Abstract
Background
Recent studies of infant siblings of children diagnosed with autism have allowed for a prospective approach to examine the emergence of symptoms and revealed behavioral differences in the broader autism phenotype within the early years. In the current study we focused on a set of functions associated with visual attention, previously reported to be atypical in autism.
Method
We compared performance of a group of 9–10-month-old infant siblings of children with autism to a control group with no family history of autism on the ‘gap-overlap task’, which measures the cost of disengaging from a central stimulus in order to fixate a peripheral one. Two measures were derived on the basis of infants’ saccadic reaction times. The first is the Disengagement effect, which measures the efficiency of disengaging from a central stimulus to orient to a peripheral one. The second was a Facilitation effect, which arises when the infant is cued by a temporal gap preceding the onset of the peripheral stimulus, and would orient faster after its onset.
Results and conclusion
Infant siblings of children with autism showed longer Disengagement latencies as well as less Facilitation relative to the control group. The findings are discussed in relation to how differences in visual attention may relate to characteristics observed in autism and the broader phenotype.
Keywords: Infancy, autism, visual attention, gap-overlap task, disengagement
For infants, visual orienting is the primary means of exploring the world. The efficiency of orienting undergoes dramatic developments in the first year of life, including the capacity to disengage attention and look away from salient or captivating stimuli impinging on the fovea (Hood, 1995; Johnson, Posner, & Rothbart, 1991). Coincident with this increasing ability to disengage, infants also improve in their ability to generate predictive eye movements (Hood & Atkinson, 1993; Johnson et al., 1991). One paradigm which measures flexibility in attentional switching in response to changes in the visual environment is known as the ‘gap-overlap task’.
Because orienting skills such as those measured in the gap-overlap task relate to the infant’s ability to switch attention flexibly and regulate emotional states (Posner & Rothbart, 1998), some have suggested that early impairments in disengagement of visual attention may relate to the social-communicative deficits found in autism (Bryson et al., 2004). Generally speaking, individuals with autism have a narrow focus of attention and interest, as well as acute perception of details (O’Riordan, Plaisted, Driver, & Baron-Cohen, 2001). It is unclear, however, whether these phenomena are related, and whether or not they share common underlying mechanisms. According to one view, the infant’s inability to flexibly switch their locus of attention could lead to a decrease in social orienting (Bryson et al., 2004). It has also been suggested that atypical processing of gaze found in autism may be a consequence of atypical visual attention (van der Geest, Kemner, Camfferman, Verbaten, & Van Engeland, 2001). Although the direction of causality is difficult to establish and it is possible that difficulties in both social and non-social areas are related to a common mechanism, this pattern appears to continue into adulthood.
Several studies have suggested that task-dependent difficulties in visual attention are present in autism across the life-span, albeit to varying degrees. In adults, these difficulties are revealed in tasks requiring rapid shifting of attention to different spatial locations (Casey, Gordon, Mannheim, & Rumsey, 1993; Courchesne et al., 1994; Townsend, Harris, & Courchesne, 1996; Wainwright-Sharp & Bryson, 1993). A recent study which used identical methods with individuals of different ages showed that the latencies of visually guided saccades were atypical in children but not in adults with autism (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007), implying that compensatory mechanisms may operate later in development. Studies specifically using the gap-overlap task have demonstrated impairments in children and adults with autism both behaviorally (Landry & Bryson, 2004; van der Geest et al., 2001) as well as neurophysiologically (Kawakubo et al., 2007).
Relatively less is understood in relation to how these attentional differences in the autism phenotype develop over time. A recent area of research focusing on infants at genetic high risk for autism has begun to address the emergent nature of autism symptoms more directly. Research on infant siblings of children diagnosed with autism spectrum disorders (ASD; hereafter ‘infant siblings’) offers this opportunity because the recurrence rate of ASD is significantly elevated above the general population (Bolton et al., 1994). Studying infant siblings offers opportunities to understand why autism emerges in some cases and not in others, and can potentially explain variations associated with the broader autism phenotype (BAP) found in genetic relatives of individuals with autism including siblings, who do not themselves have a diagnosis (Dalton, Nacewicz, Alexander, & Davidson, 2007; Dawson et al., 2002; Happé, Briskman, & Frith, 2001; Hughes, Leboyer, & Bouvard, 1997). Hence, understanding the precursors of these characteristics in infants would reveal the underlying mechanisms, which may extend to unaffected relatives.
