Abstract
The epidermal growth factor receptor (EGFR) secondary kinase domain T790M non–small cell lung cancer (NSCLC) mutation enhances receptor catalytic activity and confers resistance to the reversible tyrosine kinase inhibitors gefitinib and erlotinib. Currently, irreversible inhibitors represent the primary approach in clinical use to circumvent resistance. We show that higher concentrations of the irreversible EGFR inhibitor CL-387,785 are required to inhibit EGFR phosphorylation in T790M-expressing cells compared with EGFR mutant NSCLC cells without T790M. Additionally, CL-387,785 does not fully suppress phosphorylation of other activated receptor tyrosine kinases (RTK) in T790M-expressing cells. These deficiencies result in residual Akt and mammalian target of rapamycin (mTOR) activities. Full suppression of EGFR-mediated signaling in T790M-expressing cells requires the combination of CL-387,785 and rapamycin. In contrast, Hsp90 inhibition overcomes these limitations in vitro and depletes cells of EGFR, other RTKs, and phospho-Akt and inhibits mTOR signaling whether or not T790M is present. EGFR-T790M– expressing cells rendered resistant to CL-387,785 by a kinase switch mechanism retain sensitivity to Hsp90 inhibition. Finally, Hsp90 inhibition causes regression in murine lung adenocarcinomas driven by mutant EGFR (L858R) with or without T790M. However, efficacy in the L858R-T790M model requires a more intense treatment schedule and responses were transient. Nonetheless, these findings suggest that Hsp90 inhibitors may be effective in T790M-expressing cells and offer an alternative therapeutic strategy for this subset of lung cancers.
Introduction
Activating mutations in the kinase domain of epidermal growth factor receptor (EGFR) in non–small cell lung cancers (NSCLC) commonly arise as in-frame deletions in exon 19 and L858R exon 21 substitutions, and confer sensitivity to the tyrosine kinase inhibitors (TKI) gefitinib and erlotinib (1). Structurally, mutation impairs affinity for ATP, locks the kinase in an active conformation, and facilitates interaction with ATP-competitive inhibitors (2). In addition, responses are also likely related to oncogene addiction (3).
Mutant EGFRs induce oncogenic effects by activating signaling and antiapoptotic pathways, notably those mediated by phosphatidylinositol 3-kinase (PI3K)-Akt (4) and mammalian target of rapamycin (mTOR), which regulates translation initiation through ribosomal p70S6 kinase (p70S6K) and eukaryotic translational initiation factor 4E (eIF4E) binding proteins (4E-BPs; ref. 5). In primary lung adenocarcinomas, mTOR activation, measured indirectly by augmented levels of phospho-S6 protein, was significantly more frequent in tumors with EGFR mutation compared with those with wild-type EGFR (6).
Despite initial responses of EGFR mutant tumors to small-molecule TKIs, resistance universally emerges, associated with the acquisition of or selection for a second mutation, resulting in a threonine-to-methionine amino acid substitution at position 790 (T790M) of EGFR in ~ 50% of cases (7, 8). EGFR T790M has also been reported with L858R in some cases not exposed to gefitinib or erlotinib, such as in NCI-H1975 cells (8), established before the TKI era. Recent work using purified EGFR L858R and L858R/T790M baculoproteins expressed in Sf9 cells showed a substantial increase in autophosphorylation at several sites in the double mutant compared with the single mutant, suggesting that T790M, when combined with activating kinase domain mutations, confers enhanced catalytic phosphorylating activity and cooperates to produce a more potent kinase (9).
The L858R/T790M double mutant has recently been reported to bind gefitinib with low nanomolar affinity, but restores wild-type affinity for ATP, diminishing the effectiveness of TKIs that must compete with ATP for receptor binding (10). Therefore, the primary approach under development for NSCLCs expressing mutant EGFR harboring T790M mutation is the use of irreversible inhibitors that overcome this effect because they contain a reactive group that forms a covalent bond at the edge of the ATP-binding cleft. Preclinical studies have shown that the irreversible EGFR inhibitor CL-387,785 (11), the irreversible dual EGFR and ErbB2 inhibitor HKI-272 (12), and the irreversible pan-ErbB inhibitor CI-1033 (13) can overcome resistance to L858R-mutated EGFR harboring the T790M resistance–conferring mutation.
We have recently described an inducible murine lung adenocar-cinoma model driven by expression of EGFR harboring L858R and T790M mutations (14). In contrast to murine tumors driven by EGFR-L858R, in which only peripheral tumors occurred, both peripheral and bronchial tumors developed. Surprisingly, tumors expressing L858R-T790M responded poorly to HKI-272. Further analysis indicated that mTOR signaling was weakly suppressed by HKI-272, as measured by persistent robust phosphorylation of S6 after treatment. This limitation was overcome by the addition of rapamcyin, so that combination treatment produced cytotoxic synergy in vitro and led to regressions in vivo.
Previously, we showed that L858R and deletion mutant EGFR proteins, with or without the T790M resistance mutation, require the Hsp90 chaperone for conformational maturation and are sensitive to degradation following Hsp90 inhibition with geldanamycins (15), similar to the Hsp90 dependence of mutant B-Raf oncoproteins compared with their wild-type counterparts (16, 17). In addition, Akt has been described as an Hsp90 client (18), and mTOR-mediated signaling has been reported as diminished by Hsp90 inhibition (19). These observations prompted us to further investigate Hsp90 inhibition as a therapeutic strategy in NSCLC cells expressing kinase domain mutant EGFR harboring T790M. Our results indicate that compared with irreversible EGFR inhibition, Hsp90 inhibition causes more complete suppression of the entire EGFR-PI3K-Akt-mTOR-p70S6K-S6 signaling axis in T790M-expressing cells both in vitro and in vivo. Furthermore, we have found that cells harboring T790M may have partial dependence on other receptor tyrosine kinases (RTK) besides EGFR, which are effectively depleted by Hsp90 inhibition. This is especially true in T790M-expressing cells rendered resistant to CL-387,785 after prolonged in vitro exposure, which show increased activation of the insulin and insulin-like growth factor-IR (IGF-IR) receptors and which remain sensitive to Hsp90 inhibition.
Materials and Methods
Cell lines and drug treatments
NCI-H1975 and NCI-H820 NSCLC cell lines were obtained from the American Type Culture Collection (ATCC). HCC827 and H3255 cells were provided by Dr. Adi Gazdar (University of Texas Southwestern Medical Center, Dallas, TX) and Drs. Bruce Johnson and Pasi Jänne (Dana-Farber Cancer Institute, Boston, MA), respectively. PC9 was a gift from Dr. Takashi Owa (Eisai Co., Ltd., Tsukuba, Japan). Cells were maintained in ATCC-specified growth medium. HCC827 isogenic cell lines expressing EGFR-DelA747-S752 (HCC827_Del), EGFR-DelA747-S752/T790M (HCC827_Del/T790M), or vector were maintained in medium supplemented with 1000 µg/mL G418. Ba/F3cells stably expressing mutant EGFR were established by puromycin selection and cultured in the absence of interleukin-3(IL-3). Pooled stable cell lines transformed to IL-3 independence were used for drug sensitivity experiments. CL-387,785; AG1024; SU11274; and an irreversible inhibitor of EGFR, ErbB2, and ErbB4 [H1,2,4i; N-(4-((3-chloro-4 fluorophenyl) amino)pyrido[3,4-d]pyrimidin-6-yl)2-butynamide] were obtained from Calbiochem. Rapamycin was obtained from LC Labs. 17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) and 17-allylamino-17-demethoxygeldanamycin (17-AAG) were obtained from LC Labs or Invivogen. Erlotinib was obtained from Biaffin GmbH & Co. KG.
Cell proliferation and apoptosis assays
NSCLC cells were seeded at a density of 5,000 to 7,000 per well on 96-well plates, cultured in the presence of drugs or vehicle for 72 hours, and subjected to the CCK-8 colorimetric assay (Dojindo) in at least duplicate samples according to the manufacturer’s specifications. Combination indices were calculated by the median effect method available on Calcusyn (Biosoft). For detection of apoptosis by flow cytometry, adherent and nonadherent cells were pooled. Apoptosis was measured using an Annexin V–FLUOS Staining Kit (Roche Applied Science).
Western blot and phospho-RTK array analysis
Whole-cell lysates were prepared in lysis buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitor cocktails (Calbiochem). Protein concentrations were determined using the bicinchoninic acid protein assay kit (Pierce) and equivalent amounts (50 µg) were subjected to SDSPAGE on 4% to 20% gradient gels. Antibodies used for Western blotting were from Biosource International (phospho-MTOR, phospho-c-Met, phospho-IGF-IR, raptor), Upstate Biotechnology (c-Met), Santa Cruz Biotechnology (EGFR, ErbB2, c-Raf, cdk4), Lab Vision, (ErbB3), and Roche Applied Science (hemagglutinin). All other antibodies were from Cell Signaling Technology. Bands were quantified using ImageJ software (NIH). Phospho-RTK Arrays (R&D Systems) were performed according to the manufacturer’s instructions. Seventy-five micrograms of whole-cell lysate were applied to each array; after Western blotting, films were scanned for densitometric analysis using ImageJ Software to determine the percent control activation using equations accounting for lot variation for array strips and background.
