Abstract
Purpose
African American colorectal cancer (CRC) patients have worse survival outcomes than Caucasian patients. To determine if differences exist in the molecular mechanisms driving CRC between African Americans and Caucasians, we characterized patient tumors from a single institution by assessing genetic alterations involved in CRC progression and response to treatment.
Experimental Design
We retrospectively examined 448 African Americans and Caucasians diagnosed with CRC at The University of Chicago Medical Center between 1992 and 2002. Microsatellite instability (MSI) status was determined by genotyping the BAT25, BAT26, BAT40, D5S346, and BAX loci. Mutations in KRAS codons 12 and 13 and BRAF codon 600 were identified by direct sequencing. MSI and detected mutations were correlated with clinicopathological features.
Results
Overall, no difference existed in MSI or BRAF mutation frequencies between African Americans and Caucasians. However, African Americans with microsatellite stable (MSS)/MSI-low (MSI-L) tumors had a higher proportion of KRAS mutations than Caucasians (34% v. 23%, p=0.048) that was isolated to proximal colon cancers and primarily driven by mutations in codon 13. There was no racial/ethnic difference in receipt of chemotherapy, but African Americans with MSS/MSI-L tumors had a 73% increased risk of death over Caucasians that could not be explained by known prognostic factors.
Conclusions
The significantly higher risk of death among African Americans with MSS/MSI-L tumors may be related to differences in the distribution of factors influencing response to standard therapies. These data underscore the need for further research into the molecular mechanisms driving CRC progression in underserved and understudied populations.
Keywords: colorectal cancer, microsatellite instability, KRAS, BRAF, African Americans
INTRODUCTION
African Americans have the highest incidence and mortality rates of colorectal cancer in the U.S. Unlike Caucasians, who display declining mortality rates, African American mortality rates have stabilized over the past 20 years for reasons not well understood.[1] Although many factors are likely to play a role in the disparate survival outcomes between African American and Caucasian patients, whether tumor biology and genetics contribute to this observed health disparity have yet to be fully explored.[2–7]
African Americans with colorectal cancer are typically diagnosed at a younger age than Caucasians [8, 9], with the largest mortality rate differences between African Americans and Caucasians being among younger patients and those diagnosed at early stages.[2, 3] African Americans also have a higher incidence of proximal tumors than Caucasians for reasons that are unknown and understudied.[8–10] Whereas survival rates do no vary significantly between African Americans and Caucasians with rectal adenocarcinomas, African Americans with colonic adenocarcinomas are more likely to die of their disease than Caucasians with colonic adenocarcinomas.[6] These findings suggest that aggressive tumor phenotypes associated with poor prognostic outcomes are partly responsible for the disparities.
Colorectal cancer initiation and progression is driven by an accumulation of genetic mutations, leading to loss of genomic integrity through three identified patterns. The majority (80–85%) of colorectal tumors display chromosomal instability, leading to defects in genes (e.g. TP53, EGFR, KRAS, BRAF, APC) that guard chromosomal integrity and regulate cell proliferation, differentiation, and apoptosis. A minority (15%) of tumors, having a better prognosis, progress through the microsatellite instability (MSI) pathway and is associated with inactivation of mismatch repair (MMR) genes. A third group (15%) of tumors, somewhat overlapping with MSI tumors, undergo aberrant methylation and display the CpG island methylator phenotype (CIMP).[11, 12] The identification of these (epi)genetic alterations driving the development of colorectal tumors has been vital to understanding how tumors progress to aggressive phenotypes and in determining how these mechanisms can be targeted to improve patient outcomes.
Due to the paucity of data on colorectal cancer progression in African Americans, we conducted a comprehensive study examining the molecular pathogenesis of this disease in a cohort of patients at The University of Chicago Medical Center. We assessed MSI status and KRAS and BRAF mutations, as they are involved in disease initiation, progression, and treatment response.[11–22] Although previously studied, these molecular pathways have not been characterized in African Americans, and their potential role in the aggressive tumor phenotypes and poor survival outcomes reported in the African American population has not been fully explored. To our knowledge, this is the first study to examine whether differential distribution of biological factors contributes to outcome differences among African Americans and Caucasians in a hospital-based cohort.
METHODS
Patient and Sample Ascertainment
Our selection criteria for inclusion in this study were patients diagnosed with colorectal cancer between 1992 and 2002, who had invasive cancer, and who had accessible matching normal and tumor tissue blocks. A total of 1,539 patients, who underwent surgical resection at The University of Chicago Medical Center, were identified in The University of Chicago Tumor Registry. Of those patients, we were able to link 564 (37%) patients to The University of Chicago Department of Pathology database from which we abstracted patient pathology reports. Of these 564 patients, 448 (29%) patients had accessible normal and tumor tissue blocks for inclusion in this study (Supplemental Figure 1). This study was given approval by The University of Chicago Institutional Review Board.
All samples were fixed in 10% formalin and embedded in paraffin. Inclusion criteria included tumor location in the colon or rectum and operative resection. Clinical information included age, sex, race/ethnicity, tumor size, grade, American Joint Committee on Cancer (AJCC) stage, receipt of 5-fluorouracil (5-FU) treatment, survival, and status at last follow-up. Samples were analyzed anonymously as lab researchers were blinded to clinical and pathological data.
Pathological Assessment and DNA Extraction
Representative areas of the lesions were carefully selected from hematoxylin and eosin stained sections. DNA was extracted from matched surrounding normal and tumor 50-micron tissue sections using the PUREGENE® DNA Purification Kit (Gentra Systems, Minneapolis, MN) according to the manufacturer’s instructions. The protocol was modified with the use of octane for deparaffinization.
