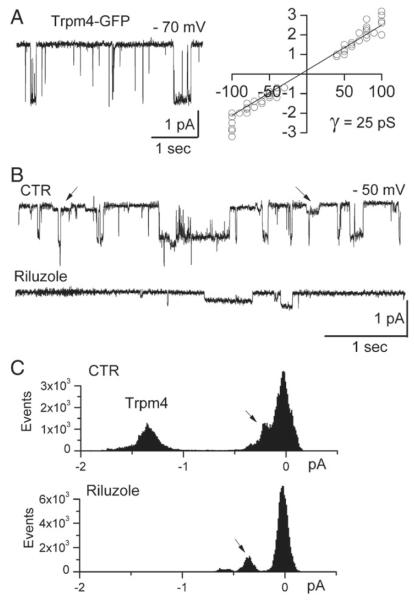
Riluzole blocks Trmp4 single channel currents. A: Single channel current in an inside-out patch from a COS-7 cell expressing Trpm4-EGFPN1; a plot of the open channel current amplitude as a function of holding potential indicates a slope conductance of 25 pS; charge carrier, Cs+; data from 15 patches. B: Single channel current in an inside-out patch from a COS-7 cell expressing Trpm4-EGFPN1, before and after addition of riluzole (100 μM); note block of the 25 pS channel, but not of the smaller conductance channel (arrows); the data shown are representative of 11 patches. C: All-point histograms of currents recorded before (above) and after (below) riluzole, confirming block of the 25 pS channel, but not of the smaller conductance channel (arrows).
Performance on the accelerating rotarod (IITC, Life Science, Woodland Hills, CA) was determined as described (Hamm et al., 1994), 6 weeks after SCI. The drum was started at 4 rpm and was accelerated at a rate of 2rpm every 5s up to a maximum of 45rpm. Three trials separated by20 minwereadministeredoneachdayoftestingfor3 days.Wereport the average latency to falling off of the drum.
To measure rearing times, rats were videotaped while in a translucent cylinder (19×20 cm) and spontaneous rearing was quantified as the number of seconds spent with both front paws elevated above shoulder-height during a 3-minute period of observation (Simard et al., 2007b). In separate experiments, assessment of rearing behavior in sham-lesioned rats showed that performance waned after 2–3weeks, presumably because the rats became habituated to the test chamber.
Grip strength was measured using a digital grip strength meter (Bioseb BP, In Vivo Research Instruments, France). Forelimb grip strength was measured separately for the ipsilateral and contralateral forepaws and for the two together. The rat was held by its trunk, the forelimbs were placed on the grasping metal bar (T shaped), and the rat was encouraged to grip the metal bar on the device attached to the force transducer. Once the grip was secured, the animal was slowly pulled away from the bar. The maximal average force exerted on the grip strength meter by both forepaws or each separately was averaged from 3 trials per day for each rat, for 2 consecutive days.
Lesion volume
At 6 weeks after SCI, the rats were deeply anesthetized, perfusion-fixed with 10% neutral buffered formalin, and the spinal cords were harvested. Serial paraffin sections were stained with hematoxylin and eosin (H&E). Lesion areas (in mm2) were measured on serial sections every 250 μm; lesion volumes (in mm3) were calculated as the sum of the areas multiplied by 0.25.
Data analysis
Like BBB scores (Barros Filho and Molina, 2008; Ferguson et al., 2004; Scheff et al., 2002), mBBB scores are derived from an ordinal scale and are not normally distributed. Therefore, we analyzed mBBB scores using a nonparametric (distribution-free) procedure based on ranks (Conover and Iman, 1981). mBBB scores were rank-transformed as described (Conover and Iman, 1981) prior to the application of parametric statistical analysis. Contusion areas were analyzed by 1-way ANOVA with Tukey post-hoc comparisons. Mortality was assessed using Fisher’s exact test (2-tailed). Serial measurements of the ranks of mBBB scores and the raw data from rearing times were analyzed using a 2-way mixed-model repeated measures ANOVA, with Tukey post-hoc comparisons. Grip strength, rotarod performance and lesion volumes were analyzed using Student’s t-test.
Results
Riluzole blocks Trpm4
The putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel, Trpm4, was heterologously expressed in COS-7 cells, where it can form functional channels even in the absence of Sur1 regulatory subunits (Gerzanich et al., 2009). For patch clamp experiments, Cs+ was used as the charge carrier to block all potassium channels, including KATP channels (Chen et al., 2003). Study of inside-out patches confirmed the expression of channels with a single channel conductance of 25 pS (Fig. 1A), which is typical of Trpm4 (Simard et al., 2007a). Application of riluzole blocked opening of the 25 pS Trpm4 channel, but not of the unidentified channel of 4–6 pS that is endogenous to COS-7 cells (Figs. 1B, C).