Generation of NCI-H1975CLR cells
NCI-H1975 were exposed to increasing concentrations of CL-387,785, starting at 550 nmol/L, the approximate IC40. Drug concentration was increased stepwise when cells returned to normal growth kinetics. Cells able to grow in 5 µmol/L CL-387,785 were obtained 4 months after the initial exposure. Cells were monitored for stable resistance by testing them after growth in drug-free medium for at least 2 passages (~6 d). After 5 months, NCI-H1975CLR cells were maintained without CL-387,785, and their sensitivity was examined routinely to ensure maintenance of resistance.
Short hairpin RNA constructs, lentiviral infection, and small interfering RNA transfection
Short hairpin RNA (shRNA) constructs cloned in pLKO.1 puro vector were designed by the Harvard RNAi consortium and distributed by Open Biosystems or Sigma-Genosys. shRNA sequences are provided with the Supplementary Data. Lentiviral production and infection were performed as specified by Sigma-Genosys using MISSION Lentiviral Packaging Mix and the 293LTV cell line (Cell Biolabs). Following puromycin selection, CCK-8 assays were performed 7 to 10 days after infection. siRNA transfections were performed with RNAiMax (Invitrogen) using commercially available pooled oligonucleotides (Dharmacon and Santa Cruz Biotechnology).
Generation of the CCSP-rtTA/Tet-op-EGFR L858R and L858R-T790M mouse cohorts and drug treatment
Bitransgenic mice with lung adenocarcinomas driven by EGFR L858R or L858R-T790M were exposed to a doxycycline-containing diet for 8 to 12 weeks, and subjected to magnetic resonance imaging (MRI) to document tumor burden. Littermates were subsequently left untreated or treated with vehicle or 17-DMAG, formulated in saline, and administered by i.p. injection at 10 mg/kg, either thrice weekly or daily. Mice underwent serial MRI imaging to assess reduction in tumor volume and were subsequently sacrificed for further histologic and biochemical studies. After Dana-Farber Cancer Institute Animal Care and Use Committee approval, all mice were housed in a pathogen-free environment at the Harvard School of Public Health and were handled in accordance with Good Animal Practice as defined by the Office of Laboratory Animal Welfare.
Histologic and immunohistochemical staining
H&E and immuno-histochemical staining were performed on formalin-fixed paraffin sections using standard procedures. Antibodies against EGFR, Hsp27, cyclin D1, and phospho-S6 were from Cell Signaling Technology. For immunohistochemical quantification, 100 to 200 cells were scored as 0, 1+, and 2+ over each of 2 to 4 high-power fields to determine the average percent strongly positive cells.
MRI scanning and tumor volume measurement
Mice were anesthetized with 1% isoflurane in an oxygen/air mixture and respiratory and heart rates were monitored using Biotrig Software. Detailed procedures Cancer Research for MRI scanning, image acquisition in coronal and axial planes, and assessment of tumor volume have been described previously (20, 21).
Statistical analysis
Statistical analyses were performed using the two-tailed, unpaired Student’s t test. P values,<0.05 were considered significant.
Results
NSCLC cell lines harboring kinase domain mutations with T790M have high levels of mTOR activity and are less sensitive to the irreversible EGFR inhibitor CL-387,785 than those with kinase domain mutation alone
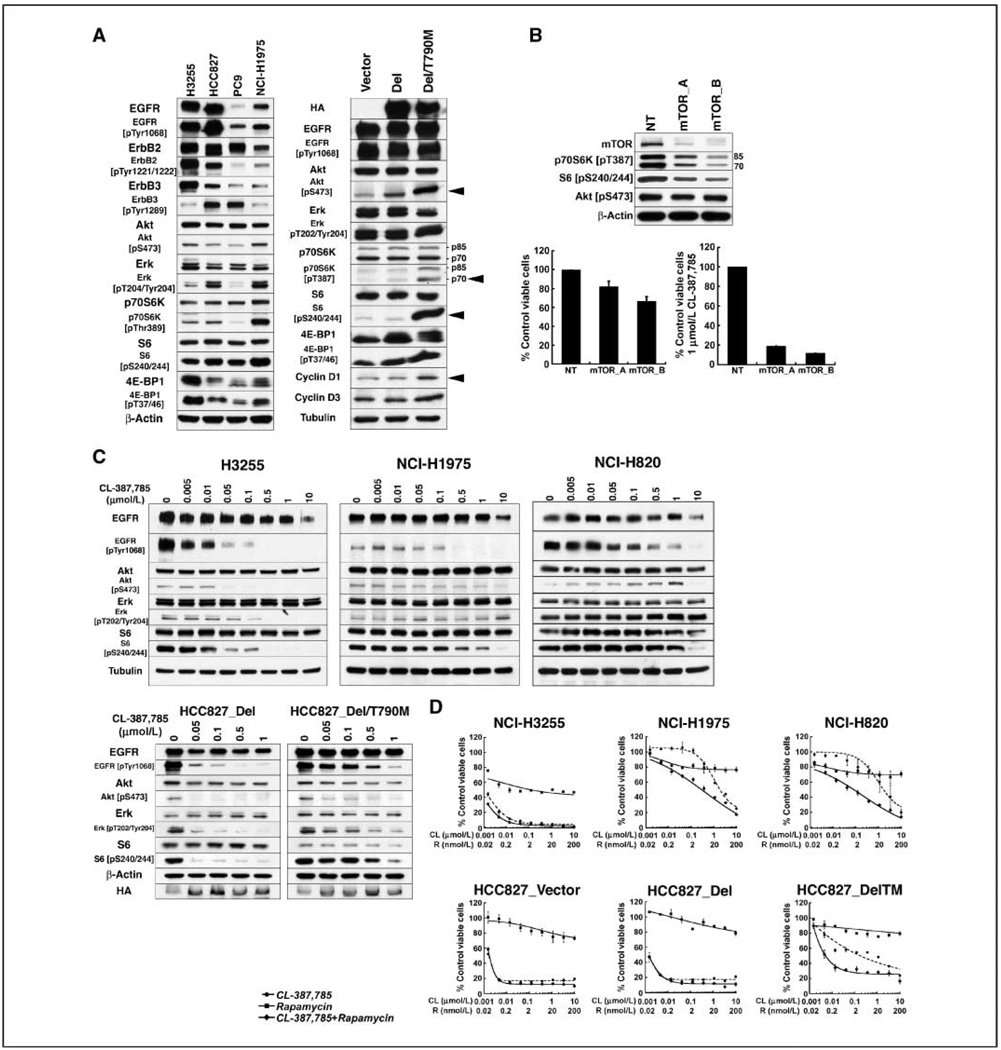
Catalytic activity of mutant EGFRs is augmented by secondary T790M mutation. To determine whether the presence of T790M enhances downstream signaling, the Western blot analyses in Fig. 1A were performed, with quantification shown in Supplementary Table S1. The data shown not only increased levels of phospho-Akt, but also abundant phospho-p70S6K and phospho-S6 in NCI-H1975 cells (L858R/T790M) compared with cells harboring kinase domain mutation without T790M. We also examined isogenic HCC827 cells engineered to express hemagglutinin-tagged EGFR A747-S752del (HCC827_Del) or EGFR A747-S752del/T790M (HCC827_Del/T790M; ref. 22). Compared with cells expressing empty vector or EGFR A747-S752del, cells expressing EGFR A747-S752del/T790M have higher levels of phospho-Akt, phospho-p70S6K, and phospho-S6, as well as phospho-4E-BP1 and cyclin D1. shRNA-mediated depletion of mTOR from NCI-H1975 cells compromises their viability, albeit after prolonged knockdown, and sensitizes them to CL-387,785 (Fig. 1B). Viability of EGFR mutant cells not expressing T790M is also compromised by mTOR pathway knockdown (data not shown). Taken together, these data suggest that mTOR-mediated signaling comprises critical events downstream of Akt in EGFR mutant cells, and occurs to a greater extent in the presence of T790M.
Figure 1.