Microsatellite Instability Analysis
We designed fluorophore-labeled primers (Integrated DNA Technologies, Iowa City, Iowa) targeting the following microsatellite loci: BAT25 forward 5’-TCGCCTCCAAGAATCTAAGT-3’, reverse 5’-TCTGCATTTTAACTATGGCTC-3’; BAT26 forward 5’-TGACTACTTTTGACTTCAGCC-3’, reverse 5’-AACCATTCAACATTTTTAACCC-3’; BAT40 forward 5’-ACAACCCTGCTTTTGTTCCT-3’, reverse 5’-GTAGAGCAAGACCACCTTG-3’; D5S346 forward 5’-ACTCACTCTAGTGATAAATCGGG-3’, reverse 5’-AGCAGATAAGACAGTATTACTAGTT-3’; BAX forward 5’-TCCATCCAGGATCGAGCAGGGCGA-3’, reverse 5’-CACTCGCTCAGCTTCTTGGTGGAC-3’. Loci were amplified by PCR and genotyped by The University of Chicago Cancer Research Center (UCCRC) DNA Sequencing Facility. Microsatellite marker stability was analyzed using GeneMapper® software (Applied Biosystems, Foster City, CA). A locus was considered unstable if allelic addition/deletion occurred in the tumor DNA compared to patient matched normal DNA. Tumors were categorized as MSS if all markers were stable, MSI-L if <30% were unstable, and MSI-H if ≥30% were unstable. MSI was evaluated independently by two researchers (BS and JZ), without knowledge of clinicopathological data and assessed twice per patient. If a discrepancy occurred, that tumor was classified as undetermined and not included within the study analysis. MSI status could not be determined for 57 (13%) tumors due to poor DNA quality (Supplemental Figure 1).
KRAS and BRAF Sequencing
We designed the following primers (Integrated DNA Technologies, Iowa City, Iowa) targeting KRAS codons 12 and 13 and BRAF codon 600 for direct sequencing: KRAS forward 5’-TGTTCTAATATAGTCACATTTTCATT-3’, reverse 5’-TCCTGCACCAGTAATATGC-3’; BRAF forward 5’-GCTTGCTCTGATACCAAAATGAG-3’, reverse 5’-CCACAAAATGGATCCAGACA-3’. Regions were amplified by PCR, sequenced by The UCCRC DNA Sequencing Facility, then analyzed using Sequencher® software (Gene Codes Corporation, Ann Arbor, MI). Mutations identified were confirmed by independent reactions using reverse sequencing. If a discrepancy occurred, that tumor was classified as undetermined and not included within the study analysis. KRAS and BRAF mutation data were not available on 28 (6%) and 30 (7%) patients, respectively, due to insufficient DNA or failed sequencing reads due to poor DNA quality (Supplemental Figure 1).
Statistical Analysis
We compared demographic and clinical characteristics between African Americans and Caucasians using Fisher’s exact tests for categorical variables such as anatomic site, t tests for continuous variables like age at diagnosis, and the Wilcoxon rank-sum test for ordinal variables such as tumor grade. We compared racial differences in MSI status, KRAS and BRAF mutation status, as well as mutation spectrum using Fisher’s exact tests. Similarly, we explored demographic and clinical factors associated with KRAS and BRAF mutations using t tests, Fisher’s exact tests, or Wilcoxon rank-sum tests as appropriate. We examined racial differences in demographic and clinical factors, as well as KRAS and BRAF mutations within the MSI strata. Survival curves were obtained by the Kaplan-Meier method, and differences in overall survival rates were analyzed with the log-rank test. Cox proportional hazard models were used to examine independent prognostic factors, including MSI status and KRAS and BRAF mutations, along with other clinicopathological factors. To explore the reasons for racial disparity in overall survival, we first conducted a univariate analysis and estimated the unadjusted hazard ratio for African Americans compared to Caucasians. Then, we added demographic factors (age and sex), pathological factors (grade, stage, and anatomic site), 5-FU treatment, and molecular markers (MSI status and KRAS and BRAF mutation status) to the Cox models in a successive manner. This model building was performed separately for all patients, for MSI-H patients, and for MSS/MSI-L patients. We tested for an interaction between race and MSI status. The final Cox model included all of these factors, plus an interaction term of race and MSI status. Patients with missing data in a particular prognostic factor were excluded from the Cox model building. A p-value <0.05 was considered statistically significant. All statistical analyses were done using Stata 11.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
For inclusion in this study, we identified 448 of 1,539 consecutive patients diagnosed with invasive colorectal cancer between 1992 and 2002 that had clinicopathological data and accessible matching normal and tumor tissues (Supplemental Figure 1). Of the 448 patients, 50% were African American and 46% were Caucasian, reflecting the general pattern of colorectal cancer patients seen at The University of Chicago Medical Center. The remaining patients were of Asian American, Hispanic American, and unknown ancestry. We compared the clinicopathological features of African Americans and Caucasians and found they were very similar, except African Americans in this hospital-based cohort were slightly older (69 v. 66 years, p=0.008). Of note, there was no difference in the receipt of chemotherapy by race/ethnicity within this cohort (Supplemental Table 1).
Molecular Characterization of Tumors
We evaluated the MSI status of all 448 cases. Of 391 tumors that could be scored (Supplemental Figure 1), 73% were microsatellite stable (MSS), 13% were MSI-low (MSI-L), and 14% were MSI-high (MSI-H). We found no difference in the frequency of MSI tumors between African Americans and Caucasians. MSS and MSI-L cases were combined for analysis, as these cases are clinically and pathologically similar.[12, 23, 24] Clinicopathological data was available for 95% of cases with MSI data as shown in Table 1. In patients with MSS/MSI-L tumors, African Americans were older and more likely to be female than Caucasians. There were trends toward significance of Caucasians with MSS/MSI-L tumors having a higher proportion of poorly differentiated tumors and African Americans with MSS/MSI-L tumors presenting more frequently with proximal colon tumors.
Table 1.