We used a whole-cell nystatin-patch configuration, again with Cs+ as the charge carrier, to study COS-7 cells expressing Trpm4. Since Trpm4 channels are opened by increasing intracellular calcium (Guinamard et al., 2010; Vennekens and Nilius, 2007), currents were activated by applying the calcium ionophore, A23187, to the bath solution in the presence of 2 mM extracellular calcium. After current activation, application of riluzole blocked both inward and outward currents (Figs. 2A, B). This pattern of block, with no rectification, is consistent with riluzole acting allosterically to inhibit channel opening, rather than acting as an open channel blocker. In COS-7 cells expressing Trpm4, block of macroscopic currents by riluzole was concentration-dependent, with an IC50 of 31.4 μM (Fig. 2C, filled circles).
Fig. 2.
Riluzole blocks Trmp4 macroscopic currents. A,B: Macroscopic whole cell currents from COS-7 cells expressing Trpm4-EGFPN1, shown at low temporal resolution (A) and higher resolution (B) during repetitive ramp pulses from –120 to +100 mV, 800 ms duration, every 15 s; Trpm4 currents were activated by adding the calcium-ionophore, A23187 (20 μM) to the bath to raise intracellular calcium; subsequent addition of riluzole (100 μM) blocked the current activated by raising intracellular calcium. C: Semi-log plot of the percent inhibition (mean± SE) of Trpm4 current in COS-7 cells (filled circles), measured at –50 mV, observed in the presence of various concentrations of riluzole; 3–7 cells at each concentration; the data for COS-7 cells were fit to a standard logistic equation with an IC50,31.4 μM; also shown is the percent block of Sur1-regulated NCCa-ATP channel currents in bEnd.3 cells by riluzole (empty circles); 5 cells at each concentration. D: Macroscopic whole cell currents from a bEnd.3 cell induced to express Sur1-regulated NCCa-ATP channels; currents were activated by depleting ATP using sodium azide (1 mM) plus 2-deoxyglucose (10 mM); subsequent addition of riluzole (100 μM) blocked the current activated by ATP depletion.
We also used a whole-cell nystatin-patch configuration, again with Cs+ as the charge carrier, to study brain microvascular endothelial (bEnd.3) cells induced to express Sur1-regulated NCCa- ATP channels, in which Trpm4 is postulated to be the pore-forming subunit (Simard et al., 2007a). The channel was induced by exposing the cells to TNFα (Simard et al., 2009a). Since Trpm4 channels are opened by depleting intracellular ATP (Guinamard et al., 2010; Vennekens and Nilius, 2007), currents were activated by applying sodium azide plus 2-deoxyglucose to the bath solution. After current activation, application of riluzole blocked both inward and outward currents (Fig. 2D) in a concentration-dependent manner (Fig. 2C, empty circles) that accorded well with observations made with heterologously expressed Trpm4 channels (Fig. 2C, filled circles).
Glibenclamide vs. riluzole – single treatment immediately after SCI
Progressive hemorrhagic necrosis
The injuries produced by weight drop (10 g, 3 cm) onto the lateral spinal cord at C7 were severe. The spinal cords from 3 groups of rats (vehicle control, glibenclamide and riluzole, administered within 2 min of impact; 3 rats per group) were examined 16 h after impact, following perfusion to remove intravascular blood. In vehicle-controls, the hemorrhagic contusion extended several millimeters rostral and caudal to the site of impact, and invariably crossed to the contralateral side (Fig. 3A). By contrast, rats treated with either glibenclamide or riluzole showed much smaller rostro-caudal expanses of hemorrhages, and largely were spared from contralateral expansion of the hemorrhage (Fig. 3A). The areas of hemorrhagic contusion in both glibenclamide- and riluzole-treated rats were significantly smaller than in vehicle-treated rats (Fig. 3A). These findings are consistent with both drugs reducing progressive hemorrhagic necrosis, as previously reported for glibenclamide (Simard et al., 2007b, 2010).
Fig. 3.
