Abstract
Trichomonad species are widespread unicellular flagellated parasites of vertebrates which interact with their hosts through carbohydrate-lectin interactions. In the past, some data has been accumulated regarding their lipo(phospho)glycans, a major glycoconjugate on their cell surfaces; on the other hand, other than biosynthetic aspects, few details about their N-linked oligosaccharides are known. In this study, we present both mass spectrometric and HPLC data about the N-glycans of different strains of Trichomonas vaginalis, a parasite of the human reproductive tract. The major structure in all strains examined is a truncated oligomannose form (Man5GlcNAc2) with α1,2-mannose residues, compatible with a previous bioinformatic examination of the glycogenomic potential of T. vaginalis. In addition, dependent on the strain, N-glycans modified by pentose residues, phosphate or phosphoethanolamine and terminal N-acetyllactosamine (Galβ1,4GlcNAc) units were found. The modification of N-glycans by N-acetyllactosamine in at least some strains is shared with the lipo(phospho)glycan and may represent a further interaction partner for host galectins, thereby playing a role in binding of the parasite to host epithelia. On the other hand, the variation in glycosylation between strains may be the result of genetic diversity within this species.
Keywords: trichomonads, N-glycan, mass spectrometry, pentose, phosphoethanolamine
Introduction
The glycoconjugates of eukaryotic parasites play important roles in host-pathogen interactions, whether it be in terms of binding, invasion, immune recognition or immunomodulation, and are often necessary for virulence. The major categories of these molecules in unicellular protozoal parasites include N-glycans, O-glycans and lipophosphoglycans (Guha-Niyogi, et al. 2001). In the case of trichomonads, a number of studies have demonstrated the presence of a lipo(phospho)glycan containing a range of monosaccharide units, such as xylose and rhamnose (Singh, et al. 2009, Ryan, et al. 2011), as well as poly-N-acetyllactosamine moieties which interact with host galectins (Okumura, et al. 2008). Indeed, lipo(phospho)glycan-mediated binding of trichomonads to mucosa is required for adherence and proinflammatory epithelial activation, which are features of Trichomonas vaginalis infections affecting large percentages of the human population worldwide (Schwebke and Burgess 2004). In cattle and cats, another sexually-transmitted trichomonad, Tritrichomonas foetus, has also been studied as regards its cell surface lipophosphoglycan, which, in contrast to that of T. vaginalis, is characterised by large numbers of fucose residues (Singh 1993). An oligoglucose O-glycan has also been identified from T. vaginalis (Grabińska, et al. 2008).
In the present study, we have focussed on the N-linked oligosaccharides of one of these organisms, T. vaginalis; to date, these glycans have not been highly characterised. As part of a bioinformatically-driven study on the enzymes required for synthesis of N-glycans, it was noted that the major structure of T. vaginalis has the composition Man5GlcNAc2, but further features of the N-glycome were not described (Samuelson, et al. 2005, Banerjee, et al. 2007); also, the presence of xylose on its N-glycans was mentioned in a publication describing potential xylosyltransferase genes, but no structural details were shown (Hwa, et al. 2007). Here, using HPLC and MALDI-TOF MS, we compare the N-glycans of different strains of T. vaginalis, thereby demonstrating strain-specific modification of their N-glycans.
Experimental Procedures
Cultivation of parasites
In this study, four different strains of Trichomonas vaginalis grown in three different laboratories were employed: C1 (ATCC 30001), G3 (ATCC PRA-98), TV2 and IR-78. The TV2 strain was isolated in 2002 from material from a patient with metronidazole susceptible trichomoniasis kindly provided by Professor A. Stary, Universitätsklinik für Dermatologie, Medizinische Universität Wien (Blaha, et al. 2006), whereas IR-78 is a metronidazole-resistant strain described first in 1979 (Meingassner and Thurner 1979). The parasites were grown in media based on Diamond’s TYM (trypticase peptone/yeast extract/maltose) medium (Diamond 1957). In the first laboratory, this was supplemented with penicillin and streptomycin and newborn calf serum; only in this case were the cells lyophilised prior to transport (sample C1/1). In the second and third laboratory, the C1 strain of T. vaginalis was cultivated in supplemented with 10% fetal bovine serum (Sigma-Aldrich) either with or without 3% Gibco MEM vitamins mix (Invitrogen) and transferred, after washing with PBS, to serum-free medium 48 hours prior to harvesting (samples C1/2 and C1/3). In a third laboratory, the C1 (sample C1/4), G3, TV2 and IR-78 strains were grown in TYM medium with antibiotics and horse serum: in this case, it was observed that the C1 and TV2 strains were adherent to the polystyrene flasks, whereas G3, the strain used for the genome sequence (Carlton, et al. 2007), and IR-78 were non-adherent.
Preparation of glycans
Parasite cells (2 g if grown in serum or 0.4 g if cultivated ‘serum-free’) or lyophilisates (50 mg) were suspended in ten millilitres boiling water and denatured for 5 minutes, prior to addition of formic acid [up to 5% (v/v)] and 1 mg porcine pepsin. Proteolysis was allowed to proceed for two days at 37 °C and the samples were centrifuged to remove insoluble material. The supernatant of the proteolysate was then incubated with 10 ml prewashed Dowex AG50 W×2 (Sigma-Aldrich) for one hour at 23 °C. The material was then poured into a column and the flowthrough fraction was reapplied; the column was then washed with 2% (v/v) acetic acid to remove unbound material and glycopeptides were eluted with 0.5 M ammonium acetate, pH 6, and lyophilised prior to gel filtration (Sephadex G25; GE Healthcare). Orcinol-positive fractions were pooled, heat treated for 5 minutes and subject todigestion with either PNGase A (peptide:N-glycosidase from almonds; Roche) in 50 mM ammonium acetate, pH 5, or PNGase F (peptide:N-glycosidase from Flavobacterium; Roche) in 100 mM ammonium carbonate, pH 8, overnight at 37 °C (in the case of the C1/4, G3, TV2 and IR-78 samples, the pooled gel filtration fractions were separated into two halves prior to glycan release, whereas the C1/3 preparation was subject to PNGase F then PNGase A treatment in series). A second round of Dowex chromatography was performed and the unbound glycans were analysed, without further purification, by MALDI-TOF MS (matrix assisted laser-desorption/ionisation time-of-flight mass spectrometry).
Pyridylamination was subsequently performed basically as described (Hase, et al. 1984). In brief, 100 mg 2-aminopyridine (Sigma-Aldrich) was dissolved in 76 μl concentrated HCl and 152 μl water; 80 μl of this solution was added to the dried glycan sample, prior to incubation in boiling water for 15 minutes. Then a solution of 4.4 mg of sodium cyanoborohydride (Sigma-Aldrich) in a mixture of 9 μl of the aforementioned 2-aminopyridine solution and 13 μl water was prepared; 4 μl of this cyanoborohydride-aminopyridine solution was added to the sample and the incubation was continued overnight at 90 °C prior to gel filtration (Sephadex G15; GE Healthcare). Fluorescence (excitation/emission 320/400nm) of the fractions was measured using a Tecan microtitre plate reader.
The glycan samples were named on the basis of the numbering of the original culture (see under Cultivation of parasites above and Supplementary Table) and the enzymes used for release: e.g. C1/1A refers to PNGase A-released glycans from sample C1/1; C1/2F refers to PNGase F released glycans from sample C1/2; C1/3FA to combined PNGase F and A release of glycans from sample C1/3. In the case of G3 and IR-78, two independent cultures of each strain were used prior to being divided for PNGase A and F digestion; only data from one culture are presented here.
Matrix-assisted laser desorption-ionisation time-of-flight mass spectrometry
Free glycans or pyridylaminated glycans (0.8 μl) were dried under vacuum onto a ground steel sample plate prior to application of 0.8 μl of either 2,5- dihydrobenzoic acid (DHB) or 6-aza-2-thiothymine (ATT) as matrices and vacuum drying once more. The samples were analysed in positive or negative ion modes using a Bruker Ultraflex I equipped with a nitrogen laser (337 nm; laser frequency of 50 Hz and pulse length of 200 ns); typically 400-1000 shots were summed. Predicted glycan species wereexamined by MS/MS (post-source decay) and all spectra were evaluated manually. A key diagnostic MS/MS fragment is that corresponding to the core reducing-terminal GlcNAc-PA (expected m/z 300.15, observed m/z values were generally in the range 299.7 - 300.2).
High performance liquid chromatography of N-glycans
Pyridylaminated N-glycans were analysed by either normal phase (NP) or reverse phase (RP) HPLC (high pressure liquid chromatography) using a Shimadzu HPLC system equipped with a fluorescence detector (RF 10 AXL). For NP-HPLC, a Tosoh Amide-80 column (4.6 × 250 mm) was used and calibrated daily using a pyridylaminated isomaltose series. Prior to injection, dried samples were taken up in 50 μl of a 25:75 mixture of buffer A (10 mM ammonium formate, pH 7) and buffer B (95% acetonitrile). The gradient was applied as follows: 0-5 mins, 75% B; 5-15 mins, 75-65% B; 15-40 mins, 65% B; 40-55 mins, 65-57% B; followed by a return to the starting conditions. For RP-HPLC, Hypersil ODS columns (5 μ, 4 × 250 mm; purchased from either MZ Analytik or Agilent) were used. For both columns, buffer A was 0.1 M ammonium acetate, pH 4, and buffer B was 30% (v/v) methanol. Gradients of increasing methanol (1% buffer B per minute) were applied. Fluorescence was recorded at 320 nm (excitation) and 400 nm (emission). For ‘2D-HPLC’ (two-dimensional HPLC) of sample C1/1A, glycans were first fractionated by NP-HPLC, collected, lyophilised and analysed by MALDI-TOF MS prior to being subject to RP-HPLC. All collected fractions were subject to MALDI-TOF MS and the compositions of glycan species (in terms of hexose, N-acetylhexose, pentose) were verified by MS/MS. Only selected spectra and chromatograms are presented here.
