Abstract
Stem cell therapy presents an opportunity to replace photoreceptors that are lost as a result of inherited and age-related degenerative disease. We have previously shown that murine postmitotic rod photoreceptor precursor cells, identified by expression of the rod-specific transcription factor Nrl, are able to migrate into and integrate within the adult murine neural retina. However, their long-term survival has yet to be determined. Here, we found that integrated Nrl.gfp+ve photoreceptors were present up to 12 months post-transplantation, albeit in significantly reduced numbers. Surviving cells had rod-like morphology, including inner/outer segments and spherule synapses. In a minority of eyes, we observed an early, marked reduction in integrated photoreceptors within 1 month post-transplantation, which correlated with increased numbers of amoeboid macrophages, indicating acute loss of transplanted cells due to an inflammatory response. In the majority of transplants, similar numbers of integrated cells were observed between 1 and 2 months post-transplantation. By 4 months, however, we observed a significant decrease in integrated cell survival. Macrophages and T cells were present around the transplantation site, indicating a chronic immune response. Immune suppression of recipients significantly increased transplanted photoreceptor survival, indicating that the loss observed in unsuppressed recipients resulted from T cell-mediated host immune responses. Thus, if immune responses are modulated, correctly integrated transplanted photoreceptors can survive for extended periods of time in hosts with partially mismatched H-2 haplotypes. These findings suggest that autologous donor cells are optimal for therapeutic approaches to repair the neural retina, though with immune suppression nonautologous donors may be effective.
Keywords: Stem cell, Progenitor cell, Photoreceptor, Retina, Cell transplantation, Immune response
INTRODUCTION
Retinal degenerations remain the largest cause of untreatable blindness in the developed world. Although encompassing a range of causes, they have in common the irreversible loss of the sensory photoreceptor cells. Many therapeutic strategies aim to slow the progression of disease. Photoreceptor cell transplantation may have great therapeutic potential, by providing the opportunity to replace the cells that are lost.
By using a fluorescent marker linked to the promoter sequence of Nrl, a transcription factor expressed in rods shortly after terminal mitosis [1], we have previously demonstrated that postmitotic rod photoreceptor precursors (herein termed Nrl.gfp+ve cells) have the ability to integrate into the adult murine retina following transplantation into the subretinal space [2]. At 3 weeks post-transplantation, Nrl.gfp+ve donor cells are correctly integrated within the outer nuclear layer (ONL) of recipient retinae and exhibit unambiguous rod morphology, including correctly orientated inner and outer segments and spherule synapses. Transplanted rods express components of the phototransduction pathway and synaptic machinery and could restore a basic light response, the pupil reflex, in a mouse model of retinal degeneration [2]. In this and similar studies [2-5], the survival of integrated photoreceptors has only been examined up to 1 month post-transplantation. It is essential to establish whether or not these cells can survive for extended periods of time post-transplantation.
Immune rejection is a major problem in many transplantation paradigms, and host responses include acute innate and adaptive immune responses. However, the eye is frequently described as an immune privileged site, a site that allows foreign grafts to survive for extended to indefinite periods of time. Anterior chamber-associated immune deviation (ACAID) is a form of immune tolerance and a state of specific immunological unresponsiveness, mediated by antigen-specific suppressor T cells [6, 7]. These cells are produced in the spleen and suppress the host immune reactions to alloantigens present in the anterior chamber of the eye [8, 9]. Of greatest relevance to photoreceptor transplantation is that the subretinal space has also been shown to elicit immune deviation [10] although such deviation may be lost if retinal pigment epithelium cell viability is compromised or the outer blood-retinal barrier is disrupted [11].
Previous investigations of transplanted cell survival in the retina have predominantly focused on the transplantation of sheets of retinal tissue or fragmented fetal neural retinal pieces into the subretinal space. These studies used histological and immunohistochemical markers and reported evidence of rejection or cell death in the transplanted population [12, 13]. Transplantation of neonatal retinal allografts to the subretinal space induces immune deviation [10]. However, these grafts deteriorate by ~1-month post implantation, apparently coinciding with loss of immune deviation and the onset of donor-specific delayed hypersensitivity [13, 14]. Transplanted retinal sheets have been shown to survive in the subretinal space for several months, although older grafts presented a loss of retinal lamination and structure, and there was little evidence of synaptic connectivity between the graft and the recipient retina [15-17]. In contrast, fragmented portions of postnatal day (P) 0 neural retina transplanted to the subretinal space of immunocompetent mice survived poorly at 5 weeks post-implantation [14]. Similarly, experiments comparing fragment and full-thickness allogeneic embryonic retinal grafts transplanted into adult recipients have shown destruction of fragmented tissue grafts within a few weeks of implantation [12]. Further analysis demonstrated the presence of major histocompatibility complex (MHC) class I and II proteins on transplanted fragmented retinal tissue but not retinal sheets, suggesting that host immune responses to fragmented and intact retinal transplants might be different [12].
Few studies have examined the survival of retinal cells transplanted to the subretinal space, although a number of studies have examined long-term neural stem/progenitor cell transplantation [18-20]. However, in these investigations very few donor cells correctly integrated within the recipient ONL and the survival of transplanted cells was evaluated by examining the mass of cells present in the subretinal space [21, 22]. By transplanting postmitotic photoreceptor precursor cells, which are known to both anatomically and functionally integrate within the recipient retina [2], it is possible to assess for the first time the ability of fully integrated transplanted photoreceptors to survive within a recipient retina. Here, we show that transplanted photoreceptor cells integrated within the adult mouse retina are subject to a host immune response but can survive for extended periods of time provided these immune responses are modulated.
MATERIALS AND METHODS
Animals
C57Bl/6J, Nrl.gfp+/+ and Cba.gfp± mice were maintained in the animal facility at University College London. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice defined as “adult” were 6–8 weeks of age at the start of the experiment. Nrl.gfp+/+ (H-2b/s) and Cba.gfp± (H-2d/b) mice were used as donors to provide dissociated non-autologous retinal cells for transplantation and C57Bl/6J (H-2b) mice as partially matched recipients.
Immune Suppression
In some experiments, recipient animals were orally immunesuppressed by addition of cyclosporine A (50 mg/kg/day) and 5% fruit cordial to the drinking water, for 1 week prior to and 3 weeks after transplantation. Immunesuppressed recipients were paired with unsuppressed recipients with regard to transplantation; specifically, the mice were anesthetized together, had alternate transplantations administered to each eye and were recovered together. This strategy was employed to reduce as many variables as possible when directly comparing different groups of transplanted animals.
Dissociation of Retinal Cells and Subretinal Transplantation
Neural retinal cells from P3–5 mice were dissociated as described previously [2] and resuspended at a concentration of 400,000 cells/μl. Animals received cell transplants (1 μl) via a transcleral injection into the subretinal space.
T-Cell Proliferation Assays
Spleens were removed and passed