Abstract
Objective
To determine rates and predictors of treatment refusal in newly identified HIV-infected individuals in Soweto, South Africa
Design
Cross-sectional Study.
Methods
We analyzed data from adult clients (> 18 years) presenting for voluntary counseling and testing (VCT) at the Zazi Testing Center, Perinatal HIV Research Unit to determine rates of antiretroviral therapy (ART) refusal among treatment-eligible, HIV-infected individuals (CD4+ <200 cells/mm3 or WHO stage 4). Multiple logistic regression models were used to investigate factors associated with refusal.
Results
From December 2008 to December 2009, 7287 adult clients were HIV tested after counseling. 2562 (35%) were HIV-infected, of whom 743 (29%) were eligible for immediate ART. One-hundred and forty-eight (20%) refused referral to initiate ART, most of whom (92%) continued to refuse after 2 months of counseling. The leading reason for ART refusal was given as “feeling healthy” (37%), despite clients having a median CD4+ cell count of 110 cells/mm3 and triple the rate of active tuberculosis as seen in non-refusers. In adjusted models, single clients (AOR= 1.80, 95% CI: 1.06–3.06) and those with active tuberculosis (AOR = 3.50, 95% CI: 1.55–6.61) were more likely to refuse ART.
Conclusion
Nearly one in five treatment-eligible HIV-infected individuals in Soweto refused to initiate ART after VCT, putting them at higher risk for early mortality. “Feeling healthy” was given as the most common reason to refuse ART, despite a suppressed CD4+ count and co-morbidities, such as tuberculosis. These findings highlight the urgent need for research to inform interventions targeting ART refusers.
Keywords: africa, HIV, refusal, treatment, VCT
Introduction
In sub-Saharan Africa, home to 68% of all people living with HIV, [1] mortality rates during the first year of antiretroviral treatment (ART) range from 8–26%, with most deaths occurring in the first few months after initiation of ART [2]. An analysis of patients who started ART in four scale-up programs in sub-Saharan Africa from 2004 to 2007 showed early mortality was associated with severity of immunodeficiency and disease status at the initiation of treatment [3]. New WHO guidelines responded to this by recommending earlier HIV diagnosis and ART initiation at a CD4+ cell count < 350 cells/mm3 to reduce early death [4].
South Africa, the country with the most HIV infected citizens globally, [5] has significantly expanded ART availability since April 2004 for individuals with a CD4+ < 200 cells/mm3 or WHO stage 4, [6] and currently has the largest ART program in the world [7]. Despite widespread availability of ART, and a National Strategic Plan to expand treatment to at least 80% of HIV-infected treatment-eligible individuals, [8] a substantial unmet need for treatment remains, and roughly only half of ART-eligible patients are actually in care [9].
Prior studies have focused on linkages to care, particularly the impact of loss to follow-up and points of delay in ART initiation on pre-treatment mortality [10–12]. While these factors need to be addressed, there is growing interest in understanding determinants of ART initiation in Sub-Saharan Africa. Little is known about whether delayed ART initiation is due to failure to present for treatment or actual treatment refusal. Our objective was to determine rates and predictors of refusal in newly identified HIV-infected individuals in Soweto, South Africa. Using data collected over the course of 2009 from a high volume testing center in Soweto, we conducted an analysis to determine the impact of ART refusal on initiation of care, and reasons for refusal in this population.
Methods
Study Setting
The Perinatal HIV Research Unit (PHRU) provides free HIV testing, treatment, prevention, and psychosocial support for HIV-infected individuals in Soweto, an urban area in South Africa with one of the highest rates of HIV transmission worldwide. The PHRU has provided on-site Voluntary Counseling and Testing (VCT), funded by USAID and PEPFAR, since 2001. Zazi Testing Center currently provides rapid HIV tests to over 300 adults (> 18 years) monthly, along with family planning, and screening for tuberculosis (TB) and sexually transmitted infections (STIs). Clients sign a consent form and are counseled by lay counselors who have undergone intensive training. They are given their HIV results, and, if positive, return for their CD4+ counts a week later, and referred for treatment as appropriate. Upon referral, clients meet with social workers to discuss ART initiation. Demographics, HIV test results, including CD4+ count, STI, and TB results are recorded in a clinical registry. Contact information is for follow-up, referral, and counseling services.