T790M-expressing NSCLC cell lines show enhanced signaling downstream of mTOR, incompletely suppressed by the irreversible EGFR inhibitor CL-387,785. A, lysates were prepared from exponentially growing cells and subjected to Western blotting with the indicated antibodies. Arrows demarcate the increased levels of phospho-Akt, phospho-p70S6K, phospho-S6, and cyclin D1 in HCC827_ Del/T790M cells. B, NCI-H1975 cells were infected with lentiviruses encoding shRNAs targeting mTOR (mTOR_A or mTOR_B) or nontargeting shRNA (NT) for 7 d before the preparation of lysates for Western blotting (top), or for 10 d before assessment of viabilityby CCK-8 assay in the absence (bottom left) or presence of 1 µmol/L CL-387,785 for an additional 72 h (bottom right). Statistically significantly decreased viability(P = 0.005, for mTOR_B shRNA expression compared with control) was associated with reduced expression of mTOR, phospho-p70S6K, and phospho-S6. Columns, mean; bars, SD. C, top, exponentially growing H3255 (L858R), NCI-H1975 (L858R/T790M), and NCI-H820 (E746_T751del/T790M) cells were treated with the indicated concentrations of CL-387,785 for 24 h, and lysates were subjected to Western blotting with the indicated antibodies. Higher concentrations of CL-387,785 are required to dephosphorylate mutant EGFR in the T790M-expressing cell lines, associated with persistent expression of phospho-Akt and phospho-S6. Bottom, isogenic HCC827_Del and HCC827_Del/T790M cells were treated with CL-387,785 at the indicated concentrations for 24 h, and lysates were subjected to Western blotting with the indicated antibodies. HA, hemagglutinin. Dephosphorylation of mutant EGFR harboring T790M requires higher drug concentration, with persistence of expression of both phospho-Akt and phospho-S6. D, cell lines were treated with a range of concentrations of CL-387,785, rapamycin, or CL-387,785 + rapamycin for 72 h in triplicate and subjected to CCK-8 assay. The viability of each sample was normalized to that of DMSO-treated cells. Points, average of normalized values for two independent experiments; bars, SD. Combination indices at the IC50 indicate synergy of EGFR and mTOR inhibition in T790M-expressing cells with values of 0.09565, 0.05488, and 0.05628 in NCI-H1975, NCI-H820, and HCC827_Del/T790M cells, respectively.
We previously showed that T790M-expressing NCI-H1975 (L858R/T790M) cells are less sensitive than H3255 (L858R) cells to the irreversible dual-specific EGFR/ErbB2 inhibitor HKI-272 (14). However, NCI-H1975 are highly sensitive to the combination of HKI-272 and rapamycin, suggesting the importance of persistent mTOR signaling in governing survival in response to HKI-272. To determine whether these results extend to another compound with greater potency and selectivity for EGFR, we treated a panel of EGFR mutant NSCLC cell lines with CL-387,785 (23, 24). The IC50s shown in Supplementary Table S2 show that cell lines with kinase domain mutation without T790M are more sensitive to CL-387,785 than those harboring a secondary T790M mutation. As shown in Fig. 1C, CL-387,875 efficiently reduces phosphorylation of EGFR, Akt, and S6 in H3255 (L858R) cells in a concentration-dependent manner. Similar data were obtained in parental HCC827 and PC9 cells (data not shown). In contrast, inhibition of EGFR phosphorylation in NCI-H1975 (L858R/T790M) and NCI-H820 (EGFR E746_T751del_insI/T790M) requires substantially higher concentration, with steady expression of phospho-Akt and downstream mTOR signaling, evidenced by persistent levels of phospho-S6. The same was true in the comparison of isogenic HCC827 cells. In HCC827_Del cells, CL-387,785 treatment abrogated phosphorylation of EGFR, Akt, and S6, whereas in HCC827_Del/T790M cells, phosphorylation of these proteins was maintained even after treatment with higher drug concentrations. Consistent with the contribution of persistent mTOR signaling to the increased viability of T790M-expressing cell lines after exposure to the irreversible EGFR inhibitor, the combination of CL-387,785 and rapamycin was synergistic in NCI-H1975 and NCI-H820 cells, but not in cell lines harboring EGFR kinase domain mutation without T790M (Fig. 1D).
The Hsp90 inhibitor 17-DMAG suppresses EGFR-Akt-mTOR-p70S6K-S6 signaling in NSCLC and Ba/F3 cell lines expressing mutant EGFR harboring T790M
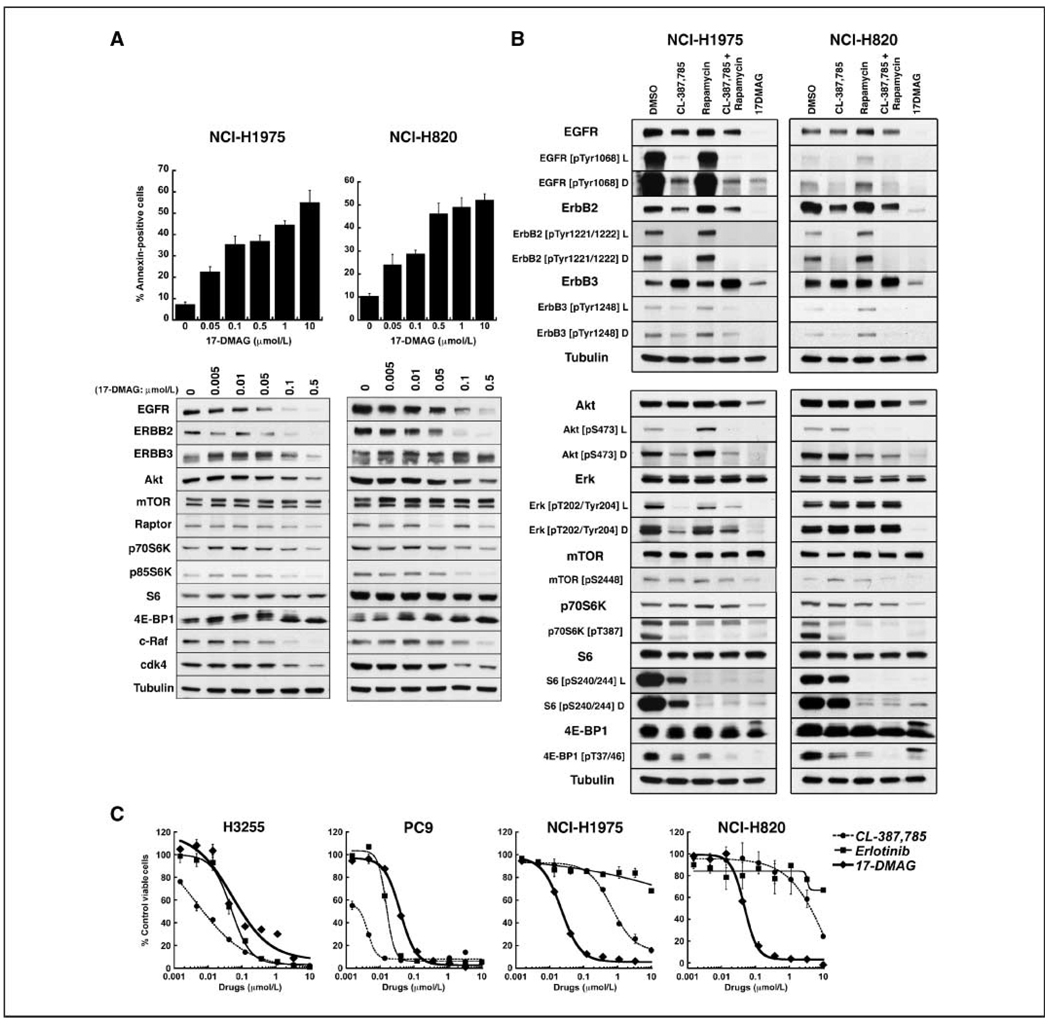
As an alternative strategy to the addition of rapamycin to CL-387,785, we asked if the same suppression of signaling could be accomplished with application of an Hsp90 inhibitor. Figure 2A shows concentration-dependent apoptosis of NCI-H1975 and NCI-H820 cells induced by treatment with 17-DMAG, a water-soluble geldanamycin currently in clinical trial (25). Western blot analysis shows that EGFRs harboring compound kinase domain/T790M mutations are depleted after 17-DMAG exposure, confirming the mutated kinases as Hsp90 clients. 17-DMAG also reduces expression of ErbB2, c-Raf, and cdk4, and also slightly reduces expression of ErbB3, raptor, and S6 kinases, indicating that the latter proteins are at least weakly sensitive to chaperone inhibition. As shown in Fig. 2B, 17-DMAG causes more reduction in expression of Akt and p70S6K and their phosphorylated forms than CL-387,785 in these lines, resulting in reduced phospho-S6 expression similar to that observed in response to rapamycin. Furthermore, for suppression of the entire signaling axis, 17-DMAG is equivalent to the combination of CL-387,785 and rapamycin in NSCLC cell lines harboring EGFR kinase domain mutations with T790M. Consistent with these data, the IC50s of EGFR kinase domain mutant cell lines to 17-DMAG were similar, whether or not T790M was present (Supplementary Table S2; Fig. 2C).
Figure 2.
17-DMAG–mediated Hsp90 inhibition induces apoptosis in EGFR kinase domain/T790M mutant cell lines and disrupts upstream and downstream signaling. A, top, exponentially growing NCI-H1975 and NCI-H820 cells were treated with 17-DMAG at the indicated concentrations for 24 h and subjected to Annexin V assay. Columns, average of three experiments; bars, SD. Bottom, cells were treated with 17-DMAG at the indicated concentrations for 24 h and lysates were subjected to Western blotting with the indicated antibodies, confirming mutant EGFR, ErbB2, c-Raf, and cdk4 as Hsp90 clients. Slight diminution of raptor and S6 kinases also occurs. B, NCI-H1975 and NCI-H820 cells were treated with 1 µmol/L CL-387,785, 25 nmol/L rapamycin, 1 µmol/L CL-387,785 + 25 nmol/L rapamycin, or 500 nmol/L 17-DMAG for 24 h. Lysates were subjected to Western blotting with the indicated antibodies. 17-DMAG depletes EGFR family members and their phosphorylated forms, as well as Akt, phospho-Akt, p70S6K and phospho-p70S6K, and phospho-S6 and phospho-4E-BP1. Treatment with 17-DMAG is equivalent to the combination of CL-387,785 + rapamycin in the inhibition of upstream and downstream signaling. L and D, light and dark exposures of the respective blots. C, cell lines were treated with CL-387,785, erlotinib, and 17-DMAG at the indicated concentrations for 72 h and viability was measured byCC K-8 assayin triplicate; the viabilityof each sample was normalized to that of DMSO-treated cells. Points, average of normalized values over two independent experiments; bars, SD. Results confirm the resistance of T790M-expressing cell lines to erlotinib and increased IC50 in response to CL-387,785. IC50s to 17-DMAG were similar whether or not EGFR harboring T790M was expressed. Data were used to generate the IC50s listed in Supplementary Table S2.
