Abstract
3,5-bis(2-fluorobenzylidine)-4-piperidone or EF24 is a potent anticancer derivative of curcumin. Using an amine derivative of EF24, we synthesized a hydrazinonicotinic acid conjugate, EFAH, for Tc-99m radiolabeling and SPECT imaging. The aqueous solubility of EFAH (3.5 mg/ml) was significantly more than that of EF24 (1.2 mg/ml); the octanol/water partition coefficient of EFAH was estimated at log P = 0.33. As an antiproliferative agent, EFAH was as effective as EF24 in suppressing the proliferation of H441, MiaPaCa-2 and Panc-1 cells. Daily intraperitoneal injection of EFAH (5 μg) for 3 weeks in mice carrying xenografts of Panc-1 pancreatic cancer showed a mean tumor volume reduction of 79%; the tumor weight decreased by 82% in the treated group. For imaging and biodistribution, EFAH was labeled with Tc-99m (98% RCY) and intravenously administered in rats. Approximately 23.7% and 14.3% of injected dose accumulated in liver and intestine, respectively, suggesting that EFAH is mostly eliminated by hepatobiliary route. The results indicate that HYNIC modification of EF24 for Tc-99m radiolabeling does not affect its anti-proliferative efficacy. For the first time, a visual biodisposition of EF24 in a live animal model has been demonstrated. Such knowledge could be of benefit in developing therapeutic curcuminoids, such as EF24.
Keywords: Curcuminoid, Tc-99m, SPECT, Cancer, EF24, Imaging
Introduction
There is a growing understanding among cancer researchers about the utility of visual, temporal and quantitative information pertaining to the delivery of drug in tumor tissue. In most instances, imaging can provide such enabling technologies where the ability to image the distribution of drug may offer an important tool in real-time management of therapy. Molecularly-targeted drugs are especially amenable to this strategy. Arguably, since drug distribution may be heterogeneous even in histologically identical tumors, image-based knowledge of drug accumulation in cancer tissue may help in predicating the drug-effect. The focus in our laboratory has been to add imaging dimension to the development of anticancer drugs by synthesizing related compounds that could be easily monitored by non-invasive imaging. The role of such technology in hastening anti-cancer drug development is acknowledged in a Critical Path Initiative by the FDA and NCI (1–3). Imaging provides both structural and functional information under physiologic conditions, and eliminates the sampling errors inherent in other methods (4). It is also possible to perform longitudinal studies in the same subject to collect time point-specific data. We describe results of our study on the development of a novel imageable curcuminoid.
Curcumin is an unsaturated polyphenolic β-ketone present in the rhizome of Indian dietary spice turmeric (Curcuma longa Linn). Several investigations have demonstrated that curcumin is chemopreventive in all three stages of carcinogenesis- initiation, progression and promotion (5–9). The pharmacological safety and efficacy of curcumin makes it an attractive therapeutic molecule, but the poor bioavailability after oral administration remains a major barrier (10). The problem of bioavailability has been attributed to its lack of water solubility, inefficient absorption and rapid metabolism (11). It is therefore of interest to modify the core structure of curcumin with a goal of enhanced bioavailability and potency. Synthetic analogs of curcumin with enhanced cytotoxicity and improved physicochemical properties are now emerging (12–14). One of these compounds is 3,5-bis(2-fluorobenzylidine)-4-piperidone (also known as EF24) which has been shown to possess potent anticancer activity, both in vitro as well as in vivo (15).
In this study, we report the synthesis of a modified EF24derivative, 1-[2-aminoethyl-(6-hydrazinopyridine-3-carbamidyl)-3,5-bis-(2-fluorobenzylidene)-4-piperidone (EFAH). EFAH could be efficiently labeled with a gamma ray-emitting Tc-99m radionuclide. We demonstrate that the modification does not affect the antiproliferative activity of 3,5-bis(benzylidine)-4-piperidone series in cancer cells, both in vitro as well as in vivo. Finally, we show that the Tc-99m-labeled analog of the compound provides quantitative information about biodistribution from single photon emission tomography (SPECT) in a rat model. To our knowledge this is the first image-based non-invasive investigation of EF24.