Study Participants, eligibility, enrollment and follow-up procedures
Patients eligible for this analysis included adults (> 18 years) who presented to Zazi outpatient center for HIV testing between December 2008 and December 2009, and were found to be HIV-infected and treatment eligible (CD4+ count < 200 cells/mm3 or WHO stage 4). All treatment eligible patients were offered free ART onsite. We captured data on patients’ willingness to accept treatment upon learning their CD4+ cell count. Those who refused were then counseled by social workers on staff regarding their CD4+ cell count and the clinical criteria associated with an AIDS diagnosis, and followed out for two months to assess if they would be willing to start treatment. Those clients who were diagnosed with active TB were counseled to initiate TB treatment before ART, according to WHO criteria.
This study and data collection instruments were approved by the University of Witwatersrand Ethics Committee (Johannesburg, South Africa) and the Partners Human Research Committee (Protocol #: 2010-P-001387/1, Boston, Massachusetts). Confidentiality was maintained by use of numbers instead of names during study proceedings and no identifying particulars were documented on interview forms.
Data elements
Patient level data were collected in the following three domains: 1) Demographic characteristics - age, gender, marital status, number of children, educational attainment, and employment status; 2) Beliefs and Behaviors - condom use, alcohol, tobacco, and drug use, willingness to disclose one’s HIV status, and history of HIV testing; and 3) Laboratory information - dates and results of HIV test, CD4+ count, and TB testing. A primary healthcare nurse performed a chart review for each participant to ascertain additional baseline data from the initial clinical evaluation, including a more extensive history for patients refusing treatment. Clients who refused ART were asked to give a single reason for refusal, which was noted by the social worker.
Statistical Analysis
We compared two groups in our analysis–specifically HIV-infected, treatment-eligible individuals who accepted ART vs. HIV-infected, treatment-eligible individuals who refused ART. The rate of ART refusal was calculated as the number of newly diagnosed HIV-infected ART-eligible study participants who actively refused to initiate ART, either upon learning their CD4+ count, or over a two month follow-up period, divided by the total number of newly diagnosed HIV-infected ART-eligible study participants.
Quantitative data were double-entered into Microsoft Office Excel, and analyses were carried out in STATA (STATACORP version 10; College Station, TX). Baseline factors were compared using chi-squared tests or Fisher’s exact test for categorical variables, while t-tests or Mann-Whitney tests were used to compare continuous variables across refusal groups. We fitted multiple logistic regression models by considering univariate factors with p-values of 0.2 or less to investigate factors associated with ART refusal. Changes in log-likelihoods were used to assess factors that significantly explained the odds of ART refusal. The Hosmer-Lemeshow goodness-of-fit test was used to asses the fit of the logistic model to our data.