Comparative effects of glibenclamide and riluzole on progressive hemorrhagic necrosis, capillary fragmentation and neuron counts. A: Representative longitudinal sections of perfusion-cleared spinal cords through the epicenter of injury 16 h after impact, in rats administered vehicle (V), riluzole (R) or glibenclamide (G) within 2 min of impact; a millimeter scale is shown at left; the bar graph (right) depicts the average lesion areas in the 3 groups; 3 rats per group; *Pb 0.05; **Pb 0.01 with respect to control. B–D: Images at low (left panels) and at high (right panels) magnification of spinal cord sections in the region of the penumbra immunolabeled for laminin, from rats administered vehicle (B), riluzole (C) or glibenclamide (D); same tissues as above; the epicenter of injury is denoted by an asterisk; the region shown at high magnification in the panel on the right is indicated by an arrow in the panel on the left. E: Histograms of the lengths of penumbral microvessels in rats administered vehicle, riluzole or glibenclamide. F: Counts of NeuN-positive neurons in contralateral gray matter in rats administered vehicle, riluzole or glibenclamide; same tissues as above; *Pb 0.05; **Pb 0.01 with respect to control.
Capillary fragmentation
Previous work established that progressive hemorrhagic necrosis after SCI is due to delayed capillary fragmentation and formation of petechial hemorrhages, and that these events are prevented by gene suppression or pharmacological block of Sur1-regulated NCCa-ATP channels in penumbral microvessels (Simard et al., 2007b, 2010). The observation that riluzole, like glibenclamide, reduced progressive hemorrhagic necrosis suggested that riluzole also might reduce capillary fragmentation.
The tissues used to evaluate progressive hemorrhagic necrosis (above) were cryosectioned and immunolabeled with antibody against laminin to identify microvessels. In vehicle-treated rats, penumbral microvessels were foreshortened and fragmented (Fig. 3B), but in riluzole-treated (Fig. 3C) as well as in glibenclamide-treated (Fig. 3D) rats, penumbral microvessels remained elongated. Histograms of the lengths of microvessels in the penumbra showed that both riluzole and glibenclamide reduced capillary fragmentation (Fig. 3E). Stratifying the data at a length of 40 μm, values of 8/536, 48/ 756 and 77/663 were obtained for vehicle, riluzole and glibenclamide treatments, respectively. The values for both drugs were significantly different from controls (Pb 0.001) and were significantly different from each other (Pb 0.001). This finding with glibenclamide accords with previous observations (Simard et al., 2007b).
Neuron counts
The same tissues also were immunolabeled for NeuN to identify viable neurons in ventral gray matter contralateral to the side of impact. Counts of viable neurons were lowest in tissues from vehicle-treated rats and were highest in glibenclamide-treated rats, with intermediate values found in riluzole-treated rats (Fig. 3F).
Glibenclamide vs. riluzole – 1-week treatment beginning 3 h after SCI
Long-term treatment outcomes were evaluated in 3 groups of rats administered vehicle, glibenclamide (10 μg/kg IP loading dose plus start of 200 ng/h subcutaneous infusion) or riluzole (2.5 mg/kg IP every 12 h). For these experiments, we began administering the drugs at the clinically relevant time of 3 h after impact.
Death
Mortality was very high in vehicle-treated rats (Fig. 4A). Death often occurred unwitnessed overnight, many hours after impact, or rats were euthanized if found in an agonal respiratory state the morning after injury. The finalcause of deathis uncertain,since physiological monitoring was not performed, but involvement of the upper thoracic spinal cord (see Fig. 3A), with injury to the sympathetic system likely resulted in spinal shock. By contrast, all rats treated with either glibenclamide or riluzole survived, and none died during the ensuing 6 weeks of observation, consistent with the reduction in progressive hemorrhagic necrosis observed in animals treated with either glibenclamide or riluzole.
Fig. 4.

Comparative effects of glibenclamide and riluzole on mortality and mBBB scores. A: Mortality was assessed in rats administered vehicle (filled squares), riluzole (empty triangles) or glibenclamide (filled circles); no animal died after day 8; 13–15 rats per group; the abscissa gives the time in days. B: Serial measurements of mBBB scores for the contralateral hindlimb (filled symbols) and for the ipsilateral hindlimb (empty symbols) in rats administered riluzole (triangles) or glibenclamide (circles); 13 rats per group; data are shown as mean ± S.E; statistical significance was determined by non-parametric analysis; *P b 0.05; the abscissa gives the time in days (d) or weeks (w). In all cases, the drugs were administered beginning 3 h after impact and were continued for 1 week.
Because of the high mortality in vehicle-treated rats, subsequent experiments on long-term outcomes were carried out with only two groups, the glibenclamide- and the riluzole-treated rats.
mBBB scores
The model we used with unilateral cervical spinal cord injury resulted in asymmetric hindlimb locomotor function, which we assessed using a modified BBB (mBBB) scoring system that maintained the scoring for each hindlimb separately (see Methods). Serial measurements were made over the course of 6 weeks after impact.
