Abstract
Infection with the malaria parasite, Plasmodium, is characterized by excessive inflammation. The establishment of a precise balance between the pro- and anti-inflammatory responses is critical to guarantee control of the parasite and survival of the host. Interleukin (IL)-10, a key regulatory cytokine produced by many cells of the immune system, has been shown to protect mice against pathology during acute Plasmodium chabaudi chabaudi AS model of malaria. However, the critical cellular source of IL-10 is still unknown. Here, we demonstrate that T cell-derived IL-10 is necessary for the control of pathology during acute malaria, as mice bearing specific deletion of Il10 in T cells fully reproduce the phenotype observed in Il10−/− mice, with significant weight loss, drop in temperature and increased mortality. Furthermore, we show that IFN-γ+ Th1 cells are the main producers of IL-10 throughout acute infection, expressing high levels of CD44 and ICOS and low levels of CD127. Although Foxp3+ regulatory CD4+ T cells produce IL-10 during infection, highly activated IFN-γ+ Th1 cells were shown to be the essential and sufficient source of IL-10 to guarantee protection against severe immune-mediated pathology. Finally, in this model of malaria we demonstrate that the generation of protective IL10+IFN-γ+ Th1 cells is dependent on IL-27 signaling, and independent of IL-21.
Introduction
The blood-stages of the malaria parasite, Plasmodium, induce a pro-inflammatory response in the host, which although important for the clearance of the parasite, can lead to severe immune-mediated pathology. In humans and in mice, high levels of the pro-inflammatory cytokines gamma interferon (IFN)-γ, tumor necrosis alpha (TNF)-α, interferon gamma-induced protein (IP)-10 (CXCL10) and interleukin-1β (IL)-1β have been shown to correlate with complications during malaria, such as severe anemia, hypoglycemia and cerebral malaria (1), (2), (3).
A successful response must strike a balance between protection from the parasite and immunopathology, and IL-10 appears to be one of the means by which this balance is established (4), (5). Down-regulation of the protective immune response by IL-10 during infection with a variety of pathogens, including Leishmania spp., Candida spp., Mycobacterium tuberculosis (6), Bordetella spp. (7) and HIV (8) is detrimental, promoting pathogen survival. Conversely, the absence or low levels of IL-10 have been shown to correlate with severe or fatal outcome in infections with P. falciparum (9), Toxoplasma gondii (10) and Trypanosoma spp. (11).
IL-10 can play either a positive or negative role in protecting the host from infectious disease or the associated immune-driven pathology. During a blood-stage Plasmodium chabaudi chabaudi AS infection in mice, the requirement of this cytokine for down-regulating the pathology-inducing inflammatory response is evident as Il10−/− mice have greater amounts of plasma IFN-γ, TNF-α and IL-12, higher mortality and more pronounced pathology than wild-type (WT) animals (12) (13). Inactivation of IFN-γ reduces the mortality (13) and pathology is ameliorated by anti-TNF-α treatment (2), confirming that IL-10 regulation of inflammatory responses is crucial in preventing severe immunopathology in this model of malaria.
IL-10 is produced by different immune cell types, including B cells, macrophages, DCs (4), (14) and several T cell subsets, such as CD8+ (15) and CD4+ T cells, including Th1 (16), (17), Th17 (18) as well as Foxp3+ Tregs (19) and regulatory type 1 cells (Tr1) (20). IL-10 from CD4+ T cells (16), CD8+ T cells (15) and myeloid cells such as dendritic cells (14) have all been shown to play important roles in regulating immunopathology in different infection models. Furthermore, within the CD4+ T cell subset, IL-10 from effector (16) or regulatory T cells (21) can have similar roles in different infections, or different roles in the same infection. For example in L. major infections, IL-10 from effector Th1 cells is necessary for suppression of inflammatory response during acute infection (16), whereas IL-10- producing antigen-specific Foxp3+ CD4+ T cells (T regs) suppress the clearance of the parasite by the effector CD4+ T cells (21), (22).
Despite intensive research on the mechanisms responsible for regulation of immunopathology in malaria, the cellular source of protective IL-10 is not known. During P. falciparum infection, greater frequencies of effector Th1 CD4+ T cells producing IL-10 have been observed in children with uncomplicated malaria (23), although their protective function has not been established. In a mouse model of non-lethal malaria, P. yoelii, IL-10 is primarily produced early in the infection by CD4+CD25−Foxp3− adaptive regulatory T cells (24). However a role for IL-10 from these cells in reducing pro-inflammatory responses, pathology or parasite clearance has not been directly demonstrated. Therefore, a clear definition of the critical protective source of IL-10 during malaria infection and the mechanisms of its induction are still to be determined. Several mechanisms have been proposed to regulate IL-10 production, including TGF-β, IL-6, IL-21 and IL-27 can mediate suppression of immune responses via the induction of IL-10 (25), (26), (27), (28), (29), (30). However, the exact mechanism involved in regulating production of protective IL-10 in many infections and inflammatory diseases including malaria is not known.
Here we show that CD4+ T cells are the major source of IL-10 throughout infection, and that mice carrying a deletion of the Il10 gene specifically in T cells fully reproduce the phenotype previously observed in Il10−/− mice (13). Importantly, immunocompromised mice reconstituted with WT, but not Il10−/− CD4+ T cells, are rescued from death and present substantially ameliorated pathology. Although Foxp3+ regulatory CD4+ T cells also produce IL-10 during this infection, adoptive transfer studies revealed that IL-10 from effector Th1 CD4+ T cells, but not from Foxp3+ Tregs, is both essential and sufficient to regulate the immunopathology of a P.c. chabaudi AS infection. We further demonstrate that the induction of IL-10 in Th1 cells requires direct IL-27 signaling, but is independent of IL-21. These findings are of the real importance for the malaria field as they define the critical cellular source of protective IL-10 and the pathways leading to it production. This knowledge has significant impact in this field and might be important for the establishment of protocols for manipulation of these pathways in order to prevent severe malaria in humans.
Materials and Methods
Animals and Infections
Female C57Bl/6 (CD45.2 and CD45.1), C57Bl/6 Il10−/− (31), Rag1−/− (32), Il21−/− (33) and Foxp3-EGFP reporter mice (34) (8-12 weeks) were bred in the specific pathogen-free facilities at the MRC National Institute for Medical Research (NIMR, London, UK). Il10flox/flox mice, which have loxP sites flanking exon 1 of Il10 (35), crossed with CD4-Cre (36), (35) and LysM-Cre (37), (38) mice were backcrossed with NIMR C57Bl/6 mice. CD19-Cre (39) mice were crossed with Il10flox/flox mice at NIMR, and backcrossed for 10 generations onto NIMR C57Bl/6. Littermate control mice (Il10flox/floxCre−) were used. TCCR−/− mice (40) were kindly provided by Dr. Nico Ghilardi, Genentech Inc. Animal experiments were performed in accordance with the UK National guidelines (Scientific Procedures) Act 1986 under license PPL80/2358 approved by the British Home Office and the Institute Ethical Review Panel.
Mice were housed in reverse light conditions and infected intraperitoneally (i.p.) with 105 RBC infected with Plasmodium chabaudi chabaudi AS (iRBC). In order to generate a fairly accurate estimate of the parasitemia and total parasite load in the circulation, blood smears were taken at the same time in all experiments, usually 4-5h after schizogony, time during the synchronous 24hs blood cycle when the sequestration is very low. The percentages of parasitized RBCs were monitored as described (41) and the total numbers of RBC and Hemoglobin concentration were determined on Vetscan (Abaxis-VetScan HM5 Hematology). The percentage of weight loss relative to the initial weight is presented. Body temperature was measured using a transponder system (Bio Medic Data Systems, IPTT-300) with microchips inserted in the scruff of the neck.
Anti-IL-10R treatment
A single dose (0.82 mg/mouse) of the monoclonal antibody (1B1.3) that binds specifically to the ligand binding domain of IL-10 receptor (42), or the IgG1 isotype control antibody (GL113) (42) was injected i.p. into C57Bl/6 mice 2 days after intravenous (i.v.) infection. Weight loss and temperature, as well as survival rate, parasitemia and total numbers of iRBC were assessed.
