Abstract
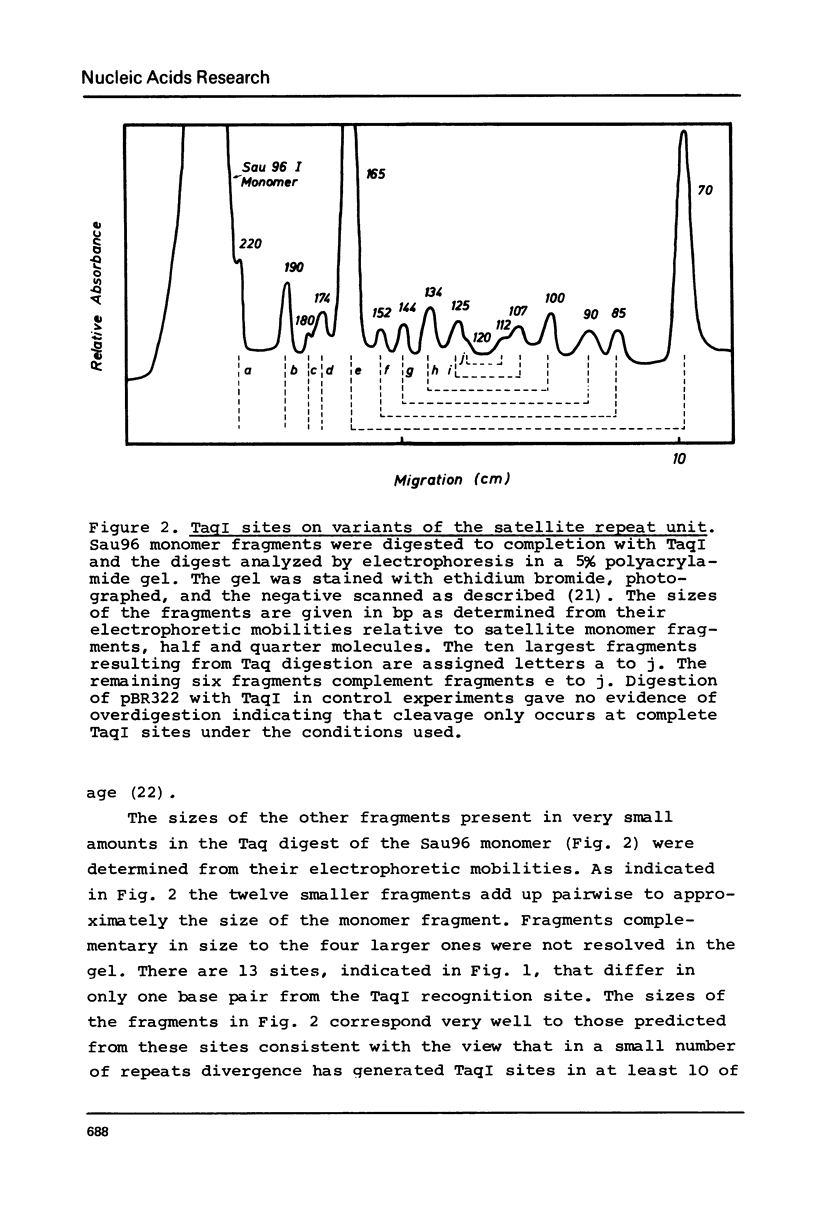
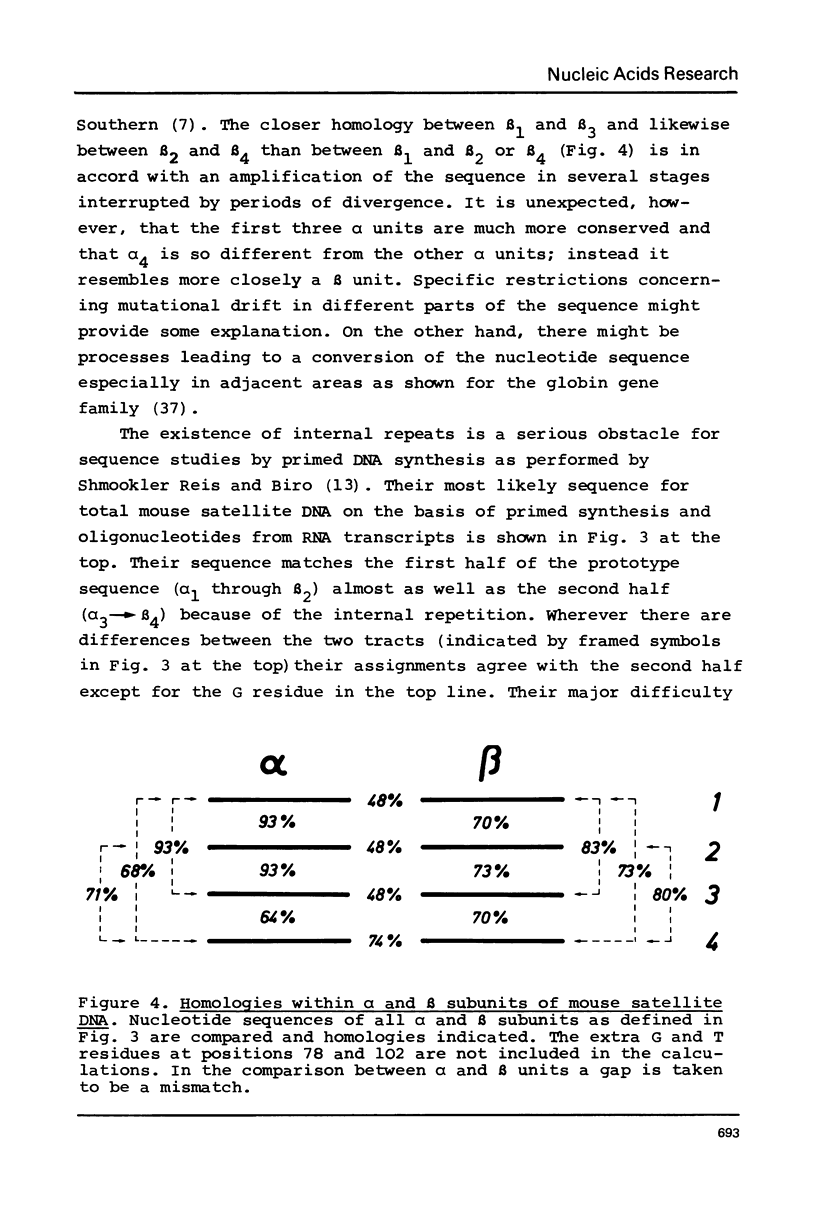
The nucleotide sequence of uncloned mouse satellite DNA has been determined by analyzing Sau96I restriction fragments that correspond to the repeat unit of the satellite DNA. An unambiguous sequence of 234 bp has been obtained. The sequence of the first 250 bases from dimeric satellite fragments present in Sau96I limit digests corresponds almost exactly to two tandemly arranged monomer sequences including a complete Sau96I site in the center. This is in agreement with the hypothesis that a low level of divergence which cannot be detected in sequence analyses of uncloned DNA is responsible for the appearance of dimeric fragments. Most of the sequence of the 5% fraction of Sau96 monomers that are susceptible to TaqI has also been determined and has been found to agree completely with the prototype sequence. The monomer sequence is internally repetitious being composed of eight diverged subrepeats. The divergence pattern has interesting implications for theories on the evolution of mouse satellite DNA.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Biro P. A., Carr-Brown A., Southern E. M., Walker P. M. Partial sequence analysis of mouse satellite DNA evidence for short range periodicities. J Mol Biol. 1975 May 5;94(1):71–86. doi: 10.1016/0022-2836(75)90405-2. [DOI] [PubMed] [Google Scholar]
- Bond H. E., Flamm W. G., Burr H. E., Bond S. B. Mouse satellite DNA. Further studies on its biological and physical characteristics and its intracellular localization. J Mol Biol. 1967 Jul 28;27(2):289–302. doi: 10.1016/0022-2836(67)90021-6. [DOI] [PubMed] [Google Scholar]
