Abstract
At fertilization, mouse sperm bind to the zona pellucida (which consists of glycoproteins ZP1, ZP2, and ZP3) that surrounds eggs. A ZP2 cleavage model of gamete recognition requires intact ZP2, and a glycan release model postulates that zona glycans are ligands for sperm. These two models were tested by replacing endogenous protein with ZP2 that cannot be cleaved (Zp2Mut) or with ZP3 lacking implicated O glycans (Zp3Mut). Sperm bound to two-cell Zp2Mut embryos despite fertilization and cortical granule exocytosis. Contrary to prediction, sperm fertilized Zp3Mut eggs. Sperm at the surface of the zona pellucida remained acrosome-intact for more than 2 hours and were displaced by additional sperm. These data indicate that sperm-egg recognition depends on the cleavage status of ZP2 and that binding at the surface of the zona is not sufficient to induce sperm acrosome exocytosis.
Mammalian fertilization requires successful recognition between ovulated eggs and acrosome-intact capacitated sperm. Most models of gamete recognition postulate that a single ligand in the extracellular zona pellucida (ZP1, ZP2, or ZP3) surrounding eggs interacts with a sperm surface receptor. Although individual zona proteins were initially considered as possible ligands, the absence of ZP1 or the replacement of ZP2 and ZP3 with human homologs does not affect the specificity of sperm-egg recognition (1–3). Carbohydrate side chains on mouse ZP2 and ZP3 that are released after fertilization have attracted greater investigative attention. Both N and O glycans have been implicated in sperm-egg recognition (4), but a particularly precise and widely embraced glycan release model proposes that O glycans attached at Ser332 and Ser334 on ZP3 act as ligands for a sperm-surface receptor (5, 6).
A more-recent ZP2 cleavage model proposes that rather than sperm binding a single ligand, sperm binding is supported by a three-dimensional zona structure. This model is predicated on the cleavage status of ZP2, rendering the zona pellucida either permissive (uncleaved ZP2) or non-permissive (cleaved ZP2) to account for sperm binding to the zona pellucida surrounding eggs, but not to that surrounding two-cell embryos (3). Both models remain controversial—the first over the presence and identity of the carbohydrate ligand (7–13) and the second for validation with human ZP2 that was fortuitously not cleaved in transgenic mice (14). To test the two models (fig. S1), transgenic mouse lines mutated to either prevent cleavage of mouse ZP2 or the attachment of implicated O glycans on ZP3 (figs. S2 and S3) were back-crossed into appropriate null backgrounds (3, 15) to establish Zp2Mut (Zp1+/+, Zp2tm/tm;mut/mut, Zp3+/+) and Zp3Mut (Zp1+/+, Zp2+/+, Zp3tm/tm;mut) lines, where tm indicates a null allele.