Developmental accounts would suggest that problems in visual orienting are likely to be present very early on in autism (e.g., Bryson et al., 2004) and it remains unknown if these extend to the BAP. However, because autism is diagnosed relatively late, rarely before two years of age (Charman & Baird, 2002), the developmental process leading to these difficulties remains poorly understood. Most retrospective studies looking back at the first two years of life consistently show less orienting towards social stimuli as early as 9 months or younger in infants later diagnosed with autism as compared to those later diagnosed with developmental delay (Palomo, Belinchon, & Ozonoff, 2006). Many of these studies also report an overall decreased level of orienting to both social and non-social stimuli, but the impairment is greater for social stimuli.
Increasingly, studies with infant siblings of children with autism have documented differences between these infants and control groups with no family history for autism within the first year of life (e.g., Elsabbagh et al., 2009; McCleery, Allman, Carver, & Dobkins, 2007). Zwaigenbaum and colleagues (2005) provided preliminary evidence that impairments in attentional disengagement emerge between 6 and 12 months of age. At 6 months, infants who were later diagnosed with autism at 2–3 years could not be distinguished on this ability from the rest of the group. However, unlike controls, these infant siblings showed either no improvement or an increase in the latency of disengagement from central stimuli between 6 and 12 months of age.
In the current study, the aim was to explore this issue further by measuring orienting skills in a group of infant siblings of children with autism. We used the Gap-overlap task, which measures differences in the efficiency of orienting towards peripheral stimuli. In this task, three trial types are contrasted: Baseline, Gap, and Overlap. The Baseline condition is used to measure reaction time in a situation where the peripheral stimulus appears immediately after the disappearance of central stimuli. In the Overlap condition, the central stimulus remains visible and overlaps with the peripheral stimulus. Finally, in the Gap condition an intervening inter-stimulus interval separates the disappearance of the central stimulus and the appearance of the peripheral one. Based on contrasting these conditions, we focused on two emerging abilities: Disengagement, defined as the difference in reaction time between the Baseline condition and the Overlap condition. This measures the ability to disengage from a central stimulus to orient to a peripheral one. The second was Facilitation, which is the difference between the Baseline condition and the Gap condition. The latter facilitation arises because the infant would be cued by the gap preceding the onset of the peripheral stimulus, and could use the offset of the central stimulus as a cue to prepare their saccade to the later-occurring peripheral one. Hence, the task measured the infants’ ability to automatically orient to visual targets against competing stimuli as well as their ability to form expectations regarding the visual environment.
Methods
Participants
A total of 19 infant siblings of children with ASD (sib-ASD) and 19 matched control infants took part in the study. Informed consent was obtained from parents of all infants taking part in the study. Infants in the sib-ASD group all had an older brother or sister who received a confirmed clinical diagnosis of an ASD by a qualified UK practitioner. The characteristics of the groups are shown in Table 1. Infants’ age range was between 261 and 375 days and there were no significant differences in age between the two groups. Infants in the sib-ASD group fell within the average range on standardized measures of general cognitive and motor skills using the Mullen Scales (Mullen, 1995; mean = 104.84, sd = 11.10). Standardized measures were not fully available for the control group, but exclusion criteria for both groups included prematurity, low birth weight, medical or neurological conditions, sensory or motor problems. None of the children in the control group had first- or second-degree relatives with autism.
Table 1.
