Abstract
Rationale
The cannabinoid system has risen to the forefront in the development of novel treatments for a number of pathophysiological processes. However, significant side effects have been observed in clinical trials raising concerns regarding the potential clinical utility of cannabinoid-based agents. Understanding the neural circuits and neurochemical substrates impacted by cannabinoids will provide a better means of gauging their actions within the central nervous system that may contribute to the expression of unwanted side effects.
Objectives
In the present study, we investigated whether norepinephrine (NE) in the limbic forebrain is a critical determinant of cannabinoid receptor agonist-induced aversion and anxiety in rats.
Methods
An immunotoxin lesion approach was combined with behavioral analysis using a place conditioning paradigm and the elevated zero maze (EZM).
Results
Our results show that the non-selective CB1/CB2 receptor agonist, WIN55,212-2, produced a significant place aversion in rats. Further, NE in the nucleus accumbens (Acb) was critical for WIN55,212-2-induced aversion but did not affect anxiety-like behaviors. Depletion of NE from the bed nucleus of the stria terminalis (BNST) was ineffective in altering WIN55,212-2-induced aversion and anxiety.
Conclusions
These results indicate that limbic, specifically accumbal, NE is required for cannabinoid-induced aversion but is not essential to cannabinoid-induced anxiety.
Keywords: Anxiety, conditioned place aversion, nucleus of the solitary tract, saporin, mood disorders
INTRODUCTION
The endocannabinoid system has been implicated in a variety of physiological functions due to abundant expression of its receptors and endogenous ligands in the central nervous system (CNS) (Herkenham et al. 1991; Mackie 2005; Mackie 2008) as well as in adipose tissue, gastrointestinal tract, skeletal muscle, heart and the reproductive system (for review, Pacher et al. 2006). The endocannabinoid system controls emotional reactivity, motivated behaviors and energy homeostasis. In the brain, the cannabinoid receptor type 1 (CB1r) is the most abundant while the cannabinoid receptor type 2 (CB2r) is found mainly in cells of the immune and hematopoietic systems (Piomelli 2003). The diverse localization of the endocannabinoid system underscores its importance as a potential target in the treatment of a variety of disorders. However, when targeting the endocannabinoid system a high number of unwanted side effects occur, as evidenced by increased incidence of anxiety and depression in obese patients treated with the CB1r antagonist, rimonabant (Steinberg and Cannon 2007). Cannabinoid agonists have also been shown to induce anxiety and dysphoria (Reilly et al. 1998; Williamson and Evans 2000). Hence, identifying neurochemical targets of cannabinoids is essential. Some studies have suggested that the dysphoric/aversive effects seen upon cannabinoid administration are due to its anxiogenic properties (McGregor et al. 1996; Ghozland et al. 2002). However, conclusive evidence is lacking to support this hypothesis.
The present study explored the role of limbic norepinephrine (NE) in cannabinoid-induced aversion and anxiety. NE is involved in cognition and attention (Aston-Jones et al. 1991) as well as in the pathophysiology of mood disorders (Heninger et al. 1996; Anand and Charney 2000). Previous studies have shown an interaction between the cannabinoid system and the NE system in areas such as the prefrontal cortex (PFC) (Oropeza et al. 2005; Oropeza et al. 2007; Page et al. 2007), nucleus accumbens (Acb) (Carvalho et al. 2010), locus coeruleus (LC) (Oropeza et al. 2005; Scavone et al. 2010) and the nucleus of the solitary tract (NTS) (Jelsing et al. 2009; Carvalho et al. 2010). Limbic regions such as the Acb and bed nucleus of the stria terminalis (BNST) have been implicated in aversive and anxiety-like behaviors (Davis 1998; Aston-Jones et al. 1999; Ventura et al. 2007; Carlezon and Thomas 2009). In the present study, we investigated the role of NE in the Acb and BNST in cannabinoid-induced aversion and anxiety. For this purpose, an immunotoxin lesion approach was used to target NE fibers in the Acb and BNST and behavioral tests were performed on rats after administration of a CB1r/CB2r agonist, WIN55,212-2.
METHODS
Subjects
Sixty four male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 220–250g were housed two or three per cage in a controlled environment (12-hour light schedule, temperature at 20°C). Food and water were provided ad libitum. The care and use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Thomas Jefferson University and were conducted in accordance with the NIH Guide for the care and use of laboratory animals. All efforts were made to minimize animal suffering and reduce the number of animals used.
Surgery
Rats were anesthetized with an intraperitoneal (i.p.) injection of a saline solution containing a cocktail of Ketamine HCl (100mg/kg; Phoenix Pharmaceutical, Inc. St. Joseph, MO) and Xyla-Ject (2mg/kg; Phoenix Pharmaceutical, Inc.) and subsequently placed in a stereotaxic surgical frame (Stoelting Corp., Wood Dale, IL). The anesthesia was maintained by administration of isoflurane (Webster Veterinary Supply, Inc., Sterling, MA) through a nose cone. Animals received bilateral injections of saporin conjugated with an antibody against dopamine-beta-hydroxylase (DSAP, Chemicon International, Inc., Temecula, CA; 0.21μg/μl in phosphate buffer (PB), pH 7.4) or control solution with non-conjugated saporin (SAP, AdvancedTargeting Systems, San Diego, CA, 0.0441μg/μl in PB) into the Acb (n= 32, 250nl bilaterally, AP: 1.7mm rostral to bregma, ML: +/− 0.8mm, DV: −7.0mm) or the BNST (n=32, 300nl bilaterally, AP: 0.4mm caudal to bregma, ML: +/−4.0mm, DV: −7.4mm, with an angle of 19.6°), according to Rat Brain Atlas of Paxinos and Watson (1997) coordinates. The dose of DSAP and SAP used was based on previously published studies (Ritter et al. 2001; Ritter et al. 2003). The volume of DSAP and SAP injected at each site was determined from pilot experiments in our laboratory using a similar protocol. Previous immunohistochemical studies indicated that a period of 2 weeks was sufficient for transport of the immunotoxin and degeneration of the affected neurons (Wrenn et al. 1996; Ritter et al. 2003). Therefore, animals were given 15 to 18 days before the start of the behavioral tests described below.
