Research highlights
▸ Adolescents with Autism and Controls report equal distress after peer rejection. ▸ Adolescents with Autism process peer rejection differently at the neural level. ▸ Adolescents with Autism display less neural evidence of distress than controls. ▸ Adolescents with Autism display less neural evidence of regulation than controls.
Keywords: Autism spectrum disorders, Peer rejection, Social exclusion, Adolescence, Functional magnetic resonance imaging
Abstract
Peer rejection is particularly pervasive among adolescents with autism spectrum disorders (ASD). However, how adolescents with ASD differ from typically developing adolescents in their responses to peer rejection is poorly understood. The goal of the current investigation was to examine neural responses to peer exclusion among adolescents with ASD compared to typically developing adolescents. Nineteen adolescents with ASD and 17 typically developing controls underwent fMRI as they were ostensibly excluded by peers during an online game called Cyberball. Afterwards, participants reported their distress about the exclusion. Compared to typically developing adolescents, those with ASD displayed less activity in regions previously linked with the distressing aspect of peer exclusion, including the subgenual anterior cingulate and anterior insula, as well as less activity in regions previously linked with the regulation of distress responses during peer exclusion, including the ventrolateral prefrontal cortex and ventral striatum. Interestingly, however, both groups self-reported equivalent levels of distress. This suggests that adolescents with ASD may engage in differential processing of social experiences at the neural level, but be equally aware of, and concerned about, peer rejection. Overall, these findings contribute new insights about how this population may differentially experience negative social events in their daily lives.
1. Introduction
1.1. Background
Autism spectrum disorders (ASD) are characterized by significant impairments in the social domain. However, among adolescents with ASD—who must tread water in a social climate ridden with concerns about peer acceptance, group norms, and popularity—deficits in social processing are likely to be particularly problematic. For example, it is common for teenagers to spend more time with peers at this age (Csikszentmihalyi and Larson, 1984), experience heightened concern about maintaining peer acceptance (Parkhurst and Hopmeyer, 1998), and place greater value on peer relationships in general (Brown, 2004). Given the range of social difficulties displayed by individuals with ASD (Frith and Hill, 2004), navigating this heightened peer salience is likely to be particularly challenging. Indeed, studies have indicated that during adolescence in particular, social skills and interactions are the biggest challenge experienced by individuals with ASD (Church et al., 2000), and the differences between individuals with ASD and their typically developing peers in social domains may be particularly pronounced at this age (Howlin, 2003).
Although there is considerable evidence that adolescents with ASD have a desire to make friends and interact with peers (Frith, 2004, Mesibov and Handlan, 1997, Volkmar and Klin, 1995), they are less likely to initiate interactions with others (Attwood, 2000, Hauck et al., 1995, Sigman and Ruskin, 1999, Volkmar and Klin, 2000) and have difficulty making friends (Prior et al., 1998). Thus, they tend to be lonelier and have lower quality friendships than their typically developing classmates (Bauminger and Kasari, 2000, Capps et al., 1996), as well as fewer close friendships (Martlew and Hodson, 1991, Sigman and Ruskin, 1999). In addition to being less able to form and keep friendships, individuals with ASD also have trouble interpreting complex social interactions and the intentions of others more generally (Baron-Cohen et al., 2001, Frith, 2004, Frith and Hill, 2004, Pierce et al., 1997), and they are less able to make quick judgments in social contexts (Volkmar and Klin, 2000), which might further undermine their social interactions with peers during adolescence.
Given the increased importance of peers during adolescence, it is not surprising that these social difficulties associated with ASD are accompanied by an increased prevalence of peer rejection among this population, compared to their typically developing counterparts. Adolescents with ASD experience high levels of bullying (van Roekel et al., 2010) and are rejected by peers up to four times more than their typically developing peers (Little, 2001, Little, 2002)—likely as a result of their poor social functioning (Attwood, 1998), stereotypical behavior (Haq and Le Couteur, 2004), and lack of close friendships, which are known to buffer individuals from the effects of social stressors (Miller and Ingham, 1976). Moreover, since they are not regularly engaging in positive interactions with peers and close friendships, they may also be missing out on a crucial resource for learning social etiquette, which might leave them more vulnerable to future peer rejection as well.
Together, this evidence indicates that adolescents with ASD are less socially skilled in peer domains and experience more frequent peer rejection than their typically developing counterparts. It is less clear, however, how adolescents with ASD differ from typically developing adolescents in their responses to peer rejection and what affective processes might underlie these differential experiences. Using experimental designs to systematically test group differences, and using neuroimaging techniques to examine differential processing in neural systems in the moment of rejection, could help reveal new insights that may help us understand how peer rejection is uniquely experienced by adolescents with ASD.
To our knowledge, only two studies have used an experimental design to test how individuals with ASD respond to the experience of social rejection, in comparison to typically developing individuals. In one of these studies, Sebastian and colleagues (2009) used a computer task called “Cyberball”—an experimental paradigm that simulates a real interactive experience of social exclusion (Williams et al., 2000, Williams et al., 2002)—to examine differences in affective responding among adolescents with ASD and typically developing controls. They found that similar to controls, adolescents with ASD reported feelings consistent with perceiving an experience of peer rejection (i.e., I felt ignored, I felt excluded, etc.). However, while controls rated their mood (e.g., I feel good, I feel friendly) as lower following exclusion, adolescents with ASD did not display a similar dampening in mood, suggesting that they may engage different affective processes than controls when they notice that they are being rejected by peers.
In another recent study, Andari and colleagues (2010) used a similar Cyberball task to examine the experience of social exclusion among adults with ASD, and added a particularly interesting manipulation; they included a condition in which participants were given oxytocin—a hormone known to enhance social affiliation and prosocial behavior (Guastella et al., 2008, Kosfeld et al., 2005). They found that oxytocin administration resulted in more socially appropriate affect and more typical behavior following exclusion (i.e., more preference and trust for socially cooperative players compared to exclusive players). In other words, the administration of oxytocin seemingly altered the neural chemistry of these individuals with ASD in a way that made them act more socially ‘typical’. Given that oxytocin is a hormone produced in areas of the brain's limbic system that are known to be involved in affect processing and emotion regulation (Ferguson et al., 2002, Huber et al., 2005, Landgraf and Neumann, 2004, Lim and Young, 2006), these findings provide promising evidence that individuals with ASD may respond differently to social exclusion due to differential processing of these social interactions at the neural level. Furthermore, they highlight the importance of exploring the neural underpinnings of social rejection experiences among individuals with ASD—particularly during adolescence when social interactions and peer rejection are prevalent and particularly difficult for this population.