Cell preparations
Single-cell suspensions obtained from spleens were incubated with FcR block (43) followed by specific antibodies (BD Pharmingen, Biolegend or eBioscience) using appropriate combinations of fluorochromes (FITC, PE, PerCP, PE Texas Red, PerCPCy5.5, PE-Cy5.5, PE-Cy7, Pacific Blue, APC and APC-Cy7): CD3 (145- 2C11), CD4 (L3T4), CD8 (53- 6.7), CD19 (MB19- 1), NK1.1 (PK 136), CD127 (A7R34), ICOS (C398.4A), CD45RB (16A), CD62L (MEL-14), CD44 (IM7), CD45.1 (A20), CD45.2 (104).
The samples were acquired on CyAn (DAKO) using Summit (Cytomation). FlowJo (Tree Star) software was used for analysis of the data.
Intracellular Staining
Intracellular cytokines were detected as described with few modifications (44). After stimulation and surface labeling, the cells were fixed with 2% paraformaldehyde (PFA; Sigma) in phosphate-buffered saline (PBS; Gibco, Invitrogen), followed by permeabilization with FACS buffer (PBS supplemented with 2% FBS and 0.1% sodium azide) containing 0.5% saponin (Sigma). To detect intracellular cytokines anti-IFN-γ-PECy7 (XMG1.2; eBiocience) and anti-IL10-APC (JES5-16E3; BD Pharmingen), or the respective isotype controls were used. Transcription factors expression were assessed by anti-Foxp3- Alexa Fluor 488 (150D; Biolegend), Foxp3- PE (FJK-16s; eBioscience) and T-bet-Pacific Blue (4B10; Biolegend) antibodies were used according to the manufacture instructions.
Cell sorting
Single-cell suspensions from spleens were incubated with anti-CD4, -CD19, -CD11b or -CD11c magnetic beads (Miltenyi Biotec). CD4+ and CD19+ cells were purified (>97%) using autoMACS (Miltenyi Biotec). CD3+CD8+, NK1.1+, CD11chi, CD11bhi cells were purified (>95%) on a MoFlo cell sorter (Cytomation) using specific antibodies anti-CD3 (145-2C11) PERCP, CD8 (53-6.7) FITC, NK1.1 (PK136) PE, CD11b (M1/ 70) PE-Cy7, CD11c (N418) APC (BD Pharmingen and eBioscience). 7-Aminoactinomycin D (7AAD) (Sigma) or PI (Sigma) was used to exclude dead cells. To obtain Foxp3-EGFP+ cells, splenocytes from Foxp3-EGFP reporter mice were incubated with anti-CD4 magnetic beads and purified using autoMACS. EGFP+ or negative cells were sorted on a MoFlo cell sorter (purity >95%)
Quantitative RT-PCR
Purified and sorted cells were placed in Trizol (Invitrogen), RNA extracted and RT-PCR assay was performed as described (43). Target gene mRNA expression was quantified using SYBR green (Fisher Scientific) and normalized to ubiquitin mRNA levels, using the 2−ΔΔCt method (45).
Il10 Forward: TTTGAATTCCCTGGGTGAGAA
Reverse: GCTCCACTGCCTTGCTCTTATT
Ubiquitin Forward: TGGCTATTAATTATTCGGTCTGCAT
Reverse: GCAAGTGGCTAGAGTGCAGAGTAAAdoptive transfer
Splenic CD4+ cells from naïve C57Bl/6 and Il10−/− mice were purified (>97%) using anti-CD4 microbeads and naïve Rag1−/− mice received 107 CD4+ T cells i.v. Mice were infected with 105 iRBC and pathology was monitored.
For adoptive transfer of effector and regulatory T cells, splenocytes from naïve C57Bl/6 (CD45.2 or CD45.1) and Il10−/− mice were obtained. CD25+ and CD25− cells were purified using the CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec) and sorted (>95% purity) on a MoFlo cell sorter. CD25+ (0.5×106) and CD25− cells (3.5×106) were injected i.v. into Rag1−/− mice. After 3 weeks the reconstituted mice were infected with 105 iRBC. Rag1−/− mice receiving 4×106 WT CD4+ cells were used as controls.
Generation of bone marrow chimeric mice
Rag1−/− mice were sub-lethally irradiated with 5 Gy using a 137Cs source and reconstituted i.v. with 1.7×106 BM cells from WT CD45.1+ mice, Il27ra−/− CD45.2+ (TCCR−/−) mice (40) or a mixture of both at ratios: 90:10, 10:90 and 50:50. Reconstitution was assessed by flow citometry seven weeks after engraftment. Two weeks thereafter all mice were infected with 105 iRBC and culled for analysis 8 days later.
Plasma cytokines
Plasma from infected mice was collected on day 7 of infection and cytokine levels were detected using Luminex xMAP Multiplexing Technology (Millipore) according to the manufacturer instructions, with limit of detection varying between 0.6 – 2.3 pg/mL.
Statistical analysis
Statistical significance was analyzed in Prism 5 (Graph Pad) using two-tailed Student’s t test and Mann Whitney test or One-Way ANOVA, where appropriate. Differences were considered statistically significant with p < 0.05.
Results
Il10−/− mice are highly susceptible to a blood-stage infection with P. chabaudi. Approximately 60% of those mice die within 15 days of infection and all mice exhibit more severe pathology than their C57Bl/6 WT counterparts (12), (13). C57Bl/6 mice treated with blocking antibodies against the IL-10 receptor (42) succumb to a lethal infection within 9 days, and exhibit severe weight loss and significant hypothermia with no effect on acute-stage parasitemia (Supplementary Figure 1). These results demonstrate that signaling through the IL-10 receptor during malaria infection is critical for the control of severe pathology but has little effect on the levels of peripheral parasitemia.
IL-10 production increases during primary infection and CD4+ T cells are the major source
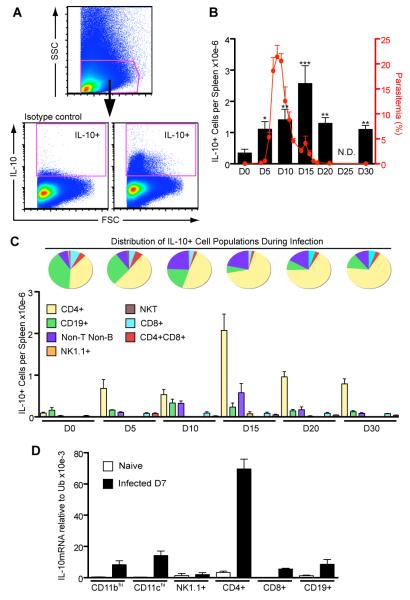
The timing of production of a regulatory cytokine such as IL-10 may be crucial for the outcome of P. chabaudi infection. Therefore, in order to define which cells produced IL-10 during the acute infection, and whether the appearance of IL-10 in these cells coincided with control of pathology and acute infection, we determined the numbers of IL-10+ splenic cells throughout the acute phase of P. chabaudi infection in C57Bl/6 mice (days 0 to 30) by FACS analysis (Figure 1). After the peak of parasitemia (characteristically around day 7), a dramatic increase in the numbers of cells producing IL-10 (gated as shown in Figure 1A) was evident, reaching a maximum of 2.5 × 106 cells per spleen on day 15 (Figure 1B). The numbers of IL-10- producing cells rapidly decreased thereafter, but spleens of animals infected for 30 days still contained more IL-10+ cells than uninfected mice. The presence of a large number of IL-10-producing cells at this time coincided with the control of pathology, which is usually observed between day 10 to 15 (13).
Figure 1. CD4+ cells are the major producers of IL-10 during acute P. chabaudi infection.
C57Bl/6 mice were infected i.p. with 105 parasitized RBC and after different time points, the splenocytes were harvested, stimulated in culture with PMA and ionomycin for 6h and IL-10 production was detected using intracellular staining.
(A) The gating strategy is shown.
(B) Total numbers of IL-10-producing cells per spleen during infection (Mean ± SEM; *p<0.05, **p<0.01 and ***p<0.001 compared to D0; t-test; n=7-9 per time point). No analysis was performed on day 25 (N.D.= not determined). The parasitemia curve is also presented.
(C) Pie charts represent the distribution of different cell subsets among IL-10+ cells during analysis. Bar graph shows total numbers of IL-10+ cells per spleen for each cell population on specific time points (Non-T Non-B cells= CD3−CD4−CD8−CD19− NK1.1− cells). Data are representative of three independent experiments.