Drug preparation and administration
WIN 55,212-2 (Sigma-Aldrich, St. Louis, MO) was dissolved in 5% dimethyl sulfoxide (DMSO)(Fisher Scientific, Fair Lawn, NJ) in 0.9% saline and injected i.p. (3.0mg/kg) in a volume of 1ml/kg body weight. Vehicle injections consisted of 5% DMSO in 0.9% saline.
Place conditioning
An unbiased place conditioning procedure was used, so that the side of the apparatus used to conditioned animals was counterbalanced in all the groups. The paradigm consisted of three phases: pre-test, conditioning and test. On pre-test day (day 1), animals were placed in the apparatus and allowed to freely explore both sides of the apparatus for 20 min. The time spent in each side was recorded by an investigator and animals with preference for one side higher than 200 sec were removed from the study (8 animals of a total of 64). During the conditioning phase (days 2–6), the rats were injected twice daily. In the morning, animals were injected with vehicle and confined to one side of the apparatus for 45 min. In the afternoon, animals were injected with WIN 55,212-2 (3.0mg/kg) and confined to the opposite side for 45 min. Control groups of animals received vehicle in both sessions. On the test day (day 7), animals were placed in the apparatus and allowed to explored both sides for 20 min. The test trial was recorded on camera and time spent in each side was measured by an investigator. No injection was given to the animals on the test day.
Spatial reference memory test
To verify that the lesion of noradrenergic input to the Acb and BNST did not alter spatial memory performance, animals were tested in the spatial reference memory test (Morris 1984). Animals were tested four days following place conditioning. WIN55,212-2 was not injected at any point during the test period. This control experiment was included to verify that spatial memory was intact in animals with a selective depletion of norepinephrine in the Acb and BNST. The test was conducted in a circular black tank (1.8m diameter) filled to a depth of 31 cm with water at 22°C and placed in a dimly lit room with extrinsic clues. The hidden platform remained at a fixed spatial location for the entire acquisition period. The acquisition phase consisted of four daily trials (inter-trial interval of 30 to 45 minutes) over four days. Each trial started with the animals being placed into the water, facing the wall of the maze, at one of four starting points: N, E, S, W. Four different starting positions were randomly used in each training block. A trial was considered complete when the rat escaped onto the platform; when this escape failed to occur within 120 s, the animal was gently guided to the platform and an escape latency of 120 s was recorded for that trial. Ratswere allowed to spend 10 s on the escape platform before being returned to home cage. Time needed to reach the platform (escape latency), length of the path described (distance swam) and swim velocity were recorded using HVS Image 2020 Plus tracking system (Version 9/05, HVS Image, Buckingham, UK).
The probe trial was assessed after the last trial of the acquisition period, removing the platform from the pool. Animals were released on the side opposite to where the platform was for a single trial of 60 sec, during which the percent time spent in each quadrant was measured. For analysis, the time spent in the target quadrant was compared with the average time spent in the remaining three quadrants.
Elevated Zero Maze (EZM)
The EZM is a modification of the elevated plus maze that is also a reliable and sensitive model of anxiety-like behavior in rodents (Shepherd et al. 1994). The EZM consists of a black ABS plastic annular platform (~120 cm diameter) elevated ~70 cm above the ground. It is divided into four equal quadrants which are ~20 cm wide: two open and two closed. The two open quadrants are opposite each other and are surrounded by a 1 cm “lip”. The two closed quadrants are enclosed by walls (~27 cm high) on both the inner and outer edges of the platform. Testing was conducted the day after the spatial reference memory test in a dimly lit room with a constant illumination on the open arms of the maze. Vehicle and WIN 55,212-2 were injected i.p. 30–35 min prior to the start of the test. At the start of the 10 min testing session, each rat was placed on the same open arm facing the center of the maze. The maze was cleaned with 65% ethanol and dried after each testing session. Time spent in the close arm and total number of entries were used as the output measure for this maze.
Locomotor activity
After the EZM, locomotor activity was assessed in a subset of animals from each treatment group to determine whether treatment influenced locomotor activity. Animals were placed in a home cage-like environment within the Home Cage Video Tracking System (Med Associates, St. Albans, VT) which includes a sound-attenuating cubicle, video tracking interface and Activity Monitor 5 software (Med Associates). Distance traveled was recorded by the video tracking system for 10 min.
Immunohistochemistry
At the conclusion of testing, animals were deeply anesthetized with an i.p. injection of sodium pentobarbital (60 mg/kg) and transcardially perfused with 50ml of heparinized saline followed by 400ml of 4% formaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1M PB (pH7.4). After perfusion, brains were removed and postfixed in the same fixative. Following post-fixation, brains were cryoprotected in a gradient of sucrose solutions (containing 0.1% sodium azide) of 10% and 20% sucrose in 0.1M PB for 1 hour each and 30% sucrose for 48–72 h. Brains were immersed in O.C.T. Embedding Compound (Electron Microscopy Sciences, Hatfield, PA) and frozen in dry ice. Coronal sections of the forebrain (35um) were cut using a Microm HM550 cryostat (Richard-Allan Scientific, Kalamazoo, MI) in multiple sets and collected in 0.1M PB. Every sixth section was processed for immunohistochemical visualization of DBH immunoreactivity to verify the DSAP-induced lesion. Free-floating sections were treated with 1% sodium borohydride in 0.1M PB for 30 min. They were then rinsed with 0.1M PB and later washed in 0.1M Tris saline buffer (TS, pH 7.6). The sections were blocked in 0.5% bovine serum albumin (BSA) in 0.1M TS for 30 min and then washed for 5 min, twice. Sections were incubated overnight at room temperature with a mouse antibody for mouse monoclonal antibody recognizing DBH (1:1000, Chemicon, Millipore) in 0.1% BSA/0.25% Triton-X 100 in 0.1M TS. The sections were then washed in 0.1M TS, three times for 10 min. Then, sections were incubated in a secondary biotin-conjugated donkey anti-mouse IgG (1:400, Jackson ImmunoResearch, West Grove, PA) in 0.1% BSA/0.25% Triton-X 100 in 0.1M TS for 30 min at room temperature. Then sections were washed in 0.1M TS, three times for 10 min. Sections were incubated in an avidin-biotin complex solution (1:200, VECTASTAIN Elite ABC Kit, Vector Laboratories, Burlingame, CA) in 0.1M TS for 30 min and then washed. Finally, a peroxidase reaction product was achieved by incubating sections in 22mg of 3-3′ diaminobenzidine (Sigma-Aldrich) containing 0.05% hydrogen peroxide.