Although no neuroimaging studies to date have examined the neural correlates of peer rejection processes among adolescents with ASD, we have previously begun to examine these processes in typically developing adolescents. In an initial study, we scanned adolescents while they were ostensibly excluded by peers during a Cyberball game, similar to that used in the two studies described above (Masten et al., 2009a). Findings indicated that typically developing adolescents who reported more social distress following exclusion displayed more activity in the subgenual portion of the anterior cingulate (subACC), a region that has been linked with both rejection sensitivity (Burklund et al., 2007) and depression (e.g., Masten et al., 2011a, Saxena et al., 2003), as well as in the anterior insula (AI), a region previously linked to adults’ experiences with social exclusion (e.g., DeWall et al., 2010, Eisenberger et al., 2003). In addition, adolescents who reported less social distress following exclusion displayed greater activity in the ventrolateral prefrontal cortex (VLPFC), a region linked with emotion regulation in adults (e.g., Eisenberger et al., 2003, Lieberman et al., 2004, Lieberman et al., 2007), as well as in the ventral striatum (VS), a region that, in addition to its well-known role in reward processing, is involved in positive reappraisals (Wager et al., 2008) and emotion regulation (Dickstein and Leibenluft, 2006). Thus, the subACC and AI appeared to be involved in the affective/distressing component of peer rejection, while the RVLPFC and VS appeared to be more involved in regulating these affective responses (Masten et al., 2009a). Follow-up studies have continued to implicate these regions in adolescents’ affective and behavioral responses to peer rejection (Masten et al., 2010a, Masten et al., 2010b, Masten et al., 2011a, Pfeifer et al., in press), suggesting that these particular regions may be important to focus on when trying to understand how adolescents with ASD differentially process peer rejection experiences.
Although they have not focused specifically on peer rejection experiences, recent studies have begun to identify some of the differential neural processes engaged by adolescents with and without ASD during other relevant aspects of social processing. For example, adolescents with ASD and controls display differential brain activity when processing social feedback and social rewards (Scott-Van Zeeland et al., 2010), and when inferring others’ emotional states (Dapretto et al., 2006, Greimel et al., 2010, Wang et al., 2004), social characteristics (Tesink et al., 2009), and true intentions (i.e., those involving irony or sarcasm; Wang et al., 2006, Wang et al., 2007). In addition, a recent meta-analysis examining differential neural engagement among individuals with ASD compared to controls across a range of studies examining social and non-social tasks, found that those with ASD consistently exhibit hypoactivation in the ACC and AI during social tasks in particular (Di Martino et al., 2009). Thus, in general, individuals with ASD display abnormal patterns of neural engagement when they are involved in complex social situations. Furthermore, the experience of peer rejection—which involves similar processes to those examined in prior work (i.e., social feedback, inferring intentions) and is particularly salient at this age and among this particular population—may also be differentially processed in the brain.
1.2. The current study
In the current study, our goal was to characterize patterns of neural functioning during experiences of peer rejection among adolescents with ASD compared to typically developing adolescents. Given previous behavioral work indicating similar self-reported feelings of rejection and distress among typically developing adolescents and those with ASD (Sebastian et al., 2009), we expected that both groups in the current study would likely show similar behavioral ratings of distress following a rejection experience. However, we expected that differences would be evident in the neural regions engaged during the actual experience of peer exclusion, given neural differences seen during other types of complex social processing (e.g., Dapretto et al., 2006, Greimel et al., 2010, Scott-Van Zeeland et al., 2010, Tesink et al., 2009, Wang et al., 2004, Wang et al., 2006, Wang et al., 2007). We hypothesized at least two potential ways that brain activity might differ in adolescents with ASD versus controls. One possibility is that adolescents with ASD might show reduced activity in regions typically engaged during adolescent peer rejection experiences (i.e., subACC, AI, RVLPFC, VS)—particularly since hypoactivation in the subACC and AI has been observed across many neuroimaging studies examining social processing in individuals with ASD (Di Martino et al., 2009). Alternatively, adolescents with ASD might show increased activity in these regions, reflecting a heightened emotional sensitivity to these experiences (i.e., greater activity in subACC and AI), given their more frequent exposure to peer rejection in daily life, as well as compensatory regulatory effort (i.e., greater activity in VLPFC and VS) to aid in controlling their heightened affective responses to these events. Finally, of course, it is also possible that peer rejection processing among adolescents with ASD involves some combination of these two alternatives.
To explore these possibilities, we simulated peer rejection using the Cyberball game during an fMRI scan (see details in Section 2.3), so that we could examine group differences in neural activity during the experience of social exclusion—one of the most common forms of peer rejection during adolescence (Coie et al., 1990). We were primarily interested in exploring differences in brain regions involved in adolescents’ affective experiences during these negative social interactions; thus, we used a region-of-interest (ROI) approach to specifically examine differential activity in regions that were found to be related to typically developing adolescents’ distress during peer exclusion in previous, independent samples (Masten et al., 2009a, Masten et al., 2011a). These regions included the subACC and AI, regions in which activity has been shown to positively relate to distress following exclusion, and the VLPFC and VS, regions in which activity has been shown to negatively relate to distress following exclusion (Masten et al., 2009a). Next, we also examined group differences in activity during peer exclusion (compared to inclusion) across the whole brain, to more specifically localize these neural activations and to explore other regions that might be differentially active for ASDs and controls. Finally, given previously established associations between neural activity during exclusion versus inclusion and participants’ self-reported distress resulting from the exclusion, we tested for these associations separately for each group, using both ROI and whole-brain analyses.
2. Method
2.1. Participants
Our sample included 19 high-functioning ASD adolescents (18 males; M = 14.0, SD = 2.4 years of age) and 17 typically developing (TD) adolescents (15 males; M = 13.6, SD = 2.5 years of age) recruited specifically for this study (i.e., TDs were not part of previously published studies using Cyberball). All participants were right handed, with the exception of 1 ASD and 2 TD adolescents who reported left-hand dominance. All participants had a full scale IQ of 80 or higher, assessed by the Wechsler Scale of Abbreviated Intelligence (Wechsler, 1999) or Wechsler Intelligence Scale for Children (Wechsler, 1991). In addition, one ASD participant was 18 at the time of the clinic assessment (although he was 17 at time of his fMRI scan), and thus his IQ was determined using the Wechsler Adult Intelligence Scale (Wechsler, 2008). The ASD and TD groups differed significantly with regard to full scale IQ (ASDs: M = 105.0, SD = 9.5; TDs: M = 112.3, SD = 11.6; t (34) = 2.098, p = .043). Thus, we controlled for IQ in all between-group analyses.