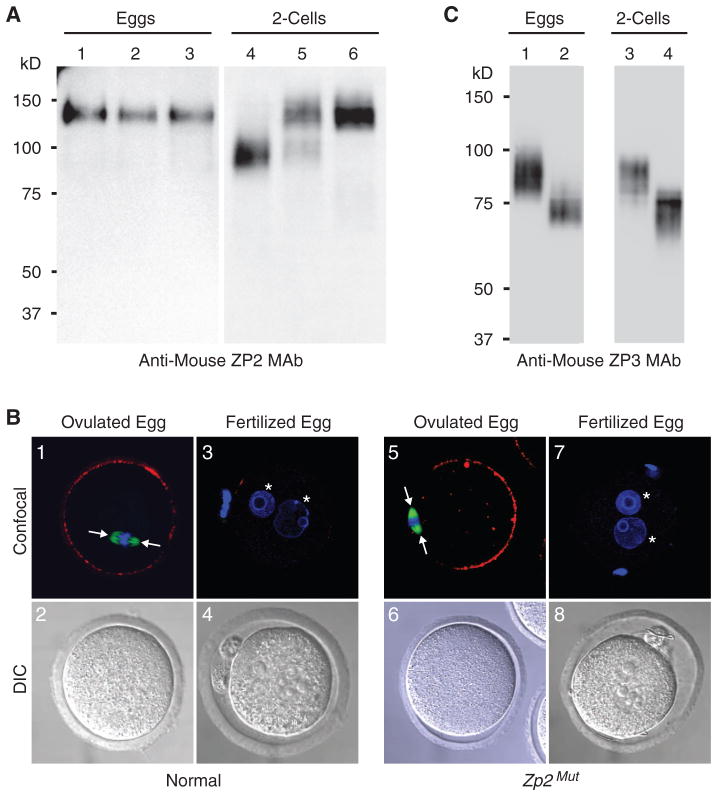
In ovulated eggs, ZP2 with a molecular mass of 120 kD is detected, reflecting uncleaved protein. After fertilization, normal ZP2 is cleaved, but mutant ZP2 is not. Thus, ZP2 was detected as a 90-kD (cleaved) protein in normal embryos and as a 120-kD (uncleaved) protein in Zp2Mut two-cell embryos. Both cleaved (endogenous) and uncleaved (mutant) ZP2 were present in mutant Zp2 transgenic mice (Zp1+/+, Zp2+/+;mut, Zp3+/+) (Fig. 1A). The inability to cleave ZP2 after fertilization could reflect the absence of cortical granule exocytosis. Therefore, ovulated and fertilized eggs were isolated from normal or Zp2Mut females and stained with Lens culinaris agglutinin (LCA) to detect cortical granules. In both normal and Zp2Mut mice, cortical granules were present at the periphery of ovulated eggs. Fertilization in the one-cell zygotes was confirmed by the presence of two pronuclei, and in each genotype, LCA staining was absent, reflecting post-fertilization cortical granule exocytosis (Fig. 1B). Thus, the inability to cleave ZP2 in embryos derived from Zp2Mut mice was independent of fertilization and cortical granule exocytosis.
Fig. 1.
Expression of mutant zona proteins. (A) Immunoblot of eggs (lanes 1 to 3) or embryos (lanes 4 to 6) from normal (lanes 1 and 4), Zp2mut transgenic (lanes 2 and 5), and Zp2Mut (lanes 3 and 6) mice using ZP2 antibodies (3). Molecular mass is at left. (B) Eggs and embryos from normal (1 to 4) or Zp2Mut (5 to 8) mice were stained with rhodamine-conjugated LCA to image cortical granules, which were present in ovulated (1 and 5) but not in fertilized (3 and 7) eggs. The metaphase spindle (arrows) was stained with a fluorescein-conjugated antibody to α-tubulin, and pronuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (asterisks). (C) Same as (A) but with eggs and embryos from normal (lanes 1 and 3) and Zp3Mut (lanes 2 and 4) mice using ZP3 antibodies (19).
No change in molecular mass was detected before and after fertilization in zonae from either normal or Zp3Mut mice. Although only Ser332 and Ser334 have been proposed as the binding sites for O glycan ligands that are required for sperm binding, the adjacent Ser329, Ser331, and Ser333 were also mutated to validate comparisons with earlier investigations (6). The loss of Ser329 and Ser332 disrupted two attachment sites [327NCS329 → 327NCA329 (where Ser329 is changed to Ala); 330NSS332 → 330NVG332 (where Ser331 is changed to Val and Ser332 is changed to Gly)] for N glycans that are occupied in native mouse ZP3 (10, 16). Thus, ZP3Mut zonae lacking two of five N glycans had a lower average molecular mass (~75 kD) than was normal (~85 kD) (Fig. 1C).