Patient clinicopathological features stratified by MSI status and race
| Factors | MSS/MSI-L | MSI-H | ||||
|---|---|---|---|---|---|---|
| African Americans |
Caucasians | P-values | African Americans |
Caucasians | P-values | |
| Overall | 170 (85%) | 150 (87%) | 0.37 | 29 (15%) | 22 (13%) | 0.37 |
| Age at diagnosis, mean ± SD | 69.1±12.5 | 65.4±13.4 | 0.01 | 66.0±14.6 | 67.1±17.3 | 0.81 |
|
Sex Female Male |
105 (62%) 65 (38%) |
72 (48%) 78 (52%) |
0.02 | 17 (59%) 12 (41%) |
13 (59%) 9 (41%) |
1.00 |
|
Grade Well differentiated Moderately differentiated Poorly differentiated |
37 (22%) 112 (68%) 16 (10%) |
25 (17%) 95 (66%) 24 (17%) |
0.07 | 2 (7%) 20 (69%) 7 (24%) |
3 (14%) 12 (54%) 7 (32%) |
0.89 |
|
AJCC stage I II III IV |
30 (18%) 50 (30%) 55 (33%) 33 (20%) |
29 (19%) 41 (28%) 45 (30%) 34 (23%) |
0.84 | 5 (17%) 12 (42%) 9 (31%) 3 (10%) |
6 (29%) 8 (38%) 7 (33%) 0 (0%) |
0.29 |
|
Anatomic site Proximal colon Distal colon Rectum Colon, unspecified |
94 (55%) 59 (35%) 4 (2%) 13 (8%) |
68 (45%) 61 (41%) 10 (7%) 11 (7%) |
0.06* | 22 (76%) 6 (21%) 1 (3%) 0 (0%) |
17 (77%) 2 (9%) 0 (0%) 3 (14%) |
0.67* |
| 5-FU treatment | 44 (28%) | 49 (34%) | 0.26 | 8 (30%) | 8 (38%) | 0.56 |
| KRAS mutation | 57 (34%) | 35 (23%) | 0.048 | 7 (24%) | 5 (23%) | 1.00 |
| BRAF mutation | 6 (4%) | 7 (5%) | 0.78 | 11 (39%) | 6 (27%) | 0.55 |
p-value was calculated after excluding patients with unspecified anatomic location of colon cancers.
MSS=microsatellite stable, MSI-L=microsatellite instability-low, MSI-H=microsatellite instability-high, SD=standard deviation, AJCC=American Joint Committee on Cancer, 5-FU=5-fluorouracil
We successfully tested for KRAS and BRAF mutations on 94% and 93% of cases, respectively (Supplemental Figure 1). We focused on KRAS codons 12 and 13 mutations and the BRAF V600E mutation, as they respectively account for 90% of all KRAS mutations and 80% of all BRAF mutations in colorectal cancers.[14, 25, 26] The overall frequency of KRAS mutations was 27% (115/420). Codon 12 mutations were more common, accounting for 77% (89/115) of all KRAS mutations identified. Twenty-four (21%) patients had codon 13 mutations, and two patients had mutations in both codons 12 and 13. The BRAF mutation frequency was 8% (32/418). Only one patient had mutations in both KRAS and BRAF, indicating co-mutation of BRAF and KRAS was uncommon (p=0.001).
Table 2 presents the correlates for KRAS or BRAF mutation status. Both KRAS (p=0.048) and BRAF (p=0.003) mutations were associated with proximal colon tumors. Patients with a BRAF mutation were older compared to patients without the mutation (73 years v. 67 years, p=0.02). Additionally, the BRAF mutation was more common in females (p=0.02), poorly differentiated tumors (p=0.005), and stage 2 and 3 cancers (p=0.01). The BRAF mutation was also highly associated with MSI-H tumors (p<0.001).
Table 2.
Clinicopathological characteristics of patients with KRAS or BRAF mutations
| Factors | Wild-type KRAS |
Mutant KRAS | P-values | Wild-type BRAF |
Mutant BRAF | P-values |
|---|---|---|---|---|---|---|
| Age at diagnosis, mean ± SD | 66.7±13.3 | 69.1±12.4 | 0.10 | 66.9±13.2 | 72.5±11.3 | 0.02 |
|
Sex Female Male |
177 (58%) 127 (42%) |
62 (54%) 53 (46%) |
0.44 | 214 (56%) 171 (44%) |
25 (78%) 7 (22%) |
0.02 |
|
Race/Ethnicity African American Caucasian |
146 (51%) 143 (49%) |
65 (59%) 45 (41%) |
0.15 | 192 (53%) 173 (47%) |
18 (56%) 14 (44%) |
0.72 |
|
Grade Well differentiated Moderately differentiated Poorly differentiated |
52 (18%) 193 (65%) 51 (17%) |
24 (22%) 72 (66%) 13 (12%) |
0.14 | 75 (20%) 242 (65%) 55 (15%) |
1 (3%) 22 (69%) 9 (28%) |
0.005 |
|
AJCC stage I II III IV |
60 (20%) 89 (30%) 102 (34%) 49 (16%) |
21 (19%) 31 (27%) 35 (31%) 26 (23%) |
0.30 | 79 (21%) 105 (28%) 122 (32%) 73 (19%) |
2 (6%) 15 (47%) 13 (41%) 2 (6%) |
0.01 |
|
Anatomic site Proximal colon Distal colon Rectum Colon, unspecified |
153 (50%) 115 (38%) 15 (5%) 21 (7%) |
71 (62%) 29 (25%) 5 (4%) 10 (9%) |
0.048* | 196 (51%) 140 (36%) 20 (5%) 29 (8%) |
26 (81%) 4 (13%) 0 (0%) 2 (6%) |
0.003* |
|
5-FU treatment No Yes |
187 (66%) 98 (34%) |
80 (72%) 31 (28%) |
0.23 | 247 (68%) 116 (32%) |
20 (62%) 12 (38%) |
0.56 |
|
MSI status MSS/MSI-L MSI-H |
240 (86%) 40 (14%) |
96 (88%) 13 (12%) |
0.62 | 323 (90%) 35 (10%) |
13 (43%) 17 (57%) |
<0.001 |
p-value was calculated after excluding patients with unspecified anatomic location of colon cancers.