(D) Several cell types (CD11bhi, CD11chi, NK1.1+, CD3+CD4+, CD3+CD8+, CD19+) were sorted on day 7 of infection and IL-10 transcription was analyzed by quantitative RT-PCR (Mean ± SEM n = 3-5).
IL-10 can be produced by cells of both the innate and adaptive immune system (5). We therefore used FACS analysis to determine which splenic cells (CD4+, CD8+, CD19+ B cells, NK cells, NKT cells) were responsible for this production during the acute P. chabaudi infection. Already from day 5, CD4+ cells were clearly the biggest IL-10+ population, accounting for nearly 50% of total IL-10-secreting cells, as shown in the pie charts (Figure 1C). On days 15, 20 and 30 post-infection, the CD4+ cells accounted for up to 70% of the IL-10+ cells with more than 2 × 106 cells per spleen. The total number of IL-10+ CD4+ T cells declined as the infection progressed to the chronic stage but at day 30 of infection the numbers were still greater than those observed in uninfected mice (0.7 × 106 on day 30 and 0.09 × 106 on day 0; p=0.0002; t-test). Regarding the frequency of IL-10+ cells in the spleen during this infection, we observed that when the whole spleen was considered these frequencies do not changed significantly (data not shown), however, we observed an approximately 10- fold increase in the frequency of IL-10+ cells within the CD4+ population (from 0.46±0.15% on day 0 to 4.19±0.08% on day 15; data not shown), which means that the increase in the total numbers of IL-10+ CD4+ T cells is not only due to the characteristic splenomegaly during this infection. Therefore, these results show CD4+ T cells to be the major source of IL-10, with maximum numbers after the peak of parasitemia.
We also found that B cells (CD19+) produced IL-10. CD19+ cells represented nearly 40% of the IL-10+ cell population in naïve mice (day 0, Figure 1C). This proportion decreased after day 10 of infection. Production of IL-10 by non-T non-B cells (CD3−CD4−CD8−CD19−NK1.1−cells) was also evident (~25% of IL-10-producing cells at day 10).
Although myeloid cells (monocytes, macrophages and DCs) can also produce IL-10 (5), (46), we could not reliably detect this by FACS analysis in these cells. Therefore, we analyzed IL-10 transcription by these cells using quantitative RT-PCR at the peak of parasitemia (day 7), when the highest antigen load is present and therefore, both myeloid and lymphoid cells are activated. The various different cell populations (CD3+CD4+, CD3+CD8+, CD19+, NK1.1+, CD11bhigh, CD11chigh) were sorted from the spleens of infected C57Bl/6 mice. In agreement with the FACS data presented in Figure 1A-C, a large up-regulation of IL-10 transcription was observed in CD4+ T cells, whilst other cell types, such as CD11bhi (43), CD11chi, CD19+ and CD8+ T cells showed much lower levels of IL-10 mRNA transcripts (Figure 1D). NK1.1+ cells showed no increase in IL-10 transcription, compared with naïve cells. Thus, despite the relative widespread up-regulation of Il10 transcription amongst cells of both the innate and adaptive immune response, CD4+ T cells expressed the highest level of IL-10 mRNA confirming them as the major source of IL-10 during P. chabaudi infection.
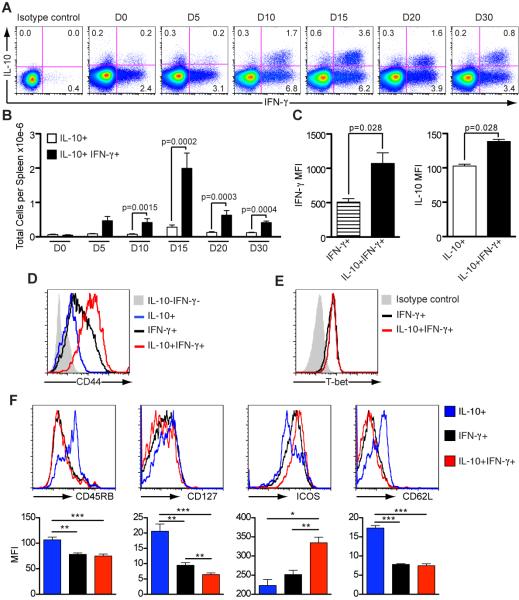
The majority of IL-10-producing CD4+ T cells also produce IFN-γ and their numbers increase transiently after the peak of parasitemia
Studies have shown that IFN-γ+ Th1 cells that co-produce IL-10 have an important function in down-regulating the immune response to T. gondii (17), L. major (16), and in controlling autoimmune responses (47). Therefore, we speculated that IL-10 produced by CD4+ T cells in conjunction with IFN-γ during infection in this malaria model also plays an important role. To examine this hypothesis, splenocytes were analyzed for cytokine production by flow cytometry at different time points after infection. We observed both IL-10+ IFN-γ+ co-producing cells and IL-10+ single producing cells. Both types of IL-10+ cells were present throughout infection but the greatest number and percentages were observed on day 15 (Figure 2A and B), coinciding with clinical signs of pathology. The frequencies of IL-10- producing single positive CD4+ T cells were low and relatively constant throughout. In fact, there were approximately 6-fold more IL-10+ IFN-γ+ double producing CD4+ T cells than those producing only IL-10, suggesting an important role for CD4+ T cells producing both IL-10 and IFN-γ in the control of immunopathology.
Figure 2. IFN-γ+ CD44hi CD4+ T cells are the main source of IL-10 during acute infection.
Spleen cells from C57Bl/6 mice were harvested on different days after infection, stimulated in culture with PMA and ionomycin, followed by intracellular staining for cytokines.
(A) Representative dot plots show gated CD4+ T cells. Production of IL-10 and IFN-γ were assessed throughout acute infection and mean percentages are presented.
(B) Total numbers of IL-10+ and IFN-γ+ IL-10+ CD4+ T cells per spleen (Mean ± SEM; n=7-9 per time point; t-test).
(C) Mean fluorescence intensity (MFI) for IFN-γ (day 5) and IL-10 (day 15) observed for the different cytokine-producing cell populations.
(D) Representative histograms show CD44 surface staining and (E) expression of transcription factor T-bet on CD4+ T cells from day 15- infected mice.
(F) CD4+ T cells from day 15-infected mice were stained for CD45RB, CD127, ICOS and CD62L, followed by intracellular staining for IFN-γ and IL-10. Representative histograms are presented and the bar graphs show the MFI for each cell population and the respective marker. (Mean ± SEM; n=6-9; t-test)
(*p<0.05; **p<0.01; ***p<0.001). Data from A-D, F are representative of three and E, two independent experiments.
The induction of these IL-10+ Th1 CD4+ T cells has been shown to require high antigen load (48). In Figure 2A we also observe this correlation, as double producing cells are induced after the peak of parasitemia, when the greatest amounts of antigen is available, and later, when parasitemia drops, the frequency of those cells also decreases.
Interestingly, IL-10+ IFN-γ+ double producing cells expressed higher levels of IFN-γ than single IFN-γ+ cells at the beginning of infection (Day 5, MFI: 1072 ± 154.1 and 509.5 ± 48.15, respectively; p=0.028; t-test) (Figure 2C). Similarly, at the time of maximum numbers of double IL-10+ IFN-γ+ cells (Day 15), MFI of IL-10 was greater in these cells compared to the single IL-10 producers (138.5 ± 3.0 and 102.5 ± 2.86, respectively; p=0.028; t-test).
The higher levels of IFN-γ and IL10 in the double positive cells may indicate that they are highly activated or more differentiated. Indeed, IL-10+ IFN-γ+ CD4+ T cells expressed more CD44 (MFI: 125.2 ± 6.3) than either IFN-γ- (66.8 ± 0.9; p=0.0022; t-test) or IL-10- (28.6 ±0.9; p=0.005; t-test) single producing CD4+ T cells (Figure 2D). These double producers also expressed the highest levels of the inducible co-stimulatory molecule (ICOS) and the lowest levels of IL-7Rα (CD127), compared with IFN-γ and IL-10 single producers (Figure 2F), demonstrating an increased activation state.