Participants were recruited through UCLA's Center for Autism Research and Treatment, fliers distributed in the local community, and by word-of-mouth through participants who had previously participated in research studies at UCLA. Parents and participants provided written consent approved by the UCLA Institutional Review Board. The TD participants had no history of psychiatric or neurological disorders, according to parental report. All ASD participants had a prior diagnosis of ASD and had their diagnosis confirmed at the UCLA Autism Evaluation Center prior to research involvement. Diagnoses were confirmed using either the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and/or the Autism Diagnostic Interview (ADI; Lord et al., 1994), as well as clinical judgment based upon DSM-IV diagnostic criteria. All 19 participants in the ASD group met criteria for Autism based on the ADI. According to the ADOS, 5 of the ASD participants met criteria for Autism, 10 met criteria for autism spectrum disorder, and 4 did not meet either of these criteria.
2.2. Procedures
On the day of their fMRI scan, participants visited UCLA's campus with their parents. They were told that during their fMRI scan they would be using the Internet to play a ball-tossing game with two other adolescents who had previously participated in our study, and it was made clear that these ‘previous participants’ did not know each other. In reality, these other ‘players’ were controlled by the computer. During this computer simulation, participants were ‘excluded’ by the other two players, and immediately following the scan, participants completed a manipulation check and self-reported their feelings of distress (see details of fMRI task and distress measure in Sections 2.3, 2.5, respectively). Finally, they were debriefed about the deception used in the study, and they were thoroughly questioned to ensure that they understood that the other players involved in the Cyberball game were not real people, and that the exclusion was fake and occurred the exact same way for all participants.
2.3. fMRI task
To simulate peer rejection during an fMRI scan, we used a computer task called “Cyberball”—an experimental paradigm that simulates a real interactive experience of social exclusion (Williams et al., 2000, Williams et al., 2002). Cyberball has been used successfully in several neuroimaging studies examining adolescents (Masten et al., 2009a, Masten et al., 2010a, Masten et al., 2010b, Masten et al., 2011a), and in behavioral studies examining youth with ASD (Sebastian et al., 2009), to simulate peer exclusion and elicit feelings of social distress. In addition, social exclusion is a particular useful proxy for peer rejection during adolescence given that isolating peers from social groups is one of the dominant methods used to reject peers at this age (Coie et al., 1990).
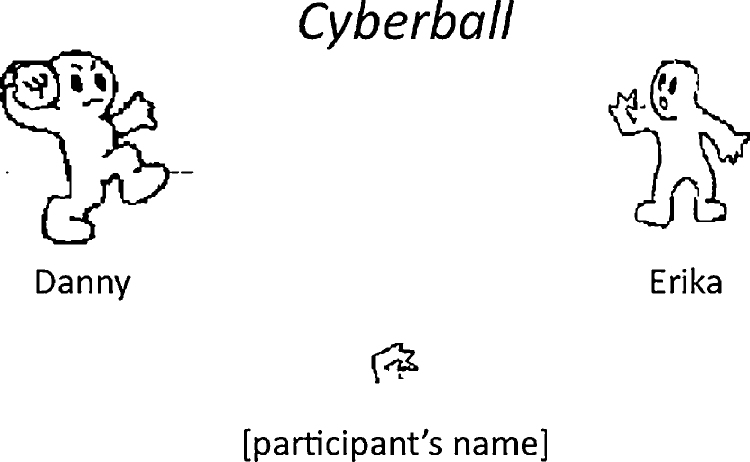
Cyberball is viewed on a computer screen through MRI compatible goggles while participants are undergoing an fMRI scan. Participants see cartoon images representing the other two players, as well as a cartoon image of their own ‘hand’ that they control using a button-box. Throughout Cyberball, the ball is thrown back and forth among the players, with the participant choosing the recipient of their own throws using the button-box, and the throws of the other two ‘players’ determined by a pre-set program (see graphical depiction of Cyberball in Fig. 1).
Fig. 1.
Graphic showing the Cyberball game as it appeared to participants. When participants received the ball in their ‘hand’ (visible on the bottom of the screen) during the inclusion portion of the game, they indicated the recipient of their next ball toss (i.e., the player on the right or left) using a button-box. During the exclusion portion of the game, the other players (i.e., ‘Danny’ and ‘Erika’) threw the ball back and forth to each other, and it was never thrown to the participant's ‘hand’.
In this study, participants played two rounds of Cyberball during two fMRI scans. Each round of Cyberball lasted 2 min and 8 s, and consisted of 60 ball tosses total, including all the participants’ tosses as well as the tosses of the two simulated players. In addition, there were 16 s preceding the game with the word “connecting” on a blank screen, during which time participants were told to wait while the connection among the players was established (to enhance the believability that players were connected via the internet). There were also 16 s of rest time following the game's conclusion, during which participants rested while viewing a blank screen. During the first round of Cyberball participants were ‘included’ throughout the game, and during the second round they were ‘excluded’ by the other players part way through the game. Throughout the inclusion round the computerized players were equally likely to throw the ball to the participant or to the other computerized player. However, during the exclusion round, the two computerized players stopped throwing the ball to the participant after the participant had received a total of 10 throws (i.e., after a total of 30 throws including those of the other players). At this point the participant watched as the other players threw the ball back and forth to each other for the remainder of the game (i.e., an additional 30 throws). Thus, the exclusion portion of the second round, following the participant's first 10 throws, consisted of half of the total number of ball tosses and lasted for 58 s.
2.4. Manipulation check
A manipulation check was administered to ensure participants’ engagement in the Cyberball game and their awareness of being excluded during the second round. Immediately following each round of Cyberball, participants were asked “How often did you get the ball thrown to you?” Participants provided answers using a scale ranging from 1 = “Never” to 7 = “All the time”.
