Abstract
Previous studies have demonstrated that curcumin induces mitochondria-mediated apoptosis. However, understanding of the molecular mechanisms underlying curcumin-induced cell death remains limited. In this study, we demonstrate that curcumin treatment of cancer cells caused dose- and time-dependent caspase 3 activation, which is required for apoptosis as confirmed using the pan-caspase inhibitor, z-VAD. Knockdown experiments and knockout cells excluded a role for caspase 8 in curcumin-induced caspase 3 activation. In contrast, Apaf-1 deficiency or silencing inhibited the activity of caspase 3, pointing to a requisite role of Apaf-1 in curcumin-induced apoptotic cell death. Curcumin treatment led to Apaf-1 upregulation, both at the protein and mRNA levels. Cytochrome c release from mitochondria to the cytosol in curcumin-treated cells was associated with upregulation of pro-apoptotic proteins, such as Bax, Bak, Bid and Bim. Cross-linking experiments demonstrated Bax oligomerization during curcumin-induced apoptosis, suggesting that induced expression of Bax, Bid and Bim causes Bax channel formation on the mitochondrial membrane. The release of cytochrome c was unaltered in p53-deficient cells, whereas absence of p21 blocked cytochrome c release, caspase activation and apoptosis. Importantly, p21 deficiency resulted in reduced expression of Apaf-1 during curcumin treatment, indicating a requirement for p21 in Apaf-1-dependent caspase activation and apoptosis. Together, our findings identify Apaf-1, Bax and p21 as novel potential targets for curcumin or curcumin-based anticancer agents.
Key words: curcumin, mitochondria, cytochrome c, Apaf-1, caspase, p21
Introduction
Turmeric has been used as a medicine for millennia in India. Curcumin (diferuloylmethane), a polyphenolic extract of turmeric, induces cell death in multiple cancer cell types with minimal toxicity to normal cells in cell culture as well as in mouse xenograft models.1–4 Clinical trials also suggest that curcumin, even at relatively high doses, is safe for humans.5,6 The mechanisms underlying curcumin-induced cancer cell death have not been clearly defined, although available evidence suggests that curcumin downregulates NFκB signaling leading to suppression of proliferation and induction of apoptosis.7,8 Curcumin induces reactive oxygen species (ROS), which cause mitochondria-mediated apoptosis.9,10 Additionally, both p53-dependent and -independent mechanisms of apoptotic cell death have been reported in multiple types of cancer cells.9,11–13 Curcumin also upregulates various pro-apoptotic proteins such as Bax/Bak, which can play critical roles in curcumin-induced apoptosis.14–16
Apoptosis is executed by caspases, which are activated by two well-known mechanisms. In the intrinsic pathway, released cytochrome c from mitochondria interacts with an adaptor protein apoptotic peptidase activating factor 1 (Apaf-1) to initiate apoptosome-mediated caspase 9 activation.17 In death receptor-mediated signaling, death ligands promote death receptor trimerization and recruitment of Fas-associated death domain (FADD)/tumor necrosis factor related (TNFR)1-associated death domain (TRADD) leading to the activation of caspase 8. Active caspase 9 or caspase 8 then activate executioner caspases such as caspase 3 to execute apoptosis.18,19 Although both caspase 8-mediated and/or caspase 9-dependent apoptosis have been reported to occur upon curcumin exposure in cancer cells,20–23 which caspase functions as initiator caspase remains unclear.
Here, we provide comprehensive evidence that curcumin induces Apaf-1-dependent caspase activation and apoptosis. The release of cytochrome c early during apoptosis further supports Apaf-1-mediated caspase signaling. Deficiency of caspase 8 does not prevent curcumin-induced caspase activation excluding death receptor signaling as an initiating event. Notably, absence of p21 leads to a marked reduction in expression of Apaf-1 upon curcumin treatment, pointing to p21 as a critical regulator of Apaf-1 levels and a key player in curcumin-induced caspase activation and apoptosis in cancer cells.
Results
Curcumin induces caspase-dependent apoptotic cell death.
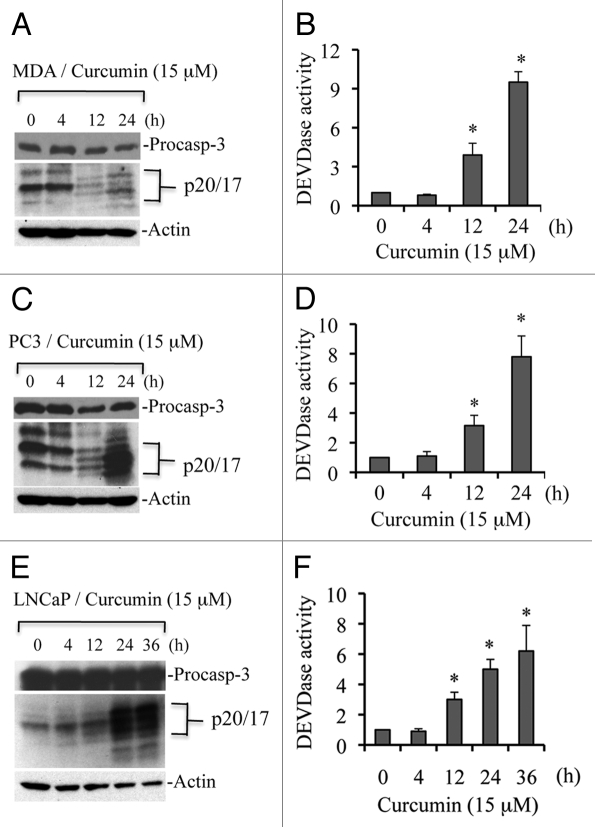
As a first step in the analysis of curcumin-induced apoptosis, we examined activation of executioner caspases. Caspase 3 is a 34-kDa protein, which can be processed to p20, 19 and 17 kDa fragments during apoptosis.24,25 To determine if curcumin treatment induces caspase 3 processing, we treated MDA-MB231, PC3 and LNCaP cells with curcumin (15 µM) for various time periods, and protein gel blotting was performed. In MDA-MB231 cells, caspase 3 was processed to multiple fragments (Fig. 1A), suggesting that curcumin induces apoptosis in a caspase-dependent manner. Since processing is not always associated with functional activation of caspases, we performed DEVDase assays, i.e., substrate cleavage assays for caspase 3/7. As shown in Figure 1B, curcumin induced time-dependent caspase activity in MDA-MB231 cells. For example, curcumin enhanced caspase activity 9-fold over control at 24 h of treatment. Similar effects were observed in PC3 and LNCaP prostate cancer cells. Caspase 3 was processed in both cell lines and caspase 3 activity was enhanced 8- and 5-fold relative to DMSO-treated controls at 24 h in PC3 and LNCaP cells, respectively (Fig. 1C–F). These findings clearly demonstrate that curcumin induces caspase activation, and suggest that caspase activity is required for execution of apoptotic cell death.
Figure 1.