Serial mBBB scores for the contralateral hindlimb showed similar recovery of function in both glibenclamide- and riluzole-treated rats (Fig. 4B, filled symbols). Serial mBBB scores for the ipsilateral hindlimb showed better recovery of function in glibenclamide-treated rats compared to the riluzole-treated rats (Fig. 4B, empty symbols). The advantage for glibenclamide was statistically significant during the first 5 weeks, but this was lost by week 6.
Rearing
Rearing is a non-coerced, complex exercise that requires balance, trunk stability, bilateral hind limb strength and coordination, and at least unilateral forelimb dexterity and strength, which together are excellent markers of cervical spinal cord function. Serial measurements of rearing during 6 weeks of recovery showed continued improvements in both glibenclamide- and riluzole-treated rats (Fig. 5A). However, through the entire course of testing, glibenclamide-treated rats exhibited consistently better performance than riluzole-treated rats (Fig. 5A).
Fig. 5.
Comparative effects of glibenclamide and riluzole on spontaneous rearing, accelerating rotarod and grip strength. A: Serial measurements of the time (mean±S.E.) spent in spontaneous rearing in rats administered riluzole (empty triangles) or glibenclamide (filled circles); 13 rats per group; *Pb 0.05; **Pb 0.01; the abscissa gives time in days (d) or weeks (w). B: Performance on the accelerating rotarod, measured 6 weeks after impact, in rats administered riluzole (RIL) or glibenclamide (GLIB), as indicated; 13 rats per group. C: Grip strength, measured 6 weeks after impact, for the contralateral forepaw (CONTRA), the ipsilateral forepaw (IPSI), and both together (BOTH), in rats administered riluzole or glibenclamide, as indicated; 13 rats per group. In all cases, the drugs were administered beginning 3 h after impact and were continued for 1 week.
Accelerating rotarod
Performance on the accelerating rotarod is a coerced exercise that requires highly dexterous, coordinated function of all limbs to prevent falling off of the rotating drum. We assessed performance on the accelerating rotarod at the end of the 6-week period of recovery. On this measure, glibenclamide-treated rats exhibited significantly better performance than riluzole-treated rats (Fig. 5B).
Grip strength
With a unilateral lower cervical spinal cord injury, forelimb grip strength is an excellent measure of the function of the lower motor neuron pool most directly affected. We assessed ipsilateral and contralateral grip strength at the end of the 6-week period of recovery. Compared to riluzole-treated rats, glibenclamide-treated rats exhibited significantly better performance on both ipsilateral and contralateral grip strength (Fig. 5C). Also, glibenclamide-treated rats exhibited significantly better performance on bilateral grip strength (Fig. 5D).
Lesion volumes
At the end of the 6-week period of testing, the rats were euthanized and the spinal cords were processed to measure lesion volumes. Glibenclamide-treated rats exhibited significantly smaller lesions compared to riluzole-treated rats (Fig. 6).
Fig. 6.

Comparative effects of glibenclamide and riluzole on lesion size. A: Representative H&E-stained sections of spinal cords at the epicenter of injury, 6 weeks after SCI, from rats treated with either riluzole or glibenclamide, as indicated. B,C: Lesion volumes (B) and lesion areas as a function of distance from the epicenter (C), 6 weeks after SCI, in rats treated with either riluzole or glibenclamide (GLIB), as indicated, beginning 3 h after impact and continuing for 1 week; 13 rats per group.
Discussion
One important finding of the present study is that riluzole blocks the putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel, Trpm4. This finding is in keeping with two previous observations: first, that riluzole is protective after SCI (Ates et al., 2007; Schwartz and Fehlings, 2001), and secondly, that gene suppression of Trpm4 prevents capillary fragmentation and progressive hemorrhagic necrosis after SCI (Gerzanich et al., 2009). Previous work suggested that the beneficial effects of riluzole in SCI might be due to: (i) block of persistent sodium currents, whose molecular origin was unknown, (ii) inhibition of glutamatergic pathways and of excitotoxicity, or (iii) an indirect neurotrophic effect. While not disputing any potential role for these mechanisms, the data presented here showing that riluzole blocks Trpm4, combined with previous molecular evidence of the critical involvement of Trpm4 in capillary fragmentation and progressive hemorrhagic necrosis in SCI (Gerzanich et al., 2009), provide compelling evidence that at least one critical mechanism for the beneficial effect of riluzole in SCI is blockade of Trpm4.