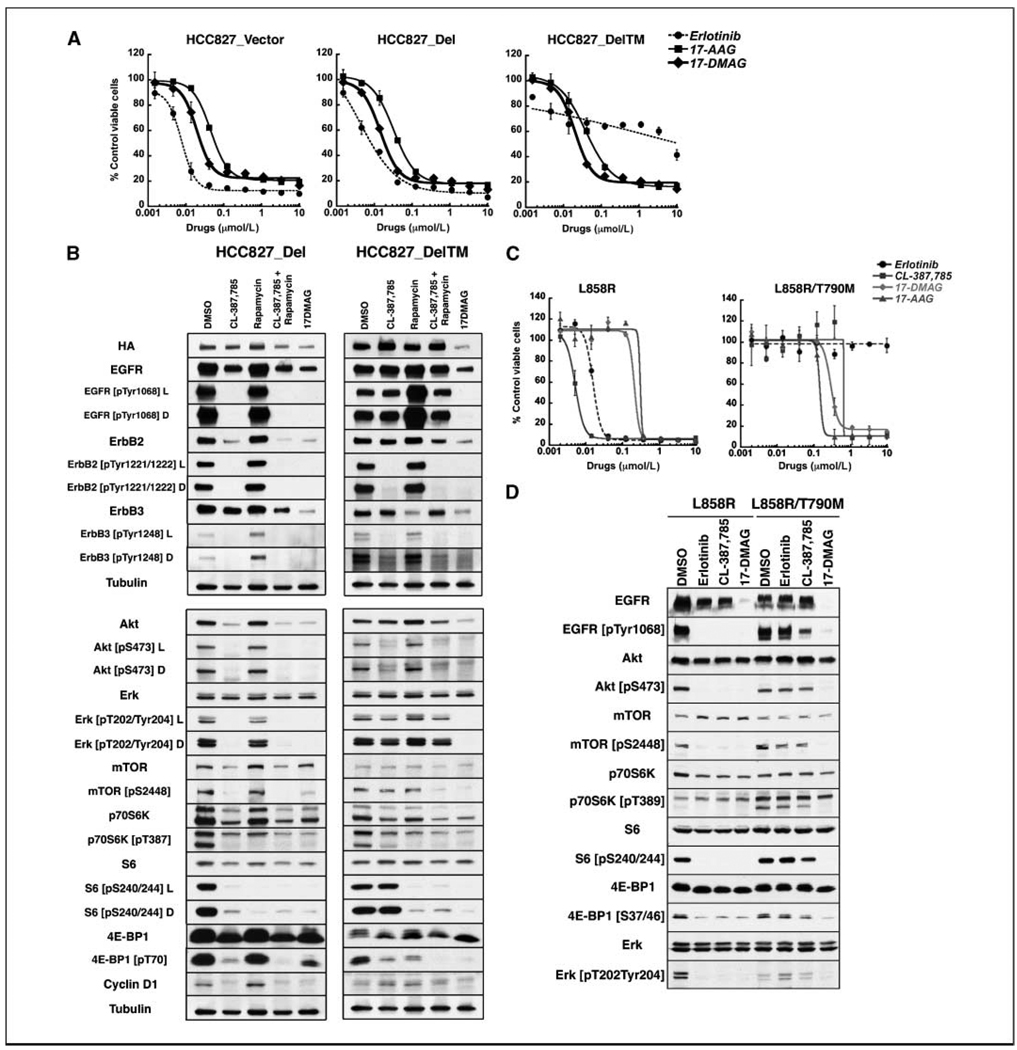
To extend our results to isogenic systems, we evaluated HCC827_Del and HCC827_Del/T790M cells. Figure 3A shows that T790M-expressing cells acquire resistance to erlotinib but retain sensitivity to the Hsp90 inhibitors 17-AAG and 17-DMAG. Although the introduction of T790M increases the baseline level of phospho-Akt, phospho-p70S6K, phospho-S6, and cyclin D1 in these cells (Fig. 1A), signaling was efficiently abrogated by Hsp90 inhibition in both HCC827_Del and HCC827_DelT790M cells (Fig. 3B). Again, in T790M-expressing cells, 17-DMAG inhibited upstream and downstream signaling as well as the combination of CL-387,785 and rapamycin, culminating in reduced S6 phosphorylation that does not occur in response to CL-387,785 alone.
Figure 3.
Analysis of isogenic cell lines confirms the potency of Hsp90 inhibition against mutant EGFR irrespective of T790M mutation. A, HCC827_Vector, HCC827_Del, and HCC827_Del/T790M cells were treated with erlotinib, 17-AAG, and 17-DMAG at the indicated concentrations for 72 h and subjected to CCK-8 assay in triplicate to assess cell viability. Viability of each sample was normalized to that of DMSO-treated cells. Points, average of normalized values from three independent experiments; bars, SD. Although T790M cells are resistant to erlotinib, theyretain sensitivity to Hsp90 inhibitors. B, HCC827_Del and HCC827_Del/T790M cells were treated with DMSO, 1 µmol/L CL-387,785, 25 nmol/L rapamycin, 1 µmol/L CL-387,785 + 25 nmol/L rapamycin, or 500 nmol/L 17-DMAG for 24 h. Lysates were subjected to Western blotting with the indicated antibodies. In HCC827_Del cells, CL-387,785, CL-387,785 + rapamycin, and 17-DMAG all suppress Akt-mTOR-p70S6K-S6 signaling. CL-387,785 is not sufficient in HCC_Del/T790M cells. In contrast, 17-DMAG depletes EGFR familymem bers and their phosphorylated forms, as well as Akt, phospho-Akt, p70S6K and phospho-p70S6K, phospho-S6, and phospho-4E-BP1, irrespective of the presence of T790M, and is equivalent to the combination of CL-387,785 + rapamycin in the inhibition of upstream and downstream signaling in T790M-expressing cells. L and D, light and dark exposures of the respective blots. C, Ba/F3 cells transformed to IL-3 independence byexpression of EGFR L858R or EGFR L858R/T790M were treated with erlotinib, CL-387,785, 17-DMAG, and 17-AAG at the indicated concentrations in triplicate. Viabilitywas normalized to that of DMSO-treated cells. Points, the average of normalized values from two independent experiments; bars, SD. T790M confers resistance to erlotinib and relative resistance to CL-387,785, whereas sensitivity to Hsp90 inhibitors is maintained. D, EGFR L858R– or EGFR L858R/T790M–expressing Ba/F3 cells were treated with DMSO, 1 µmol/L erlotinib, 1 µmol/L CL-387,785, or 500 nmol/L 17-DMAG for 24 h. Lysates were subjected to Western blotting with the indicated antibodies. Although all three drugs are effective in depletion of phospho-EGFR, phospho-Akt, phospho-p70S6K, phospho-S6, and phospho-4E-BP1 in EGFR L858R-expressing cells, only17-DMAG was effective in the inhibition of upstream and downstream signaling in EGFR L858R/T790M-expressing cells.
These results were confirmed in Ba/F3cell lines transformed to IL-3independence with kinase domain mutant EGFR with or without T790M (26). As shown in Fig. 3C, EGFR L858R-expressing cells were sensitive to erlotinib, CL-387,785, 17-DMAG, and 17-AAG, whereas EGFR L858R/T790M-expressing cells are similarly sensitive to 17-DMAG and 17-AAG, less sensitive to CL-387,785, and resistant to erlotinib. CL-387,785 abrogated signaling in L858R-expressing cells, but far less efficiently in L858R/T790M-expressing cells (Fig. 3D). In contrast, treatment with 17-DMAG suppressed upstream and downstream signaling in T790M-expressing cells, resulting in the absence of phospho-Akt and phospho-S6 expression. The IC50s of CL-387,785 ranged from 6- to 358-fold higher in T790M-expressing cells compared with their matched counterparts, whereas the presence of T790M did not substantially affect the IC50 in response to Hsp90 inhibition (Supplementary Table S3). The Ba/F3 cell models were also useful for assessing the efficacy of CL-387,785 and 17-DMAG against exon 20 insertion kinase domain mutations. These EGFR mutants are found in primary lung cancers, but have not been identified in cell lines. Like mutant EGFRs harboring T790M, they are resistant to erlotinib, variably sensitive to CL-387,785, and are routinely sensitive to Hsp90 Inhibition in T790M Mutant EGFR NSCLC 17-DMAG (Supplementary Fig. S1), adding to the group of exon 20 insertion mutants depleted by Hsp90 inhibition (27).