Data analysis
Quantification of noradrenergic fibers depletion
Noradrenergic fibers were identified using an antibody specific for DBH. Sections of SAP and DSAP-animals were labeled for DBH as described above. Sections containing the Acb and BNST were visualized using a Leica DMRBE microscope (Wetzlar, Germany), and darkfield images were acquired (at 10x) using SPOT Advanced software (Diagnostics Instruments, Inc., Sterling Heights, MI). Light intensity was kept constant for all image acquisitions. To quantify the amount of fiber depletion, two methods were used. For sections containing the Acb, two to three sections per animal (comprising different levels of the Acb as exemplified in Fig. 1c) were used for analysis. Using Image-Pro Plus (Version 5.1, Media Cybernatics, Bethesda, MD) the area of the Acb and the number of fibers per section and per side was quantified. Data was analyzed as the ratio of total number of fibers/total area analyzed and presented as percentage of control (SAP-injected animals). Since the BNST contains an extremely dense amount of noradrenergic fibers, it is not feasible to count individual DBH-immunoreactive fibers. Therefore, for sections containing the BNST, intensity of labeling was measured using Kodak Molecular Imaging Software (Version 4.5, Carestream Health Inc., Rochester, NY). Two to three sections containing the anterior BNST (ranging from approximately 0.26 posterior to bregma to a few sections posterior to 0.40mm, Fig. 1d) per animal were analyzed. Dorsal and ventral regions were analyzed separately. A region of interest (ROI) was set as a template and used to quantify all images so that the area analyzed remained constant. Thus, data is presented as percentage of control (SAP-injected animals) mean intensity. For every section analyzed, a background value was quantified in an area of the section lacking DBH-ir. The background value was subtracted to the intensity of the ROI.
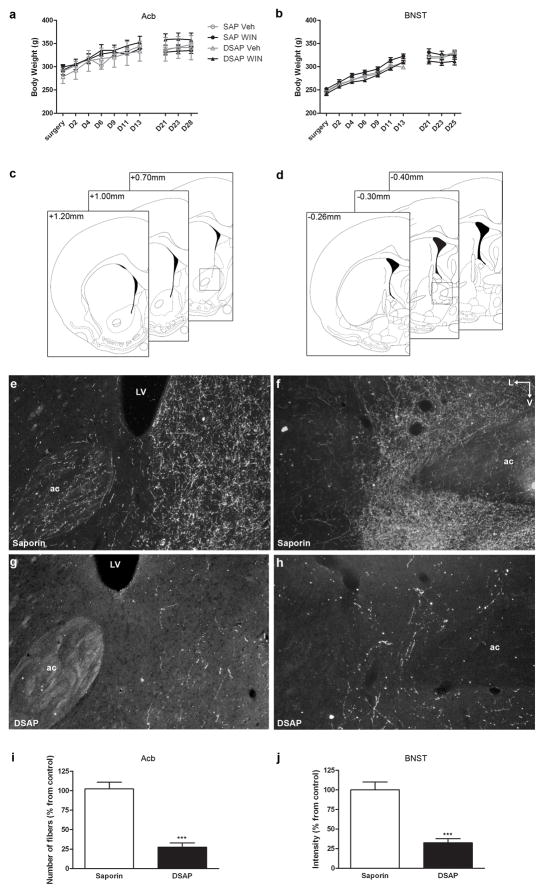
Fig. 1.
Effect of saporin conjugated with an antibody against DBH (DSAP) injection into the nucleus accumbens (Acb) and into the bed nucleus of stria terminalis (BNST). a and b. Toxin and drug treatment had no significant effect on animals’ weight throughout the experiment. c and d. Schematics adapted from the rat brain atlas of Paxinos and Watson (1997) (Paxinos, G. and Watson, C. 1997) showing the approximated levels of the Acb (c) and BNST (d) used for NE depletion quantification (note: for the BNST, a more caudal section between −0.40 and −0.80mm was analyzed). Inset in c represent the level of the photomicrographs in e and g. Inset in d represent the level of the photomicrographs in f and h. e–h. Darkfield photomicrographs showing DBH immunoreactivity in the Acb (e and g) and in the BNST (f and h) after injection of saporin or DSAP. Injection of DSAP significantly reduced the amount of DBH immunoreactivity by about 75% in both the Acb (i) and BNST (j) (*** p<0.0002). ac, anterior commissure; LV, lateral ventricle; L, lateral; V, ventral. Scale bar, 100μm
Statistical analysis
Statistical analysis was performed using SPSS 16.0 Graduate Student Version. Behavioral data were analyzed by a two-way ANOVA (Toxin × Drug). Repeated measures multivariate analysis of variance (ANOVARM) with day or period of time as the within-subject factor was also used when appropriate. One-way ANOVA, t-test and post hoc Bonferroni test were used to analyze differences between groups when appropriate. Significance was set at p < 0.05.
RESULTS
Toxin depletion of noradrenergic fibers
Animals recovered rapidly from intracranial injections without evidence of illness or abnormal behavior. DSAP and SAP animals gained weight at the same rate (Fig. 1a and b).
