Abstract
The trimethoprim-resistant dihydrofolate reductase associated with the R plasmid R388 was isolated from strains that over-produce the enzyme. It was purified to apparent homogeneity by affinity chromatography and two consecutive gel filtration steps under native and denaturing conditions. The purified enzyme is composed of four identical subunits with molecular weights of 8300. A 1100 bp long DNA segment which confers resistance to trimethoprim was sequenced. The structural gene was identified on the plasmid DNA by comparing the amino acid composition of the deduced proteins with that of the purified enzyme. The gene is 234 bp long and codes for 78 amino acids. No homology can be found between the deduced amino acid sequence of the R388 dihydrofolate reductase and those of other prokaryotic or eukaryotic dihydrofolate reductases. However, it differs in only 17 positions from the enzyme associated with the trimethoprim-resistance plasmid R67.
Full text
PDF



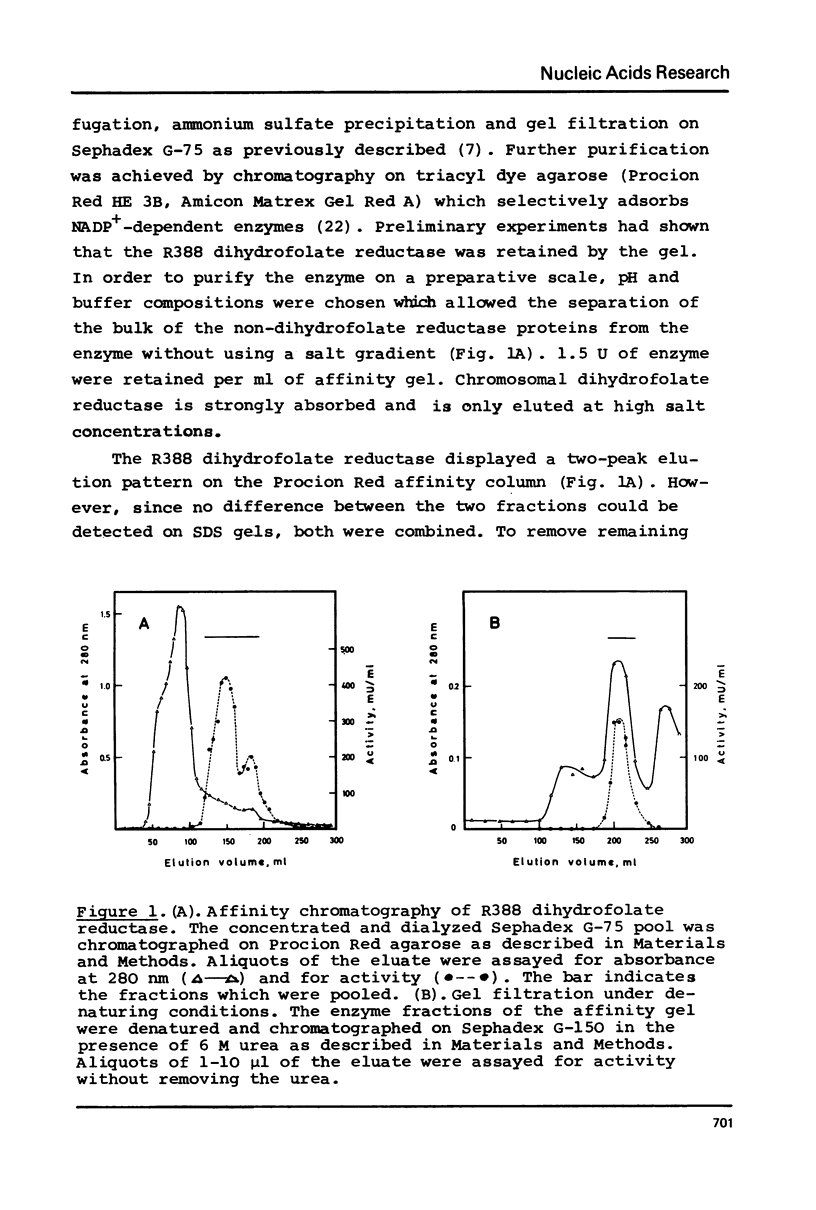
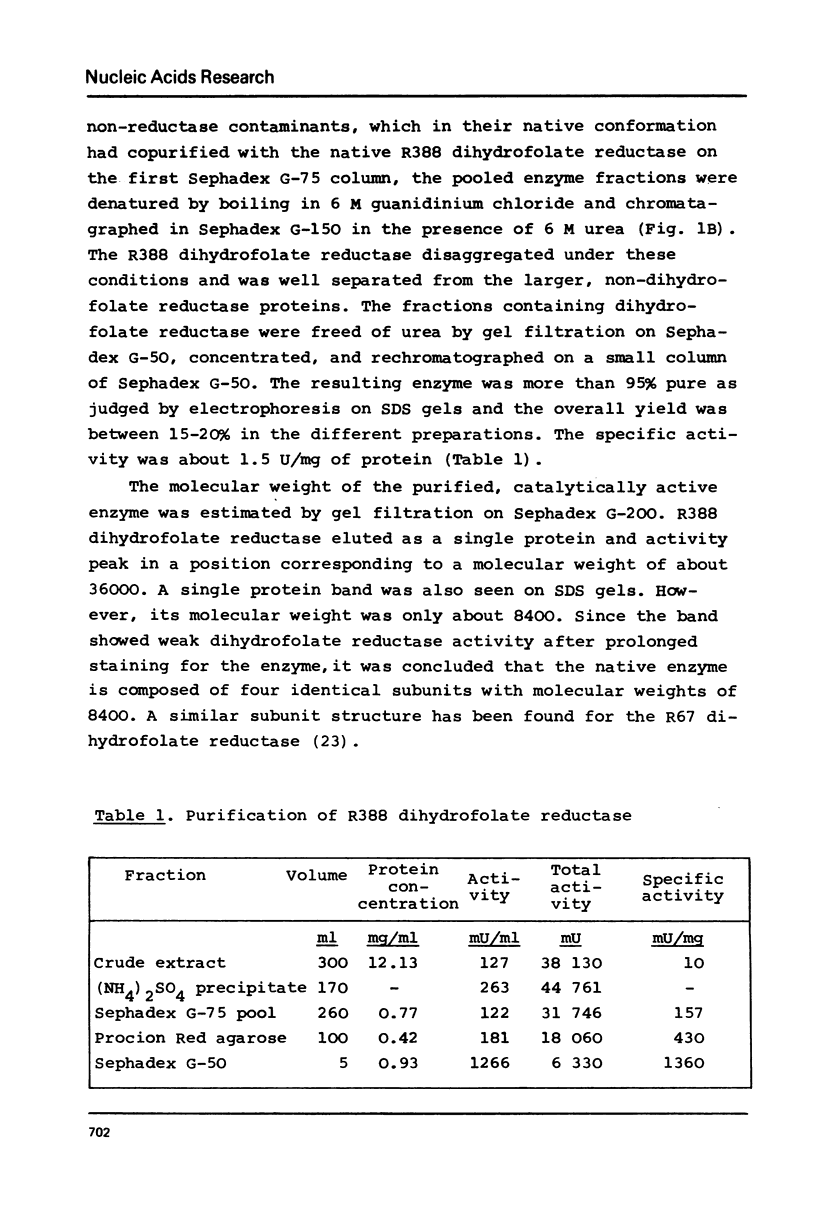
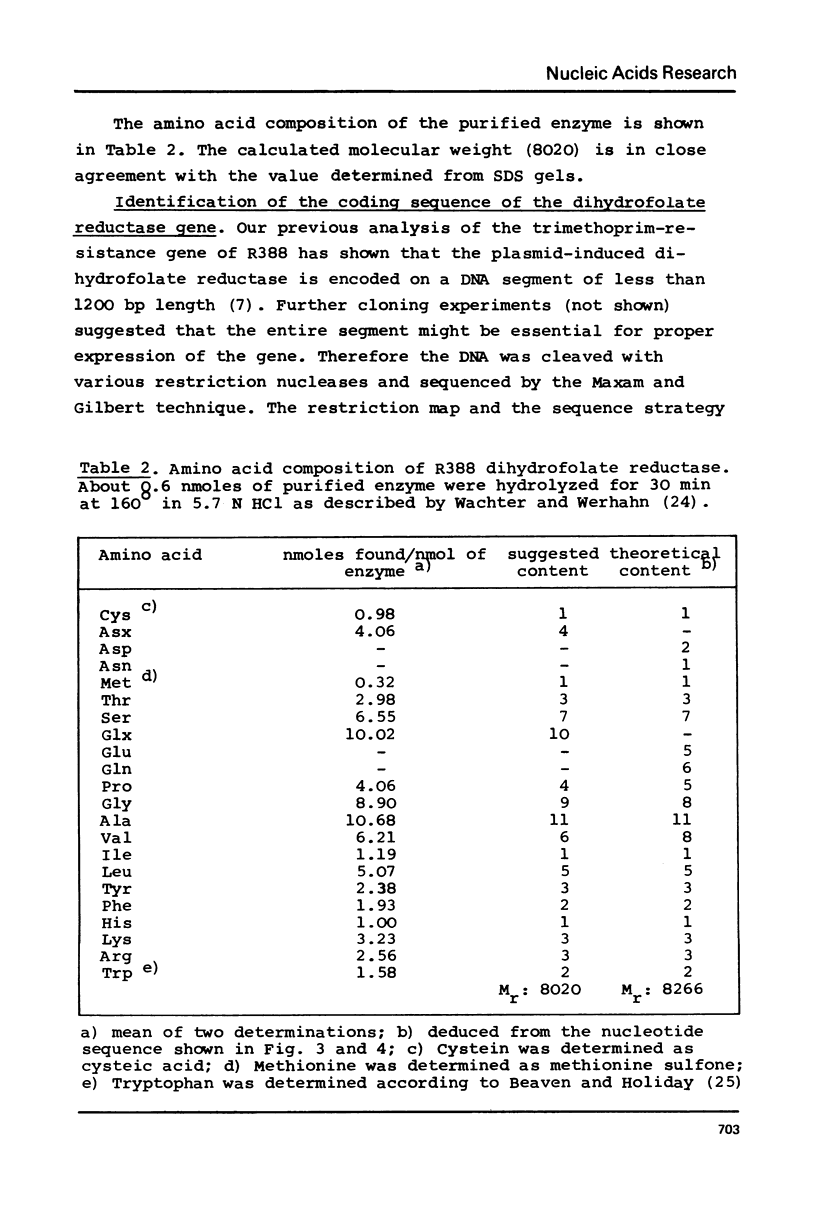







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Smith J. T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974 May 20;58(2):412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Map of plasmid RP4 derived by insertion of transposon C. J Mol Biol. 1977 Jul 5;113(3):455–474. doi: 10.1016/0022-2836(77)90233-9. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Fling M. E., Elwell L. P. Protein expression in Escherichia coli minicells containing recombinant plasmids specifying trimethoprim-resistant dihydrofolate reductases. J Bacteriol. 