Another important finding is that the effects of riluzole were qualitatively similar to those of glibenclamide in a model of severe cervical SCI. Both drugs reduced capillary fragmentation, neuronal death and progressive hemorrhagic necrosis acutely, both prevented death in this model of SCI, and both were associated with significant recovery of neurological function during the 6 weeks of assessment, as evidenced by measurements of locomotor function using mBBB scores. However, in tests of complex function requiring high level dexterity and coordination, including spontaneous rearing, performance on the accelerating rotarod and grip strength, as well as in terms of tissue sparing at 6 weeks, glibenclamide was superior to riluzole. Assuming that Trpm4 is the pore-forming subunit of the Sur1-regulated NCCa-ATP channel, as hypothesized (Gerzanich et al., 2009; Simard et al., 2007a), blockade of either the regulatory subunit or of the pore forming subunit should be molecularly equivalent, at least in principal. In practice, however, this was not the case. As discussed below, the explanation for the apparent difference in efficacy between the two drugs may reside in differences in their specificity, in their dose-limiting potency, or in their spectrum of action.
At present, specific pharmacological blockade of Trpm4 is not feasible, possibly because of structural similarities to other ion channels. Riluzole blocks Trpm4, but in a non-specific manner – many other targets are also blocked or inhibited by this drug. Riluzole blocks persistent sodium currents from channels typically not identified molecularly (Lamanauskas and Nistri, 2008; Lamas et al., 2009; Tazerart et al., 2007; Urbani and Belluzzi, 2000; Weiss and Saint, 2010; Xie et al., 2011). Riluzole blocks several molecularly identified potassium channels (Ahn et al., 2005; Cao et al., 2002; Duprat et al., 2000; Xu et al., 2001; Zona et al., 1998), and calcium channels (Huang et al., 1997; Siniscalchi et al., 1997; Stefani et al., 1997). Riluzole also inhibits glutamate release (Benavides et al., 1985; Coderre et al., 2007; Martin et al., 1993; Zona et al., 2002), and it interacts with γ-aminobutyric acid A and glycine receptor-activated channels (He et al., 2002; Martin et al., 1993; Mohammadi et al., 2001; Umemiya and Berger, 1995). Finally, riluzole directly binds to and inhibits protein kinase C (Noh et al., 2000). Most blockers of non-selective cation channels exhibit a certain degree of non-specificity (Pena and Ordaz, 2008). A high degree of non-specificity in a drug generally is undesirable, since it can be associated with untoward side-effects and undue toxicity. This may account for the acute toxicity observed with high doses of riluzole, including somnolence, coma or a moribund state, as observed by us and others (Kitzman, 2009; Mantz et al., 1992). To date, the most specific way shown to inhibit Trpm4 is by gene suppression using antisense oligodeoxynucleotide, which is highly efficacious in SCI (Gerzanich et al., 2009).
Drug specificity is less problematic with glibenclamide. Apart from high-potency block of Sur1-regulated NCCa-ATP channels (6–48 nM) (Chen et al., 2003; Simard et al., 2008), glibenclamide also blocks Sur1-regulated KATP channels in pancreatic β cells (Bryan and Aguilar-Bryan, 1999). However, at the doses used in CNS ischemia and trauma, the potential consequence of block of pancreatic KATP channels – hypoglycemia – is not observed; infusion of 200 ng/h of glibenclamide yields plasma concentrations ~5 ng/ml (Simard, unpublished observation), and has a minimal effect on serum glucose (Simard et al., 2006, 2007b, 2009c). Sur2-regulated KATP channels in cardiac and smooth muscle cells are less sensitive to block by glibenclamide, by a factor of 10 times or more (Bryan and Aguilar-Bryan, 1999; Simard et al., 2008). Other ATP-binding cassette proteins of the Abc gene family may be blocked by glibenclamide, but only at micromolar concentrations (Bessadok et al., 2011), far greater than the concentrations obtained with infusion of 200 ng/h. As with Trpm4, gene suppression of Sur1 using antisense oligodeoxynucleotide is highly specific and highly efficacious in SCI (Simard et al., 2010). Notably, in the same report, direct comparison between antisense and low-dose glibenclamide showed equivalent effects in terms of functional outcomes and lesion volumes at 6 weeks, with neither compound exhibiting any observable toxicity.