Participant characteristics and mean reaction times in the three conditions
| Control | Sib-ASD | |
|---|---|---|
| Initial group | ||
| n | 19 | 19 |
| Male: Female | 15:4 | 15:4 |
| Age in days (sd) | 304 (43) | 297 (44) |
| Group retained for the analysis | ||
| n | 16 | 16 |
| Male: Female | 14:2 | 12:4 |
| Age in days (sd) | 294 (44) | 302 (39) |
| Mean reaction times | ||
| Baseline (sd) | 320.6 ms (61.5) | 314.7 ms (93.8) |
| Overlap (sd) | 562.7 ms (166.3) | 644.4 ms (226.5) |
| Gap (sd) | 228.9 ms (26.9) | 266.9 ms (56.5) |
Stimuli and procedure
Infants were presented with the stimuli on a 40 × 60 cm monitor, while seated on their parent’s lap at 60 cm distance. Looking behavior was monitored and recorded through video from an adjacent room. All trials in this task began with a centrally presented animation. The animations, subtending around 12° × 12°, were either a cartoon of a sun or a clown (in different blocks) that expanded and contracted to attract the infant to the center prior to the onset of the trial. The peripheral target was presented randomly either to the right or the left of the central fixation stimulus at the eccentricity of 13°. Peripheral targets were always the same (a dynamic green balloon) subtending 12° × 12°. The peripheral target remained displayed until the infant looked at them or until 3 seconds elapsed. Once the infant looked to the target or if the maximum duration was reached, an attractive animation of an animal with sound replaced the peripheral target and the next trial was presented. The rate of trial presentation was controlled by the experimenter.
In the Baseline condition, the central fixation stimulus was extinguished and the peripheral target appeared simultaneously; in the Gap condition the fixation stimulus disappeared 200 ms before the peripheral target; in the Overlap condition the animated peripheral target appeared while the central fixation stimulus remained displayed (but not animated) so that the two stimuli overlapped. The three conditions were presented randomly across two blocks of 35 trials. The two blocks were identical except for the central fixation stimulus to maintain the infant’s interest in the task. Trial presentation continued until the infant became fussy or until a maximum of 70 trials was reached.
Data analysis and results
Data from three infants in each group was excluded from the analysis due to excessive fussiness or fatigue. Hence, data from 16 infants from each group was available for analysis. Video-recordings of the infants’ looking behavior overlaid in real time with input from the stimulus screen were coded off-line frame by frame. Trials were considered invalid if any of the following criteria were met: (a) the infant looked away from the screen at any point, (b) the infant did not look at the central stimulus immediately prior to the presentation of the peripheral stimulus, or (c) the infant blinked or looked away during the presentation of the peripheral stimulus. Saccadic reaction time data were analyzed for valid trials where the infant oriented towards the peripheral target after 100 to 1200 ms of its appearance (Johnson et al., 1991; Matsuzawa & Shimojo, 1997). If the infants did not look at the peripheral target within this period, reaction time was not analyzed but the trial was considered a failure to disengage. The latter were considered to be an index of the likelihood of orienting to peripheral targets rather than the speed of orienting. Inter-rater reliability calculated over 21% of the data was .9 (Cohen’s K) for the validity of trials and correlation between saccadic reaction times was .87.
The groups did not differ in the total number of trials completed (Control: mean = 60.6, sd = 11.7; Sib-ASD: mean = 59.9, sd = 12.3), nor in the number of valid trials (Control: mean = 47.1, sd = 13.4; Sib-ASD: mean = 43.0, sd = 14.1). The groups did not differ in the likelihood to orient to peripheral targets during overlap trials (Control = 84%, Sib-ASD = 91%, p = .24).
Reaction times for each group in each condition are reported in Table 1. A Kolmogorov–Smirnov test on the raw reaction time data showed that the data in two of the three conditions was not normally distributed (p < .05 for the Gap and Overlap condition in both groups). Hence, reaction time data for all three conditions were log transformed for the analysis to normalize the distribution. Figure 1 shows the log-transformed reaction times. The two groups did not differ in their reaction times during Baseline or Overlap trials (p > .05) but the sib-ASD group was slower during the Gap trials (p = .02).
Figure 1.