Critically, the expression of the transcription factor T-bet in IL-10+ IFN-γ+ Th1 cells was at similar levels to those of IFN-γ+ Th1 cells demonstrating that the major source of IL-10 during P. chabaudi infection is highly activated effector Th1 cells (Figure 2E).
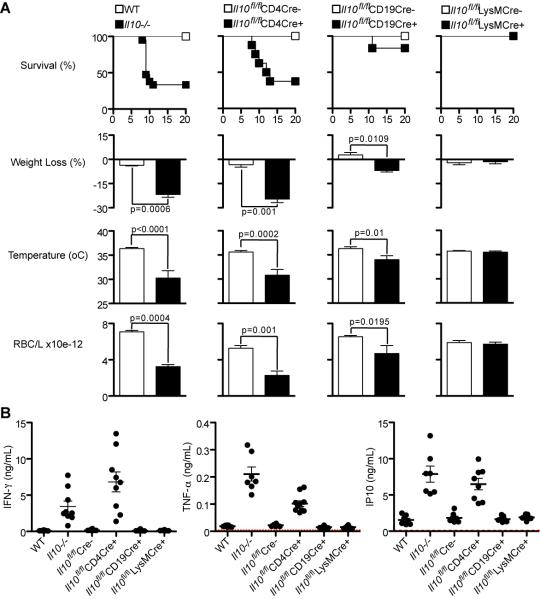
Mice with Il10 deletion specifically in T cells reproduce the phenotype observed in Il10−/− mice
As effector Th1 cells were the major source of IL-10 following the peak of infection, the next step was to determine if IL-10 from T cells was important to control immune-mediated pathology in vivo. We therefore asked whether mice carrying a deletion of Il10 specifically in T cells, CD19+ B cells or macrophages and neutrophils (LysM+ cells) would reproduce the phenotype observed in completely deficient Il10−/− mice.
For these experiments we used Il10flox/flox mice (35) crossed to CD4-Cre (36) (35), CD19-Cre (39) or LysM-Cre (37), (38) and the appropriate Il10flox/floxCre− littermates controls. The mice were infected with 105 P. chabaudi and survival rates, weight loss, temperature drop and anemia (RBC/mL) were monitored for 20 days. As shown in Figure 3A, only those mice unable to secrete IL-10 specifically from T cells (Il10fl/flCD4Cre+ mice) fully reproduced the pathological features of the infection in the Il10−/− mice, in that they exhibited substantial weight loss, hypothermia, anemia and only 30-40% of mice survived the acute infection, despite the fact that in these mice other cells are competent in secreting IL-10. Therefore, not only are T cells the major source of IL-10 during P. chabaudi infection, but they are the critical source of protective IL-10, which promotes protection from severe immunopathology. Importantly, lack of IL-10 in T cells did not affect the duration or magnitude of the low/subpatent chronic P. chabaudi AS infection that follows the acute infection (data not shown).
Figure 3. Mice with Il10 deletion specifically in T cells reproduce the phenotype observed in Il10−/− mice.
Using the Cre/loxP recombination system, specific deletions of Il10 were performed in T cells, CD19+ cells or monocytes/neutrophils (LysM+) cells. These mice are designated as Il10fl/flCD4Cre+, Il10fl/flCD19Cre+ and Il10fl/flLysMCre+ mice, respectively.
(A) Mice from these lineages were infected i.p. with 105 pRBC. Survival rates and signs of pathology were monitored, as weight loss, temperature and RBC counts. The graphs for weight loss, temperature and RBC counts present data from days 10, 9 and 11 post-infection, respectively, when the major differences were observed between the groups (Mean ± SEM; n = 10-20; t-test). Respective Cre− littermate controls for each mouse line were used. Experiments were performed four times with similar results.
(B) Plasma cytokines were detected at the peak of parasitemia (day 7) (Mean ± SEM; n = 7-10; each symbol indicates an individual mouse). Red dotted line represents the cytokine values for naïve mice.
Interestingly, mice carrying B cells unable to produce IL-10 (Il10fl/flCD19Cre+) also presented partial signs of pathology, with 15% mortality during the infection, suggesting that IL-10-producing B cells may contribute to control of malaria disease. These Il10fl/flCD19Cre+ mice have significantly lower frequencies and total numbers of IL-10+ IFN-γ+ double producing and IL-10+ single producing CD4+ T cells, compared to Il10fl/flCD19Cre− controls (Supplementary Figure 2A and B), yet comparable numbers of IFN-γ+ single producing CD4+ T cells and Foxp3+ CD4+ T cells (Supplementary Figure 2B and C). Thus, the increase in the severity of immunopathology observed in mice unable to secrete IL-10 specifically from B cells (Il10fl/flCD19Cre+ mice) during P. chabaudi infection may result from their inability to generate sufficient numbers of IL-10 secreting CD4+ T cells.
IL-10 from monocytes and neutrophils did not appear to be required for the control of immunopathology, as there was no mortality, and weight loss, anemia or hypothermia in Il10fl/flLysMCre+ mice were similar to their respective Cre− controls and WT C57Bl/6 mice. Similar to infections in Il10−/− mice (12), (13), the effects of the deletion of Il10 in any of the cell populations could not readily be ascribed to differences in parasitemias as these were similar between the Il10fl/fl Cre+ mice and their respective Cre− controls, and not significantly different from those of WT C57Bl/6 (Supplementary Figure 2D).
Similarly to Il10−/− mice (12), (13), infected Il10fl/flCD4Cre+ mice exhibited greater amounts of the pro-inflammatory cytokines IFN-γ, TNF-α and IP-10 compared to infected WT and Il10fl/flCre− control mice (Figure 3B), indicating the more inflammatory nature of the host response to P. chabaudi when IL-10 was lacking in T cells. Taken together, these results demonstrate a major protective role for IL-10 produced by T cells, most likely CD4+ T cells, against immune-mediated pathology during an acute blood-stage P. chabaudi infection.
IL-10 from WT CD4+ T cells rescues immunodeficient mice from death
Since CD4+ T cells were found to be the major producers of IL-10 during a P. chabaudi infection, and T cell-derived IL-10 is important for the regulation of pathology in this infection, we wanted to verify directly that IL-10 from CD4+ T cells prevented pathology. Therefore, WT C57Bl/6 or Il10−/− CD4+ T cells were adoptively transferred into Rag1−/− mice, which were then infected with P. chabaudi and the acute stage pathology monitored. In these experiments the aim was to observe a direct protective role for CD4-derived IL-10 in the acute stage of infection when immunopathology is most evident. Rag1−/− mice infected with P. chabaudi normally exhibit severe weight loss, hypothermia and anemia and approximately 40% die already within the first 15 days of infection (Figure 4A). As shown in Figure 4A and B, all Rag1−/− mice that received WT CD4+ T cells survived for the duration of the analysis, exhibited significantly less weight loss, less hypothermia and less anemia than Rag1−/− mice receiving Il10−/− CD4+ T cells or unreconstituted Rag1−/− mice, demonstrating that IL-10-sufficient CD4+ T cells controlled pathology in this model. By contrast, recipient mice transferred with Il10−/− CD4+ T cells showed equivalent pathology to unreconstituted Rag1−/− mice and, in fact, required longer to recover.
Figure 4. IL-10 from CD4+ T cells rescues immunodeficient mice from death.
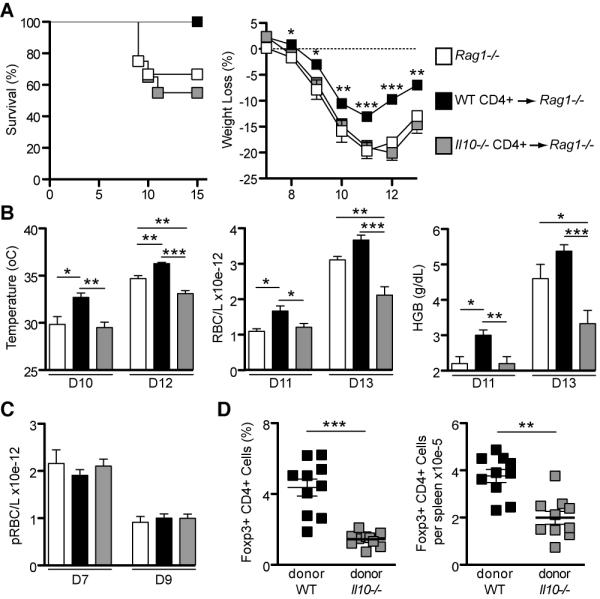
On day -1, Rag1−/− mice received 107 naive CD4+ T cells i.v. either from C57Bl/6 WT or Il10−/− mice. Unreconstituted Rag1−/− mice were used as controls.