- Brown S. D., Dover G. A. The specific organisation of satellite DNA sequences on the X-chromosome of Mus musculus: partial independence of chromosome evolution. Nucleic Acids Res. 1980 Feb 25;8(4):781–792. [PMC free article] [PubMed] [Google Scholar]
- Corneo G., Ginelli E., Soave C., Bernardi G. Isolation and characterization of mouse and guinea pig satellite deoxyribonucleic acids. Biochemistry. 1968 Dec;7(12):4373–4379. doi: 10.1021/bi00852a033. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., McCallum M., Walker P. M. The isolation of complementary strands from a mouse DNA fraction. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1729–1734. doi: 10.1073/pnas.57.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Harbers B., Spencer J. H. Nucleotide clusters in deoxyribonucleic acids. XII. The distribution of 5-methylcytosine in pyrimidine oligonucleotides of mouse L-cell satellite DNA and main band DNA. Biochem Biophys Res Commun. 1975 Sep 16;66(2):738–746. doi: 10.1016/0006-291x(75)90572-0. [DOI] [PubMed] [Google Scholar]
- Harbers K., Harbers B., Spencer J. H. Nucleotide clusters in in deoxyribonucleic acids. X. Sequences of the pyrimidine oligonucleotides of mouse L-cell satellite DNA. Biochem Biophys Res Commun. 1974 Jun 4;58(3):814–821. doi: 10.1016/s0006-291x(74)80490-0. [DOI] [PubMed] [Google Scholar]
- Harbers K., Spencer J. H. Nucleotide clusters in deoxyribonucleic acids. Pyrimidine oligonucleotides of mouse L-cell satellite deoxyribonucleic acid and main-band deoxyribonucleic acid. Biochemistry. 1974 Mar 12;13(6):1094–1101. doi: 10.1021/bi00703a006. [DOI] [PubMed] [Google Scholar]
