Abstract
Synaptic pathology and mitochondrial oxidative damage are early events in Alzheimer’s disease (AD) progression. Loss of synapses and synaptic damage are the best correlate of cognitive deficits found in AD patients. Recent research on amyloid bet (Aβ) and mitochondria in AD revealed that Aβ accumulates in synapses and synaptic mitochondria, leading to abnormal mitochondrial dynamics and synaptic degeneration in AD neurons. Further, recent studies using live-cell imaging and primary neurons from amyloid beta precursor protein (AβPP) transgenic mice revealed that reduced mitochondrial mass, defective axonal transport of mitochondria and synaptic degeneration, indicating that Aβ is responsible for mitochondrial and synaptic deficiencies. Tremendous progress has been made in studying antioxidant approaches in mouse models of AD and clinical trials of AD patients. This article highlights the recent developments made in Aβ-induced abnormal mitochondrial dynamics, defective mitochondrial biogenesis, impaired axonal transport and synaptic deficiencies in AD. This article also focuses on mitochondrial approaches in treating AD, and also discusses latest research on mitochondria-targeted antioxidants in AD.
1. Introduction
Increasing evidence suggests that mitochondria play a large role in aging and several age-related diseases, including, cancer, diabetes, cardiovascular, neurodegenerative diseases, and hereditary mitochondrial diseases [1-12]. Germline mutations in mitochondrial DNA (mtDNA) are involved in causing hereditary mitochondrial diseases, including Leigh syndrome, Parkinsonism, and Wilson disease [3]. Age-dependent accumulation of somatic mtDNA changes are involved in disease progression of neurodegenerative diseases, including Alzheimer’s (AD), Parkinson’s (PD), amyotrophic lateral sclerosis (ALS), Huntington’s (HD) [2]. It is interesting to note that age-dependent accumulation of somatic mtDNA changes are neuronal-specific for each of these degenerative diseases [13]. Dysfunction of mitochondria is linked to increased levels of reactive oxygen species (ROS) production, abnormal intracellular calcium levels and reduced mitochondrial ATP. More recent research on mitochondrial structure in tissues of brains from AD, PD and HD revealed that imbalanced mitochondrial dynamics (increased mitochondrial fission and decreased fusion) may be the primary cause of mitochondrial dysfunction and neuronal damage [14].
Tremendous progress has been made in mitochondrial therapeutics in AD mouse models and clinical trials of AD patients. Further, recently, several mitochondria-targeted molecules have been developed and currently being tested in cell and mouse models of neurodegenerative diseases. The purpose of this article is to summarize latest developments in mitochondrial research with a particular focus on AD. This article also discusses how mitochondria-targeted molecules protect mitochondria against Aβ-induced toxicity, and increase neuronal survival in neurons affected by AD.
2. Mitochondrial structure, function and physiology
Mitochondria are cytoplasmic organelles that are essential for the life and death. Mitochondria arise from a symbiotic association between glycolytic protoeukaryotic cells and oxidative bacteria 1.5 billion years ago [15]. Mitochondria change their shape rapidly according to the requirement of cell structure and function. Several features of mitochondria that reflect their endosymbiotic origin are their double-membrane structure and their circular genome with mitochondria specific transcription, translation and protein assembly systems [16]. Mitochondria reduced their genome size to 16.5 kb DNA and adopt to their new cellular environment, and the reduction of their genome probably increases their replication. The half-life of neuronal mitochondria is about one month, and half-life varies with tissue type in mammalians [4]. However, the decay of old mitochondria and the synthesis of new mitochondria are active in all mammalian cells, including neurons. Mitochondrial function is well maintained in cells because of continuous mitochondrial recycling [17].
Mitochondria are heteroplasmy in general, meaning both healthy and defective mitochondria co-exist in cells [3,15]. Mitochondrial dynamics is well maintained in healthy cells, in other words - mitochondrial division and fusion are equal and balance equally. Mitochondrial dynamics is essential for cell survival. However, in cells from a disease state and/or cells exposed to toxins and other oxidative insults, the dynamics of mitochondria are imbalanced, resulting structural and functional abnormalities leading to cell death.
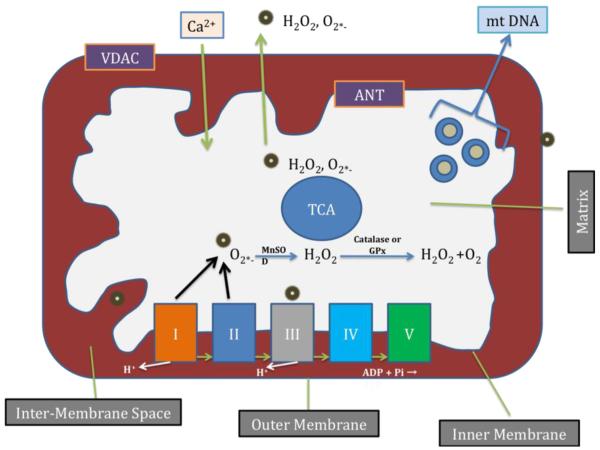
Mitochondria are compartmentalized into 2 lipid membranes: the outer and inner mitochondrial membranes. The outer membrane is highly porous and allows the passage of low molecular-weight substances between the cytosol and the inter-membrane space of mitochondria (Fig. 1) [16]. However, the inner membrane provides a highly efficient barrier to ionic flow, houses the electron transport chain (ETC) (Fig. 2) and covers the mitochondrial matrix. The mitochondrial matrix contains tricarboxylic acid (TCA) and beta-oxidation. Recent studies revealed that several proteins, including Aβ (4kDa), a 99 amino acid residues of c-terminal fragment of AβPP found in matrix [18-20]. Therefore the concept ‘inner membrane does not allow big proteins to matrix’ may not always be true, particularly in mitochondria from disease state neurons.
Figure 1.
Mitochondrial structure and sites of free radical generation. Mitochondria are bag like structures compartmentalized with two lipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane. The inner mitochondrial membrane houses the mitochondrial respiratory chain and provides a highly efficient barrier to ionic flow. The inner mitochondrial membrane houses respiratory chain or electron transport chain (ETC). In the ETC, complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. Superoxide radicals are dismutated by manganese superoxide dismuase and produce H2O2. In addition, ETC involves H2O2 reducing to H2O and O2 by catalase or glutathione peroxidase accepting electrons donated by NADH and FADH2 and then yielding energy to generate ATP from adenosine diphosphate and inorganic phosphate. Free radicals are also generated by tricarboxylic acid in the matrix. These radicals are carried to the cytoplasm via voltage-dependent anion channels, and may involve oxidation DNA and proteins in the cytoplasm.