Hsp90 inhibition also suppresses other kinases activated in T790M-expressing cell lines
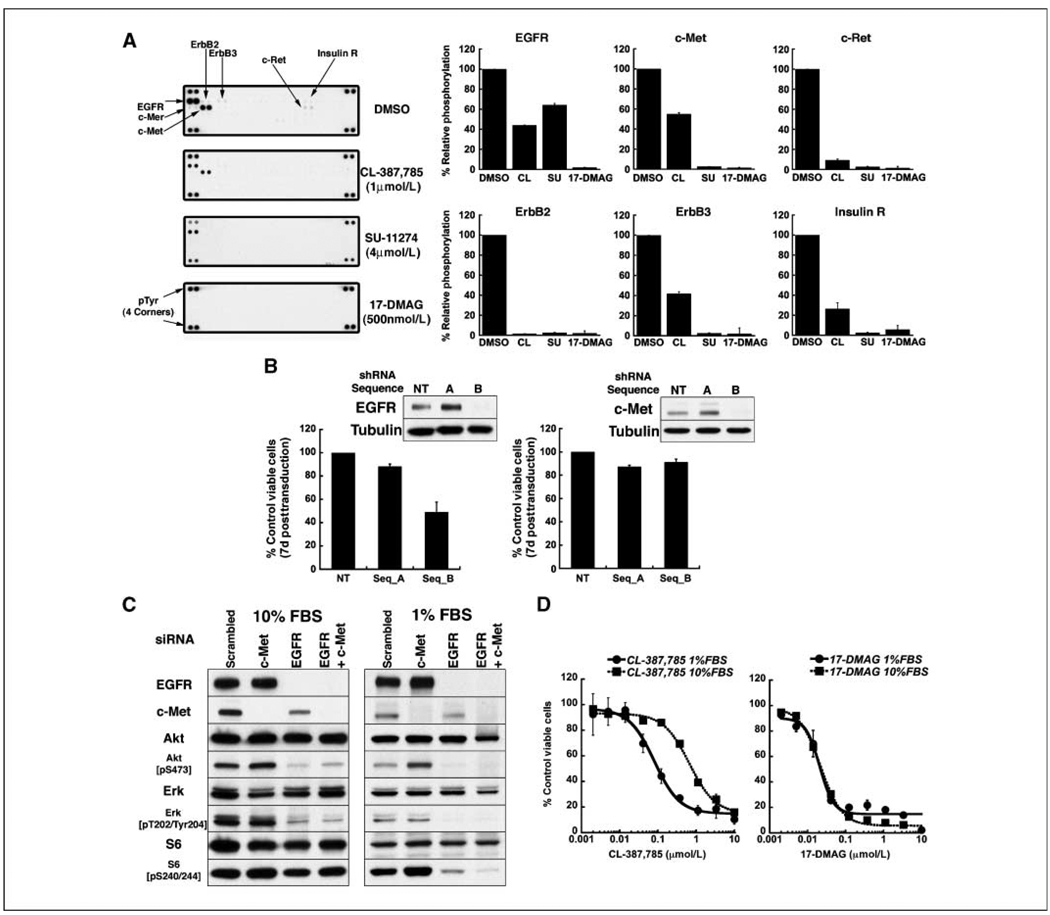
Our results in isogenic systems suggest that the deficiency of CL-387,785 is largely one of potency against mutant EGFR-expressing T790M. Residual phosphorylated mutant EGFR harboring T790M, with its enhanced ability to direct signaling, results in incomplete suppression of Akt phosphorylation, ultimately driving robust phosphorylation of S6 downstream from mTOR. However, the data in Fig. 1 also show the persistence of phospho-Akt and phospho-S6 even at high concentrations of CL-387,785, when the inhibition EGFR phosphorylation is more complete, and raise the possibility that other signaling pathways may be active in T790M-expressing cell lines. To address this possibility, we performed profiling of activated RTKs in NCI-H1975 cells (Figs. 4A; Supplementary Fig. S2). In addition to EGFR, these cells express a high level of activated c-Met. Lower levels of activation of other EGFR family members as well as c-Ret and the insulin receptor were also observed. Densitometric analyses indicate that treatment with CL-387,785 partially reduces c-Met phosphorylation, suggesting that c-Met may be activated by EGFR-mediated transphosphorylation, by other tyrosine kinases activated by EGFR, as well as autophosphorylation. Interestingly, activation of c-Met occurs in other mutant EGFR cell lines as well, both with and without T790M (Supplementary Fig. S3). In fact, signaling in NCI-H1975 cells may be dependent on multiple RTKs. These cells show substantial but incomplete dependence on EGFR, as evidenced by partial loss of viability after shRNA-mediated EGFR depletion (Fig. 4B, left). Depletion of EGFR does not abolish expression of phospho-Akt or phospho-S6 (Fig. 4C, left), similar to results obtained with CL-387,785. The IC50 of CL-387,785 against NCI-H1975 cells is reduced in low serum (Fig. 4D, left), again suggesting that in high serum, signaling pathways in addition to EGFR are functioning. In high serum, shRNA-mediated depletion of c-Met does not compromise viability and the combined siRNA-mediated depletion of EGFR and c-Met does not further reduce expression of phospho-Akt or phospho-S6. However, in low serum, combined depletion of EGFR and c-Met compromises downstream signaling more than EGFR depletion alone (Fig. 4C, right). Taken together, these results suggest that EGFR, c-Met, and other RTKs all contribute to signaling in high serum in NCI-H1975 cells; in low serum, a more pronounced dependence on EGFR and c-Met emerges.
Figure 4.
NCI-H1975 (EGFR L858R/T790M) cells express activated RTKs in addition to mutant EGFR, which contribute to signal transduction and invasiveness and which are depleted by17-DMAG. A, left, lysates from NCI-H1975 cells treated with DMSO or the indicated compounds for 24 h were subjected to RTK profiling, as described in Materials and Methods, demonstrating the activation of multiple receptors in addition to EGFR. In each array, each RTK is assayed in duplicate. Right, densitometric analysis was performed and normalized for background and array lot variations. Relative phosphorylation of receptors in drug-treated cells was compared with that in DMSO-treated cells, the latter defined as 100%. Columns, average of duplicate spots; bars, SD. B, NCI-H1975 cells were transduced with lentiviruses encoding shRNAs targeting EGFR or c-Met. CCK-8 assays were performed 7 d after infection, and viability was normalized to cells infected with a lentivirus encoding a nontargeting shRNA construct. Western blots were performed 5 d posttransduction; in each case, the nontargeting construct and sequence A did not reduce expression of EGFR or c-Met. For shRNA targeting EGFR, columns represent the average of duplicate samples; for shRNA targeting c-Met, columns represent the average of quadruplicate samples. Bars, SD. C, NCI-H1975 cells were transfected with scrambled siRNA, or siRNA targeting EGFR or c-Met either alone or together. Transfected cells were maintained in growth medium with 10% or 1% fetal bovine serum; 48 h posttransfection, lysates were subjected to Western blotting with the indicated antibodies. Depletion of EGFR and c-Met in low serum is required to reduce mTOR signaling. D, NCI-H1975 cells were treated with the indicated concentrations of CL-387,785 or 17-DMAG in medium supplemented with 10% or 1% FBS. At 72 h, CCK-8 assaywas performed and the viability of each sample was normalized to that of DMSO-treated cells. Points, average of normalized values from two independent experiments; bars, SD. The IC50 for CL-387,785 in 10% serum is 667 nmol/L and decreases to 81 nmol/L in 1% serum. The IC50s for 17-DMAG in 1% and 10% serum were 21 and 22 nmol/L, respectively.
Notably, after treatment with 17-DMAG, all RTK activity is effectively reduced (Fig. 4A), suggesting that Hsp90 inhibition abrogates upstream signaling mediated by EGFR as well as other RTKs in T790M-expressing cells. As expected, the IC50 of 17-DMAG is not reduced in low serum (Fig. 4D, right).
Although c-Met depletion alone did not compromise the viability of NCI-H1975 cells, it reduced invasiveness of these cells, measured by a Boyden chamber assay (Supplementary Fig. S4A) also shown for 17-DMAG (Supplementary Fig. S4B).