Enzymatic or chemical digestion of N-glycans
Aliquots of isolated 2D-HPLC fractions were, based on the results of MALDI-TOF MS, subject to targetted exoglycosidase digestions. Depending on the peak intensity, between one-twentieth and one-half of a fraction was incubated with either 0.2 μl Streptococcus β-hexosaminidase (0.01 U; Calbiochem) using the manufacturer’s buffer, 0.1 μl Aspergillus saitoi α1,2-mannosidase (2 μU; Prozyme) with the manufacturer’s buffer, 0.1 μl repurified Aspergillus oryzae β1,4-galactosidase (0.027 U) with 50 mM sodium citrate, pH 4.5 (Zeleny, et al. 1997), or 0.5 μl jack bean α-mannosidase (0.5 U; Sigma-Aldrich) with 50 mM sodium citrate, pH 4.5. The incubations were subject to a second round of RP-HPLC and MALDI-TOF MS.
For glycans solely fractionated by RP-HPLC, one tenth of each redissolved fraction (1 μl) was mixed with 0.5 μl 0.2 M ammonium acetate, pH 5, buffer and either 0.5 μl A. saitoi α1,2-mannosidase (10 μU; Prozyme), 0.5 μl Xanthomonas α1,2/3-mannosidase (32 U; New England Biolabs), 0.25 μl Xanthomonas β1,2-xylosidase (2.5 mU, Merck Calbiochem), 0.5 μl recombinant Bacteroides fragilis β1,4-galactosidase (2 U; New England Biolabs), 0.25 μl jack bean α-mannosidase (Sigma-Aldrich) or 0.25 μl jack bean β-hexosaminidase (0.06 U; Sigma-Aldrich); incubations were performed overnight at 37 °C and analysed directly by MALDI-TOF MS using ATT as matrix. For dephosphorylation, either whole N-glycomes or selected fractions were dried and incubated overnight at 0 °C with 3 μl 48% (v/v) hydrofluoric acid prior to evaporation; the samples were diluted in water and re-evaporated, prior to redissolving once again and analysis by MALDI-TOF MS.
Results
General analytical approach
In order to analyse the N-glycomes of different Trichomonas vaginalis strains, glycans were released using PNGase A and/or PNGase F. The glycans from the first sample of the C1 strain to be analysed (C1/1A) were fractionated first by NP-HPLC (see Supplementary Figure 1), prior to subsequent re-fractionation by RP-HPLC, analysis by MALDI-TOF MS and MS/MS and selected exoglycosidase digestions (see Table 1 for a summary of these data). In addition, considering a cautionary tale regarding data on another parasite grown in medium containing bovine serum, Giardia intestinalis (Robbins and Samuelson 2005), glycans were also prepared from the C1 strain after starvation of serum for two days prior to harvesting (samples C1/2F and C1/3FA). The subsequent N-glycomic comparisons with these ‘serum-free’ C1 samples, as well as with four strains grown in medium containing horse serum (C1, G3, TV2 and IR-78), were performed based on MALDI-TOF MS/MS of RP-HPLC fractions (see Table 2 for a summary).
Table 1. Summary of T. vaginalis PNGase A-released glycans.
Glycans from sample C1/1A were initially subject to NP-HPLC (Tosoh Amide 80; fractions A-L) prior to refractionation of each NP-HPLC fraction by RP-HPLC (Hypersil, MZ Analytik) and MALDI-TOF MS using DHB as matrix (see Supplementary Figure 2). Both calculated and observed m/z values are shown; the differences in these values vary between −0.02 and +0.31. Multiple forms of glycans are possible due to isomeric variation. Fractions marked with an asterisk (*) are considered to be derived from serum in the cultivation medium, whereas those retention times in glucose units (g.u.) marked with a double dagger (‡) are similar to those described in the literature (Tomiya, et al. 1988, Tomiya, et al. 1991).
| ‘2D’ fraction |
m/z [M+H]+ (calculated) |
m/z [M+H]+ (observed) |
RP-HPLC (g.u.) | Composition |
|---|---|---|---|---|
| A1 | 989.38 | 989.36 | 5.2 | Hex3HexNAc2 |
| A2 | 989.38 | 989.41 | 7.2‡ | Hex3HexNAc2 |
| B1 | 1121.42 | 1121.45 | 5.2 | Hex3HexNAc2Pent1 |
| B2 | 1121.42 | 1121.49 | 6.2 | Hex3HexNAc2Pent1 |
| B3 | 1121.42 | 1121.44 | 7.1 | Hex3HexNAc2Pent1 |
| B4 | 1192.46 | 1192.50 | 9.5‡ | Hex3HexNAc3 |
| C1 | 1151.44 | 1151.49 | 5.9 | Hex4HexNAc2 |
| D1 | 1151.44 | 1151.56 | 4.5 | Hex4HexNAc2 |
| D2 | 1324.50 | 1324.67 | 5.8 | Hex3HexNAc3Pent1 |
| D3 | 1151.44 | 1151.58 | 6.2 | Hex4HexNAc2 |
| D4 | 1324.50 | 1324.59 | 8.5 | Hex3HexNAc3Pent1 |
| E1 | 1253.47 | 1253.57 | 5.3 | Hex3HexNAc2Pent2 |
| E2 | 1283.48 | 1283.58 | 6.2 | Hex4HexNAc2Pent1 |
| E3 | 1354.51 | 1354.66 | 7.5 | Hex4HexNAc3 |
| E4 | 1354.51 | 1354.65 | 9.8‡ | Hex4HexNAc3 |
| F1 | 1283.48 | 1283.44 | 5.8 | Hex4HexNAc2Pent1 |
| G1 | 1313.49 | 1313.48 | 4.2 | Hex5HexNAc2 |
| G2 | 1313.49 | 1313.48 | 5.8‡ | Hex5HexNAc2 |
| G3 | 1456.54 | 1456.62 | 6.1 | Hex3HexNAc3Pent2 |
| G4 | 1486.58 | 1486.65 | 7.0 | Hex4HexNAc3Pent1 |
| G5 | 1486.58 | 1486.62 | 8.8 | Hex4HexNAc3Pent1 |
| H1 | 1415.52 | 1415.65 | 4.7 | Hex4HexNAc2Pent2 |
| H2 | 1445.53 | 1445.61 | 5.8 | Hex5HexNAc2Pent1 |
| H3 | 1516.57 | 1516.74 | 7.0 | Hex5HexNAc3 |
| H4 | 1689.64 | 1689.84 | 8.2 | Hex4HexNAc4Pent1 |
| H5 | 1719.65 | 1719.85 | >11 | Hex5HexNAc4 |
| H6 | 1719.65 | 1719.78 | >11 | Hex5HexNAc4 |
| I1 | 1475.54 | 1475.72 | 6.2 | Hex6HexNAc2 |
| I2 | 1618.60 | 1618.80 | 6.5 | Hex4HexNAc3Pent2 |
| I3* | 1719.65 | 1719.91 | 9.7‡ | Hex5HexNAc4 |
| J1* | 1865.71 | 1865.84 | >11‡ | Hex5HexNAc4dHex1 |
| K1 | 1851.69 | 1852.00 | 10.5 | Hex5HexNAc4Pent1 |
| L1* | 2084.78 | 2085.03 | >11‡ | Hex6HexNAc5 |
| L2 | 2084.78 | 2085.08 | >11 | Hex6HexNAc5 |
Table 2. Comparison of the N-glycomes of four strains of Trichomonas vaginalis.
Seven different samples of T. vaginalis representing four different strains were subject to the glycan release protocol described using either PNGase A and/or PNGase F. Two of the C1 samples (C1/2 and C1/3) were maintained for two days in the absence of serum prior to harvesting. The major glycan, Man5GlcNAc2 (abbreviated composition H5N2) is given the relative abundance ‘+++’. Glycans with MALDI-TOF MS intensities of around 10% as compared to H5N2 are indicated as ‘++’; others for which lower amounts were detected in either complete spectra or HPLC fractions are indicated as ‘+’. m/z values are indicated for the unlabelled (free ) and labelled (PA) forms.