Methods and Materials
All reagents were obtained from commercial sources, and were used directly without further purification. 1H NMR spectra and 13C NMR spectra were recorded at 300 MHz and 75 MHz on Varian VX-300 (Varian Inc., CA). The spectra were referenced to the residual protonated solvents. Abbreviations such as s, d, t, m, br and dd used in the description of NMR spectra denote singlet, doublet, triplet, multiplet, broad, and double doublet respectively. The chemical shifts and coupling constants were reported in δ parts per million (ppm) and hertz (Hz), respectively. The mass spectra were recorded by Finnigon MAT LCQ mass spectrometer (San Jose, CA). The NMR and mass spectroscopy data for the synthesized compounds is provided in the supplemental document. The reverse phase high performance liquid chromatography (RP-HPLC) was performed with Beckman Model 126 pump, 166 absorbance detector, Bioscan Model B-FC-300 radioisotope detector. HPLC solvents consisted of water and acetonitrile with 0.1 % trifluoroacetic acid. Radionuclide Tc-99m, as pertechnetate, was obtained from OUHSC Nuclear Pharmacy (Oklahoma City, OK). All intermediate and final products were monitored by thin layer chromatography (TLC) on 250 μm silica plates. Where applicable, the compounds were purified by column chromatography using 200–300 mesh silica gel columns. Melting points were recorded on an Electrothermal Mel-Temp melting point apparatus (Thermo Scientific, Waltham, MA). The reported melting points (°C) are uncorrected.
3,5-Bis-(2-fluorobenzylidene)-4-piperidone (1)
Hydrochloric Acid gas (generated in situ) was bubbled into a solution of 4-piperidone hydrochloride monohydrate (3 gm, 19.5 mmol) in glacial acetic acid (80 ml) until a clear solution was obtained (about 15 min). To the reaction mixture, 2-fluorobenzaldehyde (6 ml, 56.5 mmol) was added and the mixture was left at room temperature for 48 h. The crystals formed were filtered on a Buchner funnel, washed with absolute ethanol (50 ml) and ether (50 ml), and followed by drying to obtain 1 as yellow crystalline solid (5.71 gm, 94% yield, m.p. 185–186°C). Free base of 1 was generated by treating the acetate salt with 10% potassium carbonate solution.
1-(2-Aminoethyl)-3,5-bis-(2-fluorobenzylidene)-4-piperidone (2)
2-Bromoethylamine hydrobromide (204 mg, 1.00 mmol), compound 1 (311 mg, 1.00 mmol), cesium carbonate (325 mg, 1.00 mmol) and potassium iodide (166 mg, 1.00 mmol) were taken together in a flask containing DMF (5 ml). The reaction mixture was heated at 80 °C for 18 h. The progress of the reaction was monitored by an appearance of a new spot in silica TLC. After the reaction was complete, the reaction mixture was filtered and the solvent was dried. The residue was dissolved in chloroform and washed with saturated sodium chloride solution followed by water. The organic phase was separated, dried over anhydrous sodium sulfate and concentrated to obtain a crude yellow solid. The crude compound was passed through a silica column, and the title compound was eluted with 10% methanol in methylene chloride. The fractions containing the pure compound were collected, and concentrated to dryness to obtain the compound 2 as a yellow solid (252 mg, 53% yield, m.p. 151–152°C).
N′-(5-{2-[3,5-Bis-(2-fluorobenzylidene)-4-oxo-piperidin-1-yl]-ethylcarbamoyl}-pyridin-2-yl)-hydrazinecarboxylic acid tert-butyl ester (3)
Succunimidyl-6-boc-hydrazinonicotinic acid was synthesized in-house following a reported method (16). Succunimidyl-6-boc-hydrazinonicotinic acid (360 mg, 1.03 mmol) was added to compound 2 (280 mg, 0.79 mmol) in DMF containing diisopropyl ethylamine (650 μl, 3.95 mmol). The reaction mixture was stirred at room temperature for 48 h, and monitored by TLC where a new spot appeared with polarity in between the reactants. After completion of the reaction, the solvent was evaporated to dryness, and the pure conjugate was separated by column chromatography on silica (200–300 mesh) with 10% methanol in chloroform. The fractions containing the desired compound were collected and dried to yield compound 3 as yellow foamy solid (290 mg, 62% yield, m.p. 123–125°C). Rf (10% methanol in chloroform) = 0.40.
N-{2-[3,5-Bis-(2-fluorobenzylidene)-4-oxo-piperidin-1-yl]-ethyl}-6-hydrazinonicotinamide (4)
To deprotect compound 3, hydrochloric acid (55 μl of 11 N) was added to a solution of compound 3 in 5 ml of methylene chloride (120 mg, 0.20 mmol). The reaction mixture was stirred at room temperature for 5 h and a slower moving spot was obtained in TLC. The reaction mixture was evaporated to dryness and compound 4 was isolated as yellow solid. Methylene chloride and ether wash was given to the solid to remove impurities (86 mg, 87% yield, m.p. 222–224°C). Rf (30% MeOH in Chloroform) = 0.10.