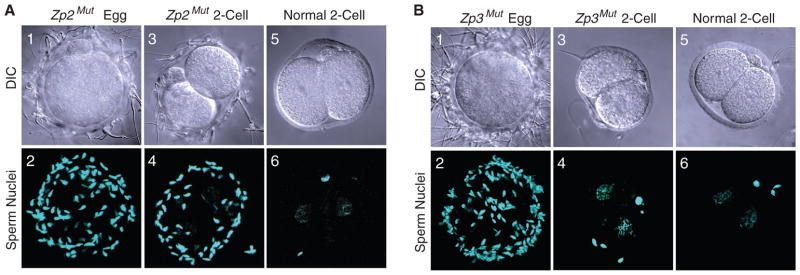
To test sperm-egg recognition, eggs and two-cell embryos were isolated from normal, Zp2Mut, and Zp3Mut mice. After insemination, sperm bound avidly to Zp2Mut eggs (67.2 ± 9.5, n = 12 eggs), as compared with normal two-cell embryos used as wash controls (7.0 ± 0.8, n = 13 embryos). However, sperm also bound (49.3 ± 5.5, n = 19 embryos) to two-cell embryos isolated from Zp2Mut females, in which ZP2 remained uncleaved (Fig. 2A). Contrary to prediction, sperm bound to Zp3Mut eggs (96.1 ± 1.9, n = 7 eggs) in assays using normal two-cell embryos as negative wash controls (Fig. 2B). Thus, sperm binding was unaffected by the ZP3 mutations (Ser332 → Ala; Ser334 → Ala), which preclude the attachment of O glycans at those sites. Rather, sperm binding to the surface of the zona pellucida required uncleaved ZP2, in a process that was independent of fertilization and cortical granule exocytosis.
Fig. 2.
Sperm binding to eggs and embryos. (A) Sperm binding to Zp2Mut eggs (1 and 2) and two-cell embryos (3 and 4) was assayed after 1 hour of incubation with normal capacitated sperm using normal two-cell embryos (5 and 6) as wash controls. The number of sperm bound to eggs and embryos was determined by confocal microscopy. (B) Same as (A) but with eggs and embryos from Zp3Mut females.
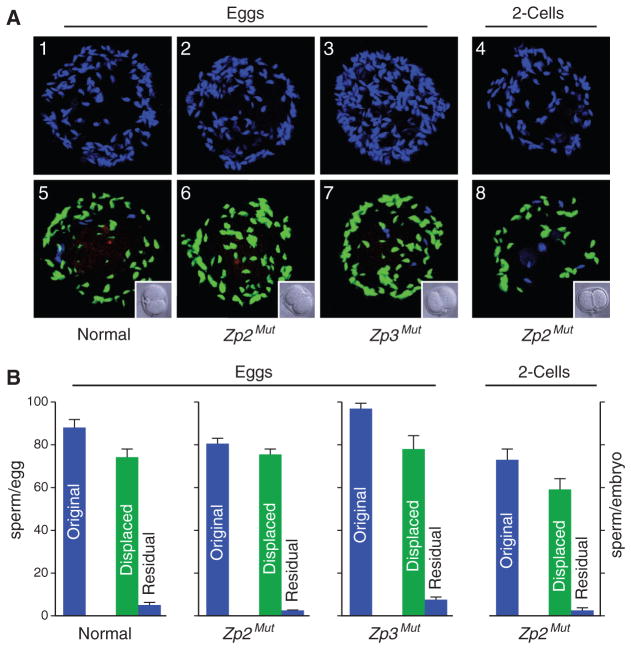
To determine the reversibility of gamete interactions on the surface of the zona pellucida, eggs and embryos were inseminated for 1 hour with normal sperm labeled with Hoechst stain. The fertilized eggs and embryos were then rinsed to remove loosely adherent sperm and challenged with capacitated Acr3-enhanced green fluorescent protein (EGFP) sperm (1 hour) followed by washing, using normal two-cell embryos as controls (Fig. 3A). Although comparable numbers of total sperm bound normal, Zp2Mut, and Zp3Mut eggs as well as Zp2Mut embryos, 91 to 97% of the initially bound sperm had been replaced with Acr3-EGFP sperm (Fig. 3B). These results indicate that the reversibility of sperm adherence to the zona pellucida for eggs (normal, Zp2Mut, and Zp3Mut) is also observed in Zp2Mut two-cell embryos in which ZP2 remains uncleaved.