Curcumin induces caspase 3 activation in multiple cell types. MDA-MB231 (A and B), PC3 (C and D) and LNCaP (E and F) were treated with curcumin (15 µM) for the indicated times. At the end of treatment, cells were harvested, washed with 1x PBS and lysed in caspase lysis buffer.26 Equal amounts of protein were subjected to protein gel blotting for detection of caspase 3 processing or used for caspase 3 activity measurements (i.e., DEVDase activity). MDA, MDA-MB231 cells; and procasp-3, procaspase 3. Data are mean ± SD of three independent experiments. *p < 0.01.
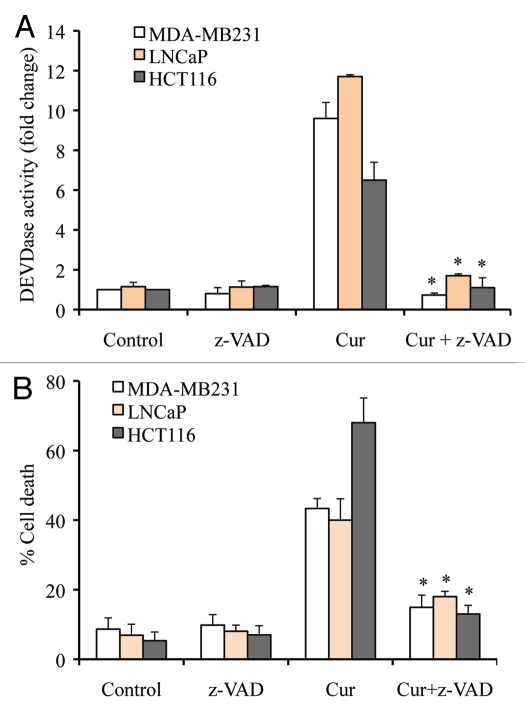
To further demonstrate that curcumin induces caspase-dependent apoptosis, we pre-treated MDA-MB231, LNCaP and HCT116 cells with the pan-caspase inhibitor z-VAD and confirmed that curcumin-induced caspase 3 activity was inhibited upon z-VAD treatment (Fig. 2A). As shown in Figure 2B, inhibition of caspase activation by z-VAD abrogated curcumin-induced cell death. Data from these experiments clearly demonstrate that curcumin induces caspase-dependent apoptotic cell death.
Figure 2.
Curcumin induces caspase-dependent apoptosis. Cells were treated with curcumin (cur; 15 µM) or vehicle (control; DMSO) for 24 h, and percentage cell death was quantified using Trypan blue dye or cells were harvested and equal amounts of protein (50 µg) were subjected to caspase 3 activity (i.e., DEVDase activity) measurements. In some experiments, pan-caspase inhibitor (z-VAD; 50 µM) was added prior to curcumin treatment. Data are mean ± SD of three independent experiments. *p < 0.01.
Deficiency of caspase 8 does not affect curcumininduced caspase activation and cell death.
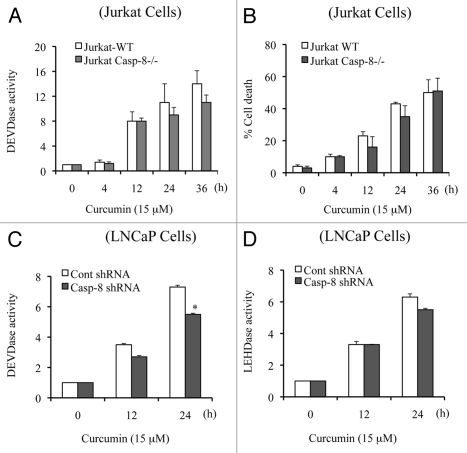
Having established that curcumin induces caspase-dependent apoptotic cell-death, we began investigating how the caspase cascade is initiated. To determine if caspase 3 is activated via death receptor-mediated signaling, we treated caspase 8-deficient Jurkat cells with curcumin and measured DEVDase activity (i.e., caspase 3 activity). As shown in Figure 3A, caspase 8 deficiency did not significantly affect curcumin induction of caspase 3 activity, indicating that death receptor-mediated caspase 8-dependent signaling is not significantly involved in curcumin-induced apoptotic cell death. Quantification of cell viability demonstrated that curcumin-induced cell death is not significantly altered in caspase 8-deficient Jurkat cells (Fig. 3B).
Figure 3.
Caspase 8 deficiency does not modulate caspase activation and apoptosis. Jurkat WT and Jurkat caspase 8-/- cells (A and B) or Caspase 8-silenced LNCaP cells (C and D) were treated with curcumin (15 µM) for the indicated times. Percentage cell death was quantified using Trypan blue dye or cells were harvested and equal amounts of protein (50 µg) were subjected to caspase 3 activity measurements. Casp-8-/-, Jurkat caspase 8-/- cells; casp-8, procaspase 8. Data are mean ± SD of three independent experiments. *p < 0.01.
To further demonstrate that caspase 8 is not an initiating caspase in curcumin-induced apoptosis, we silenced caspase 8 in LNCaP cells and treated them with curcumin for multiple times. Curcumin-induced caspase 3 activity was only modestly reduced (∼20%) at 24 h in caspase 8 silenced cells and caspase 9 activity was not affected (Fig. 3C and D), suggesting that caspase 9 activation initiates the caspase cascade. Note that the slight decrease in caspase activity at the 24 h time point (Fig. 3C and D) might reflect a minor contribution of caspase 8 activity in the amplification of caspase cascade. Together, these findings clearly demonstrate that the curcumin-induced caspase cascade and cell death do not significantly depend upon a caspase 8-activated mechanism.
Deficiency of Apaf-1 inhibits caspase activation and cell death.
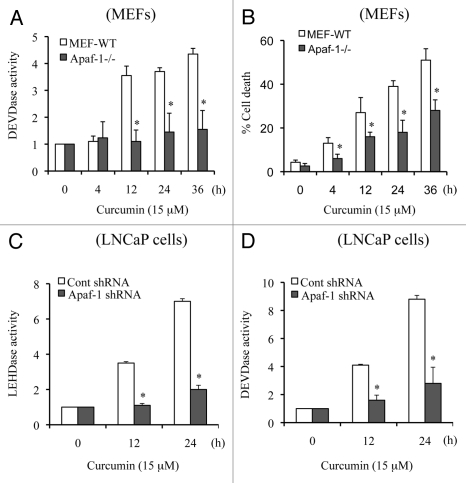
Apaf-1 plays a critical role in caspase 9 activation, which in turn activates caspase 3. We selected wild-type MEFs (MEFs WT) and Apaf-1-deficient MEFs to further understand the molecular mechanism of curcumin-induced apoptotic cell death. We treated MEFs WT and Apaf-1-deficient MEFs with curcumin for various times and observed that curcumin-induced caspase 3 activity was inhibited in Apaf-1-deficient cells (Fig. 4A), supporting a requirement for Apaf-1 apoptosome-mediated caspase 9 activation in initiation of the caspase cascade. As shown in Figure 4B, cell death was also significantly inhibited in Apaf-1-deficient cells. It is important to mention that in the absence of Apaf-1, we observed a low level of cell death, suggesting that non-apoptotic mechanisms of cell death (e.g., autophagy) may also contribute to the killing effect of curcumin. Together, our findings suggest that curcumin targets Apaf-1 to induce mitochondria-dependent apoptotic cell death.