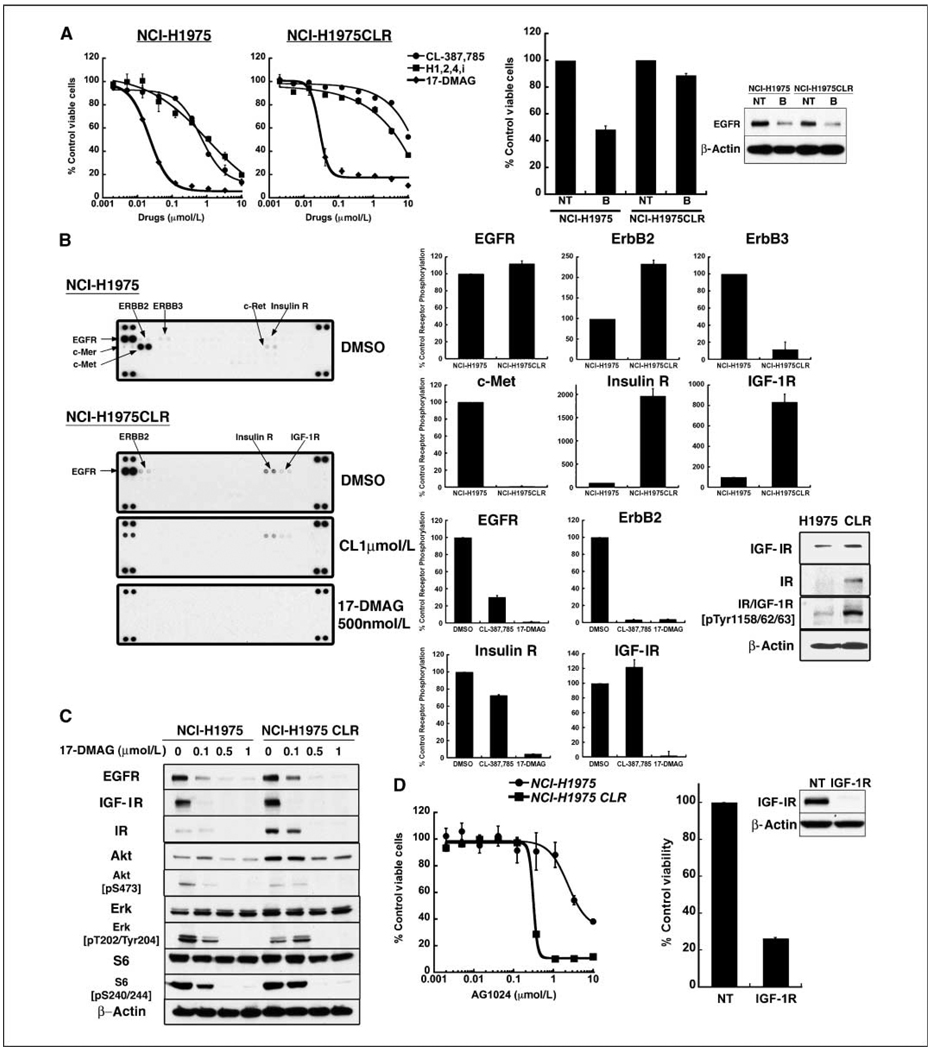
NCI-H1975 cells rendered resistant to CL-387,785 activate insulin receptor and IGF-IR pathways that are sensitive to Hsp90 inhibition
Although signaling in T790M-expressing cell lines is not fully inhibited by CL-387,785, partial suppression of signaling occurs, especially at high concentration. Nonetheless, these cells may be primed to readily develop resistance based on the activation of other RTKs. To test this possibility, we generated an NCI-H1975 cell line with acquired resistance to CL-387,785 after 5 months of passage in the presence of graded drug concentrations, ultimately yielding a cell line (NCI-H1975CLR) with an IC50 of ~ 10 µmol/L, 15-fold higher than the IC50 of parental cells (Fig. 5A, left). These cells were also resistant to an irreversible inhibitor of ErbB1, ErbB2, and ErbB4, and to the CL-387,785/rapamycin combination (data not shown). shRNA-mediated depletion of EGFR showed loss of EGFR dependence (Fig. 5A, right). Profiling and Western blot analyses of these cells revealed reduced activation of c-Met and increased activation of both insulin receptor and IGF-IR that persists in the presence of CL-387,785 (Fig. 5B; Supplementary Fig. S5). NCI-H1975CLR cells were sensitized to AG1024, an inhibitor of the insulin receptor and IGF-IR RTKs, with an IC50 reduced >7-fold compared with the IC50 of parental cells (Fig. 5D, left ), and also had compromised viability in response to shRNA-mediated IGF-IR depletion (Fig. 5D, right ). Importantly, NCI-H1975CLR cells retained sensitivity to 17-DMAG, without change in IC50 compared with parental cells (Fig. 5A), and activated insulin receptor and IGF-IR expression as well as downstream signaling pathways were extinguished by 17-DMAG in parental and CL-387,785-resistant cells with comparable efficiency (Fig. 5B and C). These data suggest that Hsp90 inhibition may have advantages in T790M cells that are both partially sensitive (NCI-H1975) and resistant (NCI-H1975CLR) to irreversible EGFR inhibitors based on the ability to deplete EGFR, other RTKs, as well as downstream signaling molecules.
Figure 5.
NCI-H1975CLR cells are resistant to irreversible EGFR inhibitors but remain sensitive to 17-DMAG. A, left, parental and CL-387,785–resistant (CLR) NCI-H1975 cells were exposed to the indicated concentrations of CL-387,785, another irreversible inhibitor (H1,2,4i) and 17-DMAG for 72 h in triplicate. Viabilityof each sample was normalized to that of DMSO-treated cells. Points, average of normalized values from two independent experiments; bars, SD. In CLR cells, the IC50s for CL-387,785 and H1,2,4i increase from 0.666 to >10 µmol/L, and 1.02 to 3.71 µmol/L, respectively, whereas the IC50 for 17-DMAG is similar in both CLR and parental cells (0.028 and 0.022 µmol/L, respectively). Right, parental and CLR NCI-H1975 cells were transduced with lentiviruses encoding nontargeting shRNA or shRNA-targeting EGFR (sequence B), followed byCCK8 assay 7 d posttransduction. Growth was normalized to that of cells transduced with the nontargeting shRNA construct. Lysates were collected 5 d posttransduction and subjected to Western blotting. CLR cells no longer show EGFR dependence. B, left, parental or CLR NCI-H1975 cells were treated with DMSO, CL-387,785, or 17-DMAG for 24 h and lysates were hybridized to phospho-RTK arrays. Right, densitometric analysis was performed for NCI-H1975 DMSO and NCI-H1975CLR DMSO strips. In the top two rows, relative phosphorylation of receptors in CLR cells was compared with that in parental cells, the latter defined as 100%. In the bottom two rows, similar analysis was performed for NCI-H1975CLR cells treated with DMSO, CL-387,785, or 17-DMAG. Relative phosphorylation of receptors in drug-treated cells was compared with that in DMSO-treated cells, the latter defined as 100%. Columns, average of duplicate spots; bars, SD. Inset, Western blot analysis demonstrating increased IGF-IR, insulin receptor, and their activated forms in NCI-H1975CLR cells compared with parental cells, confirming the results of the phospho-RTK arrays. The phospho-specific antibody recognizes phosphorylated forms of both insulin receptor and IGF-IR. C, NCI-H1975 or NCI-H1975CLR cells were treated with increasing concentrations of 17-DMAG for 24 h. Whole-cell lysates were subjected to Western blotting with indicated antibodies. 17-DMAG treatment suppressed phospho-Akt, mTOR, p70S6K, and S6 signaling equally well in both cell lines. D, left, NCI-H1975 and NCI-H1975CLR cell lines were treated with AG1024 at the indicated concentrations, and viable cells were measured after 72 h. The survival of each sample was normalized to that of DMSO-treated cells. Points, average of normalized values from three independent experiments; bars, SD. The IC50 for AG1024 is 0.314 µmol/L in CLR cells, compared with 2.31 µmol/L in parental cells. Right, NCI-H1975 CLR cells were transduced with a lentivirus encoding shRNA targeting IGF-IR. CCK-8 assays were performed 7 d after infection, and viability was normalized to cells infected with a lentivirus encoding a nontargeting shRNA construct. Columns, average of two experiments, each performed with duplicate samples; bars, SD.
Hsp90 inhibition induces regression of murine lung adenocarcinomas induced by mutant EGFR with and without T790M
To confirm that the activity of Hsp90 inhibition against tumors expressing mutant EGFR in vivo, 17-DMAG was used in the treatment of murine lung adenocarcinomas driven by either EGFR L858R (20) or EGFR L858R-T790M (14). In EGFR L858R mice (Supplementary Fig. S6), substantial regressions occurred over 2 to 4 weeks with thrice per week dosing, measured by both MRI and histology. Immunohisto-chemical staining and Western blotting showed increased expression of Hsp27, consistent with Hsp90 inhibition, and reduced expression of EGFR and cyclin D1 at 1 week. These pharmacodynamic changes were also noted after 3to 4 weeks of treatment as well.