Immunohistochemistry for DBH in the forebrain of DSAP and SAP-injected animals was performed to verify the localization and the extent of the lesion. Two animals, out of 56 without any baseline preference, were excluded from behavioral testing due to inaccurate placement of the toxin. Injection of SAP did not affect DBH immunoreactivity (ir) when compared to vehicle-injected animals, whereas DSAP-injected animals revealed a marked reduction of DBH-ir (Fig. 1e–h) in both the Acb and BNST. Surrounding areas, such as the septal nuclei for the Acb and the ventral pallidum and medial preoptic area for the BNST were intact. Depletion of DBH fibers was quantified as explained in the methods section and for both areas a significant depletion of about 75% of DBH-ir fibers was achieved using injection of DSAP- when compared to SAP-injected animals (Fig. 1i and j).
Depletion of noradrenergic fibers in the Acb reverses the aversive effects of WIN 55,212-2
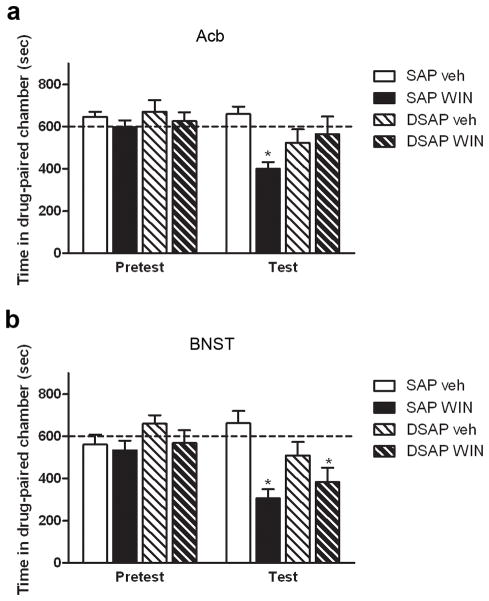
The place conditioning paradigm was used to assess the aversive effects of WIN 55,212-2. Animals were assigned to four groups: animals that received SAP injections and were injected with vehicle in both sessions (SAP/vehicle, 7 animals); animals that received SAP injections and were conditioned with WIN 55,212-2 (SAP/WIN, 6 animals); animals injected with DSAP and received vehicle in both sessions (DSAP/veh, 6 animals) and animals that received DSAP injections and were conditioned with WIN 55,212-2 (DSAP/WIN, 10 animals). Repeated measures analysis revealed that the there was an overall effect of time of testing (F(1,25)=5.849, p=0.023), meaning that the conditioning phase affected the performance of the animals on the test day. The analysis also showed an interaction between the treatments (toxin and drug) (F(1,25)=4.350, p=0.047). Further analysis showed that SAP treated animals that received WIN 55,212-2 spent less time in the drug-paired chamber than the respective vehicle group (t(11)=5.468, p<0.001), indicating that WIN 55,212-2 induced aversive-like behaviors (Fig. 2a). On the contrary, animals depleted of NE in the Acb did not show aversion to WIN 55,212-2 when compared with DSAP/vehicle-treated animals (t(14)= −0.471, p=0.645) (Fig. 2a). This suggests that noradrenergic input to the Acb is important for the development of aversion to WIN 55,212-2.
Fig. 2.
Effect of DSAP on the development of WIN 55,212-2-induced place aversion. a. Animals that received saporin injection in the Acb developed place aversion to WIN 55,212-2 (3.0mg/kg, * p<0.001 compared to SAP/Veh). This effect was blocked by injection of DSAP into the Acb (p>0.05, compared to DSAP/Veh). b. Animals that received toxin injection into the BNST developed place aversion to WIN 55,212-2 that was not blocked by DSAP injection (* p=0.05 compared to vehicle treated animals)
Depletion of noradrenergic fibers in the BNST is not implicated in the aversive effects of WIN 55,212-2
Animals injected with DSAP or SAP in the BNST were assigned to four different groups as mentioned above for Acb injections and conditioned in the same manner (6 to 8 animals a group). Repeated measures analysis revealed an effect of time of testing (F(1,21)=6.169, p=0.022), meaning that the conditioning phase affected the performance of the animals in the test day. The analysis also revealed an interaction between time of testing and drug (F(1,21)=4.324, p=0.050) (Fig. 2b) but not between time of testing and toxin (F(1,21)=3.403, p=0.079) suggesting that WIN 55,212-2 is aversive in both SAP and DSAP treated animals and that depletion of NE in the BNST does not reverse the effects of WIN 55,212-2.
Spatial reference memory is intact
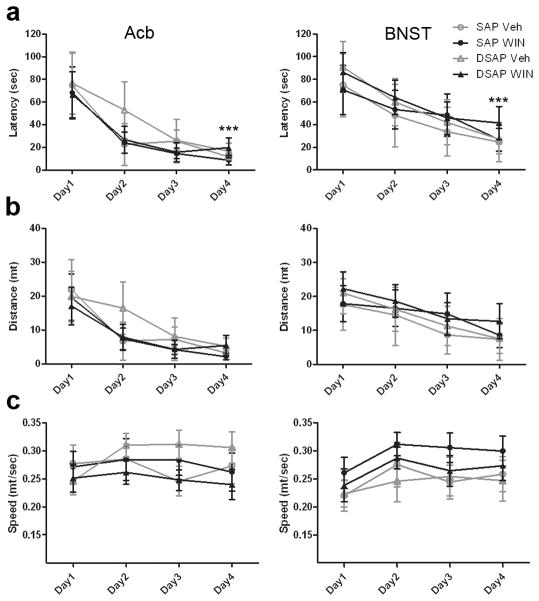
A spatial memory test was performed to ensure that depletion of NE from the target areas did not impair the animals’ ability for recall that could impact findings from the place conditioning test. To evaluate acquisition in the water maze, a two-way ANOVA for repeated measures was performed to assess any overall effects of factors toxin and drug, or their interactions on latency to the platform, distance traveled, and swim velocity during task acquisition. The analysis revealed an overall effect of trial on latency to reach the platform (F(3,36)=62.719, p<0.0001) and distance traveled (F(3,36)=55.930, p<0.0001), indicating that all animals efficiently learned where the platform was (Fig. 3a and b). No overall effect of trial on swim velocity was observed (F(3,36)=1.571, p=0.213) (Fig. 3b), suggesting that speed was constant throughout the acquisition phase. Moreover, there were no statistically significant interactions between toxin and drug for the three parameters analyzed, indicating no difference between groups on memory acquisition, distance travelled and swim velocity. Similar results were observed when the toxin was injected in the BNST (Fig. 3a–c, right column). There was an overall effect of trial on latency to reach the platform (F(3,36)=55.930, p<0.0001), distance traveled (F(3,36)=13.348, p<0.0001) and swim velocity (F(3,36)=7.274, p<0.001). Conversely, there were no significant interactions between toxin and drug for the three parameters analyzed showing that all groups had similar performances in the test.