Figure 4.
Apaf-1 deficiency inhibits curcumin-induced caspase 3 activation and apoptosis. MEFs WT and MEFs Apaf-1-/- cells (A and B) and Apaf-1 silenced LNCaP cells (C and D) were treated with curcumin (15 µM) for the indicated times. Percentage cell death was quantified using Trypan blue dye or cells were harvested and equal amounts of protein (50 µg) were subjected to caspase activity measurements. Data are mean ± SD of three independent experiments. *p < 0.01.
Since MEFs are not human cancer cells, we silenced Apaf-1 in LNCaP cells and treated them with curcumin for multiple times. As shown in Figure 4C and D, curcumin-induced caspase 9 (LEHDase) and caspase 3 (DEVDase) activities were inhibited in Apaf-1 silenced LNCaP cells. Together, these experiments clearly demonstrated that Apaf-1-mediated caspase 9 activation initiates the caspase cascade during curcumin-induced apoptosis.
Curcumin treatment causes increased expression of Apaf-1 and downregulation of FADD.
The adaptor proteins Apaf-1 and FADD regulate intrinsic and extrinsic signaling, respectively. To further demonstrate that curcumin induces intrinsic signaling to execute apoptosis, we examined the levels of Apaf-1 and FADD, in MDA-MB231 cells by protein gel blot analysis. Apaf-1 was upregulated as early as 6 h after curcumin exposure, whereas FADD was downregulated (Fig. 5A), indicating that curcumin induces the intrinsic pathway for caspase activation and apoptosis. To further demonstrate that curcumin treatment causes Apaf-1 upregulation, we isolated RNA from control and curcumin treated MDA-MB231 cells and performed real-time PCR analysis. As shown in Figure 5B, curcumin treatment induced a 3-fold upregulation of Apaf-1 mRNA, whereas mRNA levels of FADD were downregulated. Together, protein gel blot and RT-PCR analysis suggest that curcumin treatment upregulates Apaf-1 to initiate intrinsic signaling for apoptosis induction.
Figure 5.
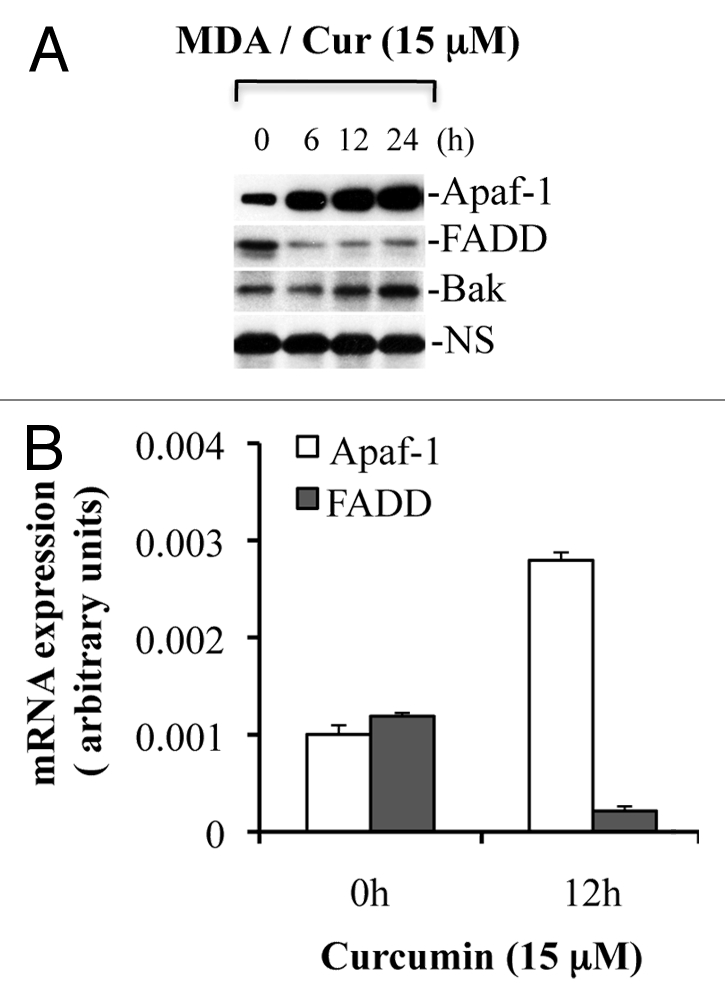
Curcumin treatment induces expression of Apaf-1 but downregulates FADD. MDA-MB231 cells were treated with curcumin (Cur) (15 µM) for the indicated times. At the end of treatment, samples were used for total RNA isolation or for the preparation of whole cell lysates. Equal amounts of protein were subjected to protein gel blotting for the detection of Apaf-1, FADD and Bak. A non-specific band serves as loading control. Isolated total RNAs were used to quantitate the expression of Apaf-1 and FADD using real-time PCR analysis. MDA, MDA-MB231 cells; NS, non-specific band serve as a loading control. Data are representative of three independent experiments.
Curcumin induces cytochrome c release in cancer cells.
Cytochrome c release from mitochondria is critical for the initiation of Apaf-1-mediated caspase 9 activation, which subsequently activates caspase 3. Therefore, we explored the effects of curcumin treatment on the subcellular distribution of cytochrome c in various cancer cell types. Cytosol and mitochondria isolated from MDA-MB231 and LNCaP cells treated with curcumin for various times were subjected to protein gel blot analysis for cytochrome c. As shown in Figure 6A, cytochrome c was mostly detected in mitochondria of untreated MDA-MB231 cells. Following treatment with curcumin, cytochrome c release was evident by 12 h and was prominent at 24 h. Similar effects were noted in LNCaP cells (data not shown). Contamination of cytosol preparations with mitochondria was excluded by examining the expression of the mitochondrial marker, cytochrome c oxidase subunit II (COX II). To further demonstrate that curcumin treatment induces cytochrome c release from mitochondria, immunofluorescence analysis was performed. As shown in Figure 6B, in untreated cells, cytochrome c showed tubular perinuclear labeling, consistent with mitochondrial localization.26,27 Upon curcumin treatment, labeling of cytochrome c became diffuse suggesting that curcumin induces cytochrome c release.
Figure 6.