Partition coefficient
The partition coefficient (KO/W) was determined in triplicate in octanol/water system. The initial oil and water phases were prepared as phases saturated in the other solvent. About 20 ml octanol was mixed with 20 ml deionized water in a screw capped glass bottle, and agitated overnight at 25 °C. The mixture was allowed to settle for 6 h to separate water-rich octanol phase (oil phase) was separated from octanol-rich water phase (water phase). About 3.0 mg of drug was dissolved in 2 ml of oil phase in a screw capped glass tube, and 2 ml of water phase was added. The tubes were agitated overnight followed by a 6 h equilibrium period. EFAH was analyzed spectrophotometrically in both the phases, and estimated against a calibration curve. KO/W was calculated as a ratio of drug in oil and water phases.
Tc-99m-labeled N-{2-[3,5-Bis-(2-fluorobenzylidene)-4-oxo-piperidin-1-yl]-ethyl}-6-hydrazinonicotinamide (5)
About 300 μl of tricine solution (100 mg/ml in water) was added to a solution of compound 4 (30 μg) in 30 μl water. Na99mTcO4 (2 mCi in 0.5 ml of saline) and 10 μl SnCl2 solution (1 mg/ml in 1N HCl) was added to the reaction mixture. The reaction mixture was incubated at room temperature for 15 min. Quantitative radiolabeling (>95%) was obtained within 15 minutes of incubation at room temperature. The labeling was followed by Whatman paper chromatography in acetone where free pertechnetate had an Rf of 0.8 and the labeled compound had an Rf of 0.1. Upon radio-RP-HPLC, compound 5 eluted at 9.2 min without any contamination of free pertechnetate (2.5 min) using a gradient of acetonitrile (20–100%) at 1.5 ml/min at 254 nm.
Stability of Tc-99m label against plasma challenge
The stability of Tc-99m labeling of EFAH was tested by challenging Tc-99m-EFAH with plasma at 37°C for up to 24 h. Briefly, about 300 μl of human plasma was incubated with 148 MBq (200 μl) of Tc-99m-EFAH. After indicated times, a 10 μl aliquot of supernatant was injected into a reverse phase HPLC column (C-18, 10 μm Sonoma) eluted under a 20 min gradient of acetonitrile (20–100%) at 1.5 ml/min.
Cell Culture and drug treatment
Human lung adenocarcinoma cell line NCI-H441 (ATCC Number: HTB-174) was obtained from the American Type Culture Collection (Manassas, VA). H441 cells were maintained at 37°C with 5% CO2 in McCoy’s 5A Medium (Invitrogen, Carlsbad, California) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and gentamicin (GIBCO Laboratories, Grand Island, NY). Pancreatic cancer cells Panc-1 and MiaPaCa-2 were maintained in RPMI 1640 1X (Catalog number 11875) in 10% heat-inactivated FBS; penicillin at 100 U and streptomycin at 100 μg per ml of medium were added as antibiotics.
To evaluate the anti-proliferative activity of synthesized compounds, the cells were seeded in a 96-well flat-bottom tissue culture plates at a density of 5×103 cells per well. The cells were allowed to attach and grow overnight. The test compounds were dissolved in dimethyl sulfoxide (DMSO) and added to cells in the culture medium supplemented with 5% FBS. The DMSO concentration was maintained at 0.1% per well. The control wells received equivalent volume of DMSO without any drugs. The cells were allowed to remain in the treatment medium for 24, 48 and 72 h.
Cell Proliferation
The total number of cells after treatment was estimated by hexosaminidase assay (17). Briefly, the medium was removed and hexosaminidase substrate solution in citrate buffer pH 5 (7.5 mM), p-nitrophenol-N-acetyl-beta-D-glucosaminidase (Calbiochem, San Diego, CA) was added at 60 μl per well. The plate was incubated at 37°C in 100% humidity for 30 minutes, before stopping the reaction by adding 90 μl of 50 mM glycine containing 5 mM of EDTA (pH 10.4); absorbance was measured at 405 nm.
Animal Studies
The animal experiments were performed according to the NIH Animal Use and Care Guidelines and were approved by the Institutional Animal Care Committee of the University of Oklahoma Health Sciences Center.
Biodistribution of Tc-99m-EFAH
Female athymic nude rats (125–150 g) were obtained from Harlan Laboratories (Indianapolis, IN), and housed in a controlled environment with 12 h day/night cycle. The animals were allowed to acclimatize at least 1 week before inoculation of lung adenocarcinoma H441 cells. On the day of tumor implantation, the animals were anesthetized with 2–3% isoflurane in oxygen stream. About 0.1 ml H441 cell suspension in phosphate buffered saline (100 million cells/ml) was subcutaneously injected in the left dorsal thigh region. The animals were returned to their cages and the tumor was allowed to grow till a palpable tumor was visible in majority of the animals.