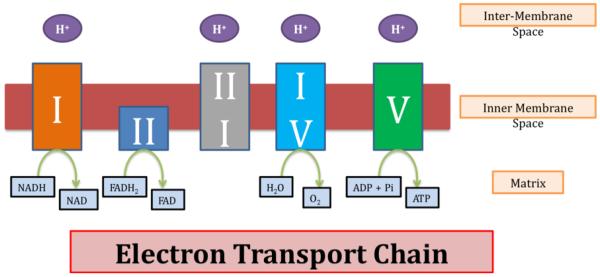
Figure 2.
The structure of electron transport of chain.
Mitochondria are controlled by both nuclear and mitochondrial genomes. MtDNA consists of a 16,571 base pair, double-stranded, circular DNA molecule [21]. The mtDNA copy number, and the number of mitochondria per cell are dependent on cell type, and ATP demand in the cell. For example, the number of mitochondrial DNA in fertilized human oocytes is about 250, 000 - while for unfertilized oocytes, the mean mitochondrial DNA number is 164,000 [22]. mtDNA contains 13 polypeptide genes that encode essential components of the ETC. mtDNA also encodes the 12S and 16S rRNA genes and the 22 tRNA genes required for mitochondrial protein synthesis. Nuclear genes encode the remaining mitochondrial proteins (approximately about over 1000 proteins), metabolic enzymes, DNA and RNA polymerases, ribosomal proteins, and mtDNA regulatory factors, such as mitochondrial transcription factor A [23]. Nuclear mitochondrial proteins are synthesized in the cytoplasm and are subsequently transported into mitochondria. The cross talk between nuclear and mitochondrial-encoded proteins is an essential process to complete oxidative phosphorylation (OXPHOS) in cells.
Mitochondria are transmitted maternally, but in rare situations, they can be transmitted paternally. They perform several cellular functions, including: the regulation of intracellular calcium, ATP production, the release of proteins that activate the caspase family of proteases, alteration of the reduction-oxidation potential of cells, and free-radical scavenging [15]. Mitochondrial ATP is generated via OXPHOS within the inner mitochondrial membrane. As shown in Fig. 1, free radicals are generated as a byproduct of OXPHOS. In the respiratory chain, complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. The superoxide radicals are dismutated by manganese superoxide dismutase, generating H2O2 and oxygen. But H2O2 is converted to H2O by antioxidants, catalase or glutathione peroxidase. The unconverted H2O2 and other radicals and superoxide radicals are carried to the cytoplasm via voltage-dependent anion channels and participate in lipid peroxidation, and protein and DNA oxidation (Fig. 1).
The presence of sufficient quantities of antioxidant enzymes in the mitochondria, scavenge free radicals and protect cells against the toxicity of oxidants. However, cells that produce more oxidants, particularly pyramidal neurons in cortex and hippocampus in AD brain – are likely to be damaged because of presence of insufficient levels of antioxidant enzymes. Thus produce oxidative stress (imbalance between oxidants and antioxidant enzymes) in neurons from AD brain.
3. Mitochondrial Defects in Alzheimer’s Disease
Alzheimer’s disease (AD) is the 6th leading cause of deaths in US and devastating mental illness in elderly population. AD is a late-onset, progressive, age-dependent neurodegenerative disease, characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality [4,24,25]. AD is also associated with the loss of synapses, synaptic function, mitochondrial structural and functional abnormalities, inflammatory responses, and neuronal loss in addition to extracellular neuritic plaques and intracellular neurofibrillary tangles. Several factors, including lifestyle, diet, environmental exposure, Apolipoprotein allele E4, and several other genetic variants reported to involve in late-onset AD.
Oxidative stress and mitochondrial dysfunction have been extensively reported in AD postmortem brains [26-32], in platelets from AD patients [33], in AD transgenic mice [19,20,30,34-43], and in cell lines that express mutant APP [35,44,45], mammalian cells that treated with Aβ [46,47] and primary neurons from AD transgenic mice [48-50]. Multiple lines of evidence suggest that mitochondrial defects play a key role in AD pathogenesis:
3. 1. Defective Glucose Metabolism in AD brains
Several positive emission tomography scan studies revealed that reduced glucose metabolism in the brains of AD patients, indicating that defective glucose utilization in AD [51,52]. Further, ApoE4 genotype is positively correlated with defective glucose utilization in the brains from AD patients.
3. 2. Reduced Mitochondrial Enzyme Activities in AD
Several biochemical studies found decreased levels of cytochrome oxidase activity, pyruvate dehydrogenase, and α-ketodehydrogenase in fibroblasts, lymphoblasts, and postmortem brains from AD patients, compared to neurons, fibroblasts, and lymphoblasts from age-matched healthy subjects [4].
3.3. Mitochondrial DNA Defects in AD
Increased mtDNA changes were found in postmortem brains from AD patients and aged-matched control subjects, compared to mtDNA changes in postmortem brain tissue from young, healthy subjects, suggesting that the accumulation of mtDNA in AD pathogenesis is age-related [53,54].
Recently, Coskun and colleagues [55] investigated whether the mtDNA copy number was related to disease progression in AD. Using molecular methods, they investigated the mtDNA copy number in DNA from patients with AD and Downs syndrome. They found increased mtDNA changes and decreased mtDNA copy number in postmortem brains from AD and Downs syndrome patients. Further, in the brain tissues from aged control subjects who did not have AD, the researchers found that mutations in the control region of mtDNA increased; and in patients with Downs syndrome, mutations in the control region of mtDNA were associated with a reduced mtDNA copy number and L-strand transcripts. The increase in mtDNA mutations was also seen in peripheral blood DNA and in lymphoblastoid cell DNAs of AD and Downs syndrome patients. In aging, Down syndrome, and Down syndrome AD, mtDNA mutations positively correlated with β-secretase activity, and the copy number of mtDNA was inversely correlated with the levels of Aβ40 and Aβ42. Therefore, mtDNA mutations may be responsible for neuropathological changes observed in AD and Down syndrome AD [55].
Lakatos et al [56] investigated mitochondrial DNA variations (haplotypes) in 138 mitochondrial polymorphisms in 358 subjects in the Caucasian Alzheimer’s Disease Neuroimaging Initiative subjects. They found that the mitochondrial ‘haplogroup UK’ may confer genetic susceptibility to AD independently of the ApoE4 allele.