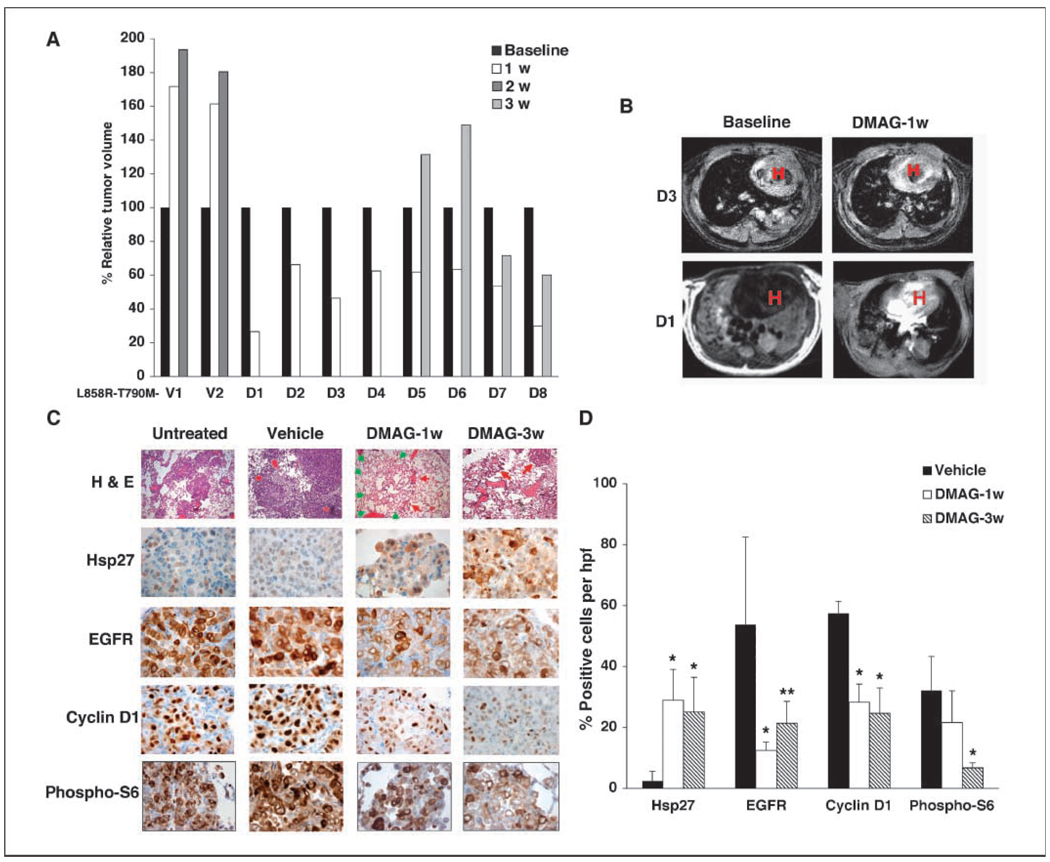
The same schedule of drug administration was not effective against EGFR L858R-T790M–driven tumors (data not shown), but a more intense daily administration of drug produced responses measured by MRI and assessed by histology (Fig. 6A–C). However, in mice treated for longer periods, and analyzed at the 3-week mark, tumors regrew on MRI scan (Fig. 6A) so that responses were transient. Low-power images (Supplementary Fig. S7) showed improvement in both peripheral and bronchial tumors after 1 week, although tumor was not eradicated. At 3week s, some mice showed continued improvement in peripheral tumors, whereas others did not; bronchial tumors more uniformly regrew (Supplementary Fig. S7). In the peripheral compartment, expected modulation of Hsp27, EGFR, cyclin D1, and phospho-S6 occurred (Fig. 6C and D). Although the significance and persistence of changes were less consistent, expected pharmacodynamic trends were also documented in the bronchial compartment (Supplementary Fig. S8).
Figure 6.
17-DMAG induces tumor regression in an EGFR L858R-T790M–driven murine lung adenocarcinoma model. A, L858R-T790M mice were treated with doxycycline for 6 to 10 wk and imaged before treatment (baseline) and after daily treatment with vehicle (V) or 17-DMAG (D) at 10 mg/kg for the indicated times, after which tumor volume was calculated. In all 17-DMAG–treated mice, there was >30% regression at 1 wk, consistent with response. B, representative MRI scans performed at 1 wk, demonstrating substantial reduction in tumor burden in two of the 17-DMAG–treated mice. C, EGFR L858R-T790M tumor-bearing mice that were left untreated, treated with vehicle, or treated with 17-DMAG for 1 or 3 wk were sacrificed for histologyand immunohistochemical analyses. Representative results for the peripheral compartment are shown. H&E staining (×100) shows histologic response. Red arrows, residual tumor. Green arrows, area of alveolar reconstruction, where normal lung architecture appears where tumor has regressed (14, 20). Immunohistochemistry panels are at ×800 magnification. D, quantification of immunohistochemical results for the peripheral compartment. Results show increased expression of Hsp27 and reduced expression of EGFR, cyclin D1, and phospho-S6 induced by17-DM AG. Columns, average of strongly positive (2+) cells scored over four high-power fields, with ~100 to 200 cells counted per high-power field; bars, SD. *, results with significant statistical difference from vehicle (P < 0.04); **, results with P < 0.07 compared with vehicle.
Discussion
Irreversible EGFR inhibitors are under active development to overcome the resistance to reversible TKIs of EGFRs harboring T790M mutation. However, chemically and biologically, these compounds have encountered major challenges. First, the dephosphorylation of EGFR induced by CL-387,785 in isogenic models indicates that the potency of irreversible EGFR inhibition is diminished against compound mutant EGFRs compared with EGFRs harboring kinase domain mutations without T790M. Second, mutant EGFRs harboring T790M direct more robust downstream signaling. The presence of T790M therefore increases the IC50 and compromises the suppression of PI3K-Akt-mTOR signaling in response to irreversible EGFR inhibition.
The importance of persistent mTOR signaling to the survival of cells expressing EGFRs harboring T790M in response to irreversible EGFR inhibitors is evidenced by synergistic cytotoxicity induced by combined EGFR inhibition and rapamycin. Our data suggest that the dephosphorylation of S6 and 4E-BP1 may serve as markers necessary but insufficient for the induction of cell death by upstream signal transduction inhibitors. However, dephosphorylation of these proteins must be coupled with inhibition of PI3K and Akt, because rapamycin does not induce cell death in EGFR mutant cells. Inhibition of TORC1 complexes by rapamycin removes negative feedback on upstream signaling and results in PI3K-Akt activation (28, 29). Therefore, the ideal signal transduction inhibitor in EGFR mutant NSCLC cells may be one that potently inhibits the mTOR pathway without inducing PI3K activation. This can be achieved with simultaneous targeting of TORC1 and TORC2 complexes (as expected upon mTOR-targeted shRNA expression shown in Fig. 1B), by EGFR inhibition alone in EGFR mutant NSCLC cells without T790M, or by the simultaneous exposure to an EGFR inhibitor and a TORC1 inhibitor such as rapamycin in T790M-expressing cells.
This requirement is also fulfilled by Hsp90 inhibition. In both NSCLC cell lines and in Ba/F3cells, 17-DMAG was able to inhibit both upstream (EGFR-PI3K-Akt) and downstream (mTORp70S6K-S6) signaling whether or not T790M was present. Mutant EGFR is a sensitive Hsp90 client, efficiently depleted to a degree similar to that of ErbB2 (30). Additional T790M mutation does not compromise interaction with the chaperone; in our previous work, the association of mutant EGFR with Hsp90 was most evident in NCI-H1975 cells compared with non–T790M-expressing cells (15). Disruption of the Hsp90-Akt interaction leads to an increase in protein phosphatase 2A–mediated dephosphorylation of Akt (31) as well as to its destabilization (18). Therefore, the depletion of phospho-Akt in response to 17-DMAG is related to both depletion of upstream mutant EGFR as well as to direct effects on Akt stability and phosphorylation. In contrast, Erk1/2 proteins are not Hsp90 clients and reduced expression of phospho-Erk in response to 17-DMAG is related entirely to reduced upstream signaling (32).
Similarly, reduced mTOR-mediated signaling in response to 17-DMAG may be secondary to depletion of mutant EGFR. It is also possible that components of the mTOR pathway are Hsp90 clients. The stability of raptor and p70S6K is slightly compromised in response to geldanamycins. In HEK293cells, ectopically expressed epitope-tagged versions of raptor and Hsp90 were readily coimmunoprecipitated (19). In these transfected cells, geldanamycin disrupted the interaction of Hsp90 and raptor without affecting the ability of raptor to bind to mTOR. Nonetheless, the kinase activity of mTOR was suppressed, suggesting that Hsp90 facilitates phosphorylation events catalyzed by mTOR through its association with raptor.
Although irreversible inhibitors may have reduced potency against EGFRs harboring T790M, lack of full suppression of the PI3K-Akt-mTOR signaling axis may also be related to the activation of other RTKs in T790M-expressing cells. Several lines of evidence suggest this possibility in NCI-H1975 cells. RTK profiling shows the presence of multiple activated kinases above background, particularly true for c-Met. Although these receptors show dephosphorylation after treatment with CL-387,785, we have been unable to completely compromise the viability of NCI-H1975 cells with shRNA targeting EGFR. Additionally, the IC50 of CL-387,785 in NCI-H1975 cells is lowered ~ 10-fold under low serum conditions. Similarly, reduced mTOR signaling in these cells requires the depletion of EGFR and c-Met, as well as their maintenance in low serum, suggesting that although EGFR may be the dominant RTK, c-Met as well as other tyrosine kinases may contribute to the activation of downstream signaling pathways. Notably, the activation of all RTKs is compromised after 17-DMAG treatment, suggesting that Hsp90 inhibition may be useful against lung cancer cells not fully dependent on signaling by a single receptor.