(A) After infection with 105 parasitized RBC i.p. on day 0, all mice were monitored for 15 days and pathology was assessed. Survival rate and weight loss are shown (weight loss graph: statistical analysis was performed using both transferred groups, but comparing mice transferred with WT CD4+ cells and unreconstituted mice showed similar results).
(B) Additional pathology measurements are presented: Temperature, RBC counts and HGB (HGB: hemoglobin).
(C) Total numbers of parasitized RBC (pRBC) per liter of blood on days 7 and 9 after infection (Mean ± SEM; n = 12-20 per group). The experiments were performed three times.
(D) Percentages and total numbers of Foxp3+ CD4+ T cells per spleen of recipient Rag1−/− mice on day 10 post-infection (each symbol indicates an individual mouse). Before infection, Rag1−/− mice receiving either WT or Il10−/− CD4+ T cells presented similar frequencies of Foxp3+ CD4+ T cells (data not shown). (Mean ± SEM; n = 10 per group)
(*p<0.05; **p<0.01; ***p<0.001; A, C and D: t-test; B: One-way ANOVA).
The differences in pathology in Rag1−/− mice receiving either WT or Il10−/− CD4+ T cells could not be explained by the differences in parasitemia, as they both showed similar numbers of parasitized RBC/L (Figure 4C). Therefore, these data confirm that IL-10 from CD4+ T cells is critical to limit the immunopathology during P. chabaudi infection, but is not important for the control of parasitemia.
Since it has been demonstrated that transfer of CD4+ T cells into lymphopenic hosts, such as Rag1−/− mice, can result in a preferential expansion of Foxp3+ Tregs (49), we assessed the numbers of Foxp3+ CD4+ T cells in the reconstituted Rag1−/− mice. Before transfer, purified cells were assessed for the expression of Foxp3 and both WT and Il10−/− CD4+ T cells presented similar frequencies of Foxp3+ cells (~14%; data not shown). Surprisingly, following transfer and infection, Rag1−/− mice receiving WT CD4+ T cells had significantly higher percentages and greater numbers of Foxp3+ CD4+ T cells per spleen compared with those mice receiving Il10−/− CD4+ T cells (Figure 4D), suggesting that Il10−/− Foxp3+ CD4+ T cells are not able to expand following infection or that the effector cells outgrew the regulatory cells. Thus it is possible that the transferred WT CD4+ T cells were better able to ameliorate immunopathology through increased Foxp3+ regulatory T cell frequencies, which may, in turn, regulate the outcome of disease directly through IL-10 production, or via other suppressor mechanisms. Therefore, the next step was to determine if Foxp3+ CD4+ Treg cells were the critical source of IL-10 for the control of pathology in this model.
Foxp3+ Treg cells produce IL-10 during infection but effector Th1 cells are the major source
We have shown that CD4+ T cells are the critical source of IL-10 for the attenuation of immunopathology in P. chabaudi infection and that IFN-γ+ IL-10+ CD4+ T cells are the major producers of IL-10 and represent highly activated effector Th1 cells. However, they may not necessarily be the critical source regulating pathology; the single IL-10+ CD4+ T cell subset may be important, and could represent a small population of suppressive Foxp3+ regulatory T cells.
To examine whether IL-10 producing Tregs could be a source of protective IL-10 during this P. chabaudi infection, mice harboring the EGFP sequence inserted into the Foxp3 gene (Foxp3-EGFP reporter mice) (34) were used to determine the contribution of Foxp3− and Foxp3+ CD4+ T cells to protective IL-10 production. It is important to point out that there was a significant increase in the total numbers of Foxp3+ Treg cells in the spleen post-infection (Figure 5A), which could be observed already from day 5 of infection. Moreover, analysis of IL-10 transcription in sorted EGFP-Foxp3+ Treg cells confirmed a large up-regulation of Il10 transcription in these cells during infection (Figure 5B). CD4+ Foxp3+ and Foxp3− splenic T cells were sorted based on EGFP protein expression and the two populations were then restimulated in vitro and stained for intracellular cytokines. Figure 5C clearly shows that Foxp3− effector CD4+ T cells are the main source of IL-10 during acute infection, reaching nearly 2 × 106 secreting cells on day 15, compared with only 0.2 × 106 Foxp3+ CD4+ Treg cells producing this cytokine.
Figure 5. Foxp3+ CD4+ T cells produce IL-10 during infection but effector CD4+ cells are the major source.
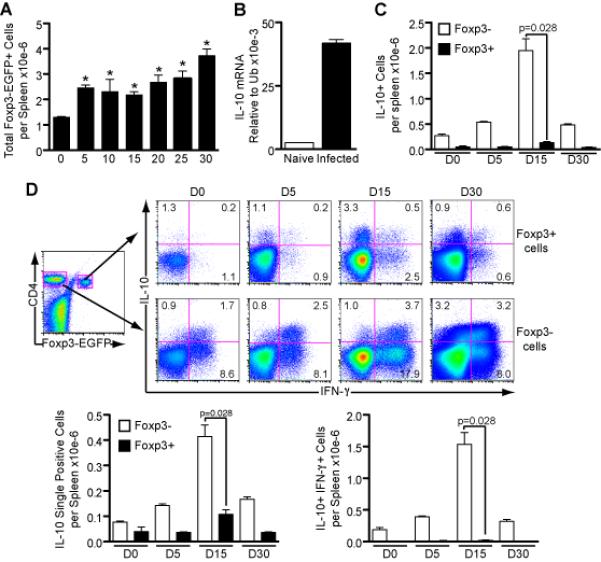
CD4+ T cells were harvested from Foxp3-EGFP reporter mice on different days after infection and Foxp3+ cells were sorted based on their expression of EGFP protein. Following stimulation with PMA and ionomycin for 6h, intracellular staining was performed for IL-10 and IFN-γ.
(A) Total numbers of Foxp3+ EGFP cells per spleen during infection with 105 parasitized RBC (Mean ± SEM; n=3-5 per time point; *p<0.05 compared to naïve; t-test).
(B) EGFP-Foxp3+ cells were sorted on day 10 of infection and IL-10 transcription was analyzed using RT-PCR (n = 3).
(C) Total numbers of Foxp3− and Foxp3+ cells producing IL-10 per spleen (n = 3-4 per time point; t-test).
(D) Representative plots show mean percentages of cytokine-producing sorted Foxp3− and Foxp3+ cells. Total numbers of Foxp3+ and Foxp3− cells, per spleen, producing only IL-10 or in combination with IFN-γ are presented. (Mean ± SEM; n = 3-4 per time point; t-test).
A and B: data are representative of two independent experiments; C and D: combined data from two or three experiments for each time point.
The majority of IL-10+ Foxp3− effector cells co-produced IFN-γ, whereas the majority of IL-10+ Foxp3+ cells did not produce IFN-γ, as observed in the representative dot plots (Figure 5D). The bar graphs showing the total numbers of cytokine producing cells confirm Foxp3− effector cells as the major source of IL-10 (Figure 5D). Therefore, whilst the total number of Foxp3+ regulatory T cells increases during infection and IL-10 production is up-regulated by these cells, double producer Foxp3− effector CD4+ T cells remain the prevailing source of IL-10. Unexpectedly, our intracellular analysis also revealed a minor population of IFN-γ+ single producing Foxp3+ cells (Figure 5D), which have also been observed in other parasitic infections (50), (51). Their function is currently unknown.