2.5. Self-reported social distress
Following completion of the Cyberball task, adolescents completed the Need-Threat Scale (NTS; Williams et al., 2000, Williams et al., 2002) in order to measure social distress associated with being excluded during the game. The NTS assesses 12 subjectively experienced consequences of being excluded during the game, including ratings of self-esteem (“I felt liked”), belongingness (“I felt rejected”), meaningfulness (“I felt invisible”), and control (“I felt powerful”), on a scale ranging from 1 = “not at all” to 5 = “very much”. Items were reverse-coded when appropriate and averaged to create a composite score with good reliability for each group (ASDs: α = .91; TDs: α = .76). This measure has been used previously to assess self-reports of distress among both typically developing adolescents (Masten et al., 2009a, Masten et al., 2011a), and those with ASD (Sebastian et al., 2009).
2.6. fMRI data acquisition
Brain images during Cyberball were collected using a Siemens Trio 3-Tesla MRI scanner. Extensive instructions and foam padding were provided to decrease motion. For each participant, an initial 2D spin-echo scout-localizing scan (TR = 8.6 ms, TE = 4.0 ms, matrix size 256 by 256, FoV = 25 cm) was acquired in order to enable prescription of slices obtained in functional scans. In addition, a high-resolution structural scan (TR = 5000 ms, TE = 34 ms, matrix size 128 by 128, FoV = 19.2 cm, 34 slices, yielding an in-plane voxel dimension of 1.5 mm × 1.5 mm, with 4 mm thick axial slices) coplanar with the functional scans was obtained for functional image registration during fMRI analysis preprocessing. The functional tasks were presented on a computer screen through MR-compatible goggles, during the functional scans lasting 2 min and 40 s each (TR = 2000 ms, TE = 28 ms, matrix size 64 × 64, FoV = 19.2 cm, 34 slices, yielding an in-plane voxel dimension of 3 mm × 3 mm, with 4 mm thick axial slices).
2.7. fMRI data analysis
2.7.1. Software and preprocessing
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK), and ROI extraction was performed using the MARsBaR toolbox within SPM (MARSeille Boîte À Région d’Intérêt; Brett et al., 2002). Preprocessing included image realignment to correct for head motion (groups did not differ in terms of head motion), normalization into a standard stereotactic space defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping, and spatial smoothing using an 8 mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio.
2.7.2. Modeling of contrasts
The inclusion and exclusion rounds of Cyberball were modeled using a block design. Each round of Cyberball was modeled as a run with each period of inclusion and exclusion modeled as a block within the run for a total of two inclusion blocks (one during the first run and one at the beginning of the second run) and one exclusion block. The “connecting” time preceding the game and the rest time after completion of the game were not modeled. Linear contrasts comparing exclusion to inclusion were calculated for each participant. These individual contrast images were then used in group-level analyses.
2.7.3. ROI analyses
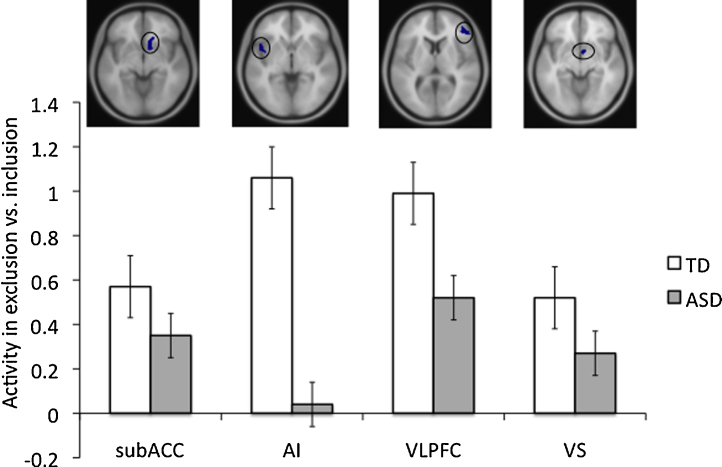
Given our interest in examining group differences in the neural substrates of peer rejection among adolescents with ASD versus typically developing adolescents, we performed region-of-interest (ROI) analyses focused on several a priori defined brain areas. Specifically, we examined four ROIs (functionally defined using the MARsBaR toolbox), in which activity during exclusion vs. inclusion was previously found to relate to self-reported social distress resulting from peer rejection (in a whole-brain regression thresholded at p < .005, 10 voxels), among two prior samples of typically developing adolescents. The adolescents in both of these prior samples ranged in age from 12 to 13, and did not overlap with any of the typically developing adolescent controls included in the current study. These ROIs included clusters in the subACC (peak voxel: [8 22 −4], t = 4.06, p = .0005, k = 151 voxels), in which activity was previously found to be positively related to social distress in a sample of 20 adolescents (see Masten et al., 2011a), and AI (peak voxel: [−46 8 −4], t = 3.72, p = .001, k = 65 voxels), in which activity was previously found to be positively related to social distress in a sample of 23 adolescents (see Masten et al., 2009a), as well as in the VLPFC (peak voxel: [42 46 14], t = 4.41, p = .0005, k = 182 voxels) and VS (peak voxel: [6 4 −8], t = 4.19, p = .000, k = 15 voxels), in which activity was previously found to be negatively related to social distress in a sample of 23 adolescents (see Masten et al., 2009a). These four ROIs are depicted visually in Fig. 2. Mean parameter estimates for each participant (that modeled the amplitude of the BOLD response during exclusion vs. inclusion) were then extracted and averaged across all voxels in each ROI. First, groups were analyzed separately to see whether each group displayed significantly more activity in each ROI during exclusion compared to inclusion. Then, group-level t-tests comparing the mean activity during exclusion versus inclusion in each ROI for TDs versus ASDs were calculated. These analyses included IQ as a covariate in order to control for between-group differences in IQ. Analyses were run in MARsBaR, and a standard statistical threshold of p < .05 was used for these ROI analyses.
Fig. 2.
Average activity during exclusion vs. inclusion in the subgenual anterior cingulate cortex (subACC), anterior insula (AI), ventrolateral prefrontal cortex (VLPFC), and ventral striatum (VS) region of interests (ROIs), for the typically developing (TD) and autism spectrum disorder (ASD) groups. Each functionally defined ROI is shown in the figures above the corresponding bar graphs. Error bars reflect standard error.