3.4. Abnormal Mitochondrial Gene Expression
Multiple studies investigated mitochondrial gene expressions in postmortem AD brains and in brain specimens from AD transgenic mice [34,57,58]. These studies found mitochondrial-encoded genes abnormally expressed in the brains AD patients and AD mice. Further, a recent, time-course global gene expression study in Tg2576 mice and age-matched non-transgenic littermates revealed an up-regulation of mitochondrial-encoded genes in 2-, 5- and 18-month-old Tg2576 mice, suggesting that mitochondrial metabolism is impaired by mutant APP and Aβ and that the up-regulation of mitochondrial genes may be a compensatory response to mitochondrial dysfunction caused mutant APP and Aβ [34]. Further, findings from this gene expression study also suggest that mitochondrial impairment is an early event in disease progression of AD. Further, Manczak et al. [31] also investigated mitochondrial-encoded genes using quantitative real-time RT-PCR in different grades of AD postmortem brains and non-demented control subjects. They found that abnormal expression of mitochondrial-encoded genes in postmortem AD brains compared to the brains of non-demented, healthy subjects [31], suggesting that impaired mitochondrial metabolism is a characteristic feature of AD patients.
The findings from these studies suggest that age-dependent production of APP and Aβ may cause mitochondrial dysfunction and mitochondrial-encoded genes were abnormally expressed to compensate the loss of mitochondria function.
3.5. Mitochondrial Dysfunction and Oxidative Stress in AD
Several studies found increased free radical production, lipid peroxidation, oxidative DNA damage, oxidative protein damage, decreased ATP production, and decreased cell viability in postmortem AD brains compared to brains from age-matched healthy subjects [26,27,29,33,59]. Further, using AD transgenic mice lines, multiple studies found increased production of free radicals, cytochrome c oxidase activity, lipid peroxidation, and reduced levels mitochondrial ATP in affected brain regions [11,19,20,30,34-43,60,61], and primary neurons AD transgenic mice or neurons expressing mutant APP and Aβ [32,47-50], further supporting mitochondrial dysfunction and oxidative stress are important features of AD pathogenesis.
3.6. Age-induced mitochondrial ROS in late-onset AD
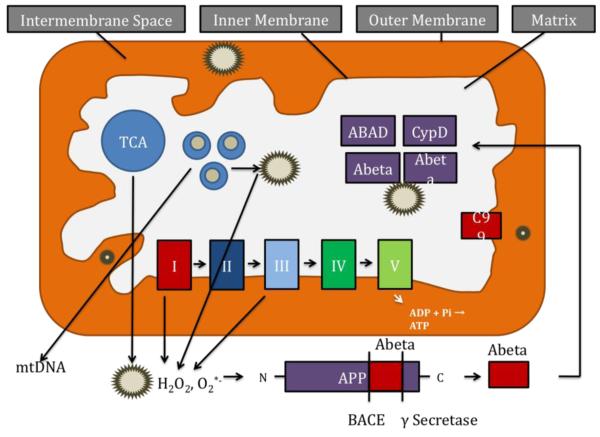
Aging and age-dependent accumulation of mtDNA plays a key role in producing mitochondrial-ROS in neurons, and as shown in Fig. 3, this mitochondrial-ROS activate beta- and γ-secretases and facilitate the cleavage of the AβPP molecule. The cleaved APP molecule (that is, Aβ) further induce free radicals, leading to the disruption of the ETC, enzyme activities, oxidized DNA, oxidized protein, and lipid peroxidation, and to the inhibition of mitochondrial ATP [4,24]. This feedback loop of age-dependent free radicals to Aβ and Aβ to free radicals ultimately leads to neuronal damage, neurodegeneration, and cognitive decline in late-onset AD patients.
Figure 3.
Age and amyloid beta-induced free radical production and cleavage of APP fragments in AD neuron. The accumulation of mtDNA changes may induce ROS production and cause oxidative damage in aged tissues. In late-onset AD, age-dependent production of ROS contribute to the secretion of Aβ peptides by activating β- and γ-secretases. These Aβ peptides enter mitochondria, induce free radicals, decrease cytochrome oxidase activity, and inhibit ATP generation. In familial AD, mutations in APP, PS1 and PS2 activate β- and γ-secretases and secrete Aβ peptides, and these Aβ peptides enter mitochondria, cause mitochondrial dysfunction and damage neurons.
3. 7. APP and Aβ Association with Mitochondria in AD
Several groups reported that AβPP, and monomeric and oligomeric forms of Aβ have been found in mitochondrial membranes [18,19,29,30,35.42,47,62,63]. Lustbader et al [19] found Aβ normally interacting with the mitochondrial matrix protein ABAD, with this interaction leading to mitochondrial dysfunction. Recently, the Reddy laboratory found Aβ monomers and oligomers in mitochondria isolated from the cerebral cortex of AβPP transgenic mice and from N2a cells expressing AβP [35]. A digitonin fractionation analysis of isolated mitochondria from AβPP transgenic mice revealed Aβ in the outer and inner membranes and matrix of mitochondria. We found that mitochondrial Aβ decreases cytochrome oxidase activity and increases free radicals and carbonyl proteins. Du et al [47] found Aβ interaction with mitochondrial matrix protein, cypclophilin D, and this abnormal interaction causes mitochondrial dysfunction in the brains of AD transgenic mice. Recently, Yao et al. [42] found Aβ in mitochondrial membranes of cortical tissues from triple transgenic mice.
More recently, Devi and Ohno [20] studied to determine, if β-cleaved C-terminal fragment C99 of APP accumulate in mitochondria of neurons affected by AD. Using immunoblotting, digitonin fractionation and immunofluorescence labeling techniques, they found that C99 is targeted to mitochondria, in particular, to the mitoplast (innermembrane and matrix compartments) in brains of AD transgenic mice (5XFAD line). Furthermore, full-length APP was also identified in mitochondrial fractions of 5XFAD mice. Remarkably, partial deletion of the β-site APP-cleaving enzyme 1 (BACE1 (+/−)) almost completely abolished mitochondrial targeting of C99 and full-length APP in 5XFAD mice at 6 months of age. However, substantial amounts of C99 and full-length APP accumulation remained in mitochondria of 12-month-old BACE1 (+/−)·5XFAD mouse brains. Consistent with these changes in mitochondrial C99/ full-length APP levels, BACE1 (+/−) deletion age-dependently rescued mitochondrial dysfunction in 5XFAD mice, as assessed by cytochrome c release from mitochondria, reduced redox or complex activities and oxidative DNA damage.
Overall, these findings together with earlier observations, suggest that Aβ, C99 fragment of APP and full-length APP are associated with mitochondria, and contribute to inducing mitochondrial dysfunction in AD neurons.
3.8. Abnormal Mitochondrial Dynamics in AD
Recent studies of mitochondrial structure in postmortem brains from AD patients and primary neurons from AD transgenic mice revealed that Aβ fragments mitochondria and causes structural changes in AD neurons [32,44,45,47-50]. Increasing evidence suggests that mitochondrial dynamics are impaired in neurons affected by AD.