Fig. 3.
Reversible sperm binding. (A) Normal (1), Zp2Mut (2), and Zp3Mut (3) eggs and Zp2Mut embryos (4) were incubated with capacitated sperm for 1 hour and stained with Hoechst before imaging by confocal microscopy. After a brief rinse, capacitated Acr3-EGFP sperm [5 × 105 ml−1 of human tubal fluid (HTF)] were added and incubated for an additional 1 hour. After washing with normal two-cell embryo controls (insets) to remove nonadherent sperm, eggs and embryos were stained with Alexa 568–SBTI before imaging by confocal microscopy (5 to 8). Acrosome-reacted and -intact sperm were labeled with Alexa 568 and EGFP, respectively. Images were modified in Adobe Photoshop to remove nuclear staining from EGFP-positive sperm; thus, Hoechst-positive, EGFP-negative sperm reflect those that were not displaced by EGFP sperm. (B) Quantification of the number of sperm bound to eggs and two-cell embryos before (blue bars labeled original) and after (green bars labeled displaced) the addition of Acr3-EGFP sperm. Residual sperm (blue bars) reflect those not displaced by Acr3-EGFP after 1 hour of incubation and removal of nonadherent sperm.
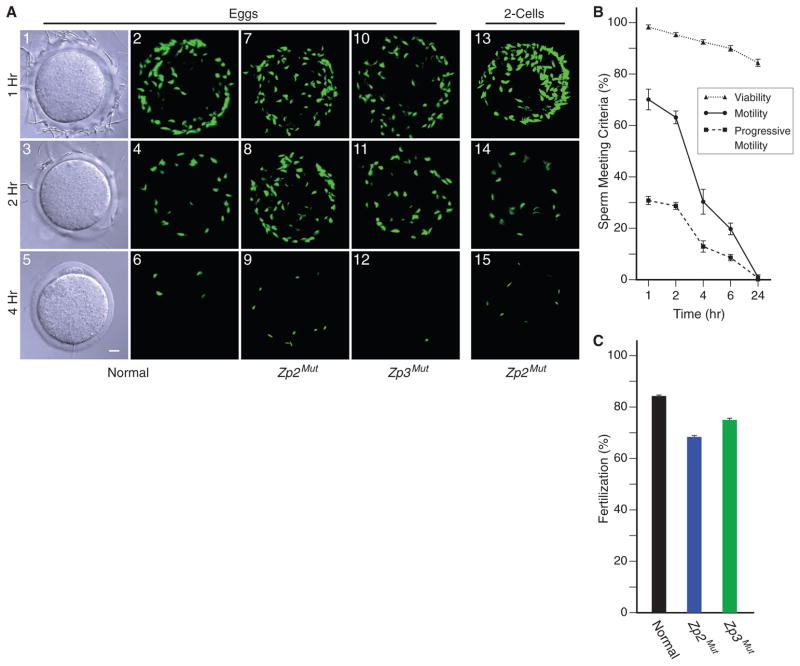
Integral to current glycan release models of sperm-egg recognition is that a zona glycan also induces exocytosis of the acrosome, which is a subcellular organelle at the head of sperm (6, 17). Using Acr-EGFP sperm to monitor acrosome status, Zp2Mut and Zp3Mut eggs were fertilized in vitro. Sperm were present at the surface of the zona pellucida for >2 hours after insemination. The number of sperm adherent to normal, Zp2Mut, and Zp3Mut eggs was comparable among the genotypes at 1 hour (80.8 ± 5.6, 76.7 ± 4.5, and 77.6 ± 13.5, respectively) and 2 hours (39.8 ± 3.6, 45.8 ± 3.3, and 43.5 ± 7.5, respectively). Sperm also remained adherent to the surface of the zona matrix surrounding Zp2Mut embryos at 1 (98.4 ± 4.1) and 2 (25.7 ± 0.9) hours (Fig. 4A). During this time period, virtually all adherent sperm remained acrosome-intact. Thus, binding to the surface of the zona pellucida is not sufficient to induce acrosome exocytosis. However, only acrosome-reacted sperm are present in the perivitelline space, which suggests that acrosome exocytosis is initiated either before arrival at the surface of the zona pellucida or during penetration of the zona matrix (18).