2.7.4. Whole-brain analyses
In order to supplement the ROI analyses, we performed a group level analysis comparing activity during exclusion versus inclusion at each voxel across the entire brain volume. First, we examined this contrast within each group separately, and then we conducted a between-groups analysis to compare the difference in activity during exclusion versus inclusion in TDs versus ASDs (controlling for IQ). Given that a large number of tests were required to examine differential activity across the whole brain volume, we employed a conservative threshold that corrected for multiple comparisons (FDR corrected in SPM5; p < .01, 10-voxel minimum cluster size). In addition, given that this threshold is highly conservative when examining social cognitive processes (Lieberman and Cunningham, 2009), for each whole-brain analysis we also performed an exploratory follow-up examination at a threshold of p < .005, 10 voxels (uncorrected). This lowered threshold provides a better balance between Type I and Type II error (Lieberman and Cunningham, 2009) and is similar to those thresholds used in previous studies examining social rejection and peer-related processing (e.g., DeWall et al., 2010, Masten et al., 2009a, Masten et al., 2010a, Masten et al., 2010b, Pfeifer et al., in press). All coordinates are reported in Montreal Neurological Institute (MNI) format.
2.7.5. Regression analyses
Finally, we examined how social distress following exclusion related to neural activity during exclusion versus inclusion, within each group. First, standard statistical software (SPSS 16.0, Chicago, IL) was used to examine how self-reported NTS scores correlated with parameter estimates of brain activity during exclusion versus inclusion in each ROI. Second, we examined the correlation between self-reported NTS scores and activity during exclusion versus inclusion at each voxel across the entire brain volume. The statistical threshold was the same as that used in the whole-brain analyses described in Section 2.7.4.
3. Results
3.1. Manipulation check
Participants’ reports of how often the ball was thrown to them (on a scale from 1 to 7) suggested that both groups were engaged in the game and noticed the exclusion during the second round. The mean for the TD group was 4.88 (SD = .93) for the inclusion round, and 2.62 (SD = 1.27) for the exclusion round. The mean for the ASD group was 4.42 (SD = .88) for the inclusion round, and 2.35 (SD = .91) for the exclusion round. There was a marginal group difference in inclusion scores (F = 1.74, p = .09) but no difference in exclusion scores (F = .45, ns) after controlling for IQ. In addition, the inclusion scores for both groups were significantly greater than the exclusion scores (for TDs: t = 6.30, p < .001; for ASDs: t = 8.61, p < .001), suggesting that both groups noticed the exclusion. Finally, this difference between the exclusion and inclusion scores did not differ across groups (F = 1.08, ns; controlling for IQ).
3.2. Self-reported distress
Participants in both groups reported moderate levels of social distress following exclusion. The mean NTS score for adolescents with ASD was 3.71 (SD = .87), while TDs reported a mean NTS score of 3.52 (SD = .57); groups did not differ significantly (F = .447, ns; controlling for IQ).
3.3. ROI analyses
3.3.1. Within-group analyses
First, we examined activity during exclusion versus inclusion separately for TDs and ASDs. The TD group displayed significantly greater activity during exclusion versus inclusion in both the subACC (t = 1.59, p < .05) and AI ([−46 8 −4]: t = 2.33, p < .05; [−34 22 0]: t = 2.50, p < .05), as well as in the VLPFC (t = 3.25, p < .01). The difference in activity in the VS was not significant, but showed a trend consistent with the other ROIs (t = 1.17, p = .13). Similarly, the ASD group displayed significantly greater activity during exclusion versus inclusion in the subACC (t = 2.83, p < .01) and AI ([−34 22 0]: t = 2.65, p < .01; [−46 8 −4]: t = .20, ns), as well as in the VLPFC (t = 2.58, p < .01) and marginally in the VS (t = 1.48, p = .08). Controlling for IQ in each of these within-group ROI analyses yielded nearly identical results.
3.3.2. Between-group analyses
To examine group differences in neural activity during peer rejection, we compared TDs’ and ASDs’ activity during exclusion versus inclusion in each of our ROIs and controlled for IQ. The TD group displayed significantly more activity in the subACC (t = 1.57, p < .05) and AI ([−46 8 −4]; t = 2.53, p < .01), that is, areas previously found to be more active to the extent that adolescents were more distressed by exclusion (Masten et al., 2009a, Masten et al., 2011a), as well as in the VLPFC (t = 1.69, p < .05), an area previously found to be more active to the extent that adolescents were less distressed by exclusion (Masten et al., 2009a). The group difference in activity in the VS was not significant, but showed a trend consistent with the other ROIs (t = 1.16, p = .13). Bar graphs illustrating these differential group means are displayed in Fig. 2.
3.4. Whole-brain analyses
3.4.1. Within-group analyses
Next, we separately examined TDs’ and ASDs’ activity during exclusion versus inclusion across the whole brain. Neither group showed a significant difference in activity in any region at the FDR-corrected threshold (similarly, neither group displayed a significant difference in activity when examining the reverse contrast, inclusion versus exclusion). However, when we performed exploratory analyses at a lowered threshold (p < .005, 10 voxels, uncorrected; see Section 2.7.4) for each group, results were consistent with the ROI analyses. The TD group showed greater activity during exclusion than inclusion in the subACC and AI, as well as in the VLPFC and VS. The ASD group similarly showed greater activity during exclusion versus inclusion in the subACC, AI, VLPFC and VS. Details of these and other areas of significant activation observed at these more liberal thresholds are listed in Supplementary Table 1, sections A (for TDs) and B (for ASDs). Again, controlling for IQ in each of these within-group whole-brain analyses yielded nearly identical results.
3.4.2. Between-group analyses
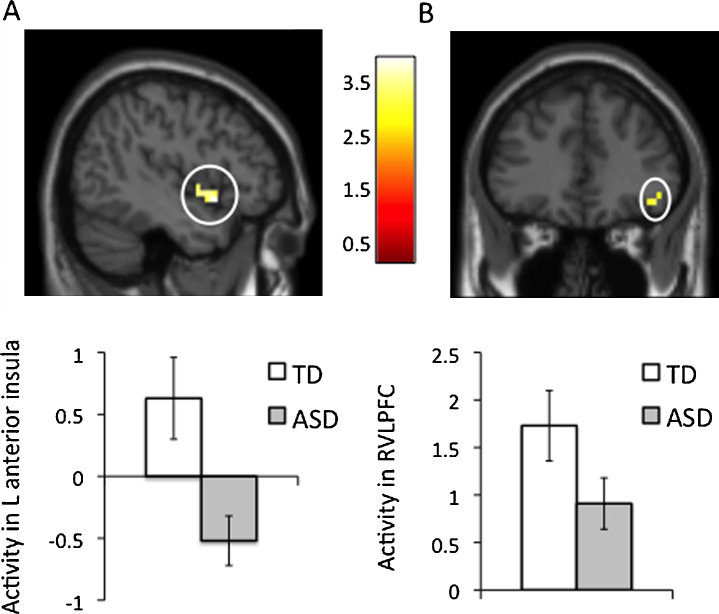
To directly compare the groups, we examined how TDs’ activity during exclusion versus inclusion compared to that of ASDs across the entire brain, controlling for IQ. There were no significant group differences at our initial FDR-corrected threshold; however, exploratory analyses at a lowered threshold (p < .005, 10 voxels, uncorrected; see Section 2.7.4) revealed group differences that were generally consistent with ROI analyses. The TD group displayed greater activity than ASDs in the left AI (see Fig. 3A) and right AI, but not in the subACC. In addition, TDs displayed greater activity than ASDs in both the VLPFC (see Fig. 3B) and VS. See Supplementary Table 1C for details of these exploratory between-group findings.