1980 Feb;141(2):779–785. doi: 10.1128/jb.141.2.779-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi U. J., Littlefield J. W. Isolation and characterization of the multiple forms of dihydrofolate reductase from methotrexate-resistant hamster cells. J Biol Chem. 1974 Mar 10;249(5):1390–1397. [PubMed] [Google Scholar]
- Kumar A. A., Blankenship D. T., Kaufman B. T., Freisheim J. H. Primary structure of chicken liver dihydrofolate reductase. Biochemistry. 1980 Feb 19;19(4):667–678. doi: 10.1021/bi00545a010. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Pech M., Streeck R. E., Zachau H. G. Patchwork structure of a bovine satellite DNA. Cell. 1979 Nov;18(3):883–893. doi: 10.1016/0092-8674(79)90140-5. [DOI] [PubMed] [Google Scholar]
- Peter G., Hänggi U. J., Zachau H. G. Localization of the dihydrofolate reductase gene on the physical map of bacteriophage T5. Mol Gen Genet. 1979 Oct 1;175(3):333–341. doi: 10.1007/BF00397233. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. A., Kalghatg K. K., Subba Rao P. V., Appaji Rao N. Isolation & properties of dihydrofolate reductase from Rhizoctonia solani. Indian J Biochem Biophys. 1976 Jun;13(2):101–105. [PubMed] [Google Scholar]
- Reddy V. A., Rao N. A. Partial purification and some properties of dihydrofolate reductase from soybean seedlings. Indian J Biochem Biophys. 1975 Jun;12(2):75–80. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Scholz K., Jaenicke L. Regelung der Synthese von Dihydrofolsäure-Reduktase bei synchronisierten Hefezellen. Eur J Biochem. 1968 May;4(4):448–457. doi: 10.1111/j.1432-1033.1968.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Smith S. L., Stone D., Novak P., Baccanari D. P., Burchall J. J. R plasmid dihydrofolate reductase with subunit structure. J Biol Chem. 1979 Jul 25;254(14):6222–6225. [PubMed] [Google Scholar]