Results
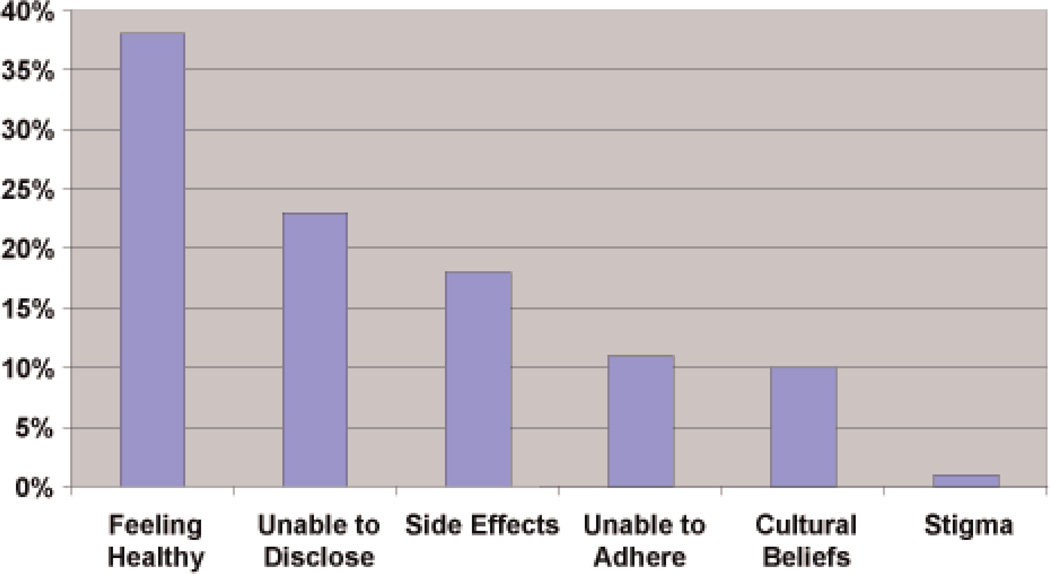
From December, 2008 to December, 2009, 7287 adult clients presented for VCT. Of these, 2562 (35%) adults were found to be HIV-infected. Within the HIV-infected cohort, 743 (29%) were eligible to start immediate ART [1]. One-hundred and forty-eight HIV-infected ART-eligible clients (20%) refused to initiate treatment upon learning their CD4+ counts, and another four clients were either hospitalized or died within a week of their HIV diagnosis. There were few differences between those who agreed to start ART and those who refused (Table 1). The median age of both groups was 34 years and the median CD4+ count was 109 cells/mm3. Sixty-three percent reported using condoms at their last sexual encounter. Ninety-two percent of ART-refusers continued to refuse after 2 months of counseling. Of the 12 clients who ultimately accepted ART after initially refusing, their median CD4 cell count was 113 (IQR 91–151); their mean age was 35 years (std 6.8 years); none had active TB; 10 (83%) had an HIV test before; and almost all of them (92%) intended to disclose their HIV status. Reasons given for ART refusal were abstracted from social worker notes and are displayed in Fig. 1. While 6 primary reasons were given by respondents, cultural beliefs encompassed both religious (Christian) and ancestral rituals (described as “super powers that will provide healing”) [2].
Table 1.
Characteristics of HIV Infected Treatment-Eligible Individuals.
| Variables | HIV+ with CD4 < 200 n = 743 |
Agreed to ART n = 595 (80%) |
Initial Refusers n = 148 (20%) |
OR (95% CI) |
Adjusted OR (95% CI) |
|---|---|---|---|---|---|
| Age in years at time of testing, median (IQR) | 34 | 33 (28–39) | 34 (28–39) | 1.00 (0.98–1.02) | |
| Gender, | |||||
| Female | 503 | 401 (67) | 102 (69) | 1.00 | |
| Male | 240 | 194 (33) | 46 (31) | 0.93 (0.63–1.37) | |
| Single/Divorce/Widow | 575 | 453 (78) | 122 (85) | 1.62 (0.98–2.69) | 1.80 (1.06–3.06)* |
| Have children | 514 | 414 (86) | 100 (87) | 1.09 (0.60–2.00) | |
| Median CD4+ (IQR)# | 109 | 109 (52–164) | 110 (58–148) | 0.92 (0.67–1.24) | |
| Level of Education | |||||
| No education | 27 | 22 (4) | 5 (4) | 1 | |
| Primary school | 51 | 38 (8) | 13 (11) | 1.50 (0.47–4.79) | |
| High school | 481 | 387 (79) | 94 (78) | 1.07 (0.39–2.90) | |
| Higher (tertiary) | 52 | 43 (8) | 9 (8) | 0.92 (0.28–3.08) | |
| Employed | 218 | 178 (36) | 40 (34) | 0.92 (0.60–1.41) | |
| First Time Testers | 232 | 179 (30) | 53 (35) | 0.76 (0.52–1.12) | |
| Intend to Disclose | 640 | 511 (92) | 129 (93) | 1.26 (0.60–2.64) | |
| Condoms at Last Sex | 398 | 324 (64) | 74 (62) | 0.92 (0.61–1.39) | |
| Use Tobacco | 155 | 127 (22) | 28 (20) | 0.87 (0.55–1.37) | |
| Use Alcohol | 264 | 210 (36) | 54 (37) | 1.07 (0.73–1.56) | |
| Use Recreational Drugs | 20 | 13 (2) | 7 (5) | 2.23 (0.87–5.70) | |
| Active TB | 34 | 20 (3) | 14 (9) | 3.00 (1.48–6.10)** | 3.20 (1.55–6.61)** |
Data are number and row percent, unless otherwise noted.
p < 0.05.
p < 0.01.