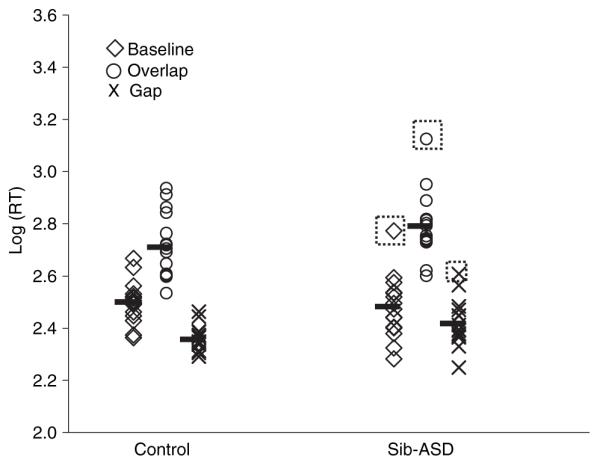
Reaction time data in the three conditions. Scatter plots show individual infant’s scores in each condition. Dark solid bars show the group means. Dotted boxes highlight cases with standardized residuals above 2.5
Disengagement was examined using ANOVA with the within-subjects factor Condition (Baseline vs. Overlap) and Group (Control, Sibs-ASD). The assumption of equality of error variance was met (Levene’s test > .1). There was a significant interaction between Group and Condition (F(1,29) = 6.11, p = .020, ηp2 = .17). Relative to the control group, the sib-ASD group showed a longer latency in the Overlap condition relative to the Baseline condition (one additional infant from the Sib-ASD group did not produce enough valid trials to allow RT to be calculated in the overlap condition). Facilitation (Gap vs. Baseline) was examined using a similar analysis. The assumption of equality of error variance was met (Levene’s test > .1). There was a significant interaction between Group and Condition (F(1,30) = 4.7, p = .018, ηp2 = .17). The sib-ASD group showed less facilitation when a temporal cue preceded the onset of the peripheral stimulus.
We were further interested in verifying whether the key significant results relating to Disengagement and Facilitation reflect the contribution of a few atypical infants in the sib-ASD group. To achieve this, we examined standardized residuals and Cook’s distance statistics generated for the two ANOVA models for individual infants. Standardized residuals (SR) within ±2.5 indicate that individual participants cannot be considered outliers and Cook’s distance (Di) values below 1 suggest that their removal from the generalized linear model (GLM) does not change the significance level. With the exception of a few infants, the ranges of the SRs across the three conditions was between −1.4 and 1.8 and all Di values were below .15. The following cases had SR scores above 2.5 (shown in dotted boxes in Figure 1): Case 22 in the baseline condition (SR = 2.8, Di = .3); Case 38 in the overlap condition (SR = 2.6, Di = .3); Case 35 in the gap condition (SR = 2.7, Di = .3). In relation to the Disengagement effect, Case 38 had the highest difference score (.53) between the Baseline and Overlap conditions, whereas in the other two cases the difference was relatively smaller than the group mean. In relation to the Facilitation effect Case 35 showed the lowest difference between the Baseline and the Gap condition (−.2), whereas the other two cases showed higher Facilitation relative to the group mean. Hence, these two infants (22 and 35) appear to have particularly pronounced difficulties in different aspects of visual orienting. On the other hand, there is no evidence that these extreme cases are driving the statistical effects observed at the group level.
Discussion
Early difficulties in attentional disengagement have been suggested as a precursor to certain aspects of the behavioral phenotype observed in autism. In previous work, developmental problems in disengagement between 6 and 12 months distinguished infants who later developed autism from infants who did not, as well as from typical controls (Zwaigenbaum et al., 2005). Our findings extend these results, revealing a more general atypical profile of visual orienting in a group of infant siblings of children with autism. This profile includes not only prolonged latency to disengage attention but also reduced facilitation arising from response preparation. We suggest that this atypicality in visual attention is part of the early expression of the broader autism phenotype found in relatives of individuals with autism. Our analysis of individual results confirmed that although a few infant siblings of children with autism showed extreme scores, the overall group effects found in Disengagement and Facilitations are not primarily driven by a few atypical cases.