| Free m/z [M+Na]+ | PA m/z [M+H]+ | PA m/z [M+Na]+ | Composition | C1/1A | C1/2F | C1/3FA | C1/4A | C1/4F | G3/A | G3/F | TV2/A | TV2/F | IR78/A | IR78/F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 771 | 827 | 849 | H2N2 | + | + | + | + | + | + | + | + | |||
| 933 | 989 | 1011 | H3N2 | + | + | + | + | + | + | ++ | ++ | + | + | |
| 1056 | 1112 | 1134 | H3N2(EtNP) | + | + | |||||||||
| 1065 | 1121 | 1143 | H3N2P1 | ++ | + | + | + | +++ | +++ | |||||
| 1095 | 1151 | 1173 | H4N2 | + | ++ | + | ++ | ++ | ++ | ++ | + | + | + | + |
| 1136 | 1192 | 1214 | H3N3 | + | + | + | + | |||||||
| 1179 | 1235 | 1257 | H3N2(EtNP)2 | + | ||||||||||
| 1197 | 1253 | 1275 | H3N2P2 | + | ||||||||||
| 1218 | 1274 | 1296 | H4N2(EtNP) | + | + | + | ||||||||
| 1227 | 1283 | 1305 | H4N2P1 | + | + | + | + | + | + | |||||
| 1257 | 1313 | 1335 | H5N2 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 1268 | 1324 | 1346 | H3N3P1 | + | ||||||||||
| 1298 | 1354 | 1376 | H4N3 | + | ++ | ++ | ++ | + | + | |||||
| 1337 | 1393 | 1415 | H5N2(Phos) | + | ||||||||||
| 1359 | 1415 | 1437 | H4N2P2 | + | ||||||||||
| 1380 | 1436 | 1458 | H5N2(EtNP) | + | + | + | + | + | + | |||||
| 1389 | 1445 | 1467 | H5N2P1 | + | + | + | + | + | + | + | + | + | ||
| 1400 | 1456 | 1478 | H3N3P2 | + | ||||||||||
| 1419 | 1475 | 1497 | H6N2 | + | + | + | + | + | + | + | + | + | + | + |
| 1430 | 1486 | 1508 | H4N3P1 | ++ | + | + | + | + | ||||||
| 1460 | 1516 | 1538 | H5N3 | + | ++ | + | ++ | ++ | ++ | ++ | ||||
| 1551 | 1607 | 1629 | H6N2P1 | + | + | |||||||||
| 1562 | 1618 | 1640 | H4N3P2 | + | + | |||||||||
| 1583 | 1639 | 1661 | H5N3(EtNP) | + | + | ++ | ||||||||
| 1592 | 1648 | 1670 | H5N3P1 | + | + | + | + | + | ||||||
| 1622 | 1678 | 1700 | H6N3 | + | + | + | + | |||||||
| 1633 | 1689 | 1711 | H4N4P1 | + | + | + | + | |||||||
| 1663 | 1719 | 1741 | H5N4 | ++ | + | + | + | + | + | + | + | + | + | + |
| 1724 | 1780 | 1802 | H5N3P2 | + | ||||||||||
| 1745 | 1801 | 1823 | H6N3(EtNP) | + | + | |||||||||
| 1784 | 1840 | 1862 | H7N3 | + | ||||||||||
| 1795 | 1851 | 1873 | H5N4P1 | + | + | + | + | + | ||||||
| 1825 | 1881 | 1903 | H6N4 | + | + | + | + | |||||||
| 1866 | 1922 | 1944 | H5N5 | + | ||||||||||
| 1957 | 2013 | 2035 | H6N4P1 | + | + | + | ||||||||
| 2028 | 2084 | 2106 | H6N5 | ++ | + | |||||||||
| 2160 | 2216 | 2238 | H6N5P1 | + | + | |||||||||
| 2190 | 2246 | 2268 | H7N5 | + | ||||||||||
| 2322 | 2378 | 2400 | H7N5P1 | + |
Analysis of the major pentamannosidic glycan
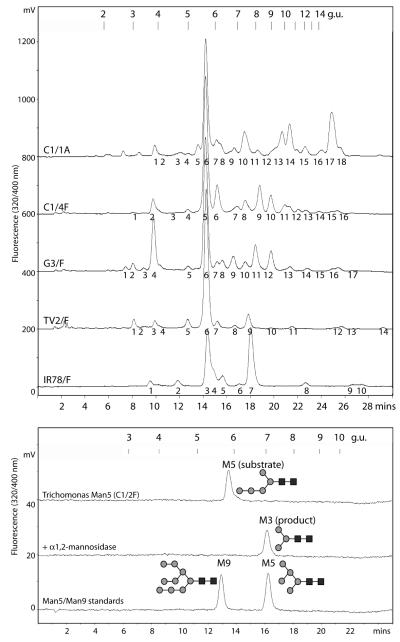
In its pyridylaminated form, the major glycan in three strains (C1, G3 and TV2) and the second most dominant glycan in the IR-78 strain has a predicted composition of Hex5HexNAc2 (m/z 1313) and an RP-HPLC retention time of 5.8 glucose units (g.u.; see Figure 1 and 2). This is unlike a typical Man5GlcNAc2 commercial standard which has a retention time corresponding to around 7 g.u. Digestion of the trichomonad Hex5HexNAc2 structure with an α1,2-specific mannosidase results in the loss of two hexose units and a shift to a Hex3HexNAc2 glycan (m/z 989) eluting at around 7 g.u.; in a mass spectrometric assay using an α1,2/3-specific mannosidase, three mannose residues were removed (Supplementary Figure 2A). Thereby, our data are compatible with the major N-glycan in T. vaginalis strains being Manα1,2Manα1,2Manα1,3(Manα1,6)Manβ1,4GlcNAcβ1,4GlcNAc.
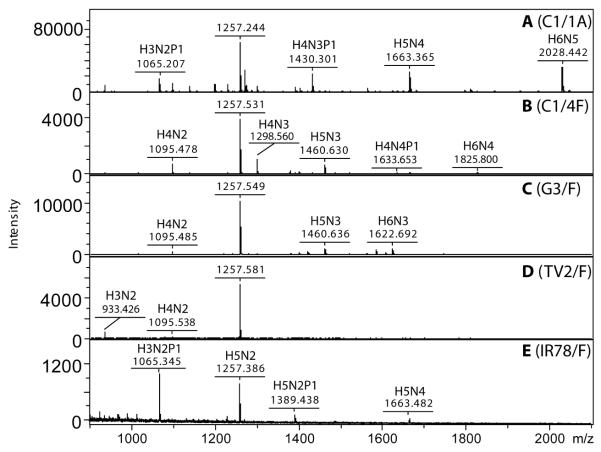
Figure 1. Comparison of unlabelled N-glycans from different samples of Trichomonas vaginalis.
Example MALDI-TOF MS spectra are shown for N-glycans prepared from two different samples of the C1 strain (A and B; sample C1/1 glycans released by PNGase A and sample C1/4 released by PNGase F), G3 strain (C, PNGase F release) and the TV2 and IR-78 clinical isolates (D and E; PNGase F release). Matrix is ATT and the samples were analysed in the positive mode. The spectra for G3/F and IR-78/F were verified for two independent preparations. The major [M+Na]+ ions are annotated along with putative compositions, where H represents hexose, N represents N-acetylhexosamine and P pentose. For comparison to the data shown in subsequent figures, the major m/z 1257 species corresponds to Man5GlcNAc2, which in pyridylaminated form has m/z values of 1313 as [M+H]+ or 1311 as [M−H]−. The intensity is in ‘arbitrary units’.
Figure 2. RP-HPLC analysis of Trichomonas vaginalis N-glycans.
Upper panel: Pyridylaminated N-glycans from the same samples as shown in Figure 1 were subject to RP-HPLC fractionation (Hypersil ODS, Agilent); the column was calibrated using an isomalto-oligosaccharide series (2-14 glucose units). Fractions were collected as indicated and subject to MALDI-TOF MS analysis; for simplicity and due to their similarity, the G3/A, TV2/A and IR-78/A chromatograms, with the same fraction numbering, are not shown. Lower panel: The major RP-HPLC fraction of T. vaginalis C1 glycans was analysed by RP-HPLC (5.8 g.u.) before and after digestion with Aspergillus α1,2-mannosidase. In comparison, commercial Man5GlcNAc2-PA (ca. 7 g.u.; Takara) and Man9GlcNAc2-PA (ca. 5.5 g.u.; Takara) as well as an isomaltose standard were run. The identity of both substrate and product was verified by MALDI-TOF MS. Based on previous data, the Man3GlcNAc2 digestion product is expected to have a retention time approximately the same as that of the commercial Man5GlcNAc2. The glycans are drawn according to the nomenclature of the Consortium for Functional Glycomics (grey circles, mannose; black squares, GlcNAc).
Plant-like xylosylation of N-glycans from the C1 strain
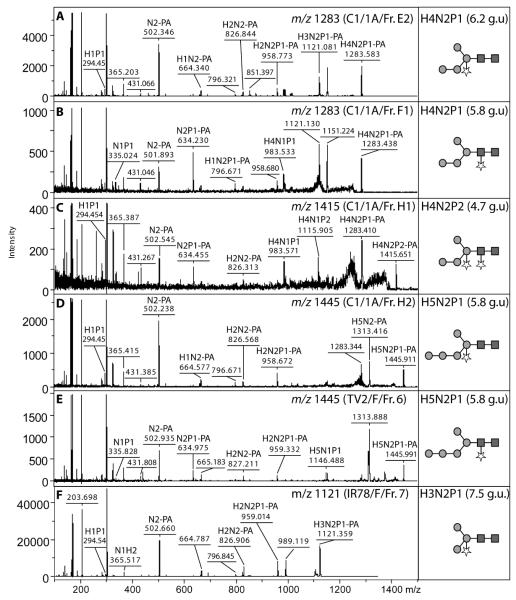
The initial mass spectrometric data were suggestive of the presence of pentose residues in three strains (C1, TV2 and IR-78). Considering the presence of a pentose (xylose) on the core β-linked mannose residue is a feature of plants and of trematodes (Paschinger, et al. 2009), a preliminary Western blotting experiment with anti-horseradish peroxidase, which recognises both core α1,3-fucose and β1,2-xylose, was performed; indeed reactivity was observed and could be reduced if the antibody was pre-incubated with a BSA conjugate carrying a plant-type N-glycan (data not shown). As apparently no core α1,3-fucosylated glycans were released by PNGase A, which unlike PNGase F can release such structures (Tretter, et al. 1991), we presumed that the reactivity with anti-HRP was due to the presence of xylose. This supposition is supported by the presence of a series of glycans in the C1/1A sample whose fragmentation patterns indicate the presence of differences of 132 (Figure 3). In particular, a fragment of m/z 796.7 is compatible with a Hex1HexNAc2Pent1 motif and is present in the glycans found in 2D-HPLC fractions E2 (m/z 1283; Hex4HexNAc2Pent1-PA) and H2 (m/z 1445; Hex5HexNAc2Pent1-PA). In the case of the IR-78 strain, the major peak in all preparations analysed had the predicted composition Hex3HexNAc2Pent1-PA (m/z 1121); the RP-HPLC elution time (7.5 g.u.; fraction 7) corresponded to that of a standard ‘MMX’ plant glycan, prepared from beans and sharing a similar fragmentation pattern upon MS/MS. This glycan was also sensitive to combined α1,2/3-mannosidase and β1,2-xylosidase digestion (Supplementary Figure 2B). The differences in the degree of the presence of the plant-like core xylosylation in the four strains tested is also apparent as judged by a comparison of anti-horseradish peroxidase reactivity (Supplementary Figure 3).
Figure 3. Analysis of pentosylated Trichomonas vaginalis N-glycans.
Glycans from the C1, TV2 and IR-78 strains separated by either 2D-HPLC (normal phase followed by reversed phase) or solely RP-HPLC were subject to selected positive-mode MS and MS/MS. Selected characteristic fragment ions are annotated, whereas common fragments such as GlcNAc1-PA (m/z 300; off-scale) are not indicated. Glycans are drawn according to the nomenclature of the Consortium for Functional Glycomics (circles, hexose; squares, HexNAc; stars, pentose).