Fig. 3.
Spatial memory acquisition is intact in animals injected with the toxin in the Acb (left column) and in the BNST (right column). Depletion of noradrenergic fibers in both the Acb and BNST did not impair memory acquisition. All groups of animals performed well in the acquisition phase of the Morris water maze test, showing low latency times to find the hidden platform by day 4 (a, *** p<0.0001, ANOVARM). All groups of animals showed similar locomotor activity, with no significant difference on distance (b) and speed of swim (c) (p>0.05, ANOVARM)
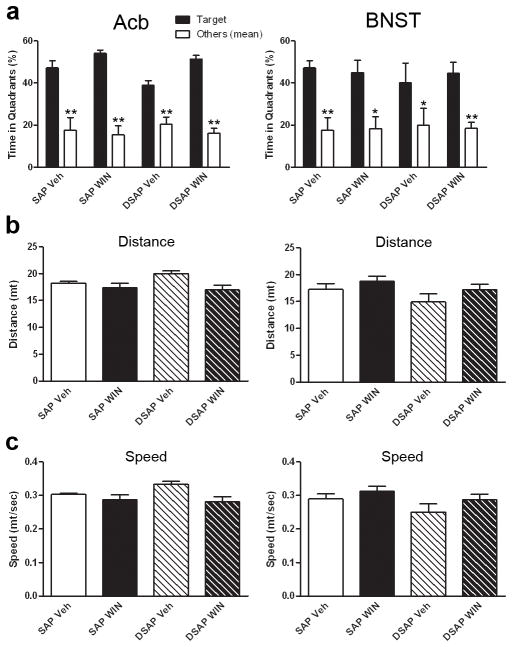
To assess memory retention, a probe trial was performed after the last trial of the acquisition phase. Repeated measures with time spent in the target quadrant and the average time spent in the other three quadrants was performed with two independent factors: toxin and drug. The analysis showed an overall effect of time spent in the quadrants (Acb, F(1,12)=410.008, p<0.0001; BNST, F(1,12)=53.960, p<0.0001). There was no interaction between drug and toxin meaning that the two factors combined did not affect the animals’ performance. T-test analysis revealed that all groups spent significantly more time in the target quadrant comparing to the non-target quadrants (Acb, p<0.001; BNST, SAP/Veh and DSAP/WIN p<0.001 and SAP/WIN and DSAP/Veh p<0.005) (Fig. 4a). Two-Away ANOVA of distance traveled and swim velocity during the probe trial revealed no significant interaction between drug and toxin (Acb, distance traveled F(1,12)=1.471, p=0.249, swim velocity F(1,12)=1.6, p=0.23; BNST, distance traveled F(1,12)=0.119, p=0.736, swim velocity F(1,12)=0.141, p=0.714) (Fig. 4b and c), suggesting no effect of drug or toxin on the animals’ locomotor activity.
Fig. 4.
Spatial memory retention is intact in animals injected the toxin in the Acb (left column) and in the BNST (right column). Depletion of noradrenergic fibers in both the Acb and BNST did not impair memory retention. During the probe trial (platform removed), all groups of animals spent significantly more time in the target quadrant (a) compared to the average time spent on the other three quadrants (* p<0.005, ** p<0.001 compared to target quadrant). No significant effect was observed in the locomotor activity shown by no changes in the distance (b) and speed of swim (c) during the probe trial
Depletion of noradrenergic fibers to the Acb or BNST has no effect on WIN 55,212-2-induced anxiety
Cannabinoid agonists are known to induce anxiety-like behaviors at high doses (Viveros et al. 2005; Rutkowska et al. 2006). To assess whether the reversal of aversive-like behaviors was due to changes in the level of anxiety, a group of animals was subjected to the EZM. A two-way ANOVA crossing toxin (SAP and DSAP) and drug (vehicle and WIN 55,212-2) treatment was performed to analyze changes in the percentage of time spent in the closed arms of the maze. This revealed an overall effect of drug in both the Acb (Fig. 5a) and BNST (Fig. 5b) experiments (F(1,12)=36.686, p<0.0001; F(1,12)=34.372, p<0.0001, respectively), indicating that WIN 55,212-2 was anxiogenic. There was no overall effect of toxin (F(1,12)=3.047, p=0.106 (for the Acb); F(1,12)=3.449, p=0.088 (for the BNST)) in the time spent in the closed arm. The analysis revealed no interaction between drug and toxin treatment (Acb, F(1,12)=0.22, p=0.647; BNST, F(1,12)=0.199, p=0.663), revealing that depletion of NE from the Acb and BNST did not affect the anxiety-like behavior induced by WIN 55,212-1.
Fig. 5.