SD=standard deviation, MSI= microsatellite instability, MSS=microsatellite stable, MSI-L=microsatellite instability-low, MSIH=microsatellite instability-high, 5-FU=5-fluorouracil
KRAS Mutation Frequency and Spectrum by Race/Ethnicity
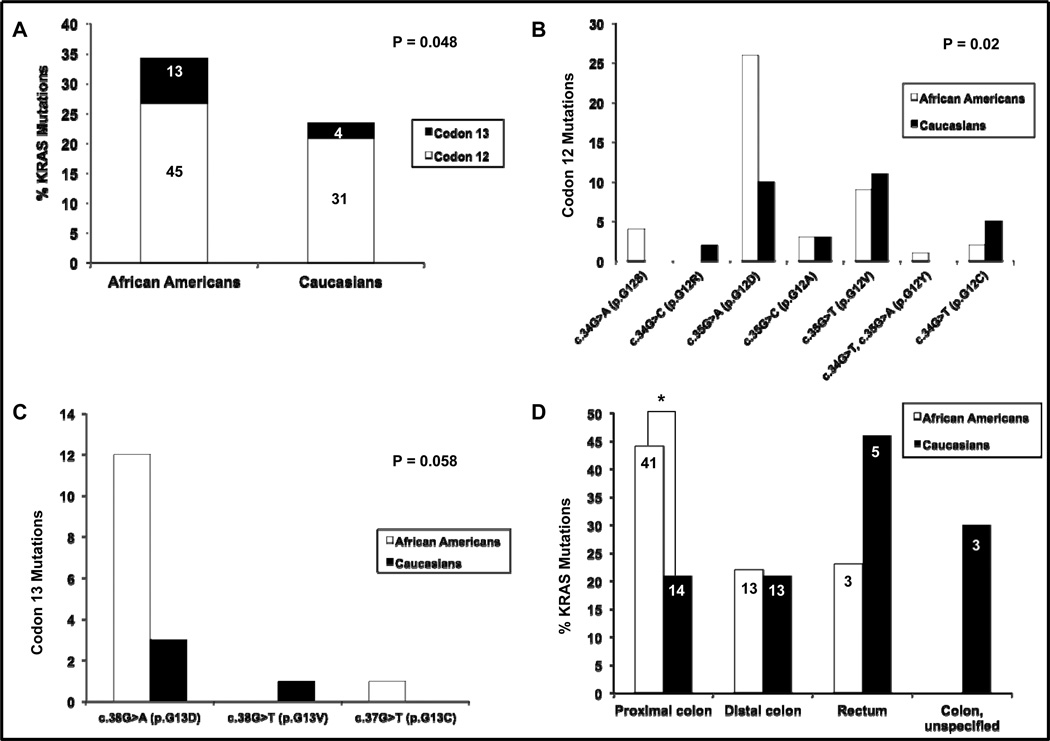
Among patients with MSS/MSI-L tumors, African Americans had a higher frequency of KRAS mutations compared to Caucasians (34% v. 23%, p=0.048), especially in codon 13 (Table 1, Figure 1A). The spectrum of mutations also varied between the two populations (Figures 1B and 1C). African Americans were more likely to have the c.35G>A (p.G12D) mutation in codon 12, while both the c.35G>A (p.G12D) and c.35G>T (p.G12V) mutations were the most common in Caucasians (p=0.02). Within codon 13, African Americans outnumbered Caucasians with the c.38G>A (p.G13D) mutation, and this was nearly statistically significant (p=0.058). Interestingly, the difference in KRAS mutation frequency between African Americans and Caucasians occurred in MSS/MSI-L tumors within the proximal colon (44% v. 21%, p=0.002); whereas in the distal colon (22% v. 21%, p=1.0), rectum (23% v. 46%, p=0.39), and colon [(unspecified) 0% v. 30%, p=0.51], the mutation frequency was similar (Figure 1D).
Figure 1.
KRAS codons 12 and 13 mutation frequencies and spectra of African American and Caucasian patients with MSS/MSI-L tumors. (A) Overall frequency of KRAS mutations by codon. Numbers on the column charts represent the raw number of KRAS-mutated cases for each population by codon. (B) Spectrum of mutations in KRAS codon 12. (C) Spectrum of mutations in KRAS codon 13. (D) Overall frequency of KRAS mutations by anatomic site. Numbers on the column charts represent the raw number of KRAS-mutated cases for each population by anatomic site. *p=0.002. MSS=microsatellite stable, MSI-L=microsatellite instability-low, P=p-value.