Pentosylation of the distal core N-acetylglucosamine residue
In other putatively mono-pentosylated glycans from the C1 strain, fragments of m/z 335.0 (HexNAc1Pent1) and m/z 634.2 (HexNAc2Pent1-PA) are observed in the case of a glycan with the same m/z value as for that in the aforementioned 2D-HPLC fraction E2, but which elutes earlier on the RP-HPLC column (fraction F1; Figure 3B); these fragments are also found in the MS/MS spectrum of the putatively doubly-pentosylated glycan found in fraction H1 (m/z 1415; Hex4HexNAc2Pent2-PA, see Figure 3C). In contrast to the C1/1A sample, the other C1 samples were hallmarked by an absence of double pentosylation; only in the case of two low abundance glycans in the C1/3FA sample (m/z 1618 and 1780; Hex4-5HexNAc3Pent2) is such a modification apparent (Table 2). Thereby, in samples C1/2F, C1/4A and C1/4F, modification by pentose residues of the core mannose and distal (second) core GlcNAc appeared to be, in general, mutually exclusive; based on the aforementioned fragmentation patterns, the pentosylation of mannose is predominantly predicted for Hex3-5HexNAc2Pent1-PA, whereas pentosylation of distal core GlcNAc is presumed for the larger Hex4-6HexNAc3-4Pent1-PA structures in these samples.
Pentosylation was not detectable amongst the glycans present in the G3 strain, but was obvious in low amounts in HPLC fractions of glycans from the TV2 strain (Figure 3E; m/z 1445); in the latter case, the pentose would appear also to be on the distal core GlcNAc residue. As the amounts of the glycans displaying this type of pentosylation are very low, there was insufficient material for compositional and linkage analyses to resolve which type of pentose was bound to the core region in which linkage.
T. vaginalis N-glycans can be modified with N-acetyllactosamine units
Another feature of the T. vaginalis C1 and G3 glycomes were masses compatible with the presence of one or more N-acetylhexosamine residues. Indeed, neutral loss of 203 mass units was observed in MS/MS spectra of a number of glycans (some with pentose residues in the case of the C1 strain) as was the serial loss of 162 and 203; the latter pattern was suggestive of the presence of terminal N-acetyllactosamine (LacNAc) units (see Supplementary Figure 4). Therefore, selected fractions were digested with β1,4-galactosidases and/or β-hexosaminidase. As described below, some glycans with compositions such as Hex4HexNAc3, Hex5HexNAc4 and Hex6HexNAc4-5 lost a single hexose when incubated with either A. oryzae β1,4-galactosidase or a recombinant β1,4-galactosidase. As shown in Figure 4, the digestion of Hex4HexNAc3Pent0-1 (Fractions C1/1A/E4 and C1/1A/G5; i.e., ‘2D-HPLC’ fractions E4 and G5 from sample C1/1A) with first β1,4-galactosidase and then β-hexosaminidase was accompanied by a diagnostic shift in RP-HPLC retention time as well as in the mass spectra - the shift to the left suggesting the presence of the LacNAc unit on the α1,6-arm.
Figure 4. Modification of Trichomonas vaginalis N-glycans with N-acetyllactosamine.
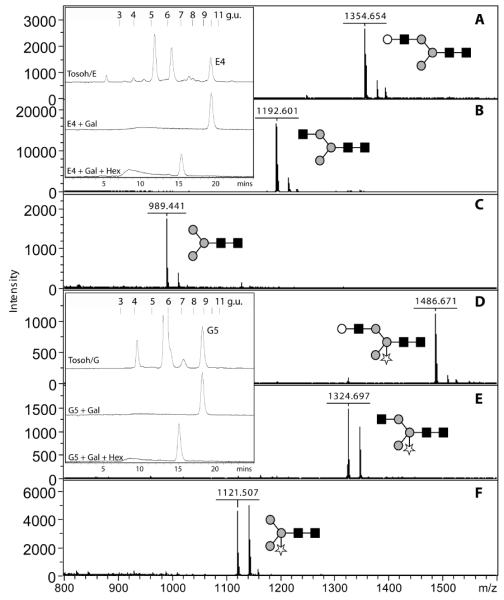
Two 2D-HPLC purified N-glycans from the C1/1A sample (E4 and G5; see Table 2 and Supplementary Figure 2) were subject to exoglycosidase treatment and refractionation by RP-HPLC (see inserts; Hypersil ODS, MZ Analytik) and positive-mode MALDI-TOF MS. Shown are spectra of untreated fractions E4 and G5 (A, D), Aspergillus β1,4-galactosidase-treated E4 and Bacteroides β1,4-galactosidase-treated G5 (B, E) as well as Aspergillus β1,4-galactosidase-treated E4 and G5 treated subsequently with jack bean β-hexosaminidase (C,F). The shifts in reversed-phase retention time are characteristic of glycans modified with LacNAc on the α1,6-arm as indicated. Glycans are drawn according to the nomenclature of the Consortium for Functional Glycomics (grey circles, mannose; white circles, galactose; black squares, GlcNAc; stars, pentose).
Amongst larger LacNAc-modified glycans in the C1/1A sample, some were also pentosylated (Hex4-5HexNAc3-4Pent1-2). For instance, the pentosylated glycans in fractions C1/1A/I2 and C1/1A/K1 (m/z 1618 and 1851; Hex4HexNAc3Pent2 and Hex5HexNAc4Pent1) lost a single hexose upon β-galactosidase treatment, a result compatible with their compositions and MS/MS spectra (see Supplementary Figure 4). Therefore, these pentosylated glycans are assumed to carry one or two N- acetyllactosamine units on one antenna; the position of the pentose can be surmised by interpretation of the fragmentation pattern as being either on the core mannose or the second GlcNAc residue.
In contrast, in the G3 glycome only glycans carrying one, but not more, N-acetyllactosamine units were found (see Supplementary Figure 4 for example MS/MS data). Galactosidase digestion as well as perdeuteromethylation of selected G3 glycans could distinguish forms of Hex5-6HexNAc3, separable by RP-HPLC (m/z 1516 and 1678), carrying either terminal galactose or terminal GlcNAc residues (data not shown) and so are predicted to have compositions such as Gal1Man4GlcNAc3, Man5GlcNAc3, Glc1Man5GlcNAc3, or Gal1Man5GlcNAc3. Other than a trace of a probable serum-derived component (Hex5HexNAc4), no modification of N-glycans by N-acetyllactosamine was obvious in the TV2 strain.
Distinguishing parasite-derived from serum-derived glycans
The presence of significant levels of larger oligosaccharides in the first sample (C1/1A), with compositions of Hex5-6HexNAc4-5Fuc0-1 was noted; however, our hypothesis was that these larger structures were in part derived from serum components such as fetuin. These peaks were indeed less conspicuous or absent in the spectra of glycans prepared from T. vaginalis C1 maintained for two days in the absence of any serum. However, ‘serum-free’ maintenance was not conducive to good cell viability (unpublished observation) and appeared, as summarised in Table 2, to result in a loss of glycomic complexity; thus the majority of our analyses were performed on glycans of the C1/1A and C1/4A samples (grown in the presence of serum). Thereby it was important to distinguish glycans of parasite and serum origin. Larger glycans containing pentose residues (Hex3-7Hex3-5Pent1-2) presumably originate from the parasite. However, glycans in low amounts containing fucose (m/z 1865, Hex5HexNAc4Fuc1; e.g. Fraction C1/1A/J1, see Table 1) or sialic acid (e.g., m/z 2010, NeuAc1Hex5HexNAc4; detected in negative mode) are considered to be of mammalian origin. These glycans are, in addition to triantennary NeuAc0-3Hex6HexNAc5, observed when glycans released from foetal calf serum proteins were directly analysed (Supplementary Figure 5). The presence of bi- and triantennarystructures in serum is consistent with previous data on the N-glycans of bovine fetuin, one of the major components of foetal calf serum (Green, et al. 1988).
In the case of the C1/4A RP fraction 11, a portion of the Hex5HexNAc4 (m/z 1719; Supplementary Figure 6) species was sensitive to β-hexosaminidase and to α1,2-mannosidase digestion, indicative of a terminal GlcNAc and a terminal α1,2-mannose residue: such a structure is, based on our understanding of parasite and mammalian N-glycosylation, most probably of T. vaginalis origin. The other portion of the glycans with m/z 1719 was β-galactosidase sensitive in a manner predicted for biantennary mammalian glycans and so suggestive of a trace of glycans derived from the medium in this fraction. On the other hand, the next two glycans in the same fraction (Hex6HexNAc4, m/z 1881, and Hex7HexNAc5, m/z 2246) are sensitive to α1,2-mannosidase and to β1,4-galactosidase and in both cases only a single hexose is removed; once β-hexosaminidase is added to the galactosidase digestion mixture, the structures ‘collapse’ down to Hex4HexNAc2. Particularly the mannosidase sensitivity of these two glycans is indicative of their parasitic origin.
Somewhat similar to the detection of multiple forms of Hex5HexNAc4 in C1/4A RP fraction 11 is the situation with the 2D-HPLC-separated fractions C1/1A/L1 and L2; in this case, two forms of Hex6HexNAc5 can be resolved by RP-HPLC (see Supplementary Figure 1). Galactosidase digestion of L1 results in loss of up to three hexose residues, whereas L2 only loses one residue (data not shown); these data suggest that approximately one half of the Hex6HexNAc5 is a triantennary glycan derived from the medium, whereas the other half is a parasite glycan carrying three N-acetyllactosamine residues in series.