Fig. 4.
Sperm binding to Zp2Mut and Zp3Mut eggs and embryos. (A) Normal (1 to 6), Zp2Mut (7 to 9), and Zp3Mut (10 to 12) eggs or Zp2Mut two-cell embryos (13 to 15) were inseminated with capacitated Acr-EGFP sperm (1 × 105 ml−1 of HTF), and fertilized eggs and embryos were imaged at 1, 2, and 4 hours by differential interference contrast and confocal microscopy. (B) Capacitated Acr3-EGFP sperm were incubated in the same media as in (A) and assayed by computer-assisted sperm analysis after 1, 2, 4, 6, and 24 hours. Each data point, which is an average of three independent biological samples ± SEM, reflects the percent of sperm meeting the specific criteria. (C) Ovulated eggs in cumulus from normal, Zp2Mut, and Zp3Mut mice were inseminated with capacitated sperm (1 × 105 sperm ml−1 of HTF), and fertilization was assayed by the presence of two-cell embryos after overnight incubation. The data reflect the average of five separate experiments for each genotype (a total of 66 to 92 eggs) ± SEM.
By 4 hours after insemination, few Acr-EGFP sperm remained on the zona surface of normal, Zp2Mut, and Zp3Mut eggs (1.8 ± 0.4, 12.0 ± 1.8, and 1.1 ± 0.3, respectively) or Zp2Mut embryos (10.9 ± 2.1). To determine whether release was secondary to acrosome exocytosis, sperm were stained with Alexa 568–conjugated soybean trypsin inhibitor (SBTI), which binds to the inner acrosomal membrane after the acrosome reaction. With rare exceptions (2 of 1929 sperm observed), no acrosome-reacted sperm were detected on the surface of the zona matrix. The disappearance of sperm from the zona surface of Zp2Mut eggs and embryos (which must be independent of ZP2 cleavage) as well as from normal and Zp3Mut eggs correlated with a pronounced decrease in progressive sperm motility (Fig. 4B), although causality has not been established.
In vitro fertilization was determined by the addition of capacitated sperm to ovulated eggs in cumulus obtained from normal, Zp2Mut, and Zp3Mut female mice. Both rescue lines had fertilization rates that were comparable to those observed with normal controls (Fig. 4C). To assess in vivo fertilization, Zp2Mut or Zp3Mut females were paired with corresponding transgenic females as controls and mated with normal male mice that were proven to be fertile. The size of Zp3Mut litters was comparable to those of co-caged control female mice (table S1), indicating that Zp3Mut mice have normal fertility both in vitro and in vivo.
However, only half (3 out of 6) of the Zp2Mut females produced pups in vivo, with 36% as many litters, the size of which were significantly smaller than those of co-caged controls. Similar numbers of eggs and one-cell embryos were recovered from the oviducts of Zp2Mut and control females after gonadotrophin stimulation or in vivo fertilization, respectively (tables S1 and S2). There was no evidence of supernumerary sperm in the perivitelline space (0.05 ± 0.03 sperm per embryo, n = 40 embryos), indicating that an effective postfertilization block to polyspermy was imposed independently of ZP2 cleavage. After flushing oviducts at embryonic day 3.5 (E3.5), significantly fewer blasto-cysts were recovered from mutant as compared with control female mice, of which mating was confirmed by the presence of a copulatory plug (table S2). Thus, early embryonic loss rather than defects in fertility appears as the major contributor to the smaller litter sizes observed in Zp2Mut females.