Prognostic Factors for Overall Survival and Racial/Ethnic Disparity
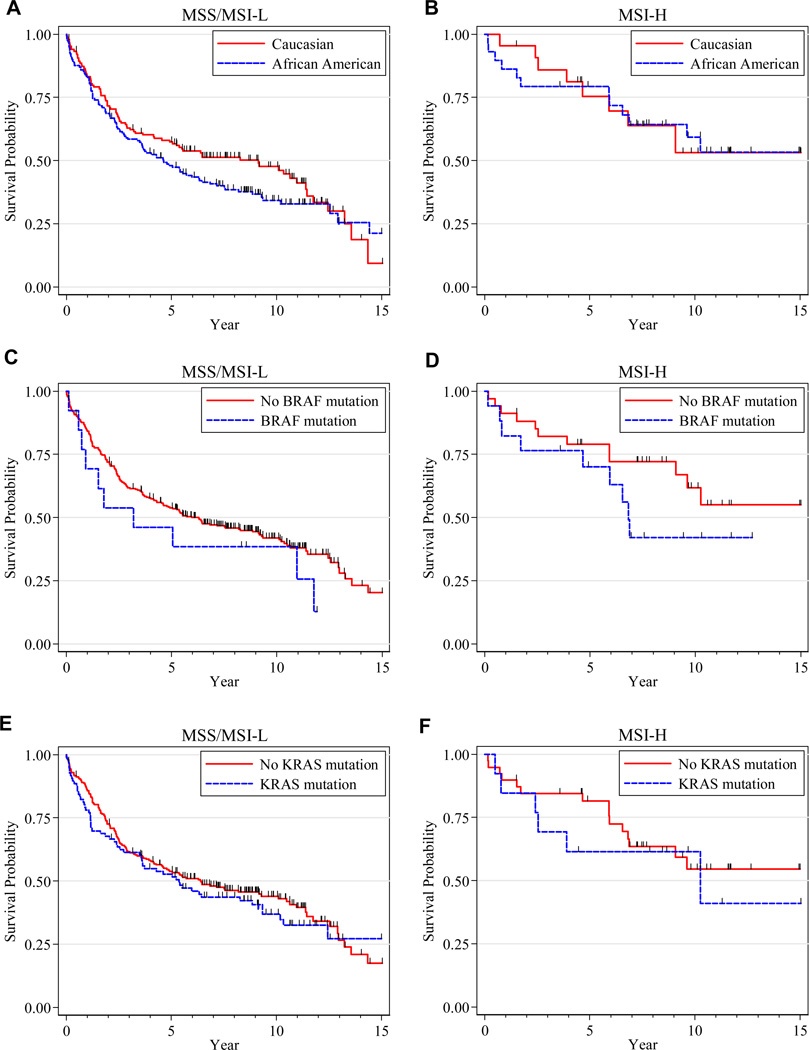
Survival outcomes were available for 446 (99%) patients. After a median follow-up of 8.8 years, the cohort median survival time was 6.5 years (95% CI:5.4–9.1). To examine the reasons for the observed racial differences in overall survival, we developed Cox models comparing African Americans to Caucasians, adjusting for clinical and molecular variables in a serial pattern among all patients and separately according to MSI status (Table 3). In the univariate analysis (Model A), African Americans had a 14% increased risk of death over Caucasians that was not statistically significant. After adjustment for age, sex, grade, stage, and anatomic site (Model C), African Americans had a 35% increased risk of death over Caucasians that was statistically significant. Survival differences between African Americans and Caucasians remained statistically significant even after adjustment for receipt of 5-FU, MSI status, and KRAS and BRAF mutations (Model D, Model E, Model F, and Full Model, respectively). In the univariate analysis, African Americans had worse survival than Caucasians among patients with MSS/MSI-L tumors and with MSI-H tumors (Figures 2A and 2B). Following adjustment for clinicopathological and molecular factors (Full Model), African Americans had a 73% increased risk of death over Caucasians among patients with MSS/MSI-L tumors, but there was no survival difference between African Americans and Caucasians with MSI-H tumors (p for interaction = 0.10).
Table 3.
Cox models: Risk of death for African American patients relative to Caucasian patients
| Models | All Patients | MSS/MSI-L | MSI-H | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-values | HR (95% CI) | P-values | HR (95% CI) | P-values | |
|
Model A: Unadjusted |
1.14 (0.89–1.46) | 0.30 | 1.24 (0.93–1.65) | 0.14 | 1.05 (0.43–2.57) | 0.92 |
|
Model B: Adjusted for age and sex |
1.07 (0.83–1.37) | 0.61 | 1.16 (0.87–1.54) | 0.31 | 1.05 (0.43–2.56) | 0.92 |
|
Model C: Model B + adjustment for grade, stage, and anatomic site |
1.35 (1.03–1.77) | 0.03 | 1.65 (1.21–2.25) | 0.002 | 0.91 (0.36–2.25) | 0.83 |
|
Model D: Model C + adjustment for 5-FU treatment |
1.32 (0.99–1.75) | 0.06 | 1.69 (1.22–2.34) | 0.002 | 0.75 (0.30–1.88) | 0.54 |
|
Model E: Model D + adjustment for MSI status |
1.56 (1.14–2.12) | 0.005 | --- | --- | ||
|
Model F: Model E + adjustment for KRAS mutation status |
1.58 (1.16–2.16) | 0.004 | 1.72 (1.24–2.39) | 0.001 | 0.75 (0.30–1.90) | 0.55 |
|
Full Model: Model F + adjustment for BRAF mutation status |
1.59 (1.16–2.18) | 0.004 | 1.73 (1.24–2.40) | 0.001 | 0.75 (0.29–1.89) | 0.54 |
HR=hazard ratio, CI=confidence interval, MSS=microsatellite stable, MSI-L=microsatellite instability-low, MSI-H= microsatellite instability-high, 5-FU=5-fluorouracil, MSI= microsatellite instability
Figure 2.
Kaplan-Meier curves displaying patient overall survival, stratified by MSI status. (A) Overall survival by race in patients with MSS/MSI-L tumors. (B) Overall survival by race in patients with MSI-H tumors. (C) Overall survival of patients with or without a BRAF mutation in MSS/MSI-L cases. (D) Overall survival of patients with or without a BRAF mutation in MSI-H cases. (E) Overall survival of patients with or without a KRAS mutation in MSS/MSI-L cases. (F) Overall survival of patients with or without a KRAS mutation in MSI-H cases. MSS=microsatellite stable, MSI-L=microsatellite instability-low, MSIH= microsatellite instability-high
Table 4 presents all prognostic factors in the Full Model. As expected, high grade and advanced stage were associated with poor overall survival. Age at diagnosis was significantly associated with overall survival specifically in a piecewise manner: before age 65, the risk of death increased 27% per 10-year age increment; after age 65, the risk of death significantly increased 40% per 10-year age increment. Encouragingly, patients who received 5-FU-based treatment had a 45% reduced risk of dying after controlling for all other prognostic factors. There was a trend toward significance that the BRAF mutation was associated with worse survival (also see Figures 2C and 2D). However, the presence of a KRAS mutation did not correlate with overall survival (also see Figures 2E and 2F).