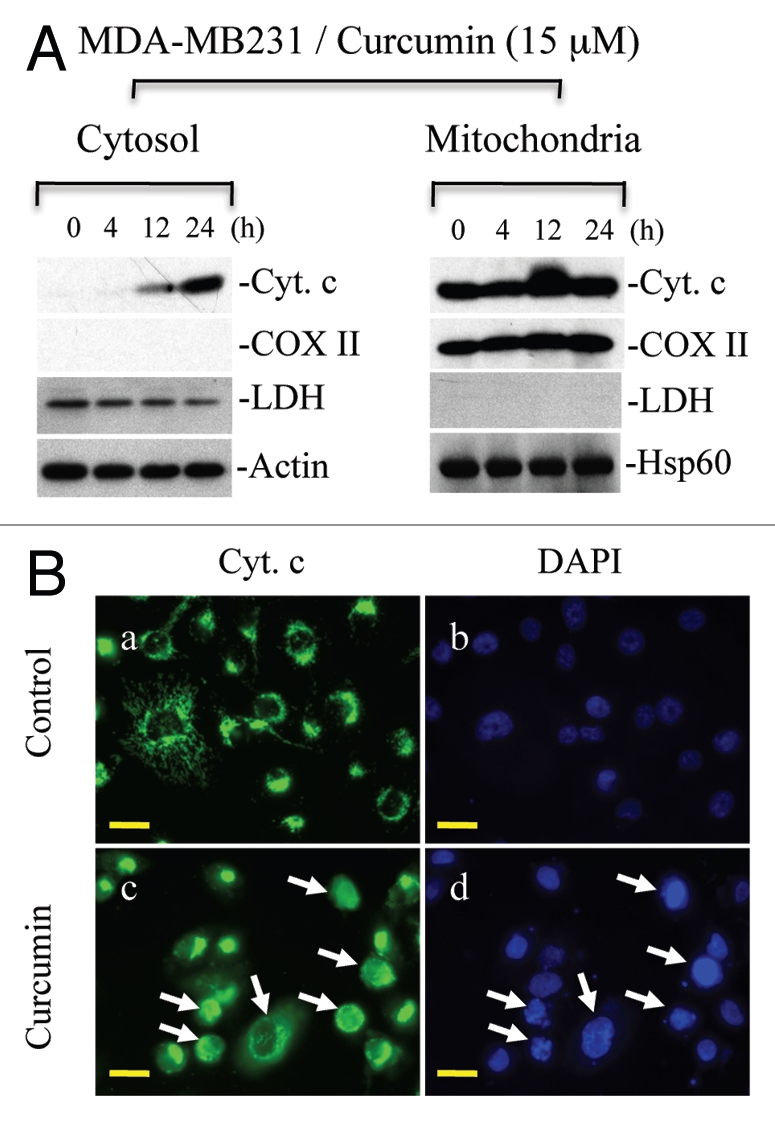
Curcumin induces cytochrome c release to the cytosol and is accompanied by nuclear fragmentation. (A) MDA-MB231 cells were treated with curcumin (15 µM) for indicated times. Cytosolic and mitochondrial fractions were isolated and equal amounts of protein were subjected to protein gel blotting for the detection of cytochrome c (Cyt. c), cytochrome c oxidase subunit II (COX II), heat shock protein 60 (Hsp60), actin or lactate dehydrogenase (LDH). Actin and Hsp60 serve as loading controls. (B) MDA-MB231 cells were treated with curcumin (15 µM) for 24 h. Following treatment, cells were incubated live with DAP I to label the nucleus and immunostained for cytochrome c (Cyt. c). Representative micrographs are shown; magnification bar represents 20 µM. Consistent with the protein gel analysis data, cytochrome c was released in individual cells as represented by diffuse cytochrome c staining. Apoptotic cells show fragmented or shiny nuclei with DAP I staining in (d). Data are representative of three independent experiments. Arrows indicate apoptotic cells showing cytochrome c release.
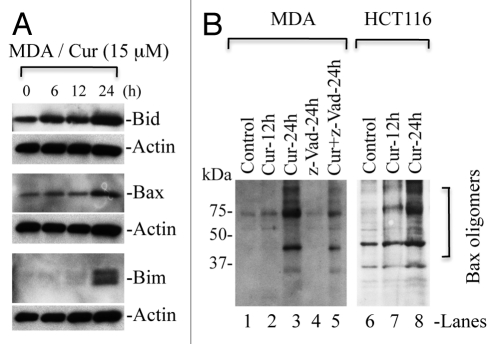
Curcumin treatment leads to upregulation of Bim, Bid, Bax and Bak, which contribute to the release of cytochrome c.
Bcl-2 family proteins play a critical role in the regulation of cytochrome c release from mitochondria. They are divided into BH3-only proteins (such as Bim and Bid), multidomain pro-apoptotic proteins (e.g., Bax and Bak) and anti-apoptotic proteins such as Bcl2 and Bcl-xl. BH3 only proteins such as Bim inhibit Bcl-2/Bcl-xl function and, thus, allow activation of Bax/Bak, which undergo oligomerization to form channels on the mitochondrial membrane.28,29 To understand whether curcumin upregulates pro-apoptotic proteins such as Bim, Bid, Bax and Bak, we treated MDA-MB231 cells with curcumin and protein gel blot analysis was performed to detect the levels of these proteins. We observed upregulation of Bid, Bim, Bax and Bak (Figs. 5A and 7A). Upregulation of Bim and Bid activates Bax/Bak and inhibits the function of Bcl-2/Bcl-xl, ultimately, resulting in oligomerization of Bax/Bak on the mitochondrial membrane to induce the release of cytochrome c.
Figure 7.
Curcumin induces upregulation of Bax, Bim and Bid, which trigger Bax oligomerization. (A) MDA-MB231 cells (MDA) were treated with curcumin (Cur) (15 µM) for the indicated times. Equal amounts of whole cell protein were separated on SDS-PA GE for detection of indicated proteins. Actin serves as loading control. (B) MDA-MB231 and HCT116 cells were treated with curcumin (15 µM) for the indicated times. In some treatment conditions, cells were pretreated with pancaspase inhibitor (z-VAD) one h prior to curcumin treatment. At the end of treatments, cells were cross-linked with BMH and subjected to protein gel blotting for the detection of Bax oligomers. Data are representative of three independent experiments.
Curcumin induces Bax oligomerization, which occurs prior to caspase activation.
To examine whether curcumin-induced upregulation of pro-apoptotic BH3-only proteins (Bim or Bid) promotes Bax oligomerization leading to pore formation on the mitochondrial membrane, unstimulated and curcumin-treated MDA-MB231 and HCT116 cells were cross-linked with BMH, an irreversible cross-linker, and protein gel analysis was performed to detect Bax oligomers. As shown in Figure 7B, curcumin induced Bax oligomerization as indicated by detection of dimers, trimers and multimers of Bax during curcumininduced apoptosis. Bax oligomerization on mitochondria leads to the release of cytochrome c, which then triggers apoptosomemediated caspase activation. To examine if Bax oligomerization is the initiating event prior to cytochrome c release and, thus, caspase activation, we pre-treated MDA-MB231 cells with pancaspase inhibitor, z-VAD. Notably, we observed that curcumin induced Bax oligomerization in the presence of z-VAD (Fig. 7B and lanes 4 and 5) suggesting that Bax activation and its oligomerization on mitochondria occur prior to caspase activation. Higher levels of Bax oligomerization were observed in the absence of z-VAD suggesting that curcumin-induced cytochrome c release triggers caspase activation, which in turn amplifies Bax activation/oligomerization.30 Collectively, these findings demonstrate that curcumin targets pro-apoptotic proteins such as Bim, Bax and Bak to permeabilize the mitochondrial membrane and execute apoptosis.