Fig. 3.
Activity found in exploratory whole-brain analyses (performed at a lowered threshold of p < .005, 10 voxels, uncorrected) to be greater during exclusion vs. inclusion among typically developing (TD) adolescents compared to those with autism spectrum disorders (ASD), after controlling for IQ, in the: (A) left anterior insula (AI; −45 12 −8), and (B) right ventrolateral prefrontal cortex (VLPFC; 45 36 −12). Bar graphs show the difference in activity during exclusion vs. inclusion, averaged across the corresponding circled cluster, for each group. Error bars reflect standard error.
3.5. Correlations with self-reported distress
Finally, within each group we investigated whether participants’ self-reported distress following their exclusion related to their brain activity during exclusion versus inclusion. Somewhat unexpectedly, there were no significant correlations between any of our ROIs and participants’ distress within the TD group (subACC: r = −.009, AI [−46 8 −4]: r = .09, AI [−34 22 0]: r = .24, VLPFC: r = −.01, VS: r = .10; all p-values > .38); however, two of these correlations were significant among the ASD group (subACC: r = .39, p < .05, AI [−46 8 −4]: r = −.003, ns, AI [−34 22 0]: r = .12, ns, VLPFC: r = .39, p < .05, VS: r = .26, ns). Whole-brain regression analyses revealed no significant correlations between NTS scores and brain activity during exclusion versus inclusion for either group (and exploratory analyses at the lowered threshold of p < .005, 10 voxels also revealed no significant correlations). Controlling for IQ did not meaningfully change these findings.
4. Discussion
This study is one of the first to examine how adolescents with ASD may differ from typically developing adolescents in their neural responsivity to peer rejection. Specifically, the findings described here indicate that adolescents with ASD may differentially process peer rejection experiences at the neural level, despite evidence of similar self-reported distress responses. In the following discussion, we interpret these findings and speculate about their meaning in order to help direct future investigations of peer-related processing among individuals with ASD.
In terms of subjective responses to peer exclusion, we found that adolescents with ASD and controls did not differ in their self-reported feelings of distress following the exclusion round of Cyberball. These findings are consistent with our hypothesis, and previous research indicating, that adolescents with ASD feel just as rejected as their typically developing counterparts when they are excluded by peers (Sebastian et al., 2009).
Despite these similar behavioral findings, neuroimaging analyses indicated that adolescents with ASD showed reduced neural engagement compared to typically developing adolescents during peer rejection. Specifically, ROI analyses (as well as exploratory whole-brain analyses performed at a lowered threshold) revealed less activity during exclusion versus inclusion among adolescents with ASD compared to controls in regions previously shown to be positively related to adolescents’ distress during exclusion (i.e., subACC, AI), as well as in regions previously shown to be negatively related to distress during exclusion (i.e., VLPFC, VS). This is consistent with many prior studies indicating that individuals with ASD display hypoactivation in brain regions involved in emotion processing—including the subACC and AI specifically—when performing a range of social cognitive tasks (see Di Martino et al., 2009 for a review). Furthermore, these findings are consistent with those reported by Sebastian and colleagues (2009) indicating that individuals with ASD may show less variation in mood as a result of peer rejection. Of course, neural activity in affective-processing regions is only one potential proxy of mood, and more direct assessments of mood (such as those collected by Sebastian and colleagues) may have impacted our reported findings; however, the heightened neural activity in subACC and AI in our control group is consistent with the notion that ASDs were less affectively impacted by the rejection. Finally, although the role of oxytocin was not the primary focus of this investigation, group differences were found in several limbic areas similar to those previously linked with oxytocin production, including portions of the insula and cingulate, as well as the amygdala (see Supplementary Table 1; Ferguson et al., 2002, Huber et al., 2005, Landgraf and Neumann, 2004, Lim and Young, 2006). Thus, our findings are consistent with those found by Andari and colleagues suggesting that neural activity related to oxytocin production may relate to the differential social processing observed among ASDs and controls.
This differential engagement of neural circuitry in response to peer rejection could also be related to a number of qualitative factors related to peer rejection experiences that adolescents with ASD have in their daily lives. For example, adolescents with ASD may be more habituated to the experience of being rejected, since it happens to them more frequently. As a result, their neural responses might be dampened, given the familiarity of the experience. A second possibility is that adolescents with ASD may actually expect to be rejected when they interact with novel groups of peers. Researchers have proposed that one important component of typical neural responses to social rejection is the detection and recognition that a social expectancy (i.e., being included) is being violated (Eisenberger and Lieberman, 2004, Somerville et al., 2006, Bolling et al., 2010). Thus, the reduced neural responses to rejection observed in adolescents with ASD may reflect the fact that they have learned through experience that they are not likely to be included, even if they experience subjective distress upon later reflection. Finally, related to this possibility, adolescents with ASD might respond to social rule-breaking in unique ways. Given that they often display difficulties following social rules and initiating positive peer interactions (Attwood, 2000, Hauck et al., 1995, Sigman and Ruskin, 1999), they may be responding in ways different than typically developing adolescents when they witness similar indiscretions being committed by others.