Recently, examples of TKI resistance have been described that are mediated by the activation of RTKs not directly targeted by drug exposure. For example, amplification of c-Met accounts for ~ 20% of cases of acquired anilinoquinazoline resistance in EGFR-mutant lung cancer cells (33). Among such tumors, concomitant T790M mutation and c-Met amplification has been described, suggesting that T790M-expressing cells may depend on tyrosine kinases other than EGFR, as we are proposing for NCI-H1975 cells (33, 34). EGFR mutant cells that develop codependency on c-Met are also expected to respond to Hsp90 inhibition. Our data also show that covalent binding of an irreversible inhibitor to the EGFR L858R-T790M receptor does not prevent the emergence of resistance and provide another example by which resistance occurs via activation of alternative tyrosine kinases. Although further genomic analysis of NCI-H1975CLR cells is required for their full characterization, these cells lose EGFR dependence, with reduction of activated c-Met and ErbB3. The latter is consistent with the loss of EGFR dependence because EGFR-driven tumors typically use ErbB3 to activate PI3K (35). The resistant cells show an increase in activated insulin receptor and IGF-IR that persists after treatment with CL-387,785, and a decrease in IC50 in response to the insulin receptor/IGF-IR inhibitor AG1024, similar to the kinase switch recently described in imatinib-resistant gastrointestinal tumors, during which there is down-regulation of c-kit expression and up-regulation of AXL (36). The presence of activated insulin receptor in parental cells may have presented a simple path to resistance. In addition, activated IGF-IR has also been associated with resistance to EGFR inhibition in EGFR wild-type cells (37). Importantly, NCI-H1975CLR cells retain sensitivity to 17-DMAG, without increase in IC50, and with depletion of total and activated insulin receptor/IGF-IR (38). Therefore, Hsp90 inhibition may have activity against cells resistant to both reversible and irreversible EGFR inhibitors.
Thus far, treatment responses in animal models of EGFR-mutant lung adenocarcinoma have been representative of drug responses in humans. Murine adenocarcinomas driven by EGFR L858R-T790M are resistant to erlotinib and only slightly sensitive to HKI-272 (14), reflecting emerging clinical data indicating variable durations of stable disease but no responses among NSCLC patients previously treated with gefitinib or erlotinib (39). Regressions induced by 17-DMAG therefore suggest potential efficacy of Hsp90 inhibition against EGFR mutant/T790M NSCLC. Additionally, 17-AAG has produced tumor growth inhibition in NCIH1975 xenografts (40). Of note, despite the relative equivalence of 17-DMAG against NSCLC cell lines regardless of the presence of T790M, we have not shown equivalence against the EGFR L858R and L858R-T790M in vivo models, because a more intense treatment schedule was required in the T790M-expressing model to achieve response. Nonetheless, substantial regressions were noted, along with expected pharmacodynamic effects including induction of Hsp27, a signature of Hsp90 inhibition (41), and depletion of mutant EGFR. Importantly, downstream signaling, assessed by expression of phospho-S6, was reduced in residual tumors in the EGFR L858R-T790M model. The results with 17-DMAG are comparable with those recently shown with 17-AAG in similar murine models (42), where responses occurred, but were also incomplete and transient.
The murine models used in the work provide a platform for the comparison of drug treatments. For example, potent nongeldanamycin Hsp90 inhibitors are under development (43), and it will be of interest to determine whether these agents provide more complete or sustained responses. Similarly, a historical comparison of mice bearing EGFR L858R-T790M–expressing tumors treated with 17-DMAG to those treated with HKI-272 and rapamycin indicates that the latter combination may be superior. Among 3mice treated with HKI-272 and rapamycin, the relative tumor burden was promptly reduced and sustained over 4 weeks, ultimately with 23%, 12%, and 50% of the baseline tumor volume remaining, respectively (P = 0.04, compared with the maximal regression at 1 week documented by 17-DMAG among 8 mice).
Nonetheless, daily administration of 17-DMAG in mice did not cause weight loss or gastrointestinal side effects. Similarly, earlyphase human studies of geldanamycins have shown good tolerability (44). Hsp90 inhibitors have shown promise in ErbB2 (Her2)-amplified breast cancer, c-kit–dependent gastrointestinal tumors, and hematologic malignancies (45). Our data suggest that EGFR-mutant NSCLC represents another tumor type for which Hsp90 inhibition should be evaluated to overcome acquired reversible or irreversible EGFR TKI resistance, adding to the armamentarium in development for this population.
Supplementary Material
Acknowledgments
Grant support: Dana-Farber/Harvard Cancer Center Specialized Program of Research Excellence (SPORE) in Lung Cancer; NIH grant P50 CA090578 (D.G. Tenen and G.I. Shapiro); a Career Development Award as part of the SPORE (T. Shimamura); NIH grants R01 CA090687 (G.I. Shapiro), K08 AG024004 (K-K. Wong), R01 AG2400401 (K-K. Wong), R01 CA122794 (K-K. Wong), R01 CA116010 (M. Meyerson), and K99 CA126026 (S. Kobayashi); as well as a grant from the Flight Attendant Medical Research Institute (K-K. Wong).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest.
T. Shimamura, D. Li, C.L. Borgman, K.K. Wong, and G.I. Shapiro: Commercial research grant, Synta Pharmaceuticals. M. Meyerson: Commercial research grant, Novartis; ownership interest, Genzyme.
References
- 1.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 2.Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein IB. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 4.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Conde E, Angulo B, Tang M, et al. Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res. 2006;12:710–717. doi: 10.1158/1078-0432.CCR-05-1362. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 8.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:1–11. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulloy R, Ferrand A, Kim Y, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 10.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Ji H, Yuza Y, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–7101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 12.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–6408. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- 16.da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005;65:10686–10691. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- 17.Grbovic OM, Basso AD, Sawai A, et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 19.Ohji G, Hidayat S, Nakashima A, et al. Suppression of the mTOR-raptor signaling pathway by the inhibitor of heat shock protein 90 geldanamycin. J Biochem (Tokyo) 2006;139:129–135. doi: 10.1093/jb/mvj008. [DOI] [PubMed] [Google Scholar]
- 20.Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Ji H, Zaghlul S, et al. Therapeutic anti-EGFR antibody 806 generates responses in murine de novo EGFR mutant-dependent lung carcinomas. J Clin Invest. 2007;117:346–352. doi: 10.1172/JCI30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi S, Shimamura T, Monti S, et al. Transcriptional profiling identifies cyclin D1 as a critical downstream effector of mutant epidermal growth factor receptor signaling. Cancer Res. 2006;66:11389–11398. doi: 10.1158/0008-5472.CAN-06-2318. [DOI] [PubMed] [Google Scholar]
- 23.Discafani CM, Carroll ML, Floyd MB, Jr, et al. Irreversible inhibition of epidermal growth factor receptor tyrosine kinase with in vivo activity by N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide (CL-387,785) Biochem Pharmacol. 1999;57:917–925. doi: 10.1016/s0006-2952(98)00356-6. [DOI] [PubMed] [Google Scholar]
- 24.Tsou HR, Overbeek-Klumpers EG, Hallett WA, et al. Optimization of 6,7-disubstituted-4-(arylamino)quino-line-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 25.Tian ZQ, Liu Y, Zhang D, et al. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg Med Chem. 2004;12:5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 26.Yuza Y, Glatt KA, Jiang J, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther. 2007;6:661–667. doi: 10.4161/cbt.6.5.4003. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Soga S, Beebe K, et al. Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer. 2007;97:741–744. doi: 10.1038/sj.bjc.6603950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 29.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Mimnaugh E, Rosser MF, et al. Sensitivity of mature ErbB2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003–4009. [PubMed] [Google Scholar]
- 33.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 34.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelman JA, Jänne PA, Mermel C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadevan D, Cooke L, Riley C, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 37.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen TO, Andrews HN, Cheang M, et al. Expression of the insulin-like growth factor I receptor and urokinase plasminogen activator in breast cancer is associated with poor survival: potential for intervention with 17-allylamino geldanamycin. Cancer Res. 2004;64:286–291. doi: 10.1158/0008-5472.can-03-1242. [DOI] [PubMed] [Google Scholar]
- 39.Wong KK, Fracasso PM, Bukowski RM, et al. HKI-272, an irreversible pan erbB receptor tyrosine kinase inhibitor: preliminary phase I results in patients with solid tumors [abstract] Proc Am Soc Clin Oncol. 2006;24:A3018. [Google Scholar]
- 40.Yang S, Qu S, Perez-Tores M, et al. Association with HSP90 inhibits Cbl-mediated down-regulation of mutant epidermal growth factor receptors. Cancer Res. 2006;66:6990–6997. doi: 10.1158/0008-5472.CAN-06-1042. [DOI] [PubMed] [Google Scholar]
- 41.Maloney A, Clarke PA, Naaby-Hansen S, et al. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2007;67:3239–3253. doi: 10.1158/0008-5472.CAN-06-2968. [DOI] [PubMed] [Google Scholar]
- 42.Regales L, Balak MN, Gong Y, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS ONE. 2007;2:e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res. 2006;95:323–348. doi: 10.1016/S0065-230X(06)95009-X. [DOI] [PubMed] [Google Scholar]
- 44.Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 45.Pacey S, Banerji U, Judson I, Workman P. Hsp90 inhibitors in the clinic. Handb Exp Pharmacol. 2006:331–358. doi: 10.1007/3-540-29717-0_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.