Differential expression of zwitterionic glycans in T. vaginalis strains
Initial negative ion mode MALDI-TOF MS data of N-glycans from G3 and TV2, but not C1 and IR-78, indicated the presence of relatively strong signals not accounted for by the major species in the positive spectra. Specifically, the negative spectrum acquired with glycans from the G3 strain was particularly striking considering the signals at m/z 1637 and 1799. In the case of TV2, signals with m/z 1110, 1272 and 1434 are present, which suggested that a typical series of Man3-5GlcNAc2 glycans may be modified by a moiety of 123 mass units (Supplementary Figure 7). Indeed, a modification of this mass was previously found, e.g., to be present on mannose residues of glycosylphosphatidylinositol anchors and was identified as phosphoethanolamine (EtNP) (Fukushima, et al. 2003). Therefore, in order to test this assumption, dephosphorylation with hydrofluoric acid (HF) (Schneider and Ferguson 1995) was performed on aliquots of these two N-glycomes. Changes in both the negative and positive mode spectra were observed (Supplementary Figure 7). The use of hydrofluoric acid resulted in the respective loss of, e.g., the m/z 1637 and 1434 [M−H]− species from the G3 and TV2 negative mode spectra; the loss of the corresponding m/z 1639 and 1436 [M+H]+ species from the positive mode spectra was also observed. Therefore, their compositions were assumed to be Hex5HexNAc3(EtNP)1 and Hex5HexNAc2(EtNP)1.
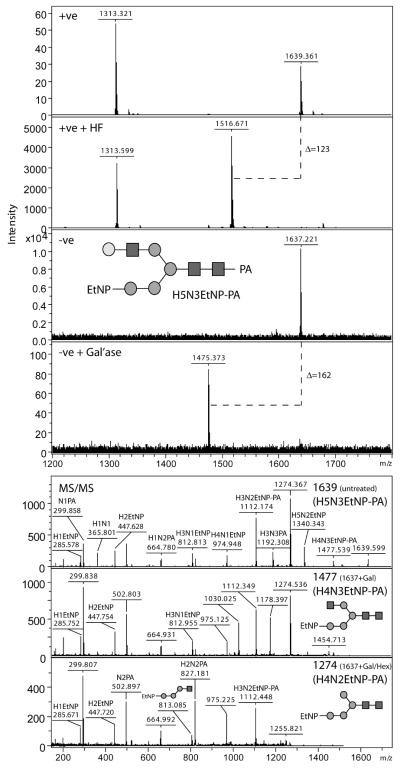
In order to examine the nature of the putative Hex5HexNAc3(EtNP)1 glycan from the G3/A sample further, an early RP-HPLC fraction (3.7 g.u.) enriched in this species was subject to treatment with HF as well as with exoglycosidases (Figure 5A). Dephosphorylation with HF resulted in the appearance of a species with m/z 1516 in positive mode (a loss of 123 mass units), whereas galactosidase digestion resulted in the loss of a single hexose residue (the product having respective m/z values of 1475 and 1477 in the negative and positive modes). On the other hand, jack bean β-hexosaminidase or Aspergillus α1,2-mannosidase digestion of the native glycan resulted in no change in the mass spectra; however, digestion by α1,2-mannosidase (to m/z 1354) was successful after HF treatment and combined hexosaminidase/galactosidase treatment resulted in removal of one hexose and one N- acetylhexosamine (data not shown).
Figure 5. Analysis of a T. vaginalis glycan modified by phosphoethanolamine.
(A) The purified RP-HPLC fraction 4 (3.7 g.u.; Figure 2) from the G3/A sample was analysed by MALDI-TOF MS in both positive and negative modes following exoglycosidase or hydrofluoric acid treatments. The fraction contains glycans with predicted compositions of Hex5HexNAc2 (m/z 1313) and Hex5HexNAc3EtNP (m/z 1639 or 1637, depending on the analysis mode). As shown by the positive mode spectrum, hydrofluoric acid treatment results in loss of 123 mass units from the latter glycan (+HF); on the other hand, treatment with Bacteroides fragilis β1,4-galactosidase results in loss of 162 mass units (+Gal’ase) in the negative mode. (B) MS/MS analysis of the m/z 1639 species (untreated), the galactosidase product (m/z 1477) and the product of combined galactosidase/hexosaminidase treatment (m/z 1274) indicated the presence of a fragment predicted to correspond to Hex3HexNAc1EtNP (m/z 813); this is compatible with a terminal location for the phosphoethanolamine moiety as shown (H, hexose; N, N-acetylhexosamine; EtNP, phosphoethanolamine; PA, pyridylamine). The proposed glycan structures of the Hex5HexNAc3EtNP and its glycosidase-digestion products are drawn according to the nomenclature of the Consortium for Functional Glycomics (grey circles, mannose; white circles, galactose; black squares, GlcNAc).
The positive mode MS/MS spectra of this glycan of m/z 1639 (also in its galactosidase and galactosidase/hexosaminidase-digested forms; Figure 5B) indicated fragments of, on average, m/z 285.7, 447.7 and 813.9, which would correspond to Hex1, Hex2 and Hex3HexNAc1 modified by phosphoethanolamine. Thus, the smallest common fragment containing both phosphoethanolamine and N-acetylhexosamine can be proposed to correspond to EtNP-Manα1,2Manα1,3Manβ1,4GlcNAc. The phosphoethanolamine moiety is thereby presumed to be linked to the terminal α1,2-mannose of the α1,3-arm; the presence of this residue would in turn indicate that the N-acetyllactosamine unit (Galβ1,4GlcNAc) is on the α1,6-arm.
Another variant of a phosphoethanolamine-modified glycan in the G3 strain is represented by a glycan of m/z 1801 with a predicted composition of Hex6HexNAc3(EtNP)1. When analysed by positive mode tandem MS, a fragment of 285.9 was also apparent (data not shown); the difference between the two zwitterionic G3 glycans is predicted to be an α1,2-mannose residue. The overall compositions of the m/z 1639 and 1801 [M+H]+ species are thereby predicted to be Gal1Man4-5GlcNAc3(EtNP)1-PA. The ‘parent’ glycans lacking the charged moiety are present in later-eluting RP-HPLC fractions (8 and 9 g.u. as compared to 3.7 g.u.; Supplementary Figure 4).
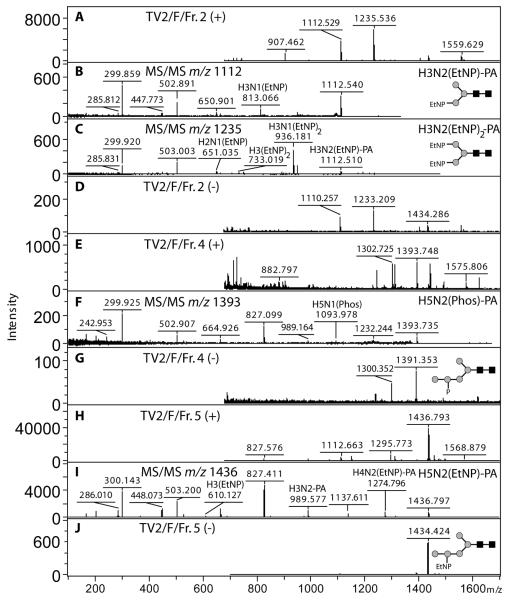
In addition to the analysis of the phosphoethanolamine-modified glycans from the G3 strain, MS/MS was also performed on a number of glycans carrying putative phosphate or phosphodiesters in three RP-HPLC fractions from the TV2 strain. Again, fragments of m/z ~286 were observed and, in one case, a glycan carrying two phosphoethanolamine moieties was detected; fragments of m/z 651 (Hex2HexNAc1EtNP) and 733 (Hex3[EtNP]2) indicate that one or both α-linked mannose residues are respectively modified in the glycans with m/z 1112 and 1235 (Figure 6). Furthermore, the positive ion MS/MS spectrum for the m/z 1393 molecular ion indicated the presence of a phosphorylated hexose (fragment of m/z 242.9) and so is compatible with a composition of Man5GlcNAc2Phos1-PA. It is noteworthy that the types of N-glycans carrying phosphoethanolamine in G3 and TV2 contrast in terms of the basic structure (either N-acetyllactosamine-containing in the former or paucimannosidic in the latter).
Figure 6. MS and MS/MS analysis of charged N-glycans from the TV2 strain.
MALDI-TOF MS of RP-HPLC fractions 2, 4 and 5 (~3, ~4 and ~4 g.u) was performed in both positive (+; A, E and H) and negative (−; D, G and J) modes; selected species were subject to positive mode MS/MS (B, C, F and I) and resulted in fragments (m/z ~243 or ~286) indicative of modification with either phosphate or phosphoethanolamine. A fragment of m/z ~300 is typical for a pyridylaminated reducing-terminal GlcNAc. The structures indicated for the m/z 1112 and 1235 species are based on the diagnostic fragments such as m/z 651 and 733; the structures shown for the m/z 1393 and 1436 species are consistent with the fragmentation pattern, but some ambiguity as to the exact positions of the phosphate/phosphodiester modifications remains.
Discussion
Key features of N-glycans from T. vaginalis
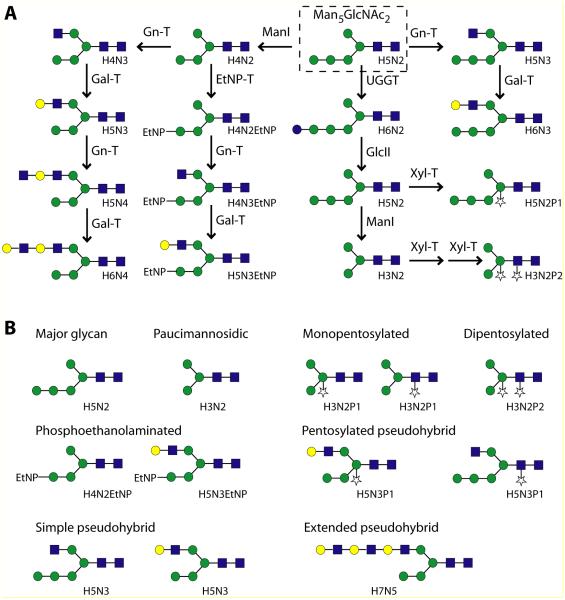
In this most exhaustive analysis of the N-glycan structures of Trichomonas vaginalis to date, a number of key findings can be summarised (see also Figure 7): first, as predicted from previous studies (Samuelson, et al. 2005, Banerjee, et al. 2007), the major glycan in all three T. vaginalis strains examined is Man5GlcNAc2 in its ‘biosynthetic’ form. This is, in part, the result of a lack of genes encoding the four luminal mannosyltransferases required for formation of the full Glc3Man9GlcNAc2 dolichol-linked precursor generally found in eukaryotes. Indeed, genes encoding putative Alg7, Alg13 and Alg 141, Alg1, Alg2, Alg 11 and Rft1 homologues can be found in the T. vaginalis genome.