Table 4.
Cox model: Multivariate analysis of prognostic factors for overall survival
| Factors | Hazard ratios (95% CI) | P-values |
|---|---|---|
|
Age at diagnosis (per 10 years) <65 year stratum ≥65 year stratum |
1.27 (0.96–1.70) 1.40 (1.13–1.74) |
<0.0001 |
| Male v. Female | 1.14 (0.84–1.55) | 0.39 |
|
Grade Well differentiated Moderately differentiated Poorly differentiated |
1.0 (ref) 1.42 (0.91–2.20) 1.97 (1.12–3.46) |
0.02 |
|
AJCC stage I II III IV |
1.0 (ref) 2.13 (1.29–3.51) 2.74 (1.59–4.73) 20.23 (11.57–35.39) |
<0.0001 |
|
Anatomic site Distal colon Proximal colon Rectum |
1.0 (ref) 1.04 (0.75–1.44) 1.23 (0.61–2.51) |
0.84 |
| 5-FU treatment | 0.55 (0.37–0.81) | 0.002 |
|
African American v. Caucasian In MSS/MSI-L In MSI-H |
1.73 (1.24–2.40) 0.75 (0.29–1.89) |
0.001 0.54 |
| BRAF mutation | 1.59 (0.88–2.86) | 0.13 |
| KRAS mutation | 0.93 (0.67–1.31) | 0.69 |
CI=confidence interval, MSI-H=microsatellite instability-high, MSS=microsatellite stable, MSI-L=microsatellite instability-low, ref=reference, AJCC=American Joint Committee on Cancer
DISCUSSION
We conducted a comprehensive analysis characterizing the colorectal tumors of self-reported African American and Caucasian patients in a hospital-based study to determine if biological factors contributed to reported poorer survival outcomes in African Americans. Overall, we found that African Americans had a 59% increased risk of death compared to Caucasians, following adjustment for demographic factors. This is similar to what is reported in U.S. population studies.[1] African Americans and Caucasians had similar demographics in this study, except age at diagnosis, in which African Americans were slightly older. The older age was likely caused by the referral pattern at The University of Chicago Medical Center, which has a tertiary cancer center that is influenced by insurance status and a higher referral rate of young Caucasians from regional hospitals. However, adjustment for known prognostic factors in the multivariate analysis, including age, did not explain the poorer survival of African Americans within this study.
To examine what factors may have contributed to the poorer prognosis of these patients, we examined overall survival stratified by MSI status, since MSI-H tumors represent a clinically and pathologically distinct disease from MSS and MSI-L tumors. Prior studies demonstrate that MSI-H tumors have a pathological, molecular, and clinical profile distinct from other colorectal cancer subtypes. These tumors tend to be proximally located, of a high histological grade, and interestingly have an improved prognosis over MSS/MSI-L tumors, which may be due to MSI-H tumors prominent infiltration of lymphocytes, tendency to be of low pathological stage, and lower propensity to metastasize.[27] As with previous studies,[12, 27, 28] our analysis showed that patients with MSI-H tumors had significantly better overall survival than patients with MSS/MSI-L tumors, and this was irrespective of race. We did not detect a difference in the frequency of MSI tumors or in the pattern of MMR protein expression (data not shown) between African Americans and Caucasians, contradicting reported differences in the loss of MMR pathway function between these two groups.[20, 29–31] It is important to note that our cohort was older in age, and extensive analysis of MSI-H cases was limited by statistical power due to the low number of cases.
Next, we examined KRAS and BRAF mutations to determine if these genetic alterations contributed to the disparate survival outcomes between African Americans and Caucasians, as defects in the mitogen-activated protein kinase (MAPK) pathway affect colorectal cancer progression, response to therapy, and survival outcome.[11, 14, 16] We found that 27% of patients had KRAS codons 12 or 13 mutations and 8% of patients had the BRAF V600E mutation. The BRAF frequency was similar to previously reported studies (10–15%).[15, 18, 32, 33] The KRAS frequency was lower than the 32–40% range reported in other studies.[22, 34, 35] The overall KRAS mutation spectrum resembled that of previous studies, with c.35G>A (p.G12D) and c.35G>T (p.G12V) being the most prevalent codon 12 mutations and c.38G>A (p.G13D) being the most prevalent codon 13 mutation.[19, 21, 22, 36] Both KRAS and BRAF mutations associated with proximal colon tumors, and BRAF mutations were more common in older patients, females, poorly differentiated tumors, stage II and III cancers, and MSI-H tumors. These observations are also aligned with previous studies.[15, 36] No differences were identified in BRAF mutation frequencies between African Americans and Caucasians; however, our analysis was limited by the low overall representation of mutant BRAF cases. We did identify a significantly higher frequency (11% difference) of KRAS mutations in African Americans than in Caucasians with MSS/MSI-L tumors. This observation was particularly interesting considering the most significant difference in mutation frequency was found in codon 13 and not in the predominantly mutated codon 12. The overall lower KRAS mutation frequency within this study may be due to the diversity of cases and the referral pattern at The University of Chicago Medical Center, and replication of our findings is warranted in a larger cohort.