CD4+ count in cells/mm3; AOR, Adjusted Odds Ratio; CI, confidence Interval; OR, Odds Ratio.
Fig. 1.
Percentage of Testers who Refuse, Treatment, by Reason.
Single clients were 1.80 times (95% CI: 1.06–3.06) more likely to refuse ART as compared to any other marital status group in adjusted models. Patients with active TB (AOR 3.20, 95% CI: 1.55–6.61) had a higher odds of refusing ART compared to clients who had no evidence of active TB.
Discussion
Despite the dramatic increase in VCT in South Africa in the past few years, with more than half of all South African adults having been tested at least once, [13] expansion of testing has not translated into earlier treatment initiation [14]. This is supported by our finding that 70% of VCT clients presenting to Zazi had been previously tested for HIV, yet nearly one in five ART-eligible adults refused treatment within two months of diagnosis, leaving them at risk for early mortality. These findings are consistent with rates of pre-ART attrition found at other sites in sub-Saharan Africa [15,16]. Despite VCT being traditionally viewed as an entry point to treatment and care, [17] these data underscore the importance of a new and unappreciated challenge in the cascade of HIV testing to sustained antiretroviral treatment.
While ART eligible patients not yet in treatment have traditionally been difficult to monitor, we were able to document both rates and reasons for refusal in this VCT population. We found that over 35% of clients who refused ART stated they were “too healthy” to initiate treatment, despite a median CD4+ count of 110 cells/mm3. In addition, those with active TB were three times more likely to refuse ART. Prior studies in sub-Saharan Africa have found similar associations between TB positivity and low acceptability of ART [18]. Early ART initiation, particularly in patients with co-morbidities such as TB, clearly remains a priority [19]. A recent South African study showing early ART initiation in conjunction with TB therapy in co-infected patients reduced mortality by 56% [20].
Despite 92% of VCT clients initially reporting they would be willing to disclose their status, over 20% of those who refused ultimately stated they were “unable to disclose.” These results show that the inability to disclose one’s status has behavioral consequences and may be more pronounced than initially acknowledged by the general population. Given patients who self-identified as being single were more likely to refuse treatment, the risk for non-disclosure remains concerning among sexually active individuals. This is particularly relevant in light of increasing evidence of the importance of ART treatment in HIV-infected individuals as a form of prevention to uninfected partners [21].
This study has a critical limitation - specifically these data were not collected to examine factors associated with ART refusal. Rather, basic clinical and demographic variables were collected concurrently on patients who accessed VCT. At the time these data were collected, the phenomenon of treatment refusal had not been identified or well understood. Given that, we have no data on clients’ understanding of HIV and AIDS and their interpretation of their test results.
As South Africa continues to expand its ART coverage, efforts will need to be made beyond simply testing and counseling to the reach the estimated 2 million people currently in need of treatment. This will require marketing the concept of ART as a life saving intervention, even for people who report feeling healthy. We have added to the growing literature on understanding factors driving treatment refusal after VCT in South Africa, [22,23] by identifying a critical barrier to ramping up accelerated treatment coverage beyond cost reductions, [24] willingness to test, and ART availability. To ensure the success of HIV treatment scale-up in South Africa, it will be essential to understand the reasons for ART refusal in individuals willing to undergo VCT, ultimately enabling the development of effective targeted interventions.
Acknowledgements
We would like to thank the clients who accessed VCT at Zazi for their time and willingness to participate in studies such as this. We also want to acknowledge the staff at Perinatal HIV Research Unit and the Zazi Staff for support for this study, and Harvard University for supporting this research. We would also like to that PEPFAR and USAID for funding their funding of the Zazi Testing Center. The opinions herein do not necessarily reflect those of USAID or the US Government.
Sources of Support: Patient care is funded by a PEPFAR grant through USAID South Africa (674-A-00–08–00009–00). The opinions expressed herein do not necessarily reflect those of the U.S. Government or USAID. ITK received support from a KL2 Medical Research Investigator Training (MeRIT) grant awarded via Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH grant #1KL2RR025757–01) and financial contributions from Harvard University and its affiliated academic health care centers. DRB receives partial funding through MH K-24 87227. NAM is partially funded by HIV and TB research training twin grants RTW007373 and RTW007370 from the Fogarty International Center.