Figure 7. Proposed major N-glycan processing pathways and glycan classes in Trichomonas vaginalis.
A non-exhaustive selection of potential N-glycosylation modification reactions are shown (A) based on the determined glycan structures, which can be classified into, e.g., paucimannosidic, pentosylated, phosphoethanolaminated, simple pseudohybrid and extended pseudohybrid types (B). Variations between strains in terms of the degree of these modifications are obvious; the identity of the pentose residue as xylose is proposed on the basis of preliminary data from others and from its presence in the lipo(phospho)glycan. Enzymes such as ManI (class I mannosidase), UGGT (UDP-Glc:glycoprotein glucosyltransferase), GlcII (glucosidase II), Gn-T (N-acetylglucosaminyltransferases), Gal-T (galactosyltransferases), Xyl-T (xylosyl- or other pentosyltransferases) and EtNP-T (phosphoethanolaminyltransferase) are either proven or proposed, but may have different or more substrate specificities than those shown. The glycans are drawn according to the nomenclature of the Consortium for Functional Glycomics (green circles, mannose; yellow circles, galactose; blue squares, GlcNAc; stars, pentose).
Second, the C1 and IR-78 strains produce pentosylated glycans: whereas the pentose attached to the core β-linked mannose can be assumed to be xylose on the basis of anti-HRP staining and xylosidase sensitivity, the pentosylation of the distal GlcNAc is reminiscent of the xylosylation of microalgal N-glycans (Levy-Ontman, et al. 2011). Furthermore, the functionality of a recombinant form of a putative UDP-xylose synthase has been demonstrated 2 and a number of β1,2-xylosyltransferase homologues have been found (Hwa, et al. 2007)3. On the other hand, there is no evidence for the presence of deoxyhexose in the N-glycans of this organism. However, both pentose (xylose) and deoxyhexose (rhamnose) residues are found in its lipo(phospho)glycan (Bastida-Corcuera, et al. 2005); the probable absence of fucose would be consistent with the lack of genes predicted to encode enzymes involved in GDP-fucose synthesis. Indeed, the absence of fucose from the lipo(phospho)glycan and N-glycans of T. vaginalis is compatible with the inability to label its cell surface with a fucose-specific lectin (Mirhaghani and Warton 1998). In contrast, T. foetus lipo(phospho)glycan contains both fucose and xylose (Singh 1993, Singh, et al. 1994); however, there is no information available to allow a genomic comparison of these species.
Third, as observed for the lipo(phospho)glycan of UR1, B7RC2 and CD-C85 strains (Singh, et al. 1994, Bastida-Corcuera, et al. 2005, Singh, et al. 2009, Ryan, et al. 2011), the C1 and G3 strains produce N-glycans modified with N-acetyllactosamine units. This moiety is an epitope for Ricinus communis aggulutinin and strains resistant to this lectin have a lower galactose content which correlates with lower adhesion to mammalian cells and a loss of the ability to bind human galectin-1 (Bastida-Corcuera, et al. 2005, Okumura, et al. 2008), a putative ‘receptor’ for a wild-type strain; we have also observed activity of a recombinant UDP-galactose epimerase2. The presence of single LacNAc units (or in the C1 strain also up to at least three repeats) on the N-glycans would indicate that these are also candidates for mediating binding to host cells; however, information as to the N-glycan structures or the relevant mutation(s) in the lectin-resistant strains is yet to be published.4 Another point when considering the glycans of this organism is that, despite the well-known production of a range of glycosidases (e.g., galactosidase and hexosaminidase) by trichomonads (Lockwood, et al. 1988, Costa e Silva Filho, et al. 1989, Connaris and Greenwell 1997), larger N-glycans can still be isolated from the cultivated cells; thus, at least in part, the N- acetyllactosamine units survive exposure to these hydrolases.
Fourth, charged glycans from the G3 and TV2 strains are modified with a moiety with 123 mass units, putatively phosphoethanolamine, which is sensitive to hydrofluoric acid treatment and results in earlier elution on RP-HPLC. The C1 strain, in contrast, displays only a minor degree of such modifications. There are precedents for the presence of this moiety on other categories of glycoconjugates; for instance, phosphoethanolamine is a component of glycosylphosphatidylinositol (GPI) anchors (Fukushima, et al. 2003, Paulick and Bertozzi 2008), insect and nematode glycolipids (Seppo, et al. 2000, Friedl, et al. 2003), insect O-glycans (Garenaux, et al. 2011) and some bacterial lipopolysaccharides (Cox, et al. 2002). To our knowledge there is only one previous report of a similar modification of an N-glycan, specifically that of locust apolipophorin with 2-aminoethylphosphonate (Hård, et al. 1993).
Although present on phospholipids and GPI anchors, phosphoethanolamine is not a component of mammalian N-glycans and so its presence on the glycans of a parasite may potentially result in a response by the adaptive or innate immune systems. Indeed, phosphoethanolamine is a ligand for the pentraxin, serum amyloid P (Schwalbe, et al. 1992), which is a mediator of classical complement pathway (Deban, et al. 2009), whereas glycans modified with its methylated form, phosphorylcholine, are associated with immunomodulation by nematode parasites (Harnett and Harnett 2001). It is therefore of interest in the future to consider whether the modification of trichomonad glycans by phosphoethanolamine has any repercussions for host-parasite interactions or the host immune system. Significantly, our preliminary data from Tritrichomonas foetus (K. Paschinger, preliminary data) indicates the modification of some N-glycans with phosphorylcholine; therefore, the modification of T. vaginalis glycans by phosphoethanolamine is not so unexpected.
Analytical lessons from the study of T. vaginalis glycans
The unambiguous analysis of heterogeneous glycans from relatively low amounts of parasite material is a challenge for a number of reasons, including the presence of unusual modifications and traces of non-parasite glycans which are, in this case, derived from serum glycoproteins. Therefore, we adopted a mixed approach based not just on mass spectrometry alone, but supported by exoglycosidase digestions and HPLC analyses of glycans released by PNGase A or F: the precaution of using both enzymes was in retrospect not necessary, due to the lack of core α1,3-fucosylated species which cannot be cleaved by PNGase F (Tretter, et al. 1991). The typical elution behaviour of some pyridylaminated glycans when analysed by RP-HPLC before and after exoglycosidase digestion is an aid in defining isomers; for instance, in our study and in accordance with previous data (Tomiya, et al. 1991), the major trichomonad Man5GlcNAc2 glycan elutes at 5.8 g.u. and contains two α1,2-linked mannose residues. In contrast, the typical protein-bound Golgi-processed Man5GlcNAc2 in multicellular eukaryotes (represented in our study by a commercial sample) elutes at around 7 g.u.
In another instance, comparison with another study of Tomiya et al. (Tomiya, et al. 1988) is also useful in order to indicate that the glycan with a composition of Gal1Man3GlcNAc3 is actually ‘GalM’ [Schachter nomenclature for Manα1,3(Galβ1,4-GlcNAcβ1,2Manα1,6)Manβ1,4GlcNAcβ1,4GlcNAc-PA; see Ref. (Schachter 1986)] as opposed to the MGal isomer; these have respective literature retention times of 8.0 and 9.9 g.u.. GalM is predicted to shift to 9.5 g.u. and 7.4 g.u. upon serial treatment with galactosidase (to GnM, as opposed to the MGn isomer) and hexosaminidase (to MM). Our results (Figure 4) are compatible with these values and indicate that, e.g., the glycan in fraction C1/1A/E4 carries a LacNAc unit on the α1,6-antenna; the definition of the galactose as being β1,4-linked on trichomonad N-glycans is based on the specificity, as quoted in the literature (Zeleny, et al. 1997) or the manufacturer’s information, of the two galactosidases employed. As core β1,2-xylose has relatively little effect on the RP-HPLC retention time (Wilson and Altmann 1998), the values for the putatively-xylosylated glycan in fraction C1/1A/G5 (‘GalMX’) are similar to those with the non-xylosylated C1/1A/E4 glycan. The presence of a residual α1,2-mannose on other structures containing N- acetyllactosamine units (Supplementary Figure 4) is also compatible with presence of Galβ1,4GlcNAc on the α1,6-arm of T. vaginalis glycans, as the α1,3-arm is thereby ‘blocked’ by the mannose residue, leaving only the other antenna free for modification by an N-acetylglucosaminyltransferase.
Despite the advantages of using diagnostic HPLC shifts, in a number of cases we rely on MS/MS data in order to propose some of the structures. Regarding pentosylated species, some care was required as a fragment of m/z 431 (HexNAc1Pent1-PA) of low abundance is apparent in a number of MS/MS spectra (Figure 3); this was also observed in a glycan prepared by incubation with a recombinant plant core β1,2-xylosyltransferase as well as a glycan purified from beans (data not shown). However, the MS/MS of these same glycans also showed a loss of 299 from the parent molecular ion, which is compatible with the loss of GlcNAc-PA alone, but not of HexNAc1Pent1-PA. Therefore, we conclude that the m/z 431 fragment is an artefact resulting from an internal rearrangement similar to that proposed for some fucosylated glycans (Wuhrer, et al. 2006).
Inter-strain differences in glycosylation
In this study, we have included data on four different strains; samples of one of these strains were also obtained from three different laboratories using three different media. We chose one typical laboratory strain (C1) as well as the strain used to sequence the genome (G3) and two locally-isolated strains (TV2 and IR-78), one of which displays metronidazole-resistance. The glycosylation of these various samples indicates that the glycome of T. vaginalis is not uniform but has variations in extent and type of pentosylation and also differences in the degree of modification with charged moieties and with N-acetyllactosamine. Thus, it may be that glycosylation is affected by a number of different factors, including the strain, cultivation method or period of time in continuous culture; indeed, loss of gene expression has been reported for other protozoa in long-term cultivation (Köhsler, et al. 2009). The effect of cultivation of C1 for two days in the absence of serum suggests some loss of complexity, but the N-glycome of the TV2 strain cultivated in the presence of serum is even less heterogeneous. The variation between the two C1 samples tested with anti-horseradish peroxidase (Supplementary Figure 3) would suggest a higher abundance of xylosylation of the core mannose in sample C1/1 as opposed to C1/4; certainly a lower number of mono- and dipentosylated structures (see Table 2) were identified in the latter sample. Hex4HexNAc3Pent1, for instance, is distinctly obvious in the complete C1/1 spectrum (Figure 1), but is only a minor structure in the other C1 spectra.