Wang and co-workers [44] investigated the effects of AβPP and Aβ on mitochondrial structural changes. They found that 40% of human neuroblastoma (M17) that express wild-type APP and 80% of M17 cells overexpressing mutant AβPP displayed alterations in mitochondrial morphology, particularly fragmented mitochondria.
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, the Reddy laboratory studied mitochondrial structure and function, and neurite outgrowth in neurons treated with Aβ [46]. In neurons treated with only Aβ, they found increased expressions of mitochondrial fission genes (Drp1 and Fis1) and decreased expressions of fusion genes (Mfn1, Mfn2, and Opa1), indicating the presence of abnormal mitochondrial dynamics in AD neurons. Transmission electron microscopy of neurons treated with Aβ revealed a significant increase in mitochondrial fragmentation, further supporting abnormal mitochondrial dynamics. They also found significantly decreased neurite outgrowth and decreased mitochondrial function in cells treated with Aβ. These findings suggest that Aβ fragments mitochondria and causes abnormal mitochondrial dynamics, leading to mitochondrial dysfunction.
Using primary neurons from a well-characterized AβPP transgenic mice (Tg2576 mouse line), for the first time, the Reddy laboratory [50] studied mitochondrial activity, including axonal transport of mitochondria, mitochondrial dynamics, morphology and function. Further, we also studied the nature of Aβ-induced synaptic alterations, and cell death in primary neurons from Tg2576 mice. Transmission electron microscopy revealed a large number of small mitochondria and structurally damaged mitochondria, with broken cristae in AβPP primary neurons. We also found an increased accumulation of oligomeric Aβ and increased apoptotic neuronal death in the primary neurons from the AβPP mice relative to the WT neurons. Our findings revealed an accumulation of intraneuronal oligomeric Aβ, leading to mitochondrial and synaptic deficiencies, and ultimately causing neurodegeneration in AβPP neurons [50].
Using postmortem brains from AD patients and control subjects, and quantitative RT-PCR and immunoblotting analyses, the Reddy laboratory [32] measured mRNA and protein levels of mitochondrial structural genes in the frontal cortex of patients with early, definite and severe AD and in control subjects. We also characterized monomeric and oligomeric forms of Aβ in these patients. We found increased expression of the mitochondrial fission genes Drp1 and Fis1 and decreased expression of the mitochondrial fusion genes Mfn1, Mfn2, Opa1 and Tomm40. The matrix gene CypD was up-regulated in AD patients. Results from our quantitative RT-PCR and immunoblotting analyses suggest that abnormal mitochondrial dynamics increase as AD progresses. Primary neurons that were found with accumulated oligomeric Aβ had lost branches and were degenerated, indicating that oligomeric Aβ may cause neuronal degeneration. These findings suggest that in patients with AD, increased production of Aβ mitochondrial fragmentation, abnormal mitochondrial dynamics and synaptic damage.
Using neurons from adult fruit flies, Zhao and colleagues [64] studied the effects of wild-type and an arctic form of Aβ42. They performed extensive time-course analyses to determine the function and structure of both axon and presynaptic terminals of individual neurons. They found Aβ accumulated intracellularly, and they found a wide range of changes typically associated with aging, including the depletion of presynaptic mitochondria, a slow-down of bi-directional transports of axonal mitochondria, decreased synaptic vesicles, increased large vacuoles, and elevated synaptic fatigue.
Overall, these findings suggest Aβ enters mitochondria and causes abnormal mitochondrial dynamics in neurons that are affected by AD, and that such abnormal mitochondrial dynamics cause mitochondrial dysfunction and abnormal mitochondrial trafficking in AD neurons.
3.9. Defective Axonal Transport of Mitochondria and Impaired Mitochondrial Biogenesis in AD
Several recent live-cell imaging studies of primary neurons treated with Aβ peptide and/or primary neurons from AD transgenic mice revealed that reduced anterograde transport of mitochondria, indicating lack of healthy mitochondria and mitochondria ATP at synapses may be an important factor that promote synaptic degeneration in AD neurons [47-50,65,66].
Using mouse hippocampal neurons and Aβ25-35 peptide, the Reddy laboratory [48] studied axonal transport of mitochondria, including mitochondrial motility, mitochondrial length and size, mitochondrial index per neurite, and synaptic alterations of the hippocampal neurons. In the PBS-treated neurons, 36.4±4.7% of the observed mitochondria were motile, with 21.0±1.3% moving anterograde and 15.4±3.4% moving retrograde and the average speed of movement was 12.1±1.8μm/min. In contrast, in the Aβ-treated neurons, the number of motile mitochondria were significantly less, at 20.4±2.6% (P<0.032), as were those moving anterograde (10.1±2.6%, P<0.016) relative to PBS-treated neurons, suggesting that the Aβ25-35 peptide impairs axonal transport of mitochondria in AD neurons. In the Aβ-treated neurons, the average speed of motile mitochondria was also less, at 10.9±1.9μm/min, and mitochondrial length was significantly decreased. Further, synaptic immunoreactivity was also significantly less in the Aβ-treated neurons relative to the PBS-treated neurons, indicating that Aβ affects synaptic viability. These findings suggest that, in neurons affected by AD, Aβ is toxic, impairs mitochondrial movements, reduces mitochondrial length, and causes synaptic degeneration.
More recently, the Reddy laboratory studied mitochondrial activity, including axonal transport of mitochondria, mitochondrial dynamics, morphology and function. Further, they also studied the nature of Aβ-induced synaptic alterations, and cell death in primary neurons from Tg2576 mice. Similar to the findings of Aβ25-35 peptide treated neurons, we found significantly decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins and defective mitochondrial function in primary neurons from AβPP mice compared with wild-type neurons.
Using 5-bromo-2-deoxyuridine (BrdU) incorporation and primary neurons, the Reddy laboratory [49] studied the mitochondrial biogenesis and mitochondrial distribution in hippocampal neurons from AβPP transgenic mice and wild-type neurons treated with oxidative stressors, rotenone and H2O2. We found that after 20h of labeling, BrdU incorporation was specific to porin-positive mitochondria. The proportion of mitochondrial area labeled with BrdU was 40.3±6.3% at 20h. The number of mitochondria with newly synthesized DNA was significantly higher in AβPP neuronal cell bodies than in the cell bodies of wild-type neurons. In neurites, the number of BrdU-positive mitochondria significantly decreased in AβPP cultures compared to wild-type neurons. Further, BrdU in the cell body significantly increased when neurons were treated with low doses of H2O2, while the neurites showed decreased BrdU staining. BrdU labeling was increased in the cell body under rotenone treatment. Additionally, under rotenone treatment, the content of BrdU labeling decreased in neurites.