Curcumin induces p53-independent but p21-mediated cytochrome c release.
In addition to Bim/Bid, p53 also modulates the activation and oligomerization of Bax/Bak leading to cytochrome c release from mitochondria.31,32 To examine the importance of p53 in curcumin-induced cytochrome c release, we selected isogenic HCT116 WT, HCT116 p53-/-, HCT116 Bax-/- and HCT116 p21-/- colon cancer cells. These isogenic cells were treated with curcumin for various times, and cytosol and mitochondria were prepared to detect the localization of cytochrome c. As shown in Figure 8A and B, curcumin induced cytochrome c release in WT as well as p53-/- cells, suggesting that p53 does not play an essential role in the release of cytochrome c. However, cytochrome c release was inhibited in HCT116 Bax-/- cells treated with curcumin (Fig. 8C), indicating that Bax plays a key role in curcumin-induced cytochrome c release in cancer cells. Surprisingly, cytochrome c release was blocked in HCT116 p21-/- cells suggesting that p21 regulates cytochrome c release during curcumin-induced apoptosis (Fig. 8D).
Figure 8.
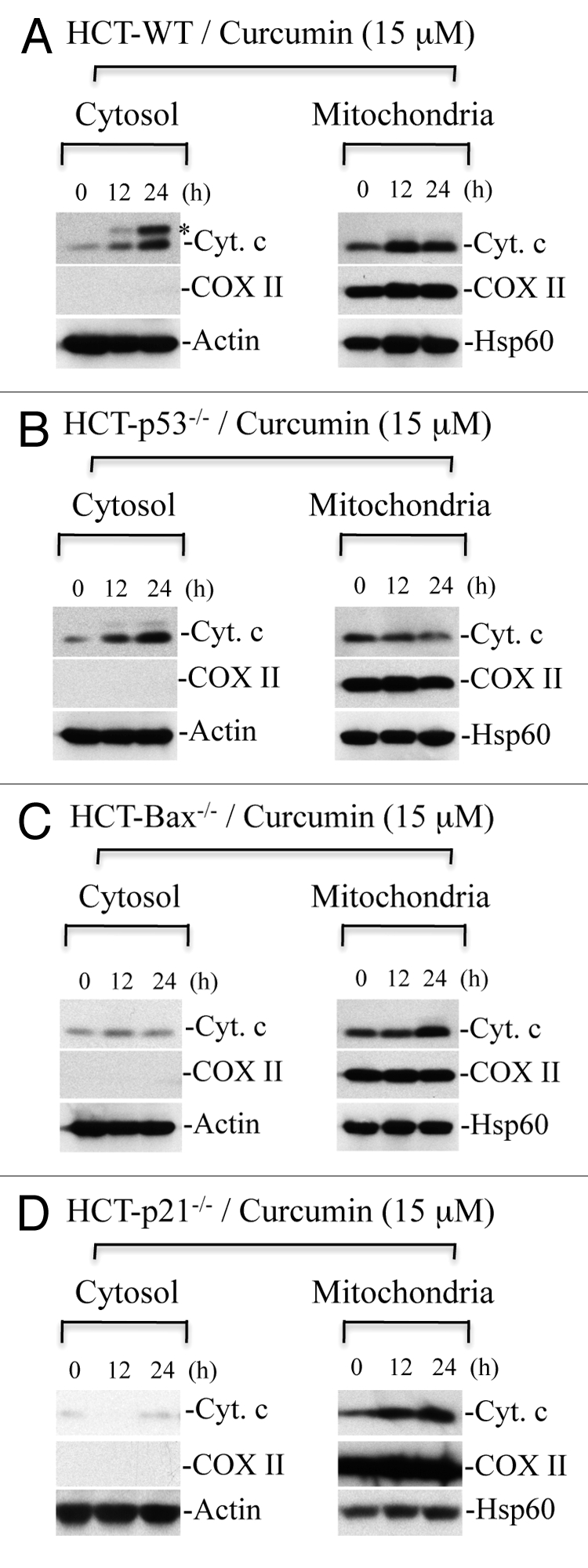
Curcumin induces Bax-dependent, p21-mediated cytochrome c release without p53 requirement. HCT116 WT (A), HCT116-p53-/- (B), HCT116-Bax-/- (C) and HCT116-p21-/- (D) cells were treated with curcumin (15 µM) for the indicated times. Cytosolic and mitochondrial fractions were isolated and equal amounts of protein were subjected to protein gel blotting for the detection of cytochrome c (Cyt. C), cytochrome c oxidase subunit II (COX II), heat shock protein 60 (Hsp60) and actin. Actin and Hsp60 serve as loading controls. *represents a non-specific band.
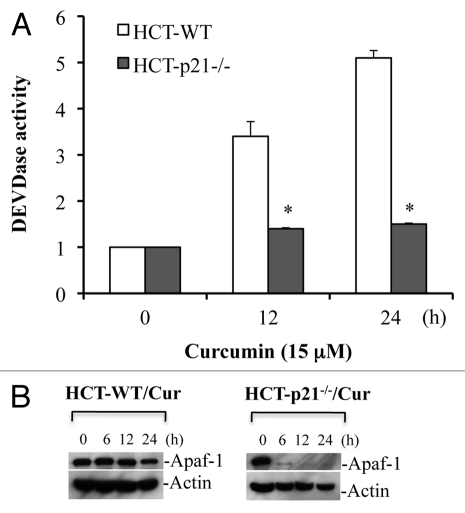
Deficiency of p21 reduces Apaf-1 protein expression and inhibits caspase activation.
Blockade of cytochrome c release in the absence of p21 would be expected to result in inhibition of caspase activation. Indeed, curcumin induced robust caspase 3 activation in HCT116-WT cells, whereas, caspase activation was inhibited in HCT116 p21-/- cells (Fig. 9A). Specifically, curcumin induced ∼5 fold increase in caspase 3 activity at 24 h treatment in WT cells, an effect that was abrogated in HCT116 p21-/- cells (Fig. 9A). These findings suggest that curcumin induces p53-independent, p21-mediated cytochrome c release and caspase activation. To further understand how deficiency of p21-/- inhibits curcumin-induced caspase activation and apoptosis, we examined the levels of Apaf-1 protein in HCT116 p21-/- cells upon curcumin treatment using protein gel blotting. Surprisingly, curcumin treatment markedly reduced expression of Apaf-1 in p21-/- cells (Fig. 9B). These findings suggest that p21 regulates the expression of Apaf-1 protein during curcumin-induced apoptosis.
Figure 9.
p21 deficiency reduces Apaf-1 expression and inhibits curcumin-induced caspase activation. HCT116 WT and HCT116-p21-/- cells were treated with curcumin (Cur) (15 µM) for the indicated times. At the end of treatment, cells were harvested and equal amounts of protein (50 µg) were subjected to caspase 3 activity measurements, or protein gel blotting for Apaf-1. Actin serves as a loading control. HCT-WT, HCT116-WT cells; HCT-p21-/-, HCT116-p21-/--deficient cells. Data are mean ± SD of three independent experiments. *p < 0.01.