Arguably the most important reason for the apparent superiority of glibenclamide over riluzole is its greater potency in blocking Sur1-regulated NCCa-ATP channels. The IC50 for block of Sur1regulated channels by glibenclamide is 6–48 nM (Chen et al., 2003; Simard et al., 2008), whereas the IC50 for block of the putative pore-forming subunit by riluzole was found here to be 31 μM. When two drugs are poorly water soluble, requiring organic agents for solubilization, a 650-fold or greater advantage in potency greatly facilitates drug formulation and drug delivery, making it easier to optimize bioavailability. Unlike glibenclamide, riluzole’s poor solubility, combined with the much higher concentrations needed, thwarted our attempts to develop a formulation that could be administered by constant infusion via mini-osmotic pump. Instead, we used twice daily injections, which likely yielded variable serum levels with peaks that were just subtoxic, and troughs that may have been insufficient to maintain constant block of Trpm4.
Another potential reason for the apparent superiority of glibenclamide over riluzole relates to glibenclamide’s non-conventional anti-inflammatory action. When CNS tissues are exposed to blood, a robust inflammatory response is induced involving both endogenous microglia and exogenous neutrophils. While important for clearing necrotic debris, free radicals generated as part of the inflammatory response can cause bystander injury to nearby healthy cells, exacerbating the overall injury (Blight, 1992; Kleinig and Vink, 2009). In the case of subarachnoid hemorrhage, a condition in which the neurotoxic effects of blood are not confounded by physical tissue destruction, it has been shown that the inflammatory response induced by extravasated blood can be ameliorated by glibenclamide (Simard et al., 2009a). The mechanism for this anti-inflammatory effect is poorly understood. It does not seem to depend on conventional anti-inflammatory pathways, but may be due to inhibition of neutrophil recruitment (Figura et al., 2009; Kleinig and Vink, 2009). Given that the inflammatory response is an important mechanism of secondary tissue injury after SCI, the non-conventional anti-inflammatory effect of glibenclamide could contribute importantly to its overall salutary effect.
The mBBB scale that we used for unilateral assessment of function was derived from the BBB locomotor rating scale, which has long been accepted as a valid measure of locomotor function, especially after mid-to-lower thoracic contusion injuries in rat models of SCI. In the model of severe unilateral cervical SCI studied here, rats treated with either glibenclamide or riluzole showed dramatic improvements in function during 6 weeks of recovery, as evidenced by mBBB scores that reached near-maximum scores of 21. The overall high scores achieved at 6 weeks, despite the severity of the initial injury, reflect the fact that lower motor neurons innervating the hindlimbs were spared from primary damage, and sufficient white matter was preserved to permit recovery of high level functioning of the hindlimbs. The similar mBBB scores for the contralateral hindlimb observed with both drugs reflected the fact that both drugs effectively prevented contralateral spread of progressive hemorrhagic necrosis. The better performance in mBBB scores for the ipsilateral hindlimb for glibenclamide-treated rats paralleled the observation that lesions, which were essentially ipsilateral, were smaller with glibenclamide than with riluzole.
Proper forelimb–hindlimb coordination is essential for effective locomotion. Some investigators have suggested that the assessment of forelimb–hindlimb coordination in the BBB scale may be inadequate (Koopmans et al., 2005). Here, because we were studying hemi-cord injury, we deliberately omitted “alternation in hindlimb stepping”, which is the principal measure of forelimb–hindlimb coordination in the BBB scale. As a substitute, we introduced performance on the accelerating rotarod, a coerced exercise that requires highly dexterous, forelimb–hindlimb coordination with synchronized alternation in hindlimb stepping to prevent falling off of the rotating drum. The better performance on the accelerating rotarod for glibenclamide-treated rats reflects better forelimb–hindlimb coordination and correlates with the smaller lesions observed with glibenclamide compared to riluzole. Overall, the mBBB scale combined with performance on the accelerating rotarod appears to have mirrored accurately the final pathology.
In summary, here we show that riluzole, a drug with demonstrated efficacy in the acute treatment of SCI, blocks the putative pore-forming subunit of the Sur1-regulated NCCa-ATP channel, which has been causally implicated in capillary fragmentation and progressive hemorrhagic necrosis following SCI. Direct comparison of riluzole and glibenclamide showed that both drugs had qualitatively similar salutary effects, but that glibenclamide was superior in terms of potency and efficacy, the latter reflected by better performance in complex locomotor tasks and in tissue preservation 6 weeks after injury. Our data indicate that block of Trpm4 is an important mechanism for the beneficial effect of riluzole in SCI.
Acknowledgment
This work was supported by grants to JMS from the Veterans Administration (Baltimore), the National Institute of Neurological Disorders and Stroke (NINDS) (NS060801), the Department of the Army (W81XWH 1010898) and the Christopher and Dana Reeve Foundation; to VG from NINDS (NS061934).