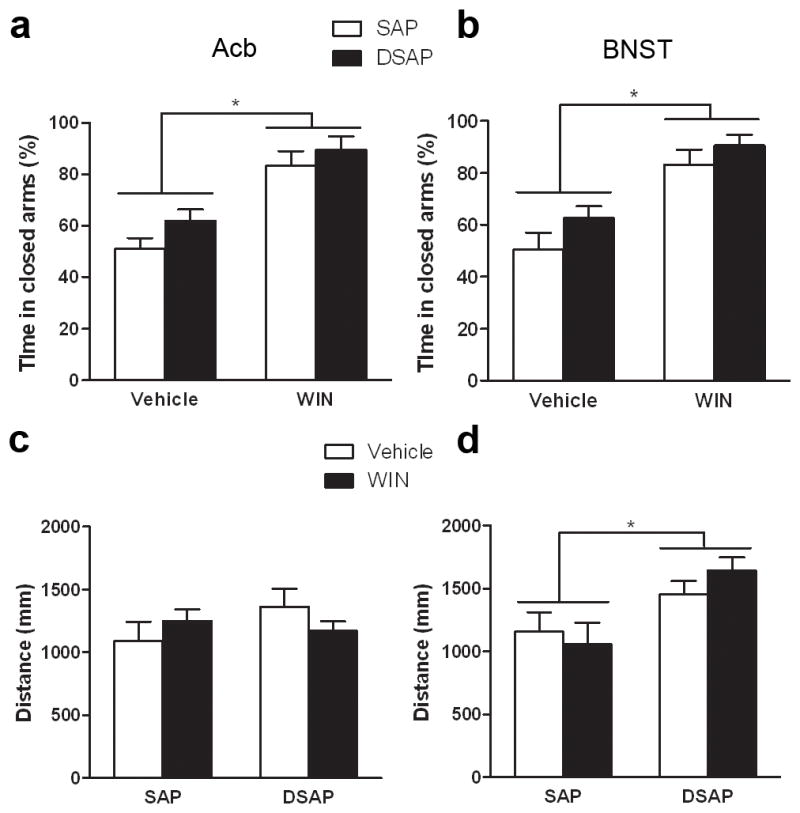
Anxiety-like behavior (a and b) and locomotor activity (c and d) of animals injected with the toxin in the Acb (a and c) and in the BNST (b and d). Animals injected WIN 55,212-2 (3.0mg/kg) showed higher anxiety levels as measured by more time spent in the close arms of the elevated zero maze (a and b)(* p<0.0001). Injection of DSAP had no effect of the anxiety levels. Toxin and drug administration in the Acb group had no effect on locomotor activity (c). DSAP injection into the BNST increased locomotor activity independently of drug administration (d, * p<0.005)
In addition, similar results were observed when analyzing the total number of entries into the arms of the maze (Table 1). There was an overall effect of drug in both the Acb and BNST experiments (F(1,12)=72.104, p<0.001), F(1,12)=11.108, p<0.01, respectively). There was no effect of toxin (Acb, F(1,12)=1.182, p=0.298; BNST, F(1,12)=0.184, p=0.184) and no interaction between drug and toxin (Acb, F(1,12)=1.748, p=0.211; BNST, F(1,12)=0.094, p=0.765). These results indicate an anxiogenic effect of WIN 55,212-2 that was not affected by depletion of NE.
Table 1.
Total number of entries in the elevated zero maze
| Acb | Vehicle | WIN 55,212-2 | Total |
|---|---|---|---|
| SAP | 38 (+/− 4.183) | 7.5 (+/− 2.63) | 45.5 |
| DSAP | 48.25 (+/− 5.921) | 6.5 (+/− 3.594) | 46.75 |
|
| |||
| Total | 78.25** | 14 | |
| BNST | Vehicle | WIN 55,212-2 | Total |
|
| |||
| SAP | 49 (+/− 8.399) | 25 (+/− 11.195) | 74 |
| DSAP | 40.25 (+/− 6.575) | 11.4 (+/− 4.874) | 51.64 |
|
| |||
| Total | 89.25* | 36.4 | |
Data represent mean of total number of entries (into opened and closed arms), +/− SEM.
p<0.001, vehicle-treated animals compared to WIN 55,212-2-treated animals
p<0.01, vehicle-treated animals compared to WIN 55,212-2-treated naimals
Locomotor activity
After completion of the EZM, the animal’s locomotor activity was assessed. A two-way ANOVA crossing toxin (SAP and DSAP) and drug (vehicle and WIN 55,212-2) treatment revealed no effect of toxin (F(1,12)=0.539, p=0.477) or drug (F(1,12)=0.727, p=0.411) treatment in the animals injected in the Acb (Fig. 5c). However, in the animals injected in the BNST it was observed an overall effect of toxin (F(1,12)=12.387, p=0.004) but not drug (F(1,12)=0.219, p=0.648) in the distance traveled, suggesting that depletion of NE from the BNST increases locomotor activity (Fig. 5d).
DISCUSSION
This study examined the neurochemical and regional substrates involved in cannabinoid-induced aversion and anxiety. The results indicate that administration of a CB1r/CB2r agonist induces conditioned place aversion and anxiety. It is reported that noradrenergic transmission within the Acb is a critical determinant for the expression of aversion-like behavior (as measured by the place conditioning paradigm) following exposure to a cannabinoid agonist. Moreover, norepinephrine depletion from the Acb and BNST did not affect anxiety-like behaviors, underscoring the involvement of differential circuitry in the expression of aversion and anxiety to a cannabinoid receptor agonist.