It is important to note that in the current study, we did not replicate previously found associations between self-reported distress and brain activity during peer exclusion within our typically developing group. Previous findings in our lab have indicated that adolescents display greater activity in the subACC and AI to the extent that they are more distressed by exclusion and greater activity in the VLPFC and VS to the extent that they are less distressed by exclusion (Masten et al., 2009a); we propose two possibilities for why we did not find similar patterns here. First, the current sample was almost all male, whereas the previously tested sample comprised slightly more females than males. Thus, one possibility is that girls and boys show differential associations between their neurobiological and subjective responses to peer-related social stimuli—a possibility that is not surprising given known gender differences in peer-related processes at this age (e.g., Guyer et al., 2009, Rose and Rudolph, 2006). Second, the current sample spanned a wide adolescent age range, whereas the previous study included only 12–13 year olds. Thus, it is possible that the correspondence between self-reported and neural affective responses to peer rejection is particularly pronounced in the years immediately following the transition to middle school (i.e., around age 13)—when peer rejection becomes particularly prevalent (e.g., Nansel et al., 2001) and the social influence of peers increases (e.g., Masten et al., 2009b). Of course, many other variables may have also contributed to the inconsistency between the current findings and those described in Masten et al. (2009a). Thus, future studies will be useful in exploring the influence of both gender and age, as well as additional factors such as pubertal status, brain development and social environment, that may impact these kinds of brain-behavior correlations.
With regard to our ASD sample, the correlational findings between self-reported distress and brain activity during peer rejection were somewhat surprising. Consistent with our previous work examining typically developing adolescents, we found a positive correlation between subACC and distress, but we also found a positive correlation between VLPFC and distress, which ran counter to previous findings in typically developing adolescents. Given that the VLPFC is typically thought to play a regulatory role in the context of social rejection (e.g., Eisenberger et al., 2003, Masten et al., 2009a, Masten et al., 2011b), one possibility is that this positive correlation reflects an ineffective attempt to regulate distress resulting from peer rejection among the ASD group. Future examination of these brain-behavior correlations and regulatory strategies specifically among ASD populations will shed additional light on this possibility.
As a whole, this study provides an important first step toward understanding how adolescents with ASD may differentially perceive and respond to peer rejection—an event that is especially salient during adolescence generally, but also particularly frequent among this population. Moreover, given that adolescents with ASD and typically developing adolescents displayed differential neural responses despite reporting similar subjective ratings, these findings highlight the importance of using neuroimaging techniques to examine processes of peer functioning in ASD. Our hope is that future investigations into the neural processes involved in social deficits in ASD will lead to better understanding of the mechanisms underlying the social atypicalities that characterize this population, and eventually inform intervention and therapy practices.
Acknowledgments
The authors wish to thank Deanna Greene. This work was supported by the National Institute of Child Health and Human Development [P50 HD055784], and a Ruth L. Kirschstein National Research Service Award (awarded to C.L. Masten). For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, and Northstar Fund. This project was in part also supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.01.004.
Appendix A. Supplementary data
References
- Andari E., Duhamel J.R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood T. Jessica Kingsley; London: 1998. Asperger's Syndrome: A Guide for Parents and Professionals. [Google Scholar]
- Attwood T. Strategies for improving the social integration of children with Asperger syndrome. Autism. 2000;4:85–100. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, mathematicians and scientists. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bauminger N., Kasari C. Loneliness and friendship in high-functioning children with Autism. Child Dev. 2000;71:447–456. doi: 10.1111/1467-8624.00156. [DOI] [PubMed] [Google Scholar]
- Bolling D.Z., Pitskel N.B., Deen B., Crowley M.J., McPartland J.C., Mayes L.C., Pelphrey K.A. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2010;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:1140–1141. [Google Scholar]
- Brown B.B. Adolescents’ relationships with peers. In: Lerner R., Steinberg L., editors. Handbook of Adolescent Psychology. John Wiley & Sons, Inc; Hoboken, NJ: 2004. pp. 363–394. [Google Scholar]
- Burklund L., Eisenberger N.I., Lieberman M.D. The face of rejection: rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc. Neurosci. 2007;2:238–253. doi: 10.1080/17470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capps L., Sigman M., Yirmiya N. Self-competence and emotional understanding in high-functioning children with autism. Dev. Psychopathol. 1996;7:137–149. [Google Scholar]
- Church C., Alisanski S., Amanullah S. The social, behavioral, and academic experiences of children with Asperger syndrome. Focus Autism Dev. Disabil. 2000;15:12–20. [Google Scholar]
- Coie J.D., Dodge K.A., Kupersmidt J.B. Peer group behavior and social status. In: Asher S.R., Coie J.D., editors. Peer rejection in childhood. Cambridge studies in social and emotional development. Cambridge University Press; New York: 1990. pp. 17–59. [Google Scholar]
- Csikszentmihalyi M., Larson R. Basic Books; New York: 1984. Being Adolescent: Conflict and Growth in the Teenage Years. [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall C.N., MacDonald G., Webster G.D., Masten C.L., Baumeister R.F., Powell C., Combs D., Schurtz D.R., Stillman T.F., Tice D.M., Eisenberger N.I. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol. Sci. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Ross K., Uddin L.Q., Sklar A.B., Castellanos F.X., Milham M.P. Functional brain correlates of social and nonsocial processes in Autism Spectrum Disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein D.P., Leibenluft E. Emotional regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Dev. Psychopathol. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.L., Lieberman M.D., Williams K.D. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ferguson J.N., Young L.J., Insel T.R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: confusion and controversies about Asperger syndrome. J. Child Psychol. Psychiatry. 2004;45:672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Frith U., Hill E.L. Oxford University Press; Oxford, England: 2004. Autism: Mind and Brain. [Google Scholar]
- Greimel E., Schulte-Ruther M., Kircher T., Kamp-Becker I., Remschmidt H., Fink G.R., Herpertz-Dahlmann B., Konrad K. Neural mechanisms of empathy in adolescents with Autism Spectrum Disorder and their fathers. Neuroimage. 2010;49:1055–1065. doi: 10.1016/j.neuroimage.2009.07.057. [DOI] [PubMed] [Google Scholar]