Footnotes
Conflict of interest JMS holds a US patent (#7,872,048), “Methods for treating spinal cord injury with a compound that inhibits a NC(Ca-ATP) channel ”. JMS is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to JMS, or for this project, by Remedy Pharmaceuticals.
References
- Ahn HS, Choi JS, Choi BH, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. Inhibition of the cloned delayed rectifier K+ channels, Kv1.5 and Kv3.1, by riluzole. Neuroscience. 2005;133:1007–1019. doi: 10.1016/j.neuroscience.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli SR, Gurses I, Turkoz Y, Tarim O, Cakir CO, Kocak A. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J. Clin. Neurosci. 2007;14:658–665. doi: 10.1016/j.jocn.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- Barros Filho TE, Molina AE. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo) 2008;63:103–108. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, Gueremy C, Le FG. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission–II. Biochemical properties. Neuropharmacology. 1985;24:1085–1092. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- Bessadok A, Garcia E, Jacquet H, Martin S, Garrigues A, Loiseau N, Andre F, Orlowski S, Vivaudou M. Recognition of sulfonylurea receptor (ABCC8/9) ligands by the multidrug resistance transporter P-glycoprotein (ABCB1): functional similarities based on common structural features between two multispecific ABC proteins. J. Biol. Chem. 2011;286:3552–3569. doi: 10.1074/jbc.M110.155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight AR. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma. 1992;9(Suppl 1):S83–S91. [PubMed] [Google Scholar]
- Bryan J, Aguilar-Bryan L. Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K(+) channels. Biochim. Biophys. Acta. 1999;1461:285–303. doi: 10.1016/s0005-2736(99)00164-9. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Dreixler JC, Couey JJ, Houamed KM. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur. J. Pharmacol. 2002;449:47–54. doi: 10.1016/s0014-2999(02)01987-8. [DOI] [PubMed] [Google Scholar]
- Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J. Neurosci. 2001;21:6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci. 2003;23:8568–8577. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J. Neurochem. 2007;100:1289–1299. doi: 10.1111/j.1471-4159.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981;35:124–133. [Google Scholar]
- Dunlop J, Beal MH, She Y, Howland DS. Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amyotrophic lateral sclerosis. J. Neurosci. 2003;23:1688–1696. doi: 10.1523/JNEUROSCI.23-05-01688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- Ferguson AR, Hook MA, Garcia G, Bresnahan JC, Beattie MS, Grau JW. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Figura M, Chilton L, Liacini A, Viskovic MM, Phan V, Knight D, Millar TM, Patel K, Kubes P, Giles WR, Tibbles LA. Blockade of K(ATP) channels reduces endothelial hyperpolarization and leukocyte recruitment upon reperfusion after hypoxia. Am. J. Transplant. 2009;9:687–696. doi: 10.1111/j.1600-6143.2009.02553.x. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, Freichel M, Simard JM. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat. Med. 2009;15:185–191. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Demion M, Launay P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 2010;25:155–164. doi: 10.1152/physiol.00004.2010. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- He Y, Benz A, Fu T, Wang M, Covey DF, Zorumski CF, Mennerick S. Neuroprotective agent riluzole potentiates postsynaptic GABA(A) receptor function. Neuropharmacology. 2002;42:199–209. doi: 10.1016/s0028-3908(01)00175-7. [DOI] [PubMed] [Google Scholar]
- Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1997;282:1280–1290. [PubMed] [Google Scholar]
- Kitzman PH. Effectiveness of riluzole in suppressing spasticity in the spinal cord injured rat. Neurosci. Lett. 2009;455:150–153. doi: 10.1016/j.neulet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr. Opin. Neurol. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Honig WM, Hamers FP, Steinbusch HW, Joosten EA. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma. 2005;22:214–225. doi: 10.1089/neu.2005.22.214. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Romero M, Reboreda A, Sanchez E, Ribeiro SJ. A riluzole- and valproate-sensitive persistent sodium current contributes to the resting membrane potential and increases the excitability of sympathetic neurones. Pflugers Arch. 2009;458:589–599. doi: 10.1007/s00424-009-0648-0. [DOI] [PubMed] [Google Scholar]
- Lips J, de HP, Bodewits P, Vanicky I, Dzoljic M, Jacobs MJ, Kalkman CJ. Neuroprotective effects of riluzole and ketamine during transient spinal cord ischemia in the rabbit. Anesthesiology. 2000;93:1303–1311. doi: 10.1097/00000542-200011000-00025. [DOI] [PubMed] [Google Scholar]