- Stone D., Smith S. L. The amino acid sequence of the trimethoprim-resistant dihydrofolate reductase specified in Escherichia coli by R-plasmid R67. J Biol Chem. 1979 Nov 10;254(21):10857–10861. [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. III. Nucleotide sequence preceding the RNA start-site on a promoter-containing fragment. Nucleic Acids Res. 1975 Nov;2(11):2091–2100. doi: 10.1093/nar/2.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter E., Werhahn R. Attachment of tryptophanyl peptides to 3-aminopropyl-glass suited for subsequent solid-phase Edman degradation. Anal Biochem. 1979 Aug;97(1):56–64. doi: 10.1016/0003-2697(79)90327-0. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Grinsted J. Mapping of functions in the R-plasmid R388 by examination of deletion mutants generated in vitro. Gene. 1978 Apr;3(2):87–95. doi: 10.1016/0378-1119(78)90053-7. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Harvey M. J., Dean P. D. The selective retardation of NADP+-dependent dehydrogenases by immobilized procion red HE-3B. Biochem J. 1978 Aug 1;173(2):591–596. doi: 10.1042/bj1730591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolg J. W., Hänggi U. J., Zachau H. G. Isolation of a small DNA fragment carrying the gene for a dihydrofolate reductase from a trimethoprim resistance factor. Mol Gen Genet. 1978 Aug 4;164(1):15–29. doi: 10.1007/BF00267594. [DOI] [PubMed] [Google Scholar]