WIN 55,212-2-induced aversion: role of limbic circuitry
The present results are in agreement with previous findings that report cannabinoid receptor agonists to be aversive to rats, as shown by the induction of conditioned place aversion (McGregor et al. 1996; Sanudo-Pena et al. 1997; Mallet and Beninger 1998; Pandolfo et al. 2009). Aversive behaviors require emotional learning and association of emotions with a context, therefore limbic areas such as the PFC, BNST and Acb have been involved in eliciting these behaviors (Gracy et al. 2001; Levita et al. 2002; Delgado et al. 2008). Gracy and colleagues (2001) have shown that place aversion to naltrexone-induced opiate withdrawal is related to neuronal activation of the shell subregion of the Acb and the central nucleus of the amygdala (CeA). Moreover, monoaminergic transmission in areas such as the amygdala, PFC, BNST and Acb has been implicated in the development of aversive behaviors (Aston-Jones et al. 1999; Delfs et al. 2000; Ventura et al. 2007; Kerfoot et al. 2008). For instance, Aston-Jones and colleagues (1999) have shown that blockade of beta adrenergic receptors in the CeA attenuates the morphine withdrawal-induced place aversion. Herein, we explored the hypothesis that NE in the Acb and BNST is a critical determinant for the establishment of cannabinoid-induced aversion. Using an immunotoxin lesion approach of two limbic areas (Acb and BNST), we were able to establish the role of selected circuits involved in the expression of aversion to cannabinoids. Both areas are important nuclei of the limbic system, integrating information arising from the amygdala (concerning affective components of the behavior), from the hippocampus and PFC (conveying contextual features from the environment), and from the ventral tegmental area (regarding reward related components of learning experiences) (Forray and Gysling 2004; Kerfoot et al. 2008). Moreover, both the Acb and BNST receive direct input from the NTS (Delfs et al. 1998; Forray et al. 2000; Forray and Gysling 2004) that conveys information regarding peripheral signals (e.g. arousal) with limbic structures. We show that this noradrenergic input from the NTS to the Acb is critical for the expression of place aversion to WIN 55,212-2. To our knowledge it is not known whether the noradrenergic neurons projecting to the Acb have collateral projections to other areas. This is a potential caveat as, when lesioning NE neurons projecting to the Acb, collateral projections to other areas could be affected. However, we consider this a remote possibility because depletion of NE from the BNST, which receives much more NE than the Acb and does have collaterals to the CeA (Roder and Ciriello 1994) and paraventricular nucleus of the hypothalamus (Terenzi and Ingram 1995), did not affect any of the behaviors analyzed. A more important consideration regarding interpretation of findings from this study relates to the fact that these animals lack NE throughout the conditioning phase and, therefore, it is not possible to discern whether NE is critical for the establishment and/or, on the other hand, for the recalling of the motivational association. However, previous studies show that impairment of NE transmission after the learning phase does not impact the expression of the behavior (Miranda et al. 2007; Kerfoot et al. 2008) suggesting that NE is not required for recalling learned associations. Nevertheless, pharmacological approaches would be needed to better clarify the time point in which NE is important for cannabinoid-induced aversion.
The ability of WIN 55,212-2 to induce aversion is most likely mediated by activation of CB1r as it has been shown that prior administration of the CB1r antagonist AM 251 prevents WIN 55,212-2-induced aversion (Pandolfo et al. 2009). CB1r has been localized to GABAergic neurons (Matyas et al. 2006) in the Acb but seldom on noradrenergic neurons (Carvalho et al. 2010). Moreover, cannabinoids have been shown to affect both glutamate and GABA transmission in the Acb (Manzoni and Bockaert 2001; Robbe et al. 2001). Interestingly, CB1r is found in noradrenergic neurons of the NTS (Carvalho et al. 2010) and WIN 55,212-2 has been shown to activate NTS neurons (Himmi et al. 1998; Jelsing et al. 2009).
We interpret the results of the present study in the following way: WIN 55,212-2 may act on CB1 receptors that are localized to noradrenergic neurons of the NTS, increasing their firing rate and subsequently increasing release of NE in the Acb. Consistent with this interpretation, WIN 55,212-2 has been shown to lead to changes in adrenergic receptor expression in the Acb (Carvalho et al. 2010). Moreover, one could speculate that activation of CB1r in glutamatergic and GABAergic terminals in the Acb may decrease the release of these amino acids, making Acb medium spiny neurons more sensitive to NE. In addition, other systems may be involved. For example, kappa opioid receptors (KOR) have been shown to be critical for THC-induced aversion (Zimmer et al. 2001; Ghozland et al. 2002). Mice lacking KOR do not show aversion to THC in the place conditioning paradigm. Dynorphin, the endogenous KOR agonist, is distributed throughout the Acb, in axon terminals that form mostly symmetric synapses (Khachaturian et al. 1982; Van Bockstaele et al. 1994). Interestingly, dynorphin is also found within NTS neurons and fibers (Fodor et al. 1994) and acute administration of the KOR synthetic agonist U-50,488H has been shown to increase c-fos activation of catecholaminergic NTS neurons (Laorden et al. 2003). This can be a potential mechanism by which dynorphin and KOR facilitate aversion to cannabinoids. Taken together, there are a number of potential interpretations and future studies are required to carefully parse out the nature of cannabinoid actions on the NTS/Acb circuit.
Others have shown that blockade of NE transmission within the BNST impairs place aversion to opiate withdrawal (Aston-Jones et al. 1999; Delfs et al. 2000). Although, in the present study, depletion of NE in the BNST did not affect WIN 55,212-2-induced aversion, the possibility exists that upon withdrawal from cannabinoid exposure, NE transmission in the BNST becomes engaged in a fashion similar to opiate withdrawal. Future studies are required to test this possibility.
Anxiogenic effects of WIN 55,212-2
Cannabinoid agonists have been shown to exert anxiogenic effects in both animals and humans (Onaivi et al. 1990; Childers and Breivogel 1998; Arevalo et al. 2001; Marco et al. 2004; Witkin et al. 2005). Taking this into consideration, we hypothesized, along with others (McGregor et al. 1996), that reduction of the aversive effects of WIN 55,212-2 observed in the present study could be due to a reduction in anxiety levels. In order to examine this, animals were tested in the EZM. Our results are in agreement with others that showed that WIN 55,212-2 administration induces anxiety-like behaviors as seen by an increased time spent in the closed arms of the maze and decreased exploration measured by a reduction in the total number of entries. None of these EZM outputs was affected by NE depletion in both the Acb and BNST. These results dissociate anxiety-like behaviors from aversive-behaviors. The results show that the same lesion that reverses the aversive behavior (depletion of NE in the Acb) had no effect on anxiety-like behavior. Though this fact cannot rule out an association between anxiety and aversion to WIN 55,212-2, it clarifies the nuclei involved in these two behaviors.
Nevertheless, it is surprising that disrupting noradrenergic transmission in the Acb, but especially in the BNST, does not affect anxiety-like behavior. The BNST is known to be a key nucleus in the expression of anxiety (Davis 1998; Davis 2006) and it is a “hot spot” of noradrenergic innervation (Forray and Gysling 2004). Hence, it is surprising that depletion of NE did not affect the expression of anxiety. However, little is known about the circuitry involved in cannabinoid-induced anxiety. The fact that other stimuli (stress, drug withdrawal) increases NE release in the BNST and this may trigger anxiety may not hold true for cannabinoid based agents. Moreover, the possibility exists that a 75% reduction of noradrenergic fibers was not sufficient to remove the noradrenergic basal tone in the BNST. Although further studies are required, the present results seem to suggest that CB1R-induced anxiety is not dependent on noradrenergic transmission.