- Mantz J, Cheramy A, Thierry AM, Glowinski J, Desmonts JM. Anesthetic properties of riluzole (54274 RP), a new inhibitor of glutamate neurotransmission. Anesthesiology. 1992;76:844–848. doi: 10.1097/00000542-199205000-00023. [DOI] [PubMed] [Google Scholar]
- Martin D, Thompson MA, Nadler JV. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur. J. Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Hughes MG, Nie L, Shah B, Clifton C, Fullwood S, Hulsebosch CE. The effect of glutamate receptor blockers on glutamate release following spinal cord injury. Lack of evidence for an ongoing feedback cascade of damage→glutamate release→damage→glutamate release→etc. Brain Res. 2005;1038:92–99. doi: 10.1016/j.brainres.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Krampfl K, Moschref H, Dengler R, Bufler J. Interaction of the neuroprotective drug riluzole with GABA(A) and glycine receptor channels. Eur. J. Pharmacol. 2001;415:135–140. doi: 10.1016/s0014-2999(01)00847-0. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol. Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Pena F, Ordaz B. Non-selective cation channel blockers: potential use in nervous system basic research and therapeutics. Mini Rev. Med. Chem. 2008;8:812–819. doi: 10.2174/138955708784912166. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J. Neurotrauma. 2002;19:1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. 2001;94:245–256. doi: 10.3171/spi.2001.94.2.0245. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim. Biophys. Acta. 2007a;1772:947–957. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J. Clin. Invest. 2007b;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Bhatta S, Gerzanich V. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr. Opin. Pharmacol. 2008;8:42–49. doi: 10.1016/j.coph.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009a;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma. 2009b;26:2257–2267. doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009c;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Norenberg MD, Tosun C, Chen Z, Ivanova S, Tsymbalyuk O, Bryan J, Landsman D, Gerzanich V. Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci. Transl. Med. 2010;2:28ra29. doi: 10.1126/scitranslmed.3000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi A. Tolerability of riluzole: a review of the literature. Clin. Ter. 2004;155:25–28. [PubMed] [Google Scholar]
- Siniscalchi A, Bonci A, Mercuri NB, Bernardi G. Effectsofriluzoleonratcortical neurones: an in vitro electrophysiological study. Br. J. Pharmacol. 1997;120:225–230. doi: 10.1038/sj.bjp.0700905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: implications for neuroprotective strategies. Exp. Neurol. 1997;147:115–122. doi: 10.1006/exnr.1997.6554. [DOI] [PubMed] [Google Scholar]
- Tazerart S, Viemari JC, Darbon P, Vinay L, Brocard F. Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J. Neurophysiol. 2007;98:613–628. doi: 10.1152/jn.00316.2007. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Inhibition by riluzole of glycinergic postsynaptic currents in rat hypoglossal motoneurones. Br. J. Pharmacol. 1995;116:3227–3230. doi: 10.1111/j.1476-5381.1995.tb15128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur. J. Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb. Exp. Pharmacol. 2007:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- Wahl F, Stutzmann JM. Neuroprotective effects of riluzole in neurotrauma models: a review. Acta Neurochir. Suppl. 1999;73(103–10):103–110. doi: 10.1007/978-3-7091-6391-7_18. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Saint DA. The persistent sodium current blocker riluzole is antiarrhythmic and anti-ischaemic in a pig model of acute myocardial infarction. PLoS One. 2010;5:e14103. doi: 10.1371/journal.pone.0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie RG, Zheng DW, Xing JL, Zhang XJ, Song Y, Xie YB, Kuang F, Dong H, You SW, Xu H, Hu SJ. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS One. 2011;6:e18681. doi: 10.1371/journal.pone.0018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Enyeart JA, Enyeart JJ. Neuroprotective agent riluzole dramatically slows inactivation of Kv1.4 potassium channels by a voltage-dependent oxidative mechanism. J. Pharmacol. Exp. Ther. 2001;299:227–237. [PubMed] [Google Scholar]
- Zona C, Siniscalchi A, Mercuri NB, Bernardi G. Riluzole interacts with voltage-activated sodium and potassium currents in cultured rat cortical neurons. Neuroscience. 1998;85:931–938. doi: 10.1016/s0306-4522(97)00604-0. [DOI] [PubMed] [Google Scholar]
- Zona C, Cavalcanti S, De SG, Siniscalchi A, Marchetti C, Gaetti C, Costa N, Mercuri N, Bernardi G. Kainate-induced currents in rat cortical neurons in culture are modulated by riluzole. Synapse. 2002;43:244–251. doi: 10.1002/syn.10040. [DOI] [PubMed] [Google Scholar]