Overall, findings from our lab together with others [47,65,66] suggest that Aβ and mitochondrial toxins enhance mitochondrial fragmentation in the cell body, and may cause impaired axonal transport of mitochondria, defective mitochondrial distribution, leading to synaptic degeneration.
4.0. Synaptic Degeneration in AD
Several recent studies focused on synapses and synaptic degeneration in AD neurons, and found Aβ abnormally accumulated in synapses and synaptic mitochondria [4,35,43,47]. This abnormal accumulation of Aβ at synapses may be important factor causing synaptic degeneration.
Recently, Dragicevic et al [43] studied synaptic mitochondrial abnormalities in the AβPPsw and AβPP+PS1 mouse lines, focusing on the hippocampus, cortex, striatum, and amygdala of 12-month-old AβPPsw and AβPP+PS1 mice as well as nontransgenic mice. They measured mitochondrial respiratory rates, ROS production, membrane potential, and cytochrome c oxidase activity. Hippocampal and cortical mitochondria showed the highest levels of mitochondrial dysfunction, while striatal mitochondria were moderately affected, and amygdala mitochondria were minimally affected. Mitochondria in affected brain tissues from AβPP+PS1 mice were more impaired than those from AβPP mice. Synaptic mitochondria were more impaired than nonsynaptic mitochondria in both the AβPPsw and AβPP+PS1 mouse models. The AβPP/PS1 mice showed more impairment in the cognitive interference task of working memory than did the AβPP mice. The correspondence between levels of mitochondrial Aβ and levels of mitochondrial dysfunction in AD mouse models supports a primary role for mitochondrial Aβ in AD pathology. Dragicevic et al [69] studied the relationship between mitochondrial Aβ levels and mitochondrial dysfunction in AD mouse models. Moreover, the degree of cognitive impairment in AD transgenic mice was linked to the extent of mitochondrial dysfunction and mitochondrial Aβ, suggesting that a mitochondrial Aβ-induced signaling cascade may contribute to cognitive impairment [43].
Recently, Du and colleagues [47] studied differences in mitochondrial properties and functions of synaptic versus non-synaptic mitochondria in the transgenic mouse brain, that overexpress the human mutant form of APP and produce Aβ. Synaptic mitochondria showed a greater degree of age-dependent accumulation of Aβ and mitochondrial alterations relative to nonsynaptic mitochondria. The synaptic mitochondrial pool of Aβ was detected at 4 months, before the onset of nonsynaptic mitochondria and Aβ deposits accumulation. Aβ-insulted synaptic mitochondria revealed early deficits in mitochondrial function, as shown by increased mitochondrial permeability transition, decline in both respiratory function and activity of cytochrome c oxidase, and increased mitochondrial oxidative damage. A low concentration of Aβ1-42 (200 nM) treated murine primary neurons showed significantly altered mitochondrial distribution and trafficking in axons.
The findings from these studies suggest that synaptic mitochondria, especially Aβ-rich synaptic mitochondria, are more susceptible to Aβ-induced damage, highlighting the importance of synaptic mitochondrial dysfunction relevant to the development of synaptic degeneration and cognitive impairments in AD.
4. Mitochondrial Approaches to Treat AD
Extensive research based on postmortem brains, cell and mouse models of AD, several cellular changes/mechanisms have been reported, including 1). Aβ production and deposits, 2). hyperphosphorylation of tau and neurofibrillary tangles, 3). inflammatory responses, 4). cholinergic inhibition, 5). loss of synapses and synaptic damage and 6). abnormal mitochondrial dynamics and mitochondrial dysfunction. Based on these cellular changes, several therapeutic approaches have been developed and currently are being tested using cell and mouse models of AD. Despite tremendous progress made in AD research, and AD therapeutics, currently there are no drugs/agents available to prevent, delay, stop disease progression in AD patients and in elderly individuals.
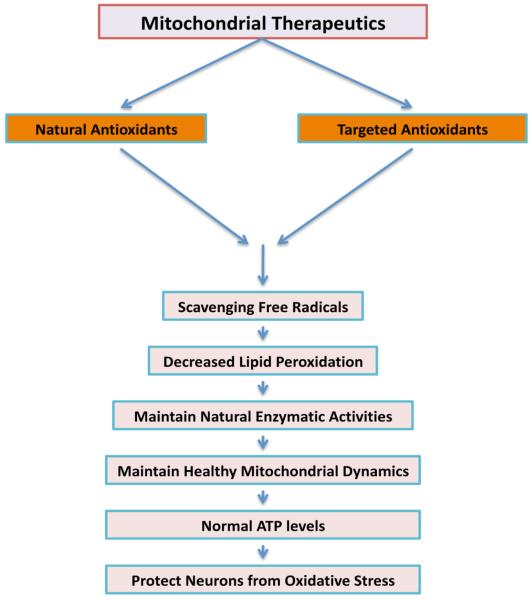
As described above, loss of synapses/synaptic damage and mitochondrial oxidative damage are early events on AD progression [18,19,28-30,32,34,35,42,44,45,47-50,63,66], and loss of synapses are the best correlate of cognitive deficits reported in AD patients. Further, impaired mitochondrial biogenesis and defective axonal transport of mitochondria are primary events that cause synaptic degeneration in AD neurons [32,48-50]. Therefore, it is critical to develop molecules that 1). scavenge free radials and decrease mitochondrial dysfunction and promote healthy mitochondrial biogenesis, 2). enhance axonal transport of organelles including mitochondria and enhance synapse formation and synaptic branches in AD neurons (Fig. 4).
Figure 4.
Schematic representation mitochondrial therapeutics for AD.
4.1. Antioxidant Therapeutics in AD
In the last decade, several groups studied efficacies of antioxidants, including vitamin E, curcumin, Ginko biloba and melatonin to determine, if antioxidants reduce Aβ and tau pathologies and enhance cognitive functions in mouse models of AD [67-72]. The outcome of these AD mice studies is positive, AD animals treated with antioxidants showed reduced soluble Aβ levels, improved mitochondrial function and cognitive behavior.
Based on encouraging outcome of AD mice studies, several clinical trials were conducted in AD patients and elderly individuals using vitamin E, vitamin C and E together, vitamin E+donepezel, Formula F+donepezel, statins and huperzine A [73-89].