How might such differences in visual orienting relate to developmental models of the emergence of autism? It has been suggested that an early deficit in social orienting, resulting in decreased input from socially relevant stimuli, may underlie the emergence of autism symptoms (Dawson et al., 2005; Johnson et al., 2005; Schultz, 2005). On the other hand, problems in visual attention e.g., prolonged disengagement, occur in various developmental disorders, including ones with markedly different cognitive and social profiles from the one found in autism, such as Williams Syndrome (Brown et al., 2003). This rules out the possibility that early differences in visual attention relate specifically to autism, unless considered with additional factors. For instance, it is possible that the proposed social orienting deficits might be compounded and amplified by the presence of other difficulties, such as those found in attentional disengagement. A problem with flexibly switching attention between different stimuli would result in ‘locking’ onto certain irrelevant aspects of the diminished input. Similarly, a limitation in the ability to use environmental events to predict and prepare for a shift in attention (anticipation) may prevent typical levels of foraging from visual scenes resulting in reduced foveation of relevant information. The infant, in this case, would not only receive decreased input from social stimuli (Bryson et al., 2004), but attentional constraints would impose qualitatively different forms of input, namely focal and irrelevant ones (Elsabbagh & Johnson, 2007). This would suggest that infants who exhibit a combination of disengagement difficulties with decreased social orienting would be at higher risk for autism than infants who exhibit one of these difficulties in isolation, a prediction requiring testing with a larger group of infants.
More generally, differences in visual orienting documented in our study are also likely to relate to differences in information processing style found in the broader autism phenotype (e.g., Happé et al., 2001). In research with typical infants, a link has been made between early variation in attentional skills and local versus global processing styles. As typically developing children begin to scan their environment flexibly and switch their attention among different stimuli, global forms are processed quickly and efficiently. Infants who exhibit a pattern of prolonged look duration rely more on local elements when processing visual stimuli (Freeseman, Colombo, & Coldren, 1993; Frick, Colombo, & Saxon, 1999). Hence, it is possible that the narrow focus of attention in autism is a developmental consequence of early difficulties in visual disengagement (Landry & Bryson, 2004). Alternatively, it can also result from atypical modulation of early visual processing areas by top-down feedback and reflected in anticipation or preparation for saccades. Moreover differences in modulation of visual attention may relate to superior performance of individuals with autism on some cognitive tasks. Owing to the lack of cognitive characterization of the current sample, the existing data does not allow us to test the direction of causality in relation to whether reduced visual disengagement is a cause or a consequence of a cognitive style focused on exploring information in great detail.
Beyond the theoretical significance of these findings in relation to developmental hypotheses of autism, to what extent can these early differences in visual attention be used as predictors for a subsequent diagnosis? One possibility is that those infants with the extreme scores identified in the infant siblings group are those who are likely to receive a diagnosis. Alternatively, early atypical visual orienting may not be sufficient for a later diagnosis, unless it is combined with other risk markers (Elsabbagh & Johnson, 2007). A limitation of our study in relation to disentangling these possibilities is the lack of follow-up data on diagnostic outcomes, and the small sample size.
In sum, our results confirmed that an atypical profile of visual attention is manifest early on in infants at risk for autism. These differences raise the possibility that certain characteristics of the broader autism phenotype are present in infancy and may relate to differences in scanning both social and non-social stimuli. Future work needs to test specific hypotheses regarding the relationship between variations in attentional abilities and scanning of the visual environment in infant siblings, which in turn would have relevant clinical implications regarding the use of such measures as predictors of later diagnosis.
Key points.
Difficulties in attentional switching have been documented in autism but little is known about how these develop over time, including in infants at risk for autism.
Relative to a control group, we found that infant siblings of children with autism showed longer latencies to disengage from a central stimulus to orient to the periphery. Moreover, their reaction times were less facilitated by a temporal gap preceding the peripheral stimulus.
It is likely that these differences in visual attention modulate visual input infants receive from the environment and may, in combination with other risk factors, be useful for predicting individual variability in the broader autism phenotype in childhood.