The normal fertility of Zp3Mut mice is not consistent with glycan release models in which O glycans attached to ZP3 Ser332 or Ser334 play an essential role in sperm-egg recognition. More generally, the ability of sperm to bind to Zp2Mut embryos after cortical granule exocytosis does not support any zona ligand in a glycan release model. Mutant mouse ZP2, which differs in only three amino acids from the native protein, has the same extent of posttranslational modifications and reconstitutes a zona pellucida in Zp2 null mice. The observed binding of sperm to Zp2Mut two-cell embryos is not consistent with glycan release models, in which a cortical granule glycosidase cleaves off a zona glycan to account for the inability of sperm to bind after fertilization. For sperm to bind to the zona pellucida after cortical granule exocytosis, the candidate glycan would have to remain accessible to sperm and yet have been inaccessible for cleavage by a cortical granule glycosidase. This inconsistency applies both to N and O glycan candidate ligands. We thus conclude that glycan release models, as currently formulated, do not offer an adequate explanation of sperm-egg recognition.
Rather, recent and accumulating data support a ZP2 cleavage model for sperm-egg recognition, in which sperm adhere to the surface of the zona pellucida if ZP2 is intact, independent of fertilization and cortical granule exocytosis. Thus, sperm bind to normal eggs but not to two-cell embryos in which ZP2 has been cleaved by a protease that is released during cortical granule exocytosis. However, mutant ZP2 protein cannot be cleaved, and sperm bind to two-cell embryos derived from Zp2Mut mice. A direct effect of ZP2 cleavage on the three-dimensional matrix provides a parsimonious explanation of these results, rendering the zona pellucida either permissive (intact ZP2) or nonpermissive (cleaved ZP2) for sperm-egg recognition.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
References and Notes
- 1.Rankin TL, et al. Development. 1998;125:2415. doi: 10.1242/dev.125.13.2415. [DOI] [PubMed] [Google Scholar]
- 2.Rankin T, Talbot P, Lee E, Dean J. Development. 1999;126:3847. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 3.Rankin TL, et al. Dev Cell. 2003;5:33. doi: 10.1016/s1534-5807(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 4.Tulsiani DR, Yoshida-Komiya H, Araki Y. Biol Reprod. 1997;57:487. doi: 10.1095/biolreprod57.3.487. [DOI] [PubMed] [Google Scholar]
- 5.Florman HM, Wassarman PM. Cell. 1985;41:313. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Litscher ES, Wassarman PM. Proc Natl Acad Sci USA. 1998;95:6193. doi: 10.1073/pnas.95.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, et al. Mol Cell Biol. 2004;24:9920. doi: 10.1128/MCB.24.22.9920-9929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. J Cell Sci. 2007;120:1341. doi: 10.1242/jcs.004291. [DOI] [PubMed] [Google Scholar]
- 9.Lopez LC, et al. J Cell Biol. 1985;101:1501. doi: 10.1083/jcb.101.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boja ES, Hoodbhoy T, Fales HM, Dean J. J Biol Chem. 2003;278:34189. doi: 10.1074/jbc.M304026200. [DOI] [PubMed] [Google Scholar]
- 11.Thall AD, Malý P, Lowe JB. J Biol Chem. 1995;270:21437. doi: 10.1074/jbc.270.37.21437. [DOI] [PubMed] [Google Scholar]
- 12.Lowe JB, Marth JD. Annu Rev Biochem. 2003;72:643. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 13.Asano M, et al. EMBO J. 1997;16:1850. doi: 10.1093/emboj/16.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jungnickel MK, Sutton KA, Florman HM. Cell. 2003;114:401. doi: 10.1016/s0092-8674(03)00648-2. [DOI] [PubMed] [Google Scholar]
- 15.Rankin T, et al. Development. 1996;122:2903. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 16.Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; G, Gly; N, Asn; S, Ser; and V, Val.
- 17.Leyton L, Saling P. J Cell Biol. 1989;108:2163. doi: 10.1083/jcb.108.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Development. 2007;134:933. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- 19.East IJ, Gulyas BJ, Dean J. Dev Biol. 1985;109:268. doi: 10.1016/0012-1606(85)90454-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.