Further, to determine the neuroprotective effects of huperzine A (an antioxidant) in AD patients, recently huperzine A was administered to randomly selected in mild to moderate AD in a multicenter trial in which 210 individuals were randomized to receive placebo (n = 70) for at least 16 weeks, with 177 subjects completing the treatment phase [90]. The primary analysis assessed the cognitive effects of huperzine A 200 μg BID at week 16 at 200 μg BID compared to placebo. Secondary analyses assessed the effect of huperzine A 400 μg BID, as well as effect on other outcomes including Mini-Mental State Examination. Huperzine A 200 μg BID did not influence change in ADAS-Cog at 16 weeks. In secondary analyses, huperzine A 400 μg BID showed a 2.27-point improvement in ADAS-Cog at 11 weeks vs 0.29-point decline in the placebo group (p = 0.001), and a 1.92-point improvement vs 0.34-point improvement in the placebo arm (p = 0.07) at week 16. Changes in clinical global impression of change, NPI, and activities of daily living were not significant at either dose. The primary efficacy analysis did not show cognitive benefit with huperzine A 200 μg BID. This study provides Class III evidence that huperzine A 200 μg BID has no demonstrable cognitive effect in patients with mild to moderate AD.
Overall, the outcome of antioxidant clinical trials is mostly negative and/or showed modest positive effect in cognitive function in AD patients or even elderly individuals. There are several possible reasons for the limited success of antioxidant clinical trials: 1). naturally occurring antioxidants might not cross the blood-brain barrier and so cannot reach mitochondria to neutralize free radicals, 2). not well-thought-out experimental design of clinical trials, and 3) most clinical trials conducted thus far in late-stage AD patients.
4.2. Mitochondria-targeted antioxidants in AD
considerable progress has been made in the last decade in developing mitochondria-targeted antioxidants. To increase the delivery of antioxidants into mitochondria, multiple mitochondria-targeted molecules have been developed: 1. triphenylphosphonium-based antioxidants - MitoQ, MitoVitE, Mito-α-lipoic acid, MitoPBN, 2. the cell-permeable, small peptide-based molecules, SS31, SS02, SS19, SS20 and 3. choline esters of glutathione and N-acetyl-l-cysteine [91-95]. However, these mitochondria-targeted molecules are not fully studied yet using cell and mouse models of AD.
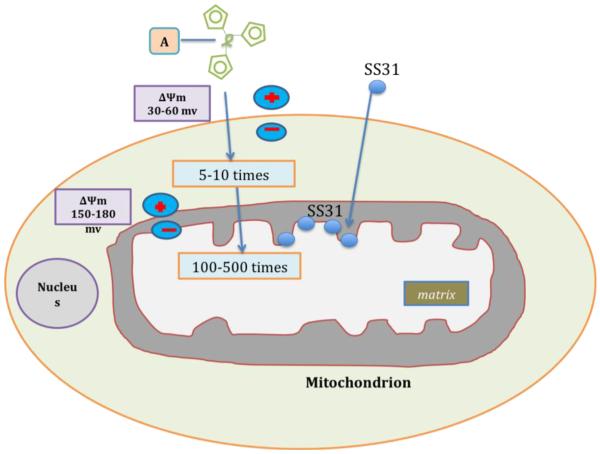
Recently, Murphy and colleagues developed a series of liphophilic triphenylphosphonium cation based antioxidants [95]. The liphophilic triphenylphosphonium cation is attached to antioxidants such as vitamin E, coenzyme Q, α-lipoic acid and these liphophilic cation attached antioxidants were preferentially taken up by mitochondria due to charge difference between mitochondria (with negative charge) and liphophilic cation based antioxidants (with positive charge) (Fig. 5). These antioxidants accumulate in the cytoplasm of cells, due to a negative plasma membrane potential and enter mitochondria and accumulate several hundred folds within the mitochondrial matrix.
Figure 5.
Schematic representation of targeting mitochondria by different molecules. A generic mitochondria-targeted antioxidant is shown constructed by the covalent attachment of an antioxidant molecule to the lipophilic triphenylphosphonium cation. Antioxidant molecules accumulate 5-10 fold in the cytoplasm, which is driven by plasma membrane potential, and then further accumulates 100-500 fold in the mitochondria. Mitochondria-targeted molecules rapidly neutralize free radicals and reduce mitochondrial toxicity. The SS31 is a cell-permeable tetra-peptide that targeted to mitochondria and protects mitochondria from oxidative damage. SS31 peptide has a sequence motif that allows them to target mitochondria several hundred fold more than natural antioxidants. Once SS peptides reach mitochondria, the SS peptides rapidly neutralize free radicals and decrease mitochondrial toxicity.
MitoQ
Among several lipophilic cation based antioxidants, MitoQ is a strong therapeutic antioxidant that has been successfully targeted to mitochondria. MitoQ excessively accumulate in the mitochondria and convert H2O2 to H2O and O2, and reduce toxic insults from free radicals in the mitochondria. This reduction may ultimately lead to the protection of neurons from age-related and AD-related mitochondrial insults [93,95]. However, higher concentrations (above 0.3μM) of MitoQ are toxic to neuronal cells.
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, the Reddy laboratory [32,46] studied mitochondrial structure and function, and neurite outgrowth in mouse neuroblastoma (N2a) cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ. In N2a cells only incubated with the Aβ, we found increased expressions of mitochondrial fission genes and decreased expression of fusion genes, and also decreased expression of peroxiredoxins, endogenous cytoprotective antioxidant enzymes. Electron microscopy of the N2a cells incubated with Aβ revealed a significantly increased number of mitochondria, indicating that Aβ fragments mitochondria. Biochemical analysis revealed that function is defective in mitochondria. Neurite outgrowth was significantly decreased in Aβ-incubated N2a cells, indicating that Aβ affects neurite outgrowth. However, in N2a cells treated with MitoQ, and SS31, and then incubated with Aβ, abnormal expression of peroxiredoxins and mitochondrial structural genes were prevented and mitochondrial function was normal; intact mitochondria were present and neurite outgrowth was significantly increased. In primary neurons from AβPP transgenic mice that were treated with MitoQ and SS31, neurite outgrowth was significantly increased and cyclophilin D expression was significantly decreased. These findings suggest that MitoQ and SS31 prevent Aβ toxicity in mitochondria from neurons affected by AD. Further research is needed using AD mouse models in order to determine MitoQ effects in cognitive behavior and AD pathology.
SS31
Recently, Szeto and Schiller developed a series of 4, small, cell-permeable antioxidant peptides (Szeto-Schiller or SS peptides) that are known to protect mitochondria from oxidative damage: 1) SS19 H-Tyr-D-Arg-Phe-Lys-NH2, 2) SS02 H-Dmt-D-Arg-Phe-Lys-NH2, 3) SS31 H-D-Arg-Dmt-Lys-Phe-NH2, and 4) SS20 H-Phe-D-Arg-Phe-Lys-NH2 [91,95-97]. These SS peptides have a sequence motif that allows them to target mitochondria. They scavenge H2O2 and ONOO-, and inhibit lipid peroxidation. Their antioxidant action can be attributed to the tyrosine, or dimethyltyrosine (Dmt), residue. Dmt is more effective than tyrosine in scavenging mitochondria for ROS. The specific location of the tyrosine or Dmt residue does not appear to be significant, as SS31 was found to be as effective as SS02 in scavenging H2O2 and in inhibiting LDL oxidation.