Without further data regarding the virulence, genomic differences or metabolic status of the different strains in the different laboratories, we do not speculate as to the significance of our comparative N-glycomic data. Certainly, those studying the glycosylation of this organism should be aware of such differences; however, it can be argued that variance between the glycoconjugate patterns of these four T. vaginalis strains reflects the considerable genetic diversity within this species (Conrad, et al. 2011). In this context, it is interesting to note that also the protein expression patterns of strains C1, G3, TV2, and IR-78, as revealed by two-dimensional gel electrophoresis, also display numerous and substantial differences (D. Leitsch, unpublished results).
Biosynthetic conclusions from glycomic data
As summarised above, the major N-glycan of T. vaginalis corresponds to the Man5GlcNAc2 isomer found during biosynthesis of the dolichol-linked precursor prior to flipping from the cytoplasmic to the lumenal face of the endoplasmic reticulum. Whereas in most eukaryotes this pentamannosyl structure is elongated by a further four mannose and three glucose residues, T. vaginalis lacks the relevant glycosyltransferase genes. Therefore, as is also the case in Entamoeba histolytica, Man5GlcNAc2-PP-Dol is the primary oligosaccharyltransferase ‘donor’ substrate for protein N-glycosylation in this organism. The oligosaccharyltransferase complex of T. vaginalis apparently consists of fewer subunits than is generally the case for eukaryotes and displays a preference for non-glucosylated glycan donors (Kelleher, et al. 2007); interestingly, in Trypanosoma brucei, there are at least two types of oligosaccharyltransferase which accept either Man5GlcNAc2-PP-Dol or Man9GlcNAc2-PP-Dol as the substrate (Izquierdo, et al. 2009c).
After transfer to protein, the presence of a UDP-Glc:glycoprotein glucosyltransferase gene in the T. vaginalis genome (Banerjee, et al. 2007) indicates that glucosylation can take place as part of a quality control system for glycoproteins (D’Alessio, et al. 2010); the occurrence of a glycan, as noted in this and an earlier study, with the composition Hex6HexNAc2 (m/z 1475) would be compatible with terminal glucose attached to Man5GlcNAc2 (Banerjee, et al. 2007). There is, though, no commercially available glucosidase II which can be used to further analyse this structure. The occurrence also of Man3-4GlcNAc2 is compatible with the presence of an active class I mannosidase (Banerjee, et al. 2007). However, other than these glucosyltransferase and mannosidase genes, there is a complete lack of knowledge regarding the genes required for the subsequent processing events in T. vaginalis.
From our data, we can predict that N-acetylglucosaminyltransferases, galactosyltransferases, xylosyltransferases and phosphoethanolaminyltransferases should be encoded by the T. vaginalis genome – while xylosyltransferase homologues have been identified (Hwa, et al. 2007), no obvious homologues for either GlcNAc-TI or -TII or β1,4-galactosyltransferase can be detected in this genome. Perhaps these activities are covered by members of other glycosyltransferase families; for instance, as lateral gene transfer from bacteria has been postulated (Carlton, et al. 2007), homologues of bacterial glycosyltransferases are candidates for being responsible for these activities. It also should be noted that a classical GlcNAc-TI or -TII cannot be expected in this organism: the partial presence of an α1,2-mannose remaining on the α1,3-arm of its N-glycans means that a typical GlcNAc-TI cannot act. As summarised in Figure 7A, although the putative location (the α1,6-arm) for the extension by the single non-reducing terminal GlcNAc residue or LacNAc repeats on some glycans is the same as that modified in other organisms by GlcNAc-TII, the inability of a classical GlcNAc-TI to act beforehand means that any GlcNAc-TII-like enzyme will have a non-classical GlcNAc-TI-independent specificity.
In summary, the glycans present in the various strains studied are somewhat akin to hybrid oligosaccharides from multicellular eukaryotes, but are different due to the presence of the ‘complex’ modifications on the α1,6-arm and of the remaining mannose residues on the α1,3-arm; thus, we propose that these glycans from T. vaginalis be defined as ‘pseudohybrid’ structures in either ‘simple’ or ‘extended’ forms (Figure 7B). From the analytical data, we surmise that the glycomic potential of different strains varies and that, for instance, the C1 strain has significant LacNAc polymerisation activity as well as pentosylation of the distal core GlcNAc. In contrast, the G3 strain may possess little or no LacNAc ‘polymerisation’ and xylosylation activities, whereas in TV2 the galactosyltransferase expression is low and in IR-78 the xylosylation of the β1,4-mannose is high. Finally, the phosphoethanolaminyltransferase activities in G3 and TV2 are probably higher than those present in C1. Our data mean that, although there are generalised structural trends regarding the N-glycosylation of T. vaginalis, there is no ‘species-specific’ glycosylation signature.
Comparisons between T. brucei and T. vaginalis
The general biosynthetic situation we propose for T. vaginalis is akin to that observed in Trypanosoma brucei: even in glucosidase II mutants in which glucose is retained on the α1,3-arm, the presence of LacNAc units on the α1,6-arm has been demonstrated (Jones, et al. 2005, Izquierdo, et al. 2009a); indeed, in T. brucei, very long polyLacNAc chains can be present (Zamze, et al. 1991, Atrih, et al. 2005). However, also no obvious GlcNAc-TI or -TII or β1,4-galactosyltransferase homologues have been identified in T. brucei (Izquierdo, et al. 2009b); to date, potentially relevant β-galactosyltransferase and dolichol-independent N- acetylglucosaminyltransferase activities have been detected only in T. brucei microsomes (Rovis and Dube 1982, Grab, et al. 1984, Pingel and Duszenko 1992). As the α1,6-arm may be the first target for modification by a N- acetylglucosaminyltransferase, prior to galactosylation, in both T. brucei and T. vaginalis, it may be attractive to propose that the relevant LacNAc-synthesising enzymes may either be related phylogenetically or at least display the same substrate specificity. However, the evolutionary distance between trypanosomes and trichomonads may mean that a set of ‘convergent’ evolutionary events has occurred so that the structural ‘result’ of these non-classical glycan processing pathways is based on disparate phyloglycogenetic mechanisms.
Supplementary Material
Acknowledgements
The authors thank Lydia Hangelmann, Peter Kysel’ and Natalie Öhl for some of the HPLC fractionation experiments. This work was supported by grants from the Austrian Fonds zur Förderung der wissenschaftlichen Forschung [P20565 to I.B.H.W. and P21946 to K.P.].
Footnotes
The presence of Alg13 and Alg14 homologues (TVAG_183240 and TVAG_038800) has been confirmed by our own databank searches.
Rosenberger, A., Hangelmann, L. and Hofinger, A. and Wilson, I.B.H., manuscript in preparation.
In a conference abstract, the same authors also suggest the presence of a novel core structure and xylose as a minor component of T. vaginalis N-glycans. (K-Y. Hwa, H. Hung and K-H. Khoo, 2006, Annual Meeting of the Society for Glycobiology, Glycobiology, 16, 1142 (Abs. 201).
Data have been presented at a recent conference which indicate the absence of LacNAc units from the N-glycans of ricin-resistant strains of T. vaginalis: Carpentieri, A., Huque, S., Costello, C.E., Robbins, P.W. and Samuelson, J.C. (2010) Conference of the American Society of Tropical Medicine and Hygiene, Abstract LB-2159.