Acknowledgements
We are grateful to the families who took part in our research. We also wish to thank Holly Garwood and Gaia Scerif for their assistance. This research was funded by the Medical Research Council (G9715587) and Autism Speaks (1292/MJ/01-201-006-065-00-00 and 06/MRE02/73).
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case–control family history study of autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Brown J, Johnson MH, Paterson S, Gilmore R, Gsödl M, Longhi E, Kamiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neurosychologia. 2003;41:1037–1046. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Landry R, Czapinski P, Mcconnell B, Rombough V, Wainwright A. Autistic spectrum disorders: Causal mechanisms and recent findings on attention and emotion. International Journal of Special Education. 2004;19:14–22. [Google Scholar]
- Casey BJ, Gordon CT, Mannheim GB, Rumsey JM. Dysfunctional attention in autistic savants. Journal of Clinical and Experimental Neuropsychology. 1993;15:933–946. doi: 10.1080/01688639308402609. [DOI] [PubMed] [Google Scholar]
- Charman T, Baird G. Practitioner review: Diagnosis of autism spectrum disorder in 2- and 3-year-old children. Journal of Child Psychology and Psychiatry. 2002;43:289–305. doi: 10.1111/1469-7610.00022. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, et al. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17:679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood M, Tucker L, Krljes S, Baron-Cohen S, Bolton P, Charman T, Baird G, Johnson MH. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Infancy and autism: Progress, prospects, and challenges. Progress in Brain Research. 2007;164:355–383. doi: 10.1016/S0079-6123(07)64020-5. [DOI] [PubMed] [Google Scholar]
- Freeseman LJ, Colombo J, Coldren JT. Individual differences in infant visual attention: Fourmonth-olds’ discrimination and generalization of global and local stimulus properties. Child Development. 1993;64:1191–1203. [PubMed] [Google Scholar]
- Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Development. 1999;70:537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Happé F, Briskman J, Frith U. Exploring the cognitive phenotype of autism: Weak ‘central coherence’ in parents and siblings of children with autism: I. Experimental tests. Journal of Child Psychology and Psychiatry. 2001;42:299–307. [PubMed] [Google Scholar]
- Hood B. Shifts of visual attention in the infant: A neuroscientific approach. In: Rovee-Collier C, Lipsitt L, editors. Advances in infancy research. Ablex; Norwood, NJ: 1995. pp. 163–216. [Google Scholar]
- Hood B, Atkinson J. Disengaging visual attention in the infant and adult. Infant Behavior and Development. 1993;16:405–422. [Google Scholar]
- Hughes C, Leboyer M, Bouvard M. Executive function in parents of children with autism. Psychological Medicine. 1997;27:209–220. doi: 10.1017/s0033291796004308. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, De Haan M, et al. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. Journal of Cognitive Neuroscience. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Okazaki S, Hosokawa-Kakurai M, Watanabe K, Kuwabara H, et al. Electrophysiological abnormalities of satial attention in adults with autism during the gap overlap task. Clinical Neurophysiology. 2007;118:1464–1471. doi: 10.1016/j.clinph.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Matsuzawa M, Shimojo S. Infants’ fast saccades in the gap paradigm and the development of visual attention. Infant Behavior and Development. 1997;20:449–455. [Google Scholar]
- McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal magnocellular (M) pathway visual processing in infants at risk for autism. Biological Psychiatry. 2007;62:1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning – AGS Edition. AGS Publishing; Circle Pines, MN: 1995. [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Palomo R, Belinchon M, Ozonoff S. Autism and family home movies: A comprehensive review. Journal of Developmental and Behavioral Pediatrics. 2006;27:S59–S68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: Delayed orienting to location. Journal of the International Neuropsychological Society. 1996;2:541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Van Der Geest JN, Kemner C, Camfferman G, Verbaten MN, Van Engeland H. Eye movements, visual attention, and autism: A saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50:614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental disorders. 1993;23:1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]