Discussion
Curcumin has been shown to possess anticancer activities but the molecular mechanisms of apoptotic cell death have not been clearly defined. Here, we provide evidence that curcumin induces caspase-dependent apoptotic cell death. Initiation of the caspase cascade is Apaf-1-dependent as curcumin failed to induce caspase 3 activation and apoptosis in Apaf-1-deficient and Apaf-1-silenced cells. In contrast, silencing or knockout of caspase 8 did not affect curcumin-induced caspase activation, indicating that caspase 8 does not play a critical role in curcumin-induced apoptosis. Upregulation of Apaf-1 along with inhibition of FADD expression further support a critical role for Apaf-1 in initiating the caspase cascade. Activation of caspases was associated with release of cytochrome c from mitochondria to the cytosol, providing further support for involvement of the Apaf-1 apoptosome-dependent caspase cascade. Curcumin treatment upregulated Bim and Bid suggesting that these pro-apoptotic proteins deactivate the antiapoptotic function of Bcl-2/Bcl-xl, thus allowing the formation of pores on the outer mitochondrial membrane and cytochrome c release from mitochondria. Indeed, curcumin promoted Bax oligomerization, via a caspase-independent mechanism, suggesting that Bax activation and oligomerization are early events during curcumin-induced caspase activation. Notably, the release of cytochrome c from mitochondria was primarily dependent upon Bax and did not require p53-mediated signaling, as indicated by the release of cytochrome c in HCT116 p53-/- cells. Surprisingly, p21 was shown to play a critical role in the release of cytochrome c from mitochondria as p21 deficiency blocked cytochrome c release and caspase activation. Importantly, p21 also plays a critical role in maintenance of Apaf-1 expression during curcumin-induced apoptosis.
Curcumin has been reported to induce caspase-dependent or caspase-independent apoptotic cell death as well as autophagy in cancer cells.20,33–35 Our findings using z-VAD demonstrate that curcumin primarily induces caspase-dependent apoptotic cell death, although caspase-independent mechanism(s) may also play a role late during apoptosis. The involvement of caspase-independent pathways is indicated by the detection of low levels of cell death in Apaf-1-deficient cells, which fail to induce caspase activation in response to curcumin treatment. It is also possible that, in the absence of caspase activation, curcumin is able to induce alternate cell death pathways such as caspase-independent apoptotic or autophagic cell death.
The initiating caspase that triggers the caspase cascade in response to curcumin has not been defined.15,20–23,36 In the current study, we provide comprehensive evidence that Apaf-1-dependent activation of caspase 9 is an initiating event in curcumin-induced apoptosis. This conclusion was further supported by the findings that curcumin-induced caspase activation and apoptosis were not significantly affected in caspase 8-deficient cells. These data are consistent with earlier reports that curcumin induces caspase 9-mediated apoptosis.22,23 However, other studies have indicated that curcumin also induces FAS or death receptor-mediated apoptosis.20,21 Since activation of caspase 8 is not sufficient to induce apoptosis in epithelial cancer cells,37 it is possible that in some cell types, curcumin induces a low level of caspase 8 activation, leading to cleavage of Bid, Bax oligomerization and cytochrome c release,20 which, in turn, triggers Apaf-1-dependent caspase 9 and 3 activation and apoptosis. In certain cell types, activation of caspase 8 by curcumin may, thus, initiate the caspase cascade; however, execution of apoptosis appears to be largely dependent on activation of a caspase 9-mediated pathway.
Upregulation of pro-apoptotic multidomain proteins such as Bax/Bak seems to play an essential role for in curcumininduced cytochrome c release.15,16 Published reports have suggested a redundant role of Bax and Bak in curcumin-induced apoptosis.15 However, whether Bax and/or Bak undergo oligomerization to permeabilize the mitochondrial membrane is not known. Our findings point to Bax as the primary mediator of cytochrome c release in curcumin-treated cells, as the effect was inhibited in HCT116-Bax-deficient cells, which still express Bak. These data suggest that Bak does not play a critical role in mediating cytochrome c release in curcumin-induced apoptosis.
How is Bax activated to undergo oligomerization and pore formation on the mitochondria during curcumin-induced apoptosis? Activation/oligomerization of Bax requires multiple mechanisms, including Bax channel formation upon activation by BH3-only proteins.28,29 Our analysis demonstrated upregulation of pro-apoptotic BH3-only proteins Bim and Bid in response to curcumin. These BH3-only proteins then contribute to Bax recruitment, activation and oligomerization. Our studies further demonstrated that Bax undergoes oligomerization in a caspase-independent manner, suggesting that Bax oligomerization is an early event in curcumin-induced apoptosis.
Although activation of p53 promotes apoptosis in cancer cells,38,39 wild-type p53 protects normal cells.40 Absence of p53-dependent apoptosis leads to cellular senescence.41 However, the involvement of p53 in curcumin-induced apoptosis remains unclear. For example, curcumin has been shown to induce apoptosis in a p53-dependent manner in multiple cancer cell types,11,14,42 and via a p53-independent mechanism in others.9,13,43,44 We recently demonstrated that the pro-apoptotic function of p53 is inhibited in multiple types of cancer cells27 due to failure of p53 to activate Bax/Bak and inhibit the anti-apoptotic functions of Bcl-2/Bcl-xL.27 Findings in this study provide further evidence that p53 is not a critical player in curcumin-induced cytochrome c release and apoptosis.
p21 protects cells by upregulating the Nrf2 signaling pathway,45 and is also implicated in regulation of apoptosis.46,47 Although p21 is a p53-target protein, the stress-induced phosphorylation pattern of p53 differs in the presence and absence of p21.48 These findings suggest that p21 regulates apoptosis via p53-dependent or-independent pathways. Previous studies have shown that silencing of p21 blocks curcumin-induced apoptosis,49 however, the underlying molecular mechanisms involved in this effect are not known. Our findings provide the first evidence that p21 promotes permeabilization of the mitochondrial membrane leading to release of cytochrome c during curcumin-induced apoptosis. Our conclusions are based on the findings that deficiency of p21 inhibited cytochrome c release and caspase activation. Importantly, we have further observed that p21 deficiency leads to reduced expression of Apaf-1. The underlying mechanisms are currently under investigation, but this finding suggests a new pathway for inhibition of caspase activation in the absence of p21 during curcumin-induced apoptotic cell death.