IL-10 from Foxp3− CD4+ T effector cells is essential and sufficient to control immune-mediated pathology during acute P. chabaudi infection
It remained to be determined whether IL-10 from Foxp3+ regulatory T cells was required to limit immunopathology, or whether IL-10 from effector Th1 cells was sufficient. In order to address this question, Foxp3+ CD4+ regulatory T cells were sorted from naïve C57Bl/6 WT mice (CD45.1 or CD45.2) or Il10−/− mice (CD45.2) based on their high level expression of CD25, whilst effector cells were sorted as CD25 negative. These two populations were co-transferred at a ratio of 7 effector CD25− CD4+ T cells (3.5 × 106) to 1 regulatory CD25+ CD4+ T cell (0.5 × 106) into Rag1−/− mice 3 weeks prior to infection with P. chabaudi. Before transfer, the sorted cells were assessed for Foxp3 expression to verify that the majority of both WT and Il10−/− CD25+ CD4+ T cells were Foxp3+ (>86%), and that CD25− CD4+ cells were almost entirely Foxp3− (Figure 6A). These results confirm that Foxp3+ Tregs can develop in the absence of IL-10 (52). Using the allotypic markers CD45.1 and CD45.2 we were able to determine that in mice receiving WT effector and WT regulatory CD4+ T cells the percentage of Foxp3+ regulatory CD25+ cells following reconstitution (Day 0) and infection (Day 10), and their ratio to effector CD25− cells, remained constant (Figure 6B). However, in mice receiving WT effector and Il10−/− regulatory CD4+ T cells, the percentage of regulatory CD25+ cells expressing Foxp3 had decreased following 3 weeks of reconstitution (Day 0) and, in addition, their ratio to effector CD25− cells decreased significantly following infection (Day 10) (p=0.05; t-test) (Figure 6B).
Figure 6. IL-10 from Foxp3− CD4+ T cells is essential and sufficient to limit pathology.
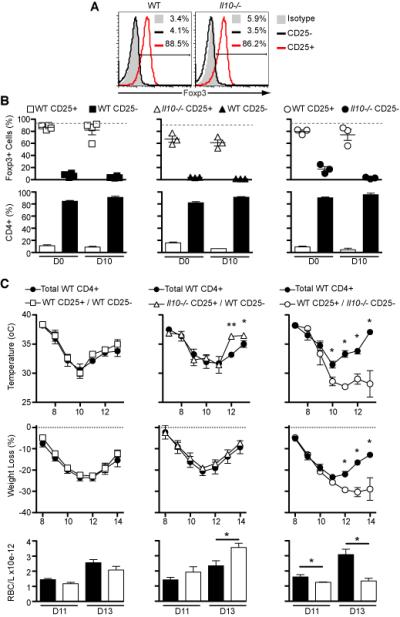
Rag1−/− mice were reconstituted with naïve cells: 0.5×106 CD4+CD25+ and 3.5×106 CD4+CD25− T cells either from C57Bl/6 WT (CD45.1 or CD45.2) or Il10−/− (CD45.2) mice. After three weeks of reconstitution, the mice were infected with 105 infected RBC and analysis of pathology was performed for 15 days.
(A) Using intranuclear staining, representative histograms show Foxp3 expression in CD4+CD25+ and CD4+CD25− T cells before transfer into immunocompromised mice.
(B) On days 0 and 10 after infection spleens from reconstituted mice were harvested and the percentages of Foxp3+ cell in the CD45.1 and CD45.2 cell populations were assessed as well as the ratio between them. The grey dotted lines represent the original frequency of Foxp3+ cells in the CD25+ population before transfer.
(C) Following infection, signs of pathology were detected using several parameters: temperature, weight loss and RBC count. [Mean ± SEM; n=3-4 (in B); n=5-7 (in C); *p<0.05; **p<0.01; t-test]. Data are representative of two independent experiments.
Infected Rag1−/− mice reconstituted with the mixture of WT effector and WT regulatory CD4+ T cells presented equivalent pathology to those reconstituted with total WT CD4+ T cells (Figure 6C), verifying the transfer model. Rag1−/− mice reconstituted with WT effector and Il10−/− regulatory T cells recovered better from the pathology, with less hypothermia and lower anemia, compared to those reconstituted with total WT CD4+ T cells (Figure 6C), demonstrating that despite a lower ratio of Foxp3-expressing CD25+ cells to effector CD4+ T cells, these mice were nevertheless protected from severe disease. Furthermore, these two groups present similar total numbers of effector cells, which is unlikely to be the reason for less severe disease in mice receiving WT effector and Il10−/− regulatory T cells when compared to those reconstituted with total WT CD4+ T cells (53.87±10.48×105 and 44.70±7.94×105 cells, respectively, p= 0.55). Therefore, IL-10 derived from Foxp3+ Treg cells is not required for protection from severe immunopathology during P. chabaudi infection. Rag1−/− mice reconstituted with Il10−/− effector and WT regulatory CD4+ T cells displayed increased hypothermia, weight loss and anemia compared to control mice receiving total WT CD4+ T cells (Figure 6C), and showed little sign of recovery from the infection. Furthermore, the survival curves clearly confirm the severe immune-mediated pathology in mice receiving Il10−/− effector and WT regulatory CD4+ T cells as they present significant higher mortality (Supplementary Figure 3). Regarding the parasite burden, no significant difference in peripheral parasitemia was observed between the groups. These data clearly demonstrate that IL-10 derived from Foxp3+ regulatory T cells is not required for protection from severe immunopathology. IL-10 from effector CD4+ T cells is the critical source, and is sufficient to limit immunopathology during P. chabaudi infection without contributing to an increase in parasite burden.
IL-27, but not IL-21, is necessary to induce optimal IL-10 production by IFN-γ+ Th1 cells
IL-27 has recently been implicated in the regulation of IL-10 production by T cells (53) and IL-21 has been shown to amplify the IL-27 signal for IL-10 production by Tr1 cells (54). We evaluated the role of these pathways in inducing IL-10 production by IFN-γ+ Th1 cells in this model of malaria. Il21−/− mice were infected with P. chabaudi and their cytokine profile was analysed by intracellular staining at day 15 post-infection. Interestingly, despite having lower total numbers of spleen cells (data not shown), IL-21-deficient mice showed greater frequencies and numbers of IL-10+ IFN-γ+ double producing Th1 cells, and IL-10+ single producing CD4+ T cells, compared with WT mice (Figure 7A), clearly demonstrating that IL-21 is not required for IL-10 production in CD4+ T cells during P. chabaudi infection.
Figure 7. Generation of IL-10+ IFN-γ+ CD4+ T cells is independent of IL-21 but requires IL-27 signaling.
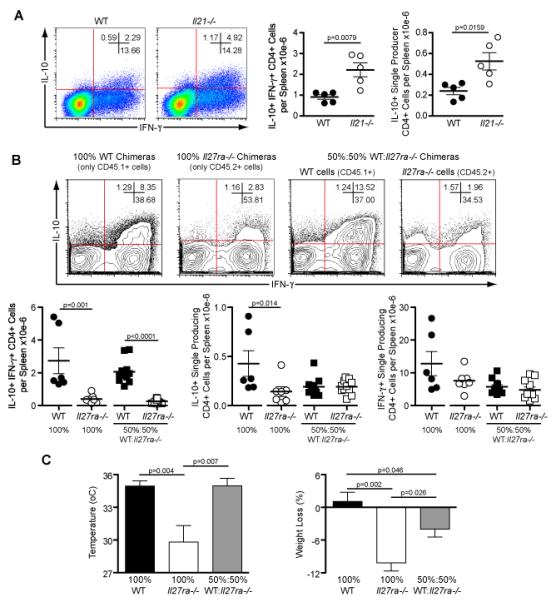
(A) Wild-type and Il21−/− mice were injected with 105 P. chabaudi infected RBC and 15 days later the splenocytes were harvested for intracellular analysis. Representative plots show IL-10 and IFN-γ production by gated CD4+ T cells. Mean percentages of cytokine-producing cells are shown. Total numbers of CD4+ T cells, per spleen, producing only IL-10 or in combination with IFN-γ are presented. (each dot represents an individual mouse). (Mean ± SEM; n=5; t-test). Data are representative of three independent experiments.
(B) After sub-lethal irradiation, Rag1−/− mice were reconstituted with 1.7×106 total bone marrow cells from WT (CD45.1+), Il27ra−/− (CD45.2+) mice or a mixture of both at ratio 50:50 and they were designated as 100% WT, 100% Il27ra−/− and 50%: 50% WT: Il27ra−/− chimeras, respectively. Nine weeks after the transplantation all mice were infected with 105 P. chabaudi and 8 days later they were culled for analysis. Representative contour plots showing IL-10 and IFN-γ production by gated CD4+ T cells. These cells were selected based on their expression of the allotypic markers CD45.1 or CD45.2. The total numbers of IL-10+ IFN-γ+ double producers, IL-10+ single producers and IFN-γ+ single producers, per spleen, are shown for WT and IL-27ra−/− cells.