We investigated the distribution of Tc-99m-EFAH by imaging in a NanoSPECT machine (Bioscan, Washington DC). The rats were anesthetized using 2–3% isoflurane in oxygen stream, and placed inside the detector. The body temperature was maintained at 37±1°C by Minerve anesthesia system (Bioscan, Washington DC). Approximately 500 μCi of Tc-99m-EFAH (~0.2 ml) was intravenously injected in the tail vein. Planar whole body images were acquired at various times over 24 h. After the final imaging session at 24 h, the rats were subjected to a biodistribution study. Briefly, the animals were euthanized by an intraperitoneal overdose of a euthanasia solution (Euthasol). Various organs were excised, washed with saline, weighed and appropriate tissue samples were counted in an automated gamma counter (Perkin-Elmer, Boston, MA). Total blood volume, bone and muscle mass were estimated as 5.7%, 10% and 40% of body weight, respectively. A diluted sample of injected Tc-99-EFAH served as a standard for comparison.
Mouse model of xenograft tumor
Panc-1 cells (ATCC, Manassas, VA) were grown in RPMI 1640, containing 10% heat-inactivated fetal bovine serum (Sigma Chemical Co.) and 1% antibiotic-antimycotic solution (Mediatech, Inc.) at 37°C in a humidified atmosphere of 5% CO2. Five-week-old male athymic nude mice (The Jackson Laboratory) were maintained with water and standard mouse chow ad libidum. The animals were injected with 1 × 106 cells in the left and right flank and allowed to form xenografts. EFAH was intraperitoneally administered daily (5 μg/injection) for 3 weeks until sacrifice. Tumors were measured weekly with a Vernier caliper and tumor volumes were calculated according to the formula length × width × depth × 0.5236. At the end of the treatment, the animals were sacrificed, and the tumors were removed and weighed.
Data Analysis
The in vitro biological data was analyzed for significance of difference at p < 0.05 using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). The in vivo biodistribution data was analyzed for presentation as percent injected dose per gram tissue as well as accumulation per organ. All data were corrected for the decay of Tc-99m radioactivity (physical decay T1/2= 6 h).
Results and Discussion
Despite recent advances in molecular and tumor biology in cancer and the introduction of several new agents, inclusive of molecularly-targeted therapies, the disease continues to be a contemporary challenge. Chemotherapy with anticancer drugs is the mainstay for the treatment of cancer.
Antineoplastic agents are cytotoxic by design and can cause severe systemic adverse effects in therapeutic doses. Curcumin and its synthetic analogs have shown some selectivity towards cancer cells. For instance, it has been shown that curcumin is antiproliferative in cancerous cells without being toxic to normal cells (18). We also showed recently that a congener of curcuminoid EF24 is not toxic to lung fibroblasts, but suppresses growth of cancer cells (19, 20). In order to enable labeling of EF24 with imageable radionuclide, we modified 3,5-bis-(2-fluorobenzylidene)-4-piperidone to carry HYNIC moiety, and investigated whether the intrinsic anticancer activity of the compound is preserved after the modification. The synthesis of EF24 was accomplished by acid-catalyzed Claisen-Schmidt condensation of 4-piperidone and 2-fluoro benzaldehyde, and has been reported elsewhere (21, 22).
Synthesis and Tc-99m radiolabeling
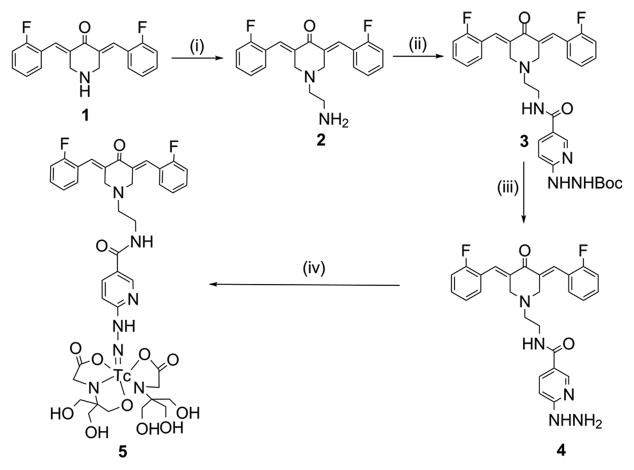
In our previous studies, we have shown that modification of piperidinyl nitrogen in EF24 does not adversely affect its activity (23). Here, we modified piperidinyl nitrogen with bromoethylamine to obtain compound 2 (82% yield). Upon a reaction of N-hydroxysuccinimidyl-6-boc-hydrazino nicotinamide with the free primary amine in 2, compound 3 containing a hydrazinonicotinoyl (HYNIC) moiety was obtained, which on deprotection afforded compound 4 (Figure 1). Compared to the precursor EF24, EFAH was found to possess more aqueous solubility (1.2 mg/ml versus 3.5 mg/ml, respectively). This may be of significance in the design of aqueous parenteral formulation, as well as in altering the oral bioavailability. According to the Lipinski’s rule, drugs with octanol/water partition coefficient (log P) less than 5.0 possess more druglikeness than the compounds with larger log P values (24). We report that the log P value of EFAH in octanol/water system is 0.33.