In summary, our study provides novel mechanistic information on curcumin-induced apoptotic cell death. We provide comprehensive evidence that curcumin induces Apaf-1-dependent caspase activation and apoptosis. Our conclusion is based on the following major findings: curcumin-induced caspase 9 and caspase 3 activation was blocked in Apaf-1-deficient MEFs and Apaf-1 silenced cancer cells, curcumin induced expression of Apaf-1 both at protein and mRNA levels, cytochrome c release is associated with increased caspase 3 activation in curcumintreated cells, and reduced expression of Apaf-1 in p21-deficient cells was associated with inhibition of caspase 3 activity and apoptosis. Caspase 8 was excluded as a major initiator of the caspase cascade, and activation and oligomerization of Bax was shown to play a critical role in curcumin-induced cytochrome c release, via a p53-independent mechanism. Importantly, p21 was shown to regulate curcumin-induced apoptosis both upstream and downstream of mitochondria. Specifically, (1) inhibition of cytochrome c release in the absence p21 suggests that p21 regulates permeabilization of the mitochondrial membrane and (2) regulation of Apaf-1 expression levels by p21 supports a role for p21 in apoptosome-mediated caspase activation.
It is critical to establish the molecular mechanisms of curcumin- induced apoptotic cell death, so that the maximum medicinal benefits of this compound can be harnessed. As shown in multiple clinical trials,5,6 curcumin is not toxic to humans even at higher doses. This agent, therefore, has therapeutic potential and could be exploited for the development of new anticancer agents with minimal toxicity to noncancerous cells.
Materials and Methods
Cells and reagents.
Most cancer cells were subcultured as previously described in reference 50. Colon cancer cells (HCT116, HCT116-Bax-KO, HCT116-p21-KO and HCT-p53-KO) were kindly provided by Dr. B. Vogelstein51,52 and cultured in McCoy's 5A media supplemented with 10% FBS. Prostate cancer (PC3 and LNCaP), breast cancer (MDA-MB231), immortalized normal human fibroblast (GM701), Jurkat WT, Jurkat caspase 8-/-, MEFs WT and MEFs Apaf-1-/- cells were obtained from the ATCC or from various investigators and were subcultured as described previously in reference 50 and 53. Primary antibodies were anti-cytochrome c (mAb, monoclonal antibody), -Apaf-1 and -Bax (Rb pAb, rabbit polyclonal antibody), -Bid, and -caspase 8 purchased from BD PharMingen. Anti-Bax N-terminus or NT (Rb pAb; Upstate), -Bak (Rb pAb; Santa Cruz), -p53 and -p21(Santa Cruz), -COX II (Mito Sciences), -Hsp60 (Millipore), -Bak NT (Rb pAb; Upstate), -Bim (Calbiochem), -caspase 3 (Rb pAbs; Biomol), -caspase 9 (Cell Signaling), -poly (ADP-ribose) polymerase (PARP), -lactate dehydrogenase (LDH) and -actin (mAb; ICN). Secondary antibodies and ECL reagents were acquired from GE Healthcare. AlexaFluor 594 or 488 conjugated goat anti-mouse or rabbit IgG (H + L) and mitochondrial dye (i.e., MitoTracker Orange, CMTMRos) were purchased from Molecular Probes (Invitrogen). The fluorogenic caspase substrates DEVD-AFC, LEHD-AFC, general caspase inhibitor z-VAD-fmk and cross-linkers were bought from Enzo Life Sciences. All other chemicals were purchased from Sigma unless specified otherwise.
Subcellular fractionation and protein gel blotting.
Whole cell lysates and mitochondrial/cytosolic fractions were prepared and protein gel blotting was performed as previously described in references 26 and 53.
Quantification of apoptosis and caspase activity measurement.
To quantify percentage apoptosis, apoptotic cells were counted based on live cell staining with DAPI to label apoptotic nuclei.26 In addition, both live and dead cells were counted using Trypan blue dye. DEVDase and LEHDase, representing caspase 3 and caspase 9 activities, respectively, were measured as previously described in references 26 and 27.
Establishment of cancer cells stably expressing Apaf-1 or caspase 8 siRNA using shRNA lentiviral vectors.
To establish stable cancer cells expressing siRNA for Apaf-1 and caspase 8, green fluorescence protein (GFP)-tagged short hairpin RNAs (shRNAs) specific to Apaf-1, caspase 8, and negative control shRNA were cloned into the pGIPZ (Open Biosystems) lentiviral vector to generate lentiviral particles. The shRNA sequences were caspase 8 (5′-GAC TTC AGC AGA AAT CTT T-3′) and Apaf-1 (5′-CCT TTG ATG GAA TCA TAA A-3′). Apaf-1, caspase 8 and control shRNA-specific lentiviral particles were obtained from the Roswell Park Cancer Institute (RPCI) shRNA core resource and were directly utilized to infect cells at a multiplicity of infection (MOI) of 3. Stable cells expressing siRNA were selected after 48 h using puromycin (1 µg/ml).27
Analysis of Apaf-1 and FADD mRNA expression in realtime.
Total RNA was isolated from DMSO- or curcumintreated cells using Tri-reagent (Molecular Research Center, Inc.). Reverse transcription was performed using superscript II (Invitrogen) with 1 mg of total RNA. 0.5 ml of cDNA was mixed with iTaq SYBR supermix with Rox (Biorad) to perform a quantitative real-time PCR reaction on an Applied Biosystems 7500. Apaf-1F, CCT CTC ATT TGC TGA TGT CG; Apaf-1R, TCA CTG CAG ATT TTC ACC AGA; FADD-F, TCT CCA ATC TTT CCC CAC AT; FADD-R, GAG CTG CTC GCC TCC CT; actin F, CTT CGT CGC ACA TTG TGT CT; and actin R, GAC AGC GCC AAG TGA AGC primers were used to amplify the mRNA expression levels. The results were then processed and Apaf-1 or FADD gene expression was normalized to actin expression in the same sample. The data are presented as fold change with respect to the DMSO-treated control.54
Immunofluorescence.
Cells were treated with curcumin and incubated live with either DAPI alone (to label the nucleus) or MitoTracker Orange (CMTMRos) and DAPI (to label mitochondria and nuclei, respectively). Cells were then fixed, permeabilized and immunolabeled for cytochrome c as described previously in references 26 and 27.
Chemical cross-linking and oligomerization assays.
Cells were suspended in 45 µl of HIM buffer (200 mM mannitol, 70 mM sucrose, 10 mM HEPES-KOH, 1 mM EGTA, pH 7.5) followed by addition of freshly prepared BMH (bismaleimidohexane) to a final concentration of 2 mM and incubated at room temperature for 30 min. Cells were then lysed in protein sample buffer and subjected to protein gel blotting.27,55
Statistical analysis.
Results are presented as mean ± standard deviation (SD) of data from at least three independent experiments. Statistical analysis was performed by ANOVA using Sigma Stat. Significant changes (p < 0.01) are represented by *.