Footnotes
Conflicts of interest
Publisher's Disclaimer: Disclaimers: There are no conflicts of interest.
These data were presented at The 18th Conference on Retroviruses and Opportunistic Infections Meetings, February 27-March 3, 2011, Boston.
References
- 1. [Accessed March 11, 2011];World Health Organization–Millennium Development Goal 6. http://www.who.int/topics/millennium_development_goals/diseases/en/index.html.
- 2.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, Keiser O, Sterne JA, Dabis F, Egger M. IeDEA Southern Africa and West Africa. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376(9739):449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation. [Accessed 6 June 2011];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 revision. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 5.Joint United Nations Program on HIV/AIDS and World Health Organization. 2010 AIDS Epidemic Update.
- 6.WHO/UNAIDS/UNICEF. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 Progress Report.
- 7.Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;24:24. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health SA. HIV and AIDS and STI Strategic Plan for South Africa. 2007:2007–2011.
- 9.Joint United Nations Programme on HIV/AIDS. [Accessed 14 June 2011];Epidemiological Fact Sheet on HIV and AIDS, South Africa. 2009 Available at: http://92.52.112.217/downloadpdf.htm?country_id = AFRZAF&lng_code = en&pdfoption = epi.
- 10.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, Lu Z, Losina E, Walensky RP, Freedberg KA. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51(2):135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, Ross D, Scott CA, Uhler LM, Katz JN, Holst H, Freedberg KA. The “ART” of Linkage: Pre-Treatment Loss to Care after HIV Diagnosis at Two PEPFAR Sites in Durban, South Africa. PLoS One. 2010;5(3):e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, Bekker LG, Wood R. Linkage to HIV Care and Antiretroviral Therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van-Wyk V, Mbelle N, Van Zyl J, Parker W, Zungu NP, Pezi S the SABSSM III Implementation Team. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town (HSRC Press) 2009:1–120. [Google Scholar]
- 14.Lawn SD, Little F, Bekker L-G, Kaplan R, Campbel E, Orrell C, Wood R. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–342. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 16.Tayler-Smith K, Zachariah R, Manzi M, Kizito W, Vandenbulcke A, Dunkley S, von Rege D, Reid T, Arnould L, Suleh A, Harries AD. Demographic characteristics and opportunistic diseases associated with attrition during preparation for antiretroviral therapy in primary health centres in Kibera, Kenya. Trop Med Int Health. 2011 doi: 10.1111/j.1365-3156.2011.02740.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.UNAIDS Report on the Global AIDS Epidemic 2010. Geneva Switzerland (WHO Library Cataloguing-in-Publication Data) 2010:1–359.
- 18.Zachariah R, Harries AD, Manzi M, Gomani P, Teck R, Phillips M, Firmenich P. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PloS ONE. 2006;1:e121. doi: 10.1371/journal.pone.0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, Sterne JA, Egger M, Fairall L. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24:2717–2725. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed June 14, 2011];HIV Prevention Trial Network 052, Interim Report. Available at: http://www.hptn.org/research_studies/hptn052.asp.
- 22.Larson BA, Brennan A, McNamara L, Long L, Rosen S, Sanne I, Fox MP. Lost opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bull World Health Organ. 2010;88(9):675–680. doi: 10.2471/BLT.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.April MD, Walensky RP, Chang Y, Pitt J, Freedberg KA, Losina E, Paltiel AD, Wood R. HIV Testing Rates and Outcomes in a South African Community, 20*01–20*06: Implications for Expanded Screening Policies. J Acquir Immune Defic Syndr. 2009;51:310–316. doi: 10.1097/qai.0b013e3181a248e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opuni M, Bishai D, Gray GE, McIntyre JA, Martinson NA. Preferences for characteristics of antiretroviral therapy provision in Johannesburg, South Africa: results of a conjoint analysis. AIDS Behav. 2010;14(4):807–815. doi: 10.1007/s10461-009-9584-4. [DOI] [PubMed] [Google Scholar]