- Guastella A.J., Mitchell P.B., Dadds M.R. Oxytocin increases gaze to the eye region of human faces. Biol. Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., McClure-Tone E.B., Shiffrin N.D., Pine D.S., Nelson E.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq I., Le Couteur A. Autism Spectrum Disorder. Medicine. 2004;32:61–63. [Google Scholar]
- Hauck M., Fein D., Waterhouse L., Feinstein C. Social initiations by autistic children to adults and other children. J. Autism Dev. Disord. 1995;25:579–595. doi: 10.1007/BF02178189. [DOI] [PubMed] [Google Scholar]
- Howlin R. Asperger syndrome in the adolescent years. In: Holliday Willey L., editor. Asperger Syndrome in Adolescence: Living with the Ups and Downs and Things in Between. Jessica Kingsley; London: 2003. pp. 13–37. [Google Scholar]
- Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P.J., Fischbacher U., Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lim M.M., Young L.J. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 2006;51:292–293. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Jarcho J.M., Berman S., Naliboff B.D., Suyenobu B.Y., Mandelkern M., Mayer E.A. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. Putting feelings into words: affect labeling disrupts amygdala activity to affective stimuli. Psychol. Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Little L. Peer victimization of children with Asperger Spectrum Disorders. J. Am. Acad. Child Psychiatry. 2001;40:995–996. doi: 10.1097/00004583-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Little L. Middle-class mothers’ perceptions of peer and sibling victimization among children with Asperger's syndrome and nonverbal learning disorders. Iss Comp Pediat Nurs. 2002;25:43–57. doi: 10.1080/014608602753504847. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E., Leventhal B., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., Le C.A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Martlew M., Hodson J. Children with mild learning difficulties in an integrated and in a special school: comparisons of behaviour, teaching and teachers’ attitudes. Brit. J. Educ. Psychol. 1991;61:355–372. doi: 10.1111/j.2044-8279.1991.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., McNealy K., Pfeifer J.H., Dapretto M. Subgenual anterior cingulate responses to peer rejection: a neural marker of adolescents’ risk for depression. Dev. Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., Pfeifer J.H., McNealy K., Mazziotta J., Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Pfeifer J.H., Dapretto M. Witnessing peer rejection during adolescence: neural correlates of empathy for experiences of social exclusion. Soc. Neurosci. 2010;5:496–507. doi: 10.1080/17470919.2010.490673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Juvonen J., Spatzier A. Relative importance of parents and peers: differences in academic and social behaviors at three grade levels spanning late childhood and early adolescence. J. Early Adolesc. 2009;29:773–799. [Google Scholar]
- Masten C.L., Telzer E.H., Fuligni A.J., Lieberman M.D., Eisenberger N.I. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc. Cogn. Affect Neurosci. 2010 doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Telzer E.H., Eisenberger N.I. An fMRI investigation of attributing negative social treatment to racial discrimination. J. Cogn. Neurosci. 2011;23:1042–1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Mesibov G.B., Handlan S. Adolescents and adults with Autism. In: Cohen D.J., Volkmar F.R., editors. Handbook of Autism and Pervasive Developmental Disorders. John Wiley & Sons, Inc.; New York: 1997. pp. 309–324. [Google Scholar]
- Miller P.M., Ingham J.G. Friends, confidants, and symptoms. Soc. Psychiatry. 1976;11:51–58. [Google Scholar]
- Nansel T.R., Overpeck M., Pilla R.S., Ruan J.W., Simons-Morton B., Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA – J. Am. Med. Assoc. 2001;285:2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst J.T., Hopmeyer A. Sociometric popularity and peer-perceived popularity: two distinct dimensions of peer status. J. Early Adolesc. 1998;18:125–144. [Google Scholar]
- Pfeifer, J.H., Masten, C.L., Moore, W.E., Oswald, T.M., Iacoboni, M., Mazziotta, J.C., Dapretto, M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, in press. [DOI] [PMC free article] [PubMed]
- Pierce K., Glad K.S., Schreibman L. Social perception in children with Autism: an attentional deficit? J. Autism Dev. Disord. 1997;27:265–282. doi: 10.1023/a:1025898314332. [DOI] [PubMed] [Google Scholar]
- Prior M., Leekam S., Ong B., Eisenmajer R., Wing L., Gould J., Dowe D. Are there subgroups with in autism spectrum? A cluster analysis of a group of children with Autism Spectrum Disorder. J. Child Psychol. Psychiatry. 1998;39:893–902. [PubMed] [Google Scholar]
- Rose A.J., Rudolph K.D. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol. Bull. 2006;132:98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Brody A.L., Ho M.L., Zohrabi N., Maidment K.M., Baxter L.R. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am. J. Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland A.A., McNealy K., Wang A.T., Sigman M., Bookheimer S.Y., Dapretto M. No neural evidence of statistical learning during exposure to artificial languages in children with Autism Spectrum Disorders. Biol. Psychiatry. 2010;68:345–351. doi: 10.1016/j.biopsych.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C.L., Blakemore S.-J., Charman T. Reactions to ostracism in adolescents with Autism Spectrum Conditions. J. Autism Dev. Disord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Sigman M., Ruskin E. Continuity and change in the social competence of children with Autism, Down Syndrome, and developmental delays. Monogr. Soc. Res. Child. 1999;64:114. doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Heatherton T.F., Kelley W.M. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Tesink C.M.J.Y., Buitelaar J.K., Petersson K.M., van der Gaag R.J., Kan C.C., Tendolkar I., Hagoort P. Neural correlates of pragmatic language comprehension in Autism Spectrum Disorders. Brain. 2009;132:1941–1952. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- van Roekel E., Scholte R.H., Didden R. Bullying among adolescents with Autism Spectrum Disorders: prevalence and perception. J. Autism Dev. Disord. 2010;40:63–73. doi: 10.1007/s10803-009-0832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar F.R., Klin A. Social development in Autism: historical and clinical perspectives. In: Baron-Cohen S., Tager-Flusberg H., Cohen D.J., editors. Understanding Other Minds: Perspectives from Autism. Oxford University Press; New York: 1995. pp. 40–55. [Google Scholar]
- Volkmar F.R., Klin A. Diagnostic issues in Asperger syndrome. In: Klin A., Volkmar F.R., Sparrow S.S., editors. Asperger Syndrome. The Guilford Press; New York: 2000. pp. 25–71. [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch. Gen. Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; New York: 1991. WISC-III Manual. [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. The Wechsler Abbreviated Scale of Intelligence-UK. [Google Scholar]
- Wechsler D. 4th ed. Pearson; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale. [Google Scholar]
- Williams K.D., Cheung C.K., Choi W. Cyberostracism: effects of being ignored over the internet. J. Pers. Soc. Psychol. 2000;79:748. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams K.D., Govan C.L., Croker V., Tynan D., Cruickshank M., Lam A. Investigations into differences between social- and cyberostracism. Group Dyn.: Theory Res. 2002;6:65–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.