Recently, the efficacy of the SS31 was studied in rodent models by several labs using different murine models of human diseases including ischemic brain injury [98], with a diabetic condition [99], undergoing myocardial infarction [100] and in ALS [101]. Researchers found that SS31 protects cells from mitochondrial toxicity in all these disease states.
The Reddy laboratory [32,48-50]extensively studied the protective properties of SS31 in neurons treated with Aβ25-35 peptide, and primary neurons from Tg2576 mice [32,48-50] and Tg2576 mice treated with SS31 (Mao and Reddy, unpublished observations).
As reported earlier, SS31 decreased the levels of mitochondrial fission proteins (Drp1, Fis1) and matrix protein, CypD and reduced mitochondrial dysfunction in neurons affected by AD. Further, SS31 enhanced the number of healthy and intact mitochondria, and increased synaptic outgrowth and neuronal branching.
Recently, the Reddy laboratory [50] studied mitochondrial activity and the nature of Aβ-induced synaptic alterations in primary neurons from Tg2576 mice. We sought to determine whether the mitochondria-targeted antioxidant SS31 could mitigate the effects of oligomeric Aβ. We found significantly decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins and defective mitochondrial function in primary neurons from AβPP mice compared with wild-type neurons. However, we found that the mitochondria-targeted antioxidant SS31 restored mitochondrial transport and synaptic viability, and decreased the percentage of defective mitochondria, indicating that SS31 protects mitochondria and synapses from Aβ toxicity.
Overall, findings our lab indicate that SS31 reduce Aβ-induced mitochondrial toxicity and increase axonal transport of mitochondria and enhance synaptic viability, and protect neurons from Aβ toxicity. Further research is needed using AD mouse models in order to determine the efficacies of SS31 and before applying for clinical trials in AD patients.
Conclusions and Future Directions
Mitochondria are essential cytoplasmic organelles that are critical for cell survival and cell death. Mitochondria are involved in aging and several age-related human diseases. Increasing evidence suggest that age-related accumulation of mtDNA changes play a large role in producing increased levels of ROS, decreased mitochondrial function, low levels of ATP production and neuronal damage in neurodegenerative diseases, including AD, PD, HD and ALS. Further, recent research on Aβ and mitochondria in AD neurons revealed that Aβ accumulates in synapses and synaptic mitochondria, leading to abnormal mitochondrial dynamics and synaptic degeneration in AD neurons. In addition, recent studies using live-cell imaging and primary neurons from AD transgenic mice revealed that reduced mitochondrial mass, defective axonal transport, impaired mitochondrial biogenesis and synaptic degeneration, indicating that Aβ is responsible for mitochondrial and synaptic deficiencies.
In terms of AD therapeutics, despite tremendous progress made in understanding disease progression and developing therapies, we still do not have drugs/agents that prevent, delay, stop AD in our elderly population. Antioxidant approaches in treating AD patients thus far are disappointing. However, mitochondria-targeted molecules appear to be promising to treat AD, and however, further research is needed to study the efficacies of mitochondria-targeted molecules.
Highlights.
Summarizes recent developments of Abeta-induced abnormal mitochondrial dynamics and synaptic degeneration in AD.
Discussed the factors that cause mitochondrial dysfunction in AD.
Highlighted the antioxidant approaches in AD.
Discussed the mitochondria-targeted antioxidant therapeutics in AD.
Acknowledgments
This research was supported by NIH grants AG028072, RR00163, and Alzheimer Association grant IIRG-09-92429
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Swerdlow RH. The neurodegenerative mitochondriopathies. J . Alzheimers. Dis. 2009;17:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [3].Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res.Brain Res.Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [4].Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reddy PH. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer’s disease. CNS Spectr. 2009;14:8–13. doi: 10.1017/s1092852900024901. discussion 16-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krzywanski DM, Moellering DR, Fetterman JL, Dunham-Snary KJ, Sammy MJ, Ballinger SW. The mitochondrial paradigm for cardiovascular disease susceptibility and cellular function: a complementary concept to Mendelian genetics. Lab.Invest. 2011;91:1122–1135. doi: 10.1038/labinvest.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kuzmicic J, Del Campo A, Lopez-Crisosto C, Morales PE, Pennanen C, Bravo-Sagua R, Hechenleitner J, Zepeda R, Castro PF, Verdejo HE, Parra V, Chiong M, Lavandero S. Mitochondrial Dynamics: a Potential New Therapeutic Target for Heart Failure. Rev.Esp.Cardiol. 2011;64:916–923. doi: 10.1016/j.recesp.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [8].Camara AK, Bienengraeber M, Stowe DF. Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front.Physiol. 2011;2:13. doi: 10.3389/fphys.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martin LJ. Mitochondrial and Cell Death Mechanisms in Neurodegenerative Diseases. Pharmaceuticals (Basel) 2010;3:839–915. doi: 10.3390/ph3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cook CC, Higuchi M. The awakening of an advanced malignant cancer: An insult to the mitochondrial genome. Biochim.Biophys.Acta. 2011 doi: 10.1016/j.bbagen.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb.Symp.Quant.Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- [13].Reddy PH, Reddy TP. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr.Alzheimer Res. 2011;8:393–409. doi: 10.2174/156720511795745401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res.Rev. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- [16].Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid.Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- [17].Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog.Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [18].Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat.Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- [20].Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the beta-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice. Neurobiol.Dis. 2011 doi: 10.1016/j.nbd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- [22].Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod.Biomed.Online. 2008;16:553–569. doi: 10.1016/s1472-6483(10)60463-4. [DOI] [PubMed] [Google Scholar]
- [23].Boengler K, Heusch G, Schulz R. Nuclear-encoded mitochondrial proteins and their role in cardioprotection. Biochim.Biophys.Acta. 2011;1813:1286–1294. doi: 10.1016/j.bbamcr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- [24].Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damageimplications for cognitive decline in aging and Alzheimer’s disease. Trends. Mol. Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- [27].Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol.Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- [28].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J.Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J.Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- [31].Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- [32].Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum.Mol.Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- [34].Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum.Mol.Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- [35].Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum.Mol.Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- [36].Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Beal M. Flint, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J.Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- [37].Eckert A, Hauptmann S, Scherping I, Rhein V, Muller-Spahn F, Gotz J, Muller WE. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- [38].Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc.Natl.Acad.Sci.U.S.A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Drago D, Cavaliere A, Mascetra N, Ciavardelli D, di Ilio C, Zatta P, Sensi SL. Aluminum modulates effects of beta amyloid(1-42) on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Res. 2008;11:861–871. doi: 10.1089/rej.2008.0761. [DOI] [PubMed] [Google Scholar]
- [40].Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic.Biol.Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- [41].Sensi SL, Rapposelli IG, Frazzini V, Mascetra N. Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer’s disease. Exp.Gerontol. 2008;43:488–492. doi: 10.1016/j.exger.2007.10.018. [DOI] [PubMed] [Google Scholar]