(C) The graphs for temperature and weight loss represent data from days 8 and 7, respectively, when the major differences were observed between the groups. (Mean ± SEM; n = 6-11; t-test)
To address the role of IL-27 for the production of IL-10 by IFN-γ+ Th1 cells we generated mixed bone marrow chimeras in which WT CD45.1+ and Il27ra−/− CD45.2+ (40) bone marrow cells were transferred to sub-lethally irradiated Rag1−/− mice at various ratios. As controls, chimeras receiving 100% WT CD45.1+ or 100% Il27ra−/− CD45.2+ bone marrow cells were also generated. After seven weeks the chimerism was determined. We selected those mixed chimeric mice with approximately 50% reconstitution with Il27ra−/− T cells (Supplementary Figure 4) for enumeration of IL-10+ IFN-γ+, IL-10+ and IFN-γ+ CD4+ T cells following infection with P. chabaudi. Pathology was also analysed. Using the gating strategy shown in Supplementary Figure 4, the WT and Il27ra−/− CD4+ T cells were gated based on the expression of their allotypic marker. IL-27R-deficient IFN-γ+ CD4+ T cells were significantly impaired in their ability to produce IL-10, with reduced frequencies and numbers of IL-10+ IFN-γ+ CD4+ T cells in chimeric mice reconstituted with 100% Il27ra−/− bone marrow compared with 100% WT bone marrow, and in Il27ra−/−, compared with WT, CD4+ T cells which co-existed in mixed chimeras (Figure 7B). Additionally, there was defective induction of IL-10+ single producer CD4+ T cells in the Il27ra−/−, compared with WT, chimeras, whilst the number of IFN-γ+ single producer CD4+ T cells per spleen was not affected by the absence of IL-27R (Figure 7B).
As IL-10+ IFN-γ+ Th1 cells are necessary to control immune-mediated pathology induced during acute P. chabaudi infection, it would be expected that chimeric mice reconstituted with only Il27ra−/− bone marrow cells, which generated the lowest number of IL-10+ IFN-γ+ Th1 cells, would present significantly increased symptoms of pathology, including hypothermia and weight loss, compared to chimeras reconstituted with 100% WT bone marrow cells. This was observed (Figure 7C). In addition, mixed bone marrow chimeric mice also presented with a partial increase in the severity of pathology (Figure 7C). In conclusion, our results clearly demonstrate that the induction of IL10+ IFN-γ+ CD4+ T cells, which provide critical protection from immunopathology during P. chabaudi infection, requires signaling through the IL-27 pathway.
Discussion
The ability to establish a precise balance between the strength of the pro-inflammatory response triggered by infection and the consecutive immune regulatory mechanisms can be crucial to guarantee efficient clearance of the pathogen without excessive tissue damage. Here we show that IL-10 production increases during acute Plasmodium chabaudi chabaudi AS infection and that IFN-γ+ Th1 CD4+ T cells are the main source throughout acute phase. As a consequence, mice with specific deletion of Il10 in T cells develop severe disease and a significant proportion succumb to infection, reproducing the phenotype observed in infected Il10−/− mice. We demonstrate that although Foxp3+ regulatory CD4+ T cells also secrete IL-10 during this malaria infection, IL-10 produced by activated effector Th1 cells co-producing IFN-γ is essential and sufficient to rescue mice from fatal immunopathology and that this mechanism is highly dependent on IL-27.
Several studies have described CD4+ T cells as the critical source of IL-10 in different infections, contributing either to protection or chronicity (16), (17), (24). In L. major infection, IL-10 from myeloid cells did not seem to play a major role, but T cell-derived IL-10 was sufficient to establish the chronic infection (16), (21), (22). However, IL-10 from CD4+ T cells is not the crucial source in all infections. In acute influenza and corona virus infections, IL-10-producing CD8+ T cells were shown to be protective, despite the fact that CD4+ T cells also constituted a significant proportion of the IL-10 producing cells (15), (55), and IL-10 produced primarily by DCs has been reported to contribute to persistence of Lymphocytic Choriomeningitis Virus (LCMV) infection (46). Therefore, the cellular origin of the essential IL-10 and its role seem to depend on the pathogen and its location (56).
B cells constituted a significant proportion of IL-10-secreting cells in the spleens of naïve mice, as described previously for other peripheral lymphoid tissues, where it has been proposed that they play a role in immune homeostasis (57). Our data show that mice unable to secrete IL-10 specifically from CD19+ B cells present significantly reduced total numbers of splenic IL-10+ IFN-γ+ CD4+ cells, suggesting that B cell-derived IL-10 is important for IL-10 production by CD4+ T cells. It has been demonstrated that chronic activation of murine CD4+ cells in the presence of IL-10 can generate IL-10-producing Tr1 cells (58). However, the requirement of IL-10 to generate IL-10+ cells is not so clear as recent data show thymic precursor cells giving rise to peripheral IL-10-expressing Treg cells in the absence of IL-10 (52). In our model of malaria the precise mechanism by which IL-10 is defectively induced in CD4+ T cells from Il10fl/flCD19Cre+ mice has to be further addressed. We cannot yet dismiss the possibility that B cell-derived IL-10 would also contribute to the control of pathology by other mechanisms.
A lack of IL-10 in T cells during P. chabaudi infection results in a greater inflammatory response, similar to that observed in infected Il10−/− mice, with high plasma levels of IFN-γ, TNF-α and IP-10. Since IFN-γ and TNF-α have both been shown to be involved in the pathology during P. chabaudi infection of Il10−/− mice (13), (2), this would suggest that the concomitant production of IL-10 by IFN-γ+ effector Th1 cells in this infection may represent an important self-limiting mechanism as proposed by Trinchieri (56) and Saraiva et al. (48), providing a highly regulated feedback loop that helps limit the collateral damage caused by exaggerated inflammation as well as allowing a protective response to different pathogens. In line with this hypothesis, it has been observed that peripheral blood mononuclear cells (PBMC) of children with uncomplicated P. falciparum malaria contain significantly greater proportions of IL-10+ IFN-γ+ double producing CD4+ T cells than PBMC from children with severe malarial disease (23), although it remains to be demonstrated whether these double producing cells are able to directly down-regulate immunopathology.
Recently, IL-10-producing Th1 cells have been identified in human blood which can suppress proliferation of naïve and memory T cells via IL-10 secretion (59), and similar to our results, these cells were highly activated, expressing high levels of ICOS, which originally was claimed to drive IL-10 secreting cells (60) and low levels of the IL-7 receptor (CD127), suggesting recent activation (61). In the P. chabaudi infection described here, these highly activated IL-10+ IFN-γ+ Th1 CD4+ T cells are present in greatest numbers following the peak of the acute infection and decline with the subsequent decrease in parasitemia, agreeing with the idea that persistence of IL-10-producing Th1 cells depends on chronic stimulation of the T cell receptor (TCR) and high antigen dose (48), (59).
IL-27 and IL-21 have been recently demonstrated to be involved in the induction of IL-10 (26), (54). Here we demonstrate that IL-27 signaling is critical for optimal production of IL-10 by IFN-γ-producing Th1 cells during acute P. c. chabaudi infection and, consequently, loss of IL-27 signaling increased the severity of immunopathology. A similar role for IL-27 has been described during autoimmune inflammation (62) and L. major infection (28). Moreover, our data on the requirement for the IL-27R in induction of IL-10 in Th1 cells would offer an explanation for the observation that IL-27R-deficient mice are highly susceptible to a P. berghei infection with exacerbated Th1 immune responses (29). IL-27 has been shown to induce IL-21, and that loss of IL-21 signaling in this situation resulted in inhibition of IL-10 production by T cells in vitro (54). The coordinated role of IL-27 and IL-21 in regulation IL-10 production is supported by the observation that elevated levels of these cytokines correlate with IL-10 responses in human visceral leishmaniasis (30). However, in this P. chabaudi infection there was no requirement for IL-21 in the production of IL-10 by CD4 T cells; indeed, IL-21 deficient mice presented increased numbers of IL-10+ IFN-γ+ CD4+ T cells.