We saw a trend among our patients in which a BRAF mutation was associated with poorer overall survival, and this data is in agreement with previously reported studies of this mutation serving as a poor prognostic indicator.[15, 32, 36–38] In the univariate analysis, KRAS mutations had no prognostic value, which is in agreement with previous studies.[34, 36, 39] Furthermore, we included KRAS mutation status in the multivariate analysis to determine its influence on disparate survival between African Americans and Caucasians, but it had no impact on survival differences. However, we did determine that the racial difference in the frequency of KRAS mutations among MSS/MSI-L cases was confined to tumors in the proximal colon. In proximal colon cancers, 44% of African Americans with MSS/MSI-L tumors had KRAS mutations compared with 21% in Caucasians with MSS/MSI-L tumors. No difference existed in the frequency of KRAS mutations among distal, rectal, or colon (unspecified) cancers in patients with MSS/MSI-L tumors. Tumors of the proximal colon have a different pathology than tumors of the distal colon and rectum, which suggest differences in causative agents of disease initiation and progression. Our data, thus, point to possible etiological differences behind disease initiation in the proximal colon between African Americans and Caucasians. Many studies have reported the effect of diet on tumor development in the colon and rectum, documenting the protective effects of insoluble fibers and phenolic substances (found in fruits, vegetables, wine, and many other foods) on colorectal carcinogenesis, along with the increased risk of adenomas, hyperplastic polyps, and colorectal cancer associated with the consumption of meat-derived mutagens found in red and processed meats cooked at high temperatures.[40–44] KRAS mutations are particularly characteristic of the type of alkylative damage that can be caused by carcinogens such as N-nitroso compounds.[42] Dietary and lifestyle patterns can vary among racial/ethnic populations, leading to varied exposures that may impact the risk and type of disease developed. Further research is needed to determine the origins of racial differences in the spectrum and frequency of KRAS mutations and to determine how environmental exposures and (epi)genetics influence tumor pathogenesis in the proximal colon versus the distal colon and rectum.
One mechanism we did not explore in this analysis, due to limited tissue availability, was the extent of widespread promoter methylation and the proportion of cases that would be classified with high or low levels of CIMP. The CIMP-high phenotype has been reported in approximately 15% of colorectal cancer cases in population-based studies [45, 46] and highly correlates with the BRAF V600E mutation, MSI-H status, proximally located tumors, and older age [45–47]. The CIMP-low phenotype correlates with a higher proportion of KRAS mutations compared to CIMP-high tumors, and patients with MSS tumors that are either CIMP-high or CIMP-low have shorter 5-year survival rates than patients with tumors containing no level of CIMP [46]. As increasing levels of CIMP are linked to poorer patient survival, especially in MSS cases, this phenotype deserves evaluation in diverse populations to determine whether its frequency varies among populations and whether it impacts disparate survival rates.
Limitations of this study were the retrospective nature and the overrepresentation of older patients seen at a single institution, thereby preventing the analysis of young onset colorectal cancer cases. Additionally, the sample size of some subgroups, like MSI-H and BRAF mutant cases, was small; hence, replication in large patient cohorts is needed. We also did not assess the influence of comorbidities. For future studies, additional data integrating lifestyle and environmental risk factors, as well as factors affecting receipt and tolerance of treatment in diverse populations, must be considered to determine the complex underlying factors that interact with tumor biology to influence poor outcomes.[48]
In the first comprehensive study of its kind that incorporates clinical, pathological, genetic, and molecular data, we found that African American colorectal cancer patients had a significantly higher risk of death than Caucasian patients that was limited to MSS/MSI-L cases and could not be explained by known prognostic factors. This study advances the science of cancer health disparities and opens up the field for further inquiry into whether observed survival differences may be related to differences in the distribution of risk factors that determine response to standard therapies. This study underscores the need for extensive characterization of cancer genomes from diverse patient populations that can lead to improved approaches to the treatment and prevention of cancer; only then can we reduce health disparities.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Despite African Americans having the highest colorectal cancer incidence and mortality rates in the U.S., there is a paucity of data driving policy to improve disparate population survival outcomes. To address the knowledge gap, we conducted molecular analysis of colorectal tumors from a cohort of African American and Caucasian patients to determine if differences existed in the distribution of genetic factors influencing colorectal cancer pathogenesis and progression. This study is relevant to the future practice of cancer medicine because it is the most comprehensive analysis of an African American cohort treated at a single institution that incorporates genetic, molecular, pathological, clinical, and treatment data to gain a better understanding of disparate survival outcomes. Our findings underscore the need for research into the science of cancer health disparities to examine the molecular mechanisms and pathways driving colorectal cancer progression in diverse patient populations.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to The University of Chicago Cancer Research Center DNA Sequencing Facility for their efforts.
GRANT SUPPORT
This work was supported by the National Cancer Institute at the National Institutes of Health - (grant number F31CA136237) and the University of Chicago Specialized Program of Research Excellence (SPORE) in Breast Cancer (grant number 5P50CA125183-04); the Doris Duke Foundation; and the Dr. Ralph and Marian Falk Medical Research Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Some of the study findings were presented at the African Organization for Research and Training in Cancer 2009 International Conference in Dar es Salaam, Tanzania and in a minisymposium at the AACR 101st Annual Meeting 2010 in Washington, DC.