Figure 1.
Synthesis of EFAH. The reactions conditions were as follows: (i) Bromoethylamine, KI, Cs2CO3, DMF, 80 °C, 5 h; (ii) HYNIC, DMF, DIPEA, room temperature, 24 h; (iii) DCM, HCl, room temperature, 2 h, and (iv) Tricine, Na99mTcO4, Stannous chloride, room temperature, 15 min.
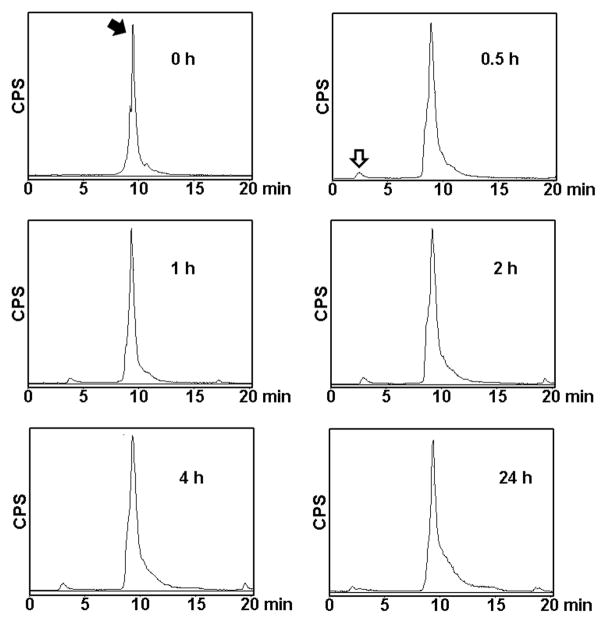
When compound 4 was labeled with Tc-99m in presence of tricine and stannous chloride, over 98% of labeling yield was obtained. Because of the ideal nuclear characteristics, Tc-99m (T1/2 = 6 h, 140 KeV) remains the most commonly used radionuclide for SPECT. It easily forms a stable complex with hydrazinonicotinamide. The labeled compound 5, was characterized by HPLC (Figure 2). The retention time of Tc-99m-labeled EFAH was measured at 9.25 min. When challenged with human plasma, there was negligible decomplexation of Tc-99m radioactivity for up to 24 h of incubation (Figure 2).
Figure 2.
The stability of Tc-99m-EFAH was analyzed by reverse-phase radioHPLC using a 20 min acetonitrile gradient (20–100%). The labeled compound was incubated with human plasma at 37 °C and the appearance of free Tc-99m-pertechnetate peak at 2.5 – 2.75 min (white arrow) was monitored over time. Labeled compound eluted at just less than 10 min (black arrow).
In vitro anticancer activity of EFAH
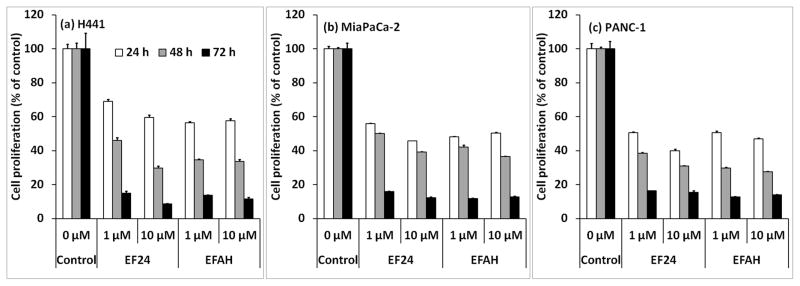
A significant concern in modifying drugs for radiolabeling purposes is the tendency of modified drug to lose activity and/or potency. Therefore, we evaluated EFAH in multiple cells lines and compared its anti-proliferative activity with that of EF24. The experimental data demonstrated that EFAH was as effective as EF24 in H441, Panc-1 and MiaPaCa-2 cell lines. Cell proliferation was studied in cancer cells in culture by estimating the decrease in the activity of lysosomal enzyme hexosaminidase (Figure 3). It is clear that EFAH possessed significant anti-proliferative activity in all the three cells tested, and the potency was comparable to that shown by EF24. The results suggest that EFAH can be further developed as an imageable anticancer compound.
Figure 3.