Concluding remarks
The endocannabinoid system is widely expressed in the central and peripheral nervous system as well as immune system. Thus, it is involved in numerous physiological processes. Understanding how cannabinoids impact multiple systems will help us to better manipulate the endocannabinoid system without engaging unwanted side effects. The present study provides new information about the neural circuits involved in cannabinoid-induced behaviors that may lead to the development of potential new pharmacotherapies for the treatment of psychiatric disorders.
Acknowledgments
This works was supported by PHS grant DA 020129. Ana Franky Carvalho was supported by the Portuguese Foundation for Science and Technology (SFRH/BD/33236/2007).
Abbreviations
- Acb
Nucleus accumbens
- ANOVARM
Repeated measures ANOVA
- BNST
Bed nucleus of stria terminalis
- BSA
Bovine serum albumin
- CB1r/CB2r
Cannabinoid receptor type1/Cannabinoid receptor type2
- CeA
Central nucleus of amygdala
- CNS
Central nervous system
- DBH
Dopamine beta hydroxylase
- DSAP
Saporin conjugated with antibody against DBH
- EZM
Elevated zero maze
- ir
Immunoreactivity
- KOR
Kappa opioid receptor
- LC
Locus coeruleus
- NE
Norepinephrine
- NTS
Nucleus of the solitary tract
- PB
Phosphate buffer
- PFC
Prefrontal cortex
- ROI
Region of interest
- SAP
Saporin
- TS
Tris saline buffer
Footnotes
Disclosure: The authors have nothing to disclose.
References
- Anand A, Charney DS. Norepinephrine dysfunction in depression. J Clin Psychiatry. 2000;61(Suppl 10):16–24. [PubMed] [Google Scholar]
- Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–131. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend. 1998;51:173–187. doi: 10.1016/s0376-8716(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor M, Pammer C, Gorcs T, Palkovits M. Neuropeptides in the human dorsal vagal complex: an immunohistochemical study. J Chem Neuroanat. 1994;7:141–157. doi: 10.1016/0891-0618(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K, Andres ME, Bustos G, Araneda S. Medullary noradrenergic neurons projecting to the bed nucleus of the stria terminalis express mRNA for the NMDA-NR1 receptor. Brain Res Bull. 2000;52:163–169. doi: 10.1016/s0361-9230(00)00229-x. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmi T, Perrin J, El Ouazzani T, Orsini JC. Neuronal responses to cannabinoid receptor ligands in the solitary tract nucleus. Eur J Pharmacol. 1998;359:49–54. doi: 10.1016/s0014-2999(98)00630-x. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Galzin AM, Guillot E, Pruniaux MP, Larsen PJ, Vrang N. Localization and phenotypic characterization of brainstem neurons activated by rimonabant and WIN55,212–2. Brain Res Bull. 2009;78:202–210. doi: 10.1016/j.brainresbull.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Kerfoot EC, Chattillion EA, Williams CL. Functional interactions between the nucleus tractus solitarius (NTS) and nucleus accumbens shell in modulating memory for arousing experiences. Neurobiol Learn Mem. 2008;89:47–60. doi: 10.1016/j.nlm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H. Dynorphin immunocytochemistry in the rat central nervous system. Peptides. 1982;3:941–954. doi: 10.1016/0196-9781(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Batkai S, Sharkey KA, Makriyannis A. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Castells MT, Milanes MV. Effects of U-50488H and U-50488H withdrawal on c-fos expression in the rat paraventricular nucleus. Correlation with c-fos in brainstem catecholaminergic neurons. Br J Pharmacol. 2003;138:1544–1552. doi: 10.1038/sj.bjp.0705179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behav Brain Res. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;(168):299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci. 1998;62:2431–2439. doi: 10.1016/s0024-3205(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, File SE, Viveros MP. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–27. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Miranda MA, Ferry B, Ferreira G. Basolateral amygdala noradrenergic activity is involved in the acquisition of conditioned odor aversion in the rat. Neurobiol Learn Mem. 2007;88:260–263. doi: 10.1016/j.nlm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat-an animal model of attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2009;205:319–326. doi: 10.1007/s00213-009-1542-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and Mechanisms of Action of Cannabinoid Receptors at the Glutamatergic Synapses of the Mouse Nucleus Accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder S, Ciriello J. Collateral axonal projections to limbic structures from ventrolateral medullary A1 noradrenergic neurons. Brain Res. 1994;638:182–188. doi: 10.1016/0006-8993(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jamontt J, Gliniak H. Effects of cannabinoids on the anxiety-like response in mice. Pharmacol Rep. 2006;58:200–206. [PubMed] [Google Scholar]
- Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett. 1997;223:125–128. doi: 10.1016/s0304-3940(97)13424-3. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Steinberg BA, Cannon CP. Cannabinoid-1 receptor blockade in cardiometabolic risk reduction: safety, tolerability, and therapeutic potential. Am J Cardiol. 2007;100:27P–32P. doi: 10.1016/j.amjcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Terenzi MG, Ingram CD. A combined immunocytochemical and retrograde tracing study of noradrenergic connections between the caudal medulla and bed nuclei of the stria terminalis. Brain Res. 1995;672:289–297. doi: 10.1016/0006-8993(94)01453-o. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Sesack SR, Pickel VM. Dynorphin-immunoreactive terminals in the rat nucleus accumbens: cellular sites for modulation of target neurons and interactions with catecholamine afferents. J Comp Neurol. 1994;341:1–15. doi: 10.1002/cne.903410102. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Res. 1996;740:175–184. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, Valverde O, Maldonado R. Absence of delta -9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci. 2001;21:9499–9505. doi: 10.1523/JNEUROSCI.21-23-09499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]