REFERENCES
- 1.U.S._Cancer_Statistics_Working_Group. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2009. United States cancer statistics: 1999–2005 incidence and mortality web-based report. [Google Scholar]
- 2.Polite B, Dignam J, Olopade O. Colorectal cancer model of health disparities: Understanding mortality differences in minority populations. J Clin Oncol. 2006;24:2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 3.Polite B, Dignam J, OIopade O. Colorectal cancer and race: Understanding the differences in outcomes between African Americans and Whites. Med Clin N Am. 2005;89:771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Carethers J. Racial and ethnic factors in the genetic pathogenesis of colorectal cancer. J Assoc Acad Minor Phys. 1999;10:59–67. [PubMed] [Google Scholar]
- 5.Troisi R, Freedman A, Devesa S. Incidence of colorectal carcinoma in the U.S.: An update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85:1670–1676. [PubMed] [Google Scholar]
- 6.Alexander D, Chatla C, Funkhouser E, Meleth S, Grizzle W, Manne U. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101:66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander D, Jhala N, Chatla C, Steinhauer J, Funkhouser E, Coffey C, et al. High-grade tumor differentiation is an indicator of poor prognosis in African Americans with colonic adenocarcinomas. Cancer. 2005;103:2163–2170. doi: 10.1002/cncr.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rim S, Seeff L, Ahmed F, King J, Coughlin S. Colorectal cancer incidence in the United States, 1999–2004. Cancer. 2009;115:1967–1976. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 9.Fairley T, Cardinez C, Martin J, Alley L, Friedman C, Edwards B, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998–2001. Cancer. 2006;107(5 Suppl):1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 10.Cheng X, Chen V, Steele B, Ruiz B, Fulton J, Liu L, et al. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age grouping the United States, 1992–1997. Cancer. 2001;92:2547–2554. doi: 10.1002/1097-0142(20011115)92:10<2547::aid-cncr1606>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz S, Bertagnolli M. Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribic C, Sargent D, Moore M, Thibodeau S, French A, Goldberg R, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T, Wu T, Catalano P, Ueki T, Satriano R, Haller D, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies H, Bignell G, Cox C, Stephens P, Edkins S, Clegg S, et al. Muations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 15.Samowitz W, Sweeney C, Herrick J, Albertsen H, Levin T, Murtaugh M, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6070. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 16.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. JNCI. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 18.Fransen K, Klintenas M, Osterstrom A, Dimberg J, Monstein H, Soderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 19.Yuen S, Davies H, Chan T, Ho J, Bignell G, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 20.Ashktorab H, Smoot D, Farzanmehr H, Fidelia-Lambert M, Momen B, Hylind L, et al. Clincopathological features and microsatellite instability (MSI) in colorectal cancers from African Americans. Int J Cancer. 2005;116:914–919. doi: 10.1002/ijc.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Tang R, Wang J, Changchien C, Hsieh L. Frequency and spectrum of K-RAS codons 12 and 13 mutations in colorectal adenocarcinomas from Taiwan. Cancer Genet Cytogen. 2005;158:55–60. doi: 10.1016/j.cancergencyto.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Brink M, de Goeij A, Weijenberg M, Roeman G, Lentjes M, Pachen M, et al. K-ras oncogenes mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003;24:703–710. doi: 10.1093/carcin/bgg009. [DOI] [PubMed] [Google Scholar]
- 23.Boland C, Thibodeau S, Hamilton S, Sidransky D, Eshleman J, Burt R, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 24.Jenkins M, Hayashi S, O'Shea A, Burgart L, Smyrk T, Shimizu D, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: A population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos J, Fearon E, Hamilton S, Verlaan-de Vries M, van Boom J, van der Eb A, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan H, Bardelli A, Lengauer C, Kinzler K, Vogelstein B, Velculescu V. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 27.Vilar E, Gruber S. Microsatellite instability in colorectal cancer - the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sargent D, Marsoni S, Monges G, Thibodeau S, Labianca R, Hamilton S, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ionov Y, Peinado M, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 30.Brim H, Mokarram P, naghibalhossaini F, Saberi-Firoozi M, Al-Mandhari M, Al-Mawaly K, et al. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol Cancer. 2008;7:68–78. doi: 10.1186/1476-4598-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar K, Brim H, Giardiello F, Smoot D, Nouraie M, Lee E, et al. Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in African Americans. Clin Cancer Res. 2009;15:1155–1161. doi: 10.1158/1078-0432.CCR-08-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogino S, Nosho K, Kirkner G, Kawasaki T, Meyerhardt J, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes L, Simons C, van den Brandt P, Goldbohm R, de Goeij A, de Bruine A, et al. Body size, physical activity and risk of colorectal cancer with or without the CpG island methylator phenotype (CIMP) PLoS One. 2011;6(4):e18571. doi: 10.1371/journal.pone.0018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samowitz W, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery M. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: A population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1193–1197. [PubMed] [Google Scholar]
- 35.Nosho K, Irahara N, Shima K, Kure S, Kirkner G, Schernhammer E, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3(11):e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth A, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 37.French A, Sargent D, Burgart L, Foster N, Kabat B, Goldberg R, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14(11):3408–3415. doi: 10.1158/1078-0432.CCR-07-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richman S, Seymour M, Chambers P, Elliott F, Daly C, Meade A, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do no preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J Clin Oncol. 2009;35:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Meyerhardt J, Irahara N, Niedzwiecki D, Hollis D, Saltz L, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15(23):7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young F, Hu Y, Le Leu R, Nyskohus L. Dietary fibre and colorectal cancer: A model for environment-gene interactions. Mol Nutr Food Res. 2005;49:571–584. doi: 10.1002/mnfr.200500026. [DOI] [PubMed] [Google Scholar]
- 41.Johnson I. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutation Res. 2004;551:9–28. doi: 10.1016/j.mrfmmm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Cross A, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 43.Le Marchand L, Donlon T, Seifried A, Wilkens L. Red meat intake, CYP2E1 genetic polymorphisms, and colorectal cancer risk. Cancer Epidem Biomar. 2002;11:1019–1024. [PubMed] [Google Scholar]
- 44.Fu Z, Shrubsole M, Smalley W, Wu H, Chen Z, Shyr Y, et al. Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev Res. 2011;4:1686–1697. doi: 10.1158/1940-6207.CAPR-11-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka N, Huttenhower C, Nosho K, Baba Y, Shima K, Quackenbush J, et al. Novel application of structural equation modeling to correlation structure analysis of CpG island methylation in colorectal cancer. Am J Pathol. 2010;177(6):2731–2740. doi: 10.2353/ajpath.2010.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barault L, Charon-Barra C, Jooste V, Funes de la Vega M, Martin L, Roignot P, et al. Hypermethylator phenotype in sporadic colon cancer: Study on a population-based series of 582 cases. Cancer Res. 2008;68(20):8541–8546. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 47.Samowitz W, Albertsen H, Herrick J, Levin T, Sweeney C, Murtaugh M, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129(3):837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Polite B, Sylvester B, Olopade O. Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011;103:1486–1488. doi: 10.1093/jnci/djr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.