References
- Atrih A, Richardson JM, Prescott AR, Ferguson MA. Trypanosoma brucei glycoproteins contain novel giant poly-N-acetyllactosamine carbohydrate chains. J Biol Chem. 2005;280:865–871. doi: 10.1074/jbc.M411061200. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Vishwanath P, Cui J, Kelleher DJ, Gilmore R, Robbins PW, Samuelson J. The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation. Proc Natl Acad Sci U S A. 2007;104:11676–11681. doi: 10.1073/pnas.0704862104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot Cell. 2005;4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha C, Duchene M, Aspock H, Walochnik J. In vitro activity of hexadecylphosphocholine (miltefosine) against metronidazole-resistant and -susceptible strains of Trichomonas vaginalis. J Antimicrob Chemother. 2006;57:273–278. doi: 10.1093/jac/dki417. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM, Jr., Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaris S, Greenwell P. Glycosidases in mucin-dwelling protozoans. Glycoconjugate Journal. 1997;14:879–882. doi: 10.1023/a:1018554408558. [DOI] [PubMed] [Google Scholar]
- Conrad M, Zubacova Z, Dunn LA, Upcroft J, Sullivan SA, Tachezy J, Carlton JM. Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol. 2011;175:30–38. doi: 10.1016/j.molbiopara.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa e Silva Filho F, Breier-Saraiva EM, Tosta MX, de Souza W. Trichomonas vaginalis and Tritrichomonas foetus secrete neuraminidase into the culture medium. Mol Biochem Parasitol. 1989;35:73–78. doi: 10.1016/0166-6851(89)90144-8. [DOI] [PubMed] [Google Scholar]
- Cox AD, Li J, Brisson JR, Moxon ER, Richards JC. Structural analysis of the lipopolysaccharide from Neisseria meningitidis strain BZ157 galE: localisation of two phosphoethanolamine residues in the inner core oligosaccharide. Carbohydr Res. 2002;337:1435–1444. doi: 10.1016/s0008-6215(02)00161-1. [DOI] [PubMed] [Google Scholar]
- D’Alessio C, Caramelo JJ, Parodi AJ. UDP-Glc:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol. 2010;21:491–499. doi: 10.1016/j.semcdb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deban L, Bottazzi B, Garlanda C, de la Torre YM, Mantovani A. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. Biofactors. 2009;35:138–145. doi: 10.1002/biof.21. [DOI] [PubMed] [Google Scholar]
- Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Friedl CH, Lochnit G, Zähringer U, Bahr U, Geyer R. Structural elucidation of zwitterionic carbohydrates derived from glycosphingolipids of the porcine parasitc nematode Ascaris suum. Biochem. J. 2003;369:89–102. doi: 10.1042/BJ20021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Ikehara Y, Kanai M, Kochibe N, Kuroki M, Yamashita K. A β-N-acetylglucosaminyl phosphate diester residue is attached to the glycosylphosphatidylinositol anchor of human placental alkaline phosphatase: a target of the channel-forming toxin aerolysin. J Biol Chem. 2003;278:36296–36303. doi: 10.1074/jbc.M304341200. [DOI] [PubMed] [Google Scholar]
- Garenaux E, Maes E, Leveque S, Brassart C, Guerardel Y. Structural characterization of complex O-linked glycans from insect-derived material. Carbohydr Res. 2011;346:1093–1104. doi: 10.1016/j.carres.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Grab DJ, Ito S, Kara UA, Rovis L. Glycosyltransferase activities in Golgi complex and endoplasmic reticulum fractions isolated from African trypanosomes. J Cell Biol. 1984;99:569–577. doi: 10.1083/jcb.99.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabińska KA, Ghosh SK, Guan Z, Cui J, Raetz CR, Robbins PW, Samuelson J. Dolichyl-phosphate-glucose is used to make O-glycans on glycoproteins of Trichomonas vaginalis. Eukaryot Cell. 2008;7:1344–1351. doi: 10.1128/EC.00061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ED, Adelt G, Baenziger JU, Wilson S, Van Halbeek H. The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1988;263:18253–18268. [PubMed] [Google Scholar]
- Guha-Niyogi A, Sullivan DR, Turco SJ. Glycoconjugate structures of parasitic protozoa. Glycobiology. 2001;11:45R–59R. doi: 10.1093/glycob/11.4.45r. [DOI] [PubMed] [Google Scholar]
- Hård K, van Doorn JM, Thomas-Oates JE, et al. Structure of the Asn-linked oliogsaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry. 1993;32:766–775. doi: 10.1021/bi00054a005. [DOI] [PubMed] [Google Scholar]
- Harnett W, Harnett MM. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim Biophys Acta. 2001;1539:7–15. doi: 10.1016/s0167-4889(01)00101-x. [DOI] [PubMed] [Google Scholar]
- Hase S, Ibuki T, Ikenaka T. Reexamination of the pyridylamination used for fluorescence labelling of oligosaccharides and its application to glycoproteins. J Biochem (Tokyo) 1984;95:197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- Hwa K-Y, Hung H-H, Chen C-H. Phylogenetic analysis of trichomonad xylosyltransferases. Frontiers in the Convergence of Bioscience and Information Technologies. IEEE. 2007:299–304. [Google Scholar]
- Izquierdo L, Atrih A, Rodrigues JA, Jones DC, Ferguson MA. Trypanosoma brucei UDP-glucose:glycoprotein glucosyltransferase has unusual substrate specificity and protects the parasite from stress. Eukaryot Cell. 2009a;8:230–240. doi: 10.1128/EC.00361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo L, Nakanishi M, Mehlert A, Machray G, Barton GJ, Ferguson MA. Identification of a glycosylphosphatidylinositol anchor-modifying β1-3 N-acetylglucosaminyl transferase in Trypanosoma brucei. Mol Microbiol. 2009b;71:478–491. doi: 10.1111/j.1365-2958.2008.06542.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo L, Schulz BL, Rodrigues JA, Güther ML, Procter JB, Barton GJ, Aebi M, Ferguson MA. Distinct donor and acceptor specificities of Trypanosoma brucei oligosaccharyltransferases. EMBO J. 2009c;28:2650–2661. doi: 10.1038/emboj.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Mehlert A, Güther ML, Ferguson MA. Deletion of the glucosidase II gene in Trypanosoma brucei reveals novel N-glycosylation mechanisms in the biosynthesis of variant surface glycoprotein. J Biol Chem. 2005;280:35929–35942. doi: 10.1074/jbc.M509130200. [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol. 2007;177:29–37. doi: 10.1083/jcb.200611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhsler M, Leitsch D, Duchene M, Nagl M, Walochnik J. Acanthamoeba castellanii: growth on human cell layers reactivates attenuated properties after prolonged axenic culture. FEMS Microbiol Lett. 2009;299:121–127. doi: 10.1111/j.1574-6968.2009.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Ontman O, Arad S. Malis, Harvey DJ, Parsons TB, Fairbanks A, Tekoah Y. Unique N-glycan moieties of the 66-KDa cell-wall glycoprotein from the red microalga Porpyridium sp. J Biol Chem. 2011;286:21340–21352. doi: 10.1074/jbc.M110.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood BC, North MJ, Coombs GH. The release of hydrolases from Trichomonas vaginalis and Tritrichomonas foetus. Mol Biochem Parasitol. 1988;30:135–142. doi: 10.1016/0166-6851(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Meingassner JG, Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob Agents Chemother. 1979;15:254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhaghani A, Warton A. Involvement of Trichomonas vaginalis surface-associated glycoconjugates in the parasite/target cell interaction. A quantitative electron microscopy study. Parasitol Res. 1998;84:374–381. doi: 10.1007/s004360050413. [DOI] [PubMed] [Google Scholar]
- Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008;10:2078–2090. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Rendić D, Wilson IBH. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj J. 2009;26:385–395. doi: 10.1007/s10719-008-9155-3. [DOI] [PubMed] [Google Scholar]
- Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel S, Duszenko M. Identification of two distinct galactosyltransferase activities acting on the variant surface glycoprotein of Trypanosoma brucei. Biochemical Journal. 1992;283:479–485. doi: 10.1042/bj2830479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PW, Samuelson J. Asparagine linked glycosylation in Giardia. Glycobiology. 2005;15:15G–16G. doi: 10.1093/glycob/cwi073. [DOI] [PubMed] [Google Scholar]
- Rovis L, Dube S. Identification and characterisation of two N-acetylglucosaminyltransferases associated with Trypanosoma brucei microsomes. Mol Biochem Parasitol. 1982;5:173–187. doi: 10.1016/0166-6851(82)90019-6. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Mehlert A, Richardson JM, Ferguson MAJ, Johnson PJ. Chemical Structure of Trichomonas vaginalis Surface Lipoglycan: a Role for Short Galactose (β1-4/3) N-acetylglucosamine Repeats in Host Cell Interaction. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.280578. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J, Banerjee S, Magnelli P, Cui J, Kelleher DJ, Gilmore R, Robbins PW. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc Natl Acad Sci U S A. 2005;102:1548–1553. doi: 10.1073/pnas.0409460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986;64:163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Schneider P, Ferguson MAJ. Microscale analysis of glycosylphosphatidylinositol structures. Methods Enzymol. 1995;250:614–630. doi: 10.1016/0076-6879(95)50100-2. [DOI] [PubMed] [Google Scholar]
- Schwalbe RA, Dahlback B, Coe JE, Nelsestuen GL. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry. 1992;31:4907–4915. doi: 10.1021/bi00135a023. [DOI] [PubMed] [Google Scholar]
- Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppo A, Moreland M, Schweingruber H, Tiemeyer M. Zwitterionic and acidic glycosphingolipids of the Drosophila melanogaster embryo. Eur. J. Biochem. 2000;267:3549–3558. doi: 10.1046/j.1432-1327.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Singh BN. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1993;57:281–294. doi: 10.1016/0166-6851(93)90204-b. [DOI] [PubMed] [Google Scholar]
- Singh BN, Beach DH, Lindmark DG, Costello CE. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch Biochem Biophys. 1994;309:273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- Singh BN, Hayes GR, Lucas JJ, Sommer U, Viseux N, Mirgorodskaya E, Trifonova RT, Sassi RR, Costello CE, Fichorova RN. Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function. Glycoconj J. 2009;26:3–17. doi: 10.1007/s10719-008-9157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiya N, Awaya J, Kurono M, Endo S, Arata Y, Takahashi N. Analyses of N-linked oligosaccharides using a two-dimensional mapping technique. Anal Biochem. 1988;171:73–90. doi: 10.1016/0003-2697(88)90126-1. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Lee YC, Yoshida T, Wada Y, Awaya J, Kurono M, Takahashi N. Calculated two-dimensional sugar map of pyridylaminated oligosaccharides: Elucidation of the jack bean α-mannosidase digestion pathway of Man9GlcNAc2. Analytical Biochemistry. 1991;193:90–100. doi: 10.1016/0003-2697(91)90047-w. [DOI] [PubMed] [Google Scholar]
- Tretter V, Altmann F, März L. Peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase F cannot release glycans with fucose attached α1→3 to the asparagine-linked N-acetylglucosamine residue. Eur. J. Biochem. 1991;199:647–652. doi: 10.1111/j.1432-1033.1991.tb16166.x. [DOI] [PubMed] [Google Scholar]
- Wilson IBH, Altmann F. Structural analysis of N-glycans from allergenic grass, ragweed and tree pollens: Core α1,3-linked fucose and xylose present in all pollens examined. Glycoconjugate Journal. 1998;15:1055–1070. doi: 10.1023/a:1006960401562. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CA, Hokke CH, Deelder AM. Mass spectrometry of proton adducts of fucosylated N-glycans: fucose transfer between antennae gives rise to misleading fragments. Rapid Commun Mass Spectrom. 2006;20:1747–1754. doi: 10.1002/rcm.2509. [DOI] [PubMed] [Google Scholar]
- Zamze SE, Ashford DA, Wooten EW, Rademacher TW, Dwek RA. Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei type II and type III variant surface glycoproteins. J Biol Chem. 1991;266:20244–20261. [PubMed] [Google Scholar]
- Zeleny R, Altmann F, Praznik W. A capillary electrophoretic study on the specificity of β-galactosidases from Aspergillus oryzae, Escherichia coli, Streptococcus pneumoniae, and Canavalia ensiformis (jack bean) Anal Biochem. 1997;246:96–101. doi: 10.1006/abio.1996.9973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.