Foxp3+ regulatory T cells are known to suppress immune responses using several mechanisms, which include those targeting the T cells directly (via suppressor cytokines, IL-2 consumption, cytolysis), or indirectly by suppressing APC function (via reduction of costimulation or antigen presentation) (63). Although some studies have shown that secretion of IL-10 by Treg cells can constitute an important component of their suppressive capacity (21), (22), (64), (65), our results here provide strong evidence that IL-10 from Foxp3+ regulatory T cells is not required to limit immunopathology elicited during acute P. chabaudi malaria. Lymphopenic mice receiving effector CD4+ T cells lacking IL-10 and Foxp3+ CD4+ Treg cells able to produce IL-10 experience severe disease, whereas reciprocal co-transfer of Tregs lacking IL-10 together with IL-10 sufficient effector CD4+ T cells did reduce pathology. However, a role for Tregs in the modulation of immunopathology in this model of malaria through a mechanism independent of IL-10 production cannot be dismissed. Such a role for Tregs in malaria is supported by the observations that Foxp3+ cell-mediated protection against P. berghei induced experimental cerebral malaria in mice is dependent upon CTLA-4 and not IL-10 (66), and that IFN-γ production by human PBMC stimulated with P. falciparum blood-stage antigens can be suppressed in vitro by CD25+ Foxp3+ regulatory T cells independently of IL-10 (67).
Interestingly, we observed that after adoptive transfer of Foxp3+ CD4+ T cells followed by infection, the loss of Foxp3 was more pronounced when Il10−/− Foxp3+ CD4+ T cells were transferred. Foxp3 expression can be lost after adoptive transfer into lymphopenic mice, however, it has been shown that IL-2 produced by co-transferred conventional T cells prevents Foxp3 down-modulation (68). Thus, in our case loss of Foxp3 expression because of lack of IL-2 is unlikely as conventional CD4+ T cells were co-transferred. Tregs require IL-10R signaling to maintain Foxp3 expression (69). It is possible that the observed decrease of Foxp3 frequencies in our model is due to the lack of IL-10 in Foxp3+ T cells themselves.
In summary, our work has identified IL-10+ IFN-γ+ Th1 cells as the prevalent source of IL-10 during infection with P. chabaudi. These effector cells are extremely activated, expressing high levels of CD44 and ICOS and low levels of CD127. In the beginning of infection, IL-10+ IFN-γ+ effector CD4 T cells secret more IFN-γ than single producers and later on, they secrete more IL-10 than the respective single producers, pointing to a possible self-feedback regulatory mechanism, in order to minimize immunopathology. Using cell transfer model into lymphopenic mice, we show clearly that IL-10 from highly activated Th1 cells is necessary and sufficient to regulate immune-mediated pathology elicited during this infection. Moreover, we demonstrate that IL-27, but not IL-21, signaling is required for induction of IL-10 by IFN-γ-producing CD4+ T cells, a critical mechanism for the control of immune-mediated pathology in mice. The next obvious step will be to validate these observations in human malaria in endemic areas. Given the already existing evidence for a protective role of IL-10 in severe malaria in humans, our identification of the potential cellular source of this cytokine and the requirement of the IL-27 receptor for its induction in this malaria model may aid in the development of intervention strategies to promote this pathway of regulation and thus potentiate immune responses that are protective against both disease and infection.
Supplementary Material
Acknowledgments
We thank Leona Gabrysova and George Kassiotis for careful reading of the manuscript, and Dr Bernard Malissen for the Foxp3-EGFP reporter mice. We also thank the Biological and Procedure Services and the Flow cytometry facility at NIMR for their skilled technical assistance.
Footnotes
This work was supported by the Medical Research Council, United Kingdom (MRC file reference: U117584248), and is part of the EviMalar European Network of Excellence supported by a European Union.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1β), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infection and Immunity. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Sanni LA, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10- deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infection and Immunity. 2003;71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stiles JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KW, De Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. In Annual Review of Immunology. 2001:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 5.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 6.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, Barnes PF. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infection and Immunity. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagamatsu K, Kuwae A, Konaka T, Nagai S, Yoshida S, Eguchi M, Watanabe M, Mimuro H, Koyasu S, Abe A. Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN. The Journal of experimental medicine. 2009;206:3073–3088. doi: 10.1084/jem.20090494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alter G, Kavanagh D, Rihn S, Luteijn R, Brooks D, Oldstone M, van Lunzen J, Altfeld M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtzhals JAL. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma, and TNF-alpha. Journal of Immunology. 1996;157:798–805. [PubMed] [Google Scholar]
- 11.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG. IL-10 Is Required to Prevent Immune Hyperactivity during Infection with Trypanosoma cruzi. Journal of Immunology. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 12.Linke A, Kuhn R, Muller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infection and Immunity. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature Medicine. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25- Foxp3- Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. Journal of Experimental Medicine. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet+Foxp3- Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. Journal of Experimental Medicine. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nature Immunology. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 19.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 21.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 22.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS pathogens. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huss DJ, Winger RC, Cox GM, Guerau-de-Arellano M, Yang Y, Racke MK, Lovett-Racke AE. TGF-beta signaling via smad4 drives IL-10 production in effector Th1 cells and reduces T cell trafficking in EAE. Eur J Immunol. 2011 doi: 10.1002/eji.201141666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 27.Spolski R, Leonard WJ. IL-21 is an immune activator that also mediates suppression via IL-10. Crit Rev Immunol. 2010;30:559–570. doi: 10.1615/critrevimmunol.v30.i6.50. [DOI] [PubMed] [Google Scholar]
- 28.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Findlay EG, Greig R, Stumhofer JS, Hafalla JC, de Souza JB, Saris CJ, Hunter CA, Riley EM, Couper KN. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol. 2010;185:2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 30.Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, Sacks D. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. 2011;186:3977–3985. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 32.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 33.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S, Genton C, Lucas B, DiSanto JP, Acha-Orbea H, Malissen B, Malissen M. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. The Journal of Immunology. 2008;180:1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
- 35.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Müller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. Journal of Experimental Medicine. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 37.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 38.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. European Journal of Immunology. 2006;36:3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 39.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Research. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 41.Freitas do Rosário AP, Muxel SM, Rodríguez-Málaga SM, Sardinha LR, Zago CA, Castillo-Méndez SI, Alvarez JM, D’Império Lima MR. Gradual decline in malaria-specific memory T cell responses leads to failure to maintain long-term protective immunity to Plasmodium chabaudi AS despite persistence of B cell memory and circulating antibody. The Journal of Immunology. 2008;181:8344–8355. doi: 10.4049/jimmunol.181.12.8344. [DOI] [PubMed] [Google Scholar]
- 42.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–6091. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 43.Sponaas AM, Freitas do Rosario AP, Voisine C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M, Potocnik AJ, Langhorne J. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 44.Stephens R, Langhorne J. Effector memory Th1 CD4 T cells are maintained in a mouse model of chronic malaria. PLoS Pathog. 2010;6:e1001208. doi: 10.1371/journal.ppat.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrysova L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, Wraith DC. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10- secreting Th1 cells. Journal of Experimental Medicine. 2009;206:1755–1767. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 50.Bueno LL, Morais CG, Araujo FF, Gomes JA, Correa-Oliveira R, Soares IS, Lacerda MV, Fujiwara RT, Braga EM. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nature Immunology. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 53.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trandem K, Zhao J, Fleming E, Perlman S. Highly Activated Cytotoxic CD8 T Cells Express Protective IL-10 at the Peak of Coronavirus-Induced Encephalitis. J Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. Journal of Experimental Medicine. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. Journal of Immunology (Baltimore, Md. : 1950) 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 59.Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med. 2009;206:1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 61.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 63.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 65.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 66.Haque A, Best SE, Amante FH, Mustafah S, Desbarrieres L, de Labastida F, Sparwasser T, Hill GR, Engwerda CR. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathog. 2010;6:e1001221. doi: 10.1371/journal.ppat.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human Cord Blood CD4+CD25hi Regulatory T Cells Suppress Prenatally Acquired T Cell Responses to Plasmodium falciparum Antigens. J Immunol. 2011;186:2780–2791. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 68.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 69.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.