- Hörz W., Hess I., Zachau H. G. Highly regular arrangement of a restriction-nuclease-sensitive site in rodent satellite DNAs. Eur J Biochem. 1974 Jun 15;45(2):501–512. doi: 10.1111/j.1432-1033.1974.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Hörz W., Zachau H. G. Characterization of distinct segments in mouse satellite DNA by restriction nucleases. Eur J Biochem. 1977 Mar 1;73(2):383–392. doi: 10.1111/j.1432-1033.1977.tb11329.x. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r63–r80. doi: 10.1093/nar/8.1.197-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Kaye A. M., Herzberg M. Mouse nuclear satellite DNA: 5-methylcytosine content, pyrimidine isoplith distribution and electron microscopic appearance. J Mol Biol. 1969 Aug 14;43(3):581–592. doi: 10.1016/0022-2836(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Biro P. A. Sequence and evolution of mouse satellite DNA. J Mol Biol. 1978 May 25;121(3):357–374. doi: 10.1016/0022-2836(78)90369-8. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Zachau H. G. A long-range and two short-range periodicities are superimposed in the 1.706-g/cm3 satellite DNA from calf thymus. Eur J Biochem. 1978 Aug 15;89(1):267–279. doi: 10.1111/j.1432-1033.1978.tb20923.x. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M., Britten R. J. Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science. 1966 Nov 11;154(3750):791–794. doi: 10.1126/science.154.3750.791. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Dingman C. W., Peacock A. C. Role of base composition in the electrophoresis of heat-treated deoxyribonucleic acid from HeLa and mouse cells in composite polyacrylamide gels. Biochemistry. 1974 Jul 30;13(16):3388–3393. doi: 10.1021/bi00713a032. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Peacock A. C. Isolation of mouse satellite deoxyribonucleic acid by composite polyacrylamide gel electrophoresis. Biochemistry. 1971 Nov;10(23):4219–4223. doi: 10.1021/bi00799a010. [DOI] [PubMed] [Google Scholar]