EFAH inhibits proliferation of (a) lung adenocarcinoma H441 cells, and pancreatic cancer cells (b) MiaPaCa-2 and (c) Panc-1. The cells were treated with 1 and 10 μM EFAH for 24–72 h, and the cell proliferation was measured by estimating hexosaminidase activity as described in the text.
Biodistribution and imaging of Tc-99m-EFAH
The importance of imaging in cancer drug development is increasing with advances in instrumentation, chemistry and imaging technologies (25). The objective behind this strategy has been to enable monitoring of the adequacy and selectivity of drug accumulation in pathologic tissue. A textbook example may be the pre-therapy elucidation of ibritumomab distribution by imaging before initiating radioimmunotherapy of non-Hodgkin’s lymphoma with Y-90-labeled ibritumomab or Zevalin (26). In the presented work, we report a similar approach to the development of synthetic curcuminoids.
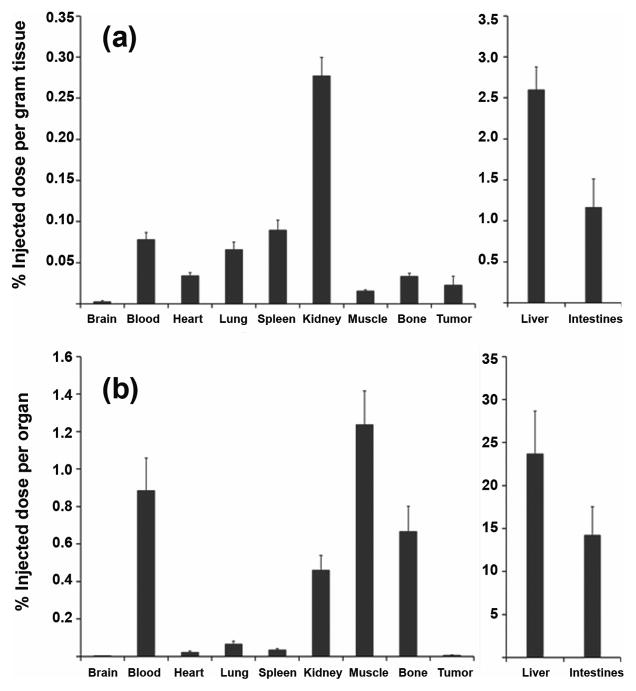
We followed up our in vitro study by investigating in vivo biodisposition of Tc-99m-EFAH in a rat model of xenograft lung tumor. Figure 4 shows the accumulation of radioactivity in various organs after 24 h of injection. The accompanying whole body images are shown in Figure 5, and they confirm the overall results obtained from biodistribution data. In the sequential images through 24 h, the radiolabeled compound appeared to travel from liver to intestine and into the feces. No other organ except liver and intestines accumulated significant radioactivity, suggesting that its clearance depended on hepatobiliary route. As expected, the tumor tissue accumulated measurable, but little radiolabeled compound. The same observation was made in the scintigraphic images. Previously, Ravidranath and Chandrasekhara reported that the majority of tritiated (H-3) curcumin administered in rats eliminated in feces (27). The in vivo behavior of Tc-99m-EFAH is suggestive of a stable complex of Tc-99m. When the rat urine collected after 30 min of injection was injected in HPLC column, no free Tc-99m radioactivity was found (data not shown).
Figure 4.
Biodistribution of Tc-99m-EFAH in nude rats carrying H441 xenograft tumors. (a) Percent injected dose per gram of tissue, and (b) percent injected dose per organ. The rats were intravenously injected with about 500 μCi of Tc-99m-EFAH, and euthanized after 24 h for biodistribution.
Figure 5.
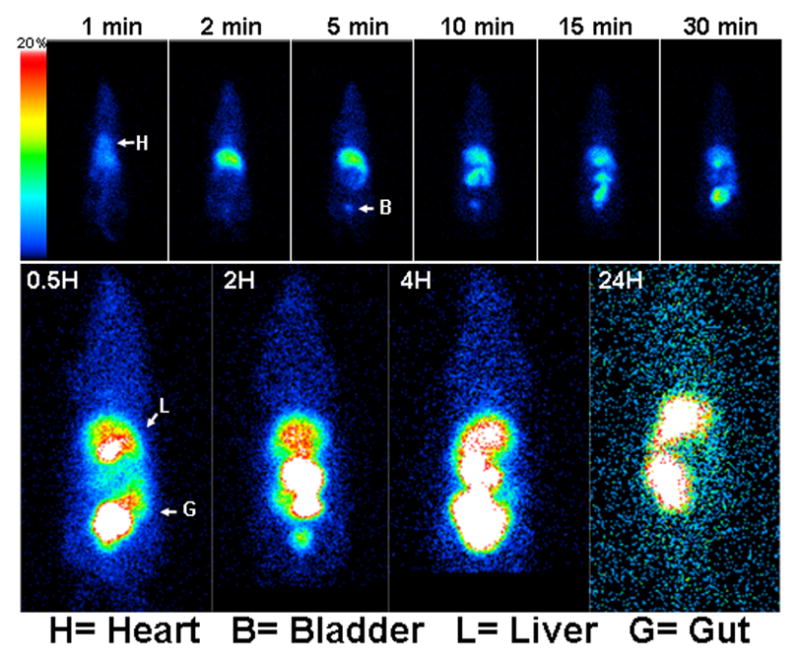
Gamma camera images of rats injected with Tc-99m-EFAH: Rats injected with approximately 500 μCi of Tc-99m-EFAH were imaged in a NanoSPECT system. A dynamic image acquisition of Tc-99m-EFAH kinetics in the first 30 min of intravenous administration (upper panel) was followed by static images (at 0.5, 2, 4 and 24 h) showing accumulation of Tc-99m-EFAH in liver and gut.