- [42].Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc.Natl.Acad.Sci.U.S.A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J.Alzheimers Dis. 2010;20(Suppl 2):S535–50. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- [44].Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc.Natl.Acad.Sci.U.S.A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J.Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J.Alzheimers Dis. 2010;20(Suppl 2):S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc.Natl.Acad.Sci.U.S.A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum.Mol.Genet. 2011 doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Calkins MJ, Reddy PH. Assessment of newly synthesized mitochondrial DNA using BrdU labeling in primary neurons from Alzheimer’s disease mice: Implications for impaired mitochondrial biogenesis and synaptic damage. Biochim.Biophys.Acta. 2011;1812:1182–1189. doi: 10.1016/j.bbadis.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim.Biophys.Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N.Engl.J.Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- [52].Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced Mitochondria Cytochrome Oxidase Activity in Adult Children of Mothers with Alzheimer’s Disease. J.Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum.Mol.Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- [54].Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc.Natl.Acad.Sci.U.S.A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J.Alzheimers Dis. 2010;20(Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G, Reglodi D, Saykin A, Weiner M, Macciardi F, Schork N, Wallace DC, Potkin SG. Alzheimer’s Disease Neuroimaging Initiative, Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol.Aging. 2010;31:1355–1363. doi: 10.1016/j.neurobiolaging.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res.Mol.Brain Res. 1994;24:336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- [58].Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J.Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- [59].Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends. Mol. Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- [60].Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J.Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- [61].Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J.Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J.Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Petersen C.A. Hansson, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc.Natl.Acad.Sci.U.S.A. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, Wang YH, YangCheng HY, Zhu HL, Sun XJ, Huang FD. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J.Neurosci. 2010;30:1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J.Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nakashima H, Ishihara T, Yokota O, Terada S, Trojanowski JQ, Lee VM, Kuroda S. Effects of alpha-tocopherol on an animal model of tauopathies. Free Radic.Biol.Med. 2004;37:176–186. doi: 10.1016/j.freeradbiomed.2004.04.037. [DOI] [PubMed] [Google Scholar]
- [68].Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Pratico D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J.Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- [69].Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- [70].Matsubara E, Bryant-Thomas T, Quinto J. Pacheco, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J.Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- [71].Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp.Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- [72].Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J.Biol.Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- [73].Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA, Field TS, Evans DA. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis.Assoc.Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- [74].Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch.Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- [75].Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am.J.Clin.Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- [76].Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ, Alzheimer’s Disease Cooperative Study Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch.Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- [77].Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener Dis. 2010;7:193–202. doi: 10.1159/000295663. [DOI] [PubMed] [Google Scholar]
- [78].Schneider LS, Raman R, Schmitt FA, Doody RS, Insel P, Clark CM, Morris JC, Reisberg B, Petersen RC, Ferris SH. Characteristics and performance of a modified version of the ADCS-CGIC CIBIC+ for mild cognitive impairment clinical trials. Alzheimer Dis.Assoc.Disord. 2009;23:260–267. doi: 10.1097/WAD.0b013e31819cb760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL, Alzheimer’s Disease Cooperative Study Group Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology. 2009;72:2115–2121. doi: 10.1212/WNL.0b013e3181aa52d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J.Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- [81].Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann.N.Y.Acad.Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- [82].Isaac MG, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst.Rev. 2008;(3) doi: 10.1002/14651858.CD002854.pub2. CD002854. [DOI] [PubMed] [Google Scholar]
- [83].DeCarli C, Frisoni GB, Clark CM, Harvey D, Grundman M, Petersen RC, Thal LJ, Jin S, Jack CR, Jr, Scheltens P, Alzheimer’s Disease Cooperative Study Group Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch.Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- [84].Burns A, O’Brien J, BAP Dementia Consensus group. Auriacombe S, Ballard C, Broich K, Bullock R, Feldman H, Ford G, Knapp M, McCaddon A, Iliffe S, Jacova C, Jones R, Lennon S, McKeith I, Orgogozo JM, Purandare N, Richardson M, Ritchie C, Thomas A, Warner J, Wilcock G, Wilkinson D, British Association for Psychopharmacology Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J.Psychopharmacol. 2006;20:732–755. doi: 10.1177/0269881106068299. [DOI] [PubMed] [Google Scholar]
- [85].Sparks DL, Sabbagh M, Connor D, Soares H, Lopez J, Stankovic G, Johnson-Traver S, Ziolkowski C, Browne P. Statin therapy in Alzheimer’s disease. Acta Neurol.Scand.Suppl. 2006;185:78–86. doi: 10.1111/j.1600-0404.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- [86].Ciabattoni G, Porreca E, Di Febbo C, Di Iorio A, Paganelli R, Bucciarelli T, Pescara L, Del Re L, Giusti C, Falco A, Sau A, Patrono C, Davi G. Determinants of platelet activation in Alzheimer’s disease. Neurobiol.Aging. 2007;28:336–342. doi: 10.1016/j.neurobiolaging.2005.12.011. [DOI] [PubMed] [Google Scholar]
- [87].Pham DQ, Plakogiannis R. Vitamin E supplementation in Alzheimer’s disease, Parkinson’s disease, tardive dyskinesia, and cataract: Part 2. Ann.Pharmacother. 2005;39:2065–2072. doi: 10.1345/aph.1G271. [DOI] [PubMed] [Google Scholar]
- [88].Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann.Pharmacother. 2005;39:2073–2080. doi: 10.1345/aph.1E495. [DOI] [PubMed] [Google Scholar]
- [89].Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ, Alzheimer’s Disease Cooperative Study Group Vitamin E and donepezil for the treatment of mild cognitive impairment. N.Engl.J.Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- [90].Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, Jin S, Thomas R, Aisen PS, Alzheimer’s Disease Cooperative Study A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76:1389–1394. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–31. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J.Biomed.Biotechnol. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim.Biophys.Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [95].Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu.Rev.Pharmacol.Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- [96].Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–83. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann.N.Y.Acad.Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- [98].Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron.Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- [99].Thomas DA, Stauffer C, Zhao K, Yang H, Sharma VK, Szeto HH, Suthanthiran M. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. J.Am.Soc.Nephrol. 2007;18:213–222. doi: 10.1681/ASN.2006080825. [DOI] [PubMed] [Google Scholar]
- [100].Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J.Biol.Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- [101].Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J.Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]