Acknowledgments
We thank Drs. B. Vogelstein and Terry Beerman for providing reagents. This work was supported in part by a National Institutes of Health K01 Award CA123142 to D.C., NIH R01 award DK60632 and DK54909 to J.D.B. and National Cancer Institute Center Support Grant P30 CA016056 to the Roswell Park Cancer Institute. We apologize to those colleagues whose publications could not be cited due to space constraints.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Anto RJ, Maliekal TT, Karunagaran D. L-929 cells harboring ectopically expressed RelA resist curcumin-induced apoptosis. J Biol Chem. 2000;275:15601–15604. doi: 10.1074/jbc.C000105200. [DOI] [PubMed] [Google Scholar]
- 2.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–1108. [PubMed] [Google Scholar]
- 3.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 5.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–364. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 7.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) downregulates the constitutive activation of nuclear factor kappaB and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 8.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–1305. [PubMed] [Google Scholar]
- 9.Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010;297:1–8. doi: 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Hail N., Jr Mitochondrial reactive oxygen species affect sensitivity to curcumin-induced apoptosis. Free Radic Biol Med. 2008;44:1382–1393. doi: 10.1016/j.freeradbiomed.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 12.Jee SH, Shen SC, Tseng CR, Chiu HC, Kuo ML. Curcumin induces a p53-dependent apoptosis in human basal cell carcinoma cells. J Invest Dermatol. 1998;111:656–661. doi: 10.1046/j.1523-747.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Watson JL, Greenshields A, Hill R, Hilchie A, Lee PW, Giacomantonio CA, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol Carcinog. 2010;49:13–24. doi: 10.1002/mc.20571. [DOI] [PubMed] [Google Scholar]
- 14.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 15.Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–1286. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- 16.Rashmi R, Kumar S, Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005;26:713–723. doi: 10.1093/carcin/bgi025. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Du F, Wang X. TNFalpha induces two distinct caspase 8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase 8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase 8 pathway independent of p53. Exp Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 22.Yang CL, Ma YG, Xue YX, Liu YY, Xie H, Qiu GR. Curcumin Induces Small Cell Lung Cancer NCI-H446 Cell Apoptosis via the Reactive Oxygen Species-Mediated Mitochondrial Pathway and Not the Cell Death Receptor Pathway. DNA Cell Biol. 2011 doi: 10.1089/dna.2011.1300. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, et al. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 26.Chandra D, Liu JW, Tang DG. Early mitochondrial activation and cytochrome c upregulation during apoptosis. J Biol Chem. 2002;277:50842–50854. doi: 10.1074/jbc.M207622200. [DOI] [PubMed] [Google Scholar]
- 27.Gogada R, Prabhu V, Amadori M, Scott R, Hashmi S, Chandra D. Resveratrol Induces p53-independent, X-linked Inhibitor of Apoptosis Protein (XIAP)-mediated Bax Protein Oligomerization on Mitochondria to Initiate Cytochrome c Release and Caspase Activation. J Biol Chem. 2011;286:28749–28760. doi: 10.1074/jbc.M110.202440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed JC. Pro-apoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 29.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- 31.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 32.Pietsch EC, Perchiniak E, Canutescu AA, Wang G, Dunbrack RL, Murphy ME. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J Biol Chem. 2008;283:21294–21304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipriani B, Borsellino G, Knowles H, Tramonti D, Cavaliere F, Bernardi G, et al. Curcumin inhibits activation of Vgamma9Vdelta2 T cells by phosphoantigens and induces apoptosis involving apoptosis-inducing factor and large scale DNA fragmentation. J Immunol. 2001;167:3454–3462. doi: 10.4049/jimmunol.167.6.3454. [DOI] [PubMed] [Google Scholar]
- 34.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Betacatenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 35.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 36.Deeb D, Xu YX, Jiang H, Gao X, Janakiraman N, Chapman RA, et al. Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther. 2003;2:95–103. [PubMed] [Google Scholar]
- 37.Algeciras-Schimnich A, Pietras EM, Barnhart BC, Legembre P, Vijayan S, Holbeck SL, et al. Two CD95 tumor classes with different sensitivities to antitumor drugs. Proc Natl Acad Sci USA. 2003;100:11445–11450. doi: 10.1073/pnas.2034995100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriano R, Patton JT, Mayo LD, Jackson MW. Inactivation of p53 signaling by p73 or PTEN ablation results in a transformed phenotype that remains susceptible to Nutlin-3 mediated apoptosis. Cell Cycle. 2010;9:1373–1379. doi: 10.4161/cc.9.7.11193. [DOI] [PubMed] [Google Scholar]
- 39.Trinh DL, Elwi AN, Kim SW. Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis. Oncotarget. 2010;1:396–404. doi: 10.18632/oncotarget.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halasi M, Schraufnagel DP, Gartel AL. Wild-type p53 protects normal cells against apoptosis induced by thiostrepton. Cell Cycle. 2009;8:2850–2851. doi: 10.4161/cc.8.17.9414. [DOI] [PubMed] [Google Scholar]
- 41.Tavana O, Benjamin CL, Puebla-Osorio N, Sang M, Ullrich SE, Ananthaswamy HN, et al. Absence of p53-dependent apoptosis leads to UV radiation hypersensitivity, enhanced immunosuppression and cellular senescence. Cell Cycle. 2010;9:3328–3336. doi: 10.4161/cc.9.16.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee M, Singh P, Panda D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010;277:3437–3448. doi: 10.1111/j.1742-4658.2010.07750.x. [DOI] [PubMed] [Google Scholar]
- 43.Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization and radiosensitization effects by downregulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 45.Villeneuve NF, Sun Z, Chen W, Zhang DD. Nrf2 and p21 regulate the fine balance between life and death by controlling ROS levels. Cell Cycle. 2009;8:3255–3256. doi: 10.4161/cc.8.20.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu J, Cai XF, Yan G. Alphavirus M1 induces apoptosis of malignant glioma cells via downregulation and nucleolar translocation of p21WAF1/CIP1 protein. Cell Cycle. 2009;8:3328–3339. doi: 10.4161/cc.8.20.9832. [DOI] [PubMed] [Google Scholar]
- 47.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 48.Neise D, Sohn D, Budach W, Janicke RU. Evidence for a differential modulation of p53-phosphorylating kinases by the cyclin-dependent kinase inhibitor p21WAF1/CIP1. Cell Cycle. 2010;9:3575–3583. doi: 10.4161/cc.9.17.12799. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(WAF1/CIP1) Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 50.Chandra D, Bratton SB, Person MD, Tian Y, Martin AG, Ayres M, et al. Intracellular nucleotides act as critical prosurvival factors by binding to cytochrome C and inhibiting apoptosome. Cell. 2006;125:1333–1346. doi: 10.1016/j.cell.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 52.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Gogada R, Yadav N, Lella RK, Badeaux M, Ayres M, et al. Defective molecular timer in the absence of nucleotides leads to inefficient caspase activation. PLoS ONE. 2011;6:16379. doi: 10.1371/journal.pone.0016379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its proapoptotic or pro-survival functions involve differential interactions with caspase 3. J Biol Chem. 2007;282:31289–31301. doi: 10.1074/jbc.M702777200. [DOI] [PubMed] [Google Scholar]
- 55.Chandra D, Choy G, Daniel PT, Tang DG. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J Biol Chem. 2005;280:19051–19061. doi: 10.1074/jbc.M501391200. [DOI] [PubMed] [Google Scholar]