Efficacy of EFAH as an antiproliferative agent
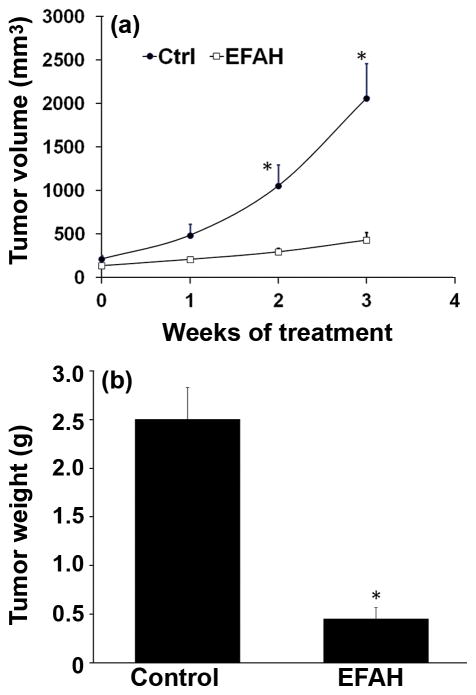
In order to test if EFAH is active as an antiproliferative agent in vivo, we administered EFAH in a mouse model of xenograft Panc-1 tumors. The xenograft tumors were allowed to grow to a size of approximately 500 mm3, following which EFAH was intraperitoneally administered on daily basis for 3 weeks. We observed a remarkable reduction in tumor size after treatment with EFAH. EFAH inhibited the growth of the tumors xenografts (Figure 6), whereas the control tumors grew during the treatment period, reaching a size of over 2,000 mm3. Panc-1 tumors in the EFAH-treated animals were smaller throughout, measuring about 434 mm3 at the end of the treatment period. The excised tumors from the EFAH-treated mice averaged 0.45 g, whereas those from the control group weighed about 2.5 g (Figure 6b).
Figure 6.
Treatment of nude mice (n = 4 per group) carrying xenograft Panc-1 tumors with EFAH inhibits the growth of tumors- (a) tumor volume and (b) tumor weight at necropsy. The mice were intraperitoneally injected with 5 μg of EFAH on daily basis for three weeks. Asterisk (*) is p <0.05 compared to untreated control.
Although the mechanism of action of EF24 is not clearly understood, evidences suggest that it causes apoptosis via a redox-dependent mechanism (28, 29). EF24 and its analogs containing dienone moiety are capable of serving as Michael acceptors. In cisplatin-resistant ovarian cancer cells, EF24 was found to cause apoptotic cell death by induction of PTEN and inhibition of AKT activities (30). In a more elaborate in vivo study by Subramaniam, et al., EF24 was found to induce caspase-mediated apoptosis in HCT-116 colon cancer xenografts (15). At the same time, marked reduction in AKT activity, as well as decreased cyclooxygenase-2, interleukin-8, and vascular endothelial growth factor mRNA and protein expression was reported (15). All these studies show that EF24-induced apoptosis is accompanied by G2-M cell cycle arrest. Further, EF24 has been shown to suppress NF-kB signaling pathway through a direct action on IκB kinase in lung, breast, ovarian and cervical cancer cells (31). NF-kB plays an important role in oncogenesis, and there is good amounts of evidence to hypothesize that the anticancer activity of curcuminoids may be mediated by inhibition of NF-kB activity (32). Further mechanistic studies may be needed for complete understanding of the specific mechanistic pathways responsible for the tumor suppressive properties of EFAH and EF24 in pancreatic cancer.
Conclusions and Future Directions
Anticancer therapy combined with image-derived knowledge of drug accumulation enables more realistic and effective treatment planning, and may be of benefit in predicating tumor response as well as timely change of therapeutic course. We synthesized a derivative of curcuminoid EF24 amenable to radiolabeling with Tc-99m. The derivative was tested in various cancer lines to ensure that the anticancer activity of EF24 is not compromised by chemical modification. We have previously shown that the putative pharmacophore of EF24 is the enone portion (23). Because this pharmacophore is preserved in EFAH, we speculate that EFAH retains the same mechanism of action. Nevertheless, it needs to be proven by empirical data which is beyond the scope of this study. The biodistribution and imaging results suggest that because of the natural clearance into the intestine, EFAH might have an added advantage in treatment of colon cancer. The application of oral delivery and the use of EFAH in a mouse model of colon cancer is a subject of another investigation being pursued in our laboratory.
Supplementary Material
Acknowledgments
The authors are thankful to the OUHSC-Nuclear Pharmacy for supplying Tc-99m-pertechnetate radioactivity. The authors also acknowledge the funding from National Cancer Institute (R03 CA143614-01).
References
- 1.Altar CA. The Biomarkers Consortium: on the critical path of drug discovery. Clin Pharmacol Ther. 2008;83:361–4. doi: 10.1038/sj.clpt.6100471. [DOI] [PubMed] [Google Scholar]
- 2.Altar CA, Amakye D, Bounos D, Bloom J, Clack G, Dean R, et al. A prototypical process for creating evidentiary standards for biomarkers and diagnostics. Clin Pharmacol Ther. 2008;83:368–71. doi: 10.1038/sj.clpt.6100451. [DOI] [PubMed] [Google Scholar]
- 3.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 4.Pomper MG. Can small animal imaging accelerate drug development? J Cell Biochem Suppl. 2002;39:211–20. doi: 10.1002/jcb.10443. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–38. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 9.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–50. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 11.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–65. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 12.Pati HN, Das U, Quail JW, Kawase M, Sakagami H, Dimmock JR. Cytotoxic 3,5-bis(benzylidene)piperidin-4-ones and N-acyl analogs displaying selective toxicity for malignant cells. Eur J Med Chem. 2008;43:1–7. doi: 10.1016/j.ejmech.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson TP, Hubbard RBt, Ehlers TJ, Arbiser JL, Goldsmith DJ, Bowen JP. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg Med Chem. 2005;13:4007–13. doi: 10.1016/j.bmc.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Sun A, Shoji M, Lu YJ, Liotta DC, Snyder JP. Synthesis of EF24-tripeptide chloromethyl ketone: a novel curcumin-related anticancer drug delivery system. J Med Chem. 2006;49:3153–8. doi: 10.1021/jm051141k. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK, Houchen CW, Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–9. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 16.Abrams MJ, Juweid M, tenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med. 1990;31:2022–8. [PubMed] [Google Scholar]
- 17.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–88. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 18.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahoo K, Dozmorov MG, Anant S, Awasthi V. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9610-4. In Press. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agashe H, Sahoo K, Lagisetty P, Awasthi V. Cyclodextrin-mediated entrapment of curcuminoid 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] or CLEFMA in liposomes for treatment of xenograft lung tumor in rats. Colloids Surf B Biointerfaces. 2011;84:329–37. doi: 10.1016/j.colsurfb.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–83. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Lagisetty P, Powell DR, Awasthi V. Synthesis and structural determination of 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J Mol Str. 2009;936:23–8. [Google Scholar]
- 23.Lagisetty P, Vilekar P, Sahoo K, Anant S, Awasthi V. CLEFMA- An Anti-Proliferative Curcuminoid from Structure Activity Relationship Studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg Med Chem. 2010;18:6109–20. doi: 10.1016/j.bmc.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths GL. The imaging probe development center and the production of molecular imaging probes. Curr Chem Genomics. 2008;1:65–9. doi: 10.2174/1875397300801010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iagaru A, Gambhir SS, Goris ML. 90Y-ibritumomab therapy in refractory non-Hodgkin’s lymphoma: observations from 111In-ibritumomab pretreatment imaging. J Nucl Med. 2008;49:1809–12. doi: 10.2967/jnumed.108.052928. [DOI] [PubMed] [Google Scholar]
- 27.Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology. 1981;22:337–44. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 28.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–75. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Sun A, Lu YJ, Hu H, Shoji M, Liotta DC, Snyder JP. Curcumin analog cytotoxicity against breast cancer cells: exploitation of a redox-dependent mechanism. Bioorg Med Chem Lett. 2009;19:6627–31. doi: 10.1016/j.bmcl.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, Hideg K, Kuppusamy P. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–18. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, Wang CY, Shoji M, Sun A, Snyder JP, Liotta D, Fu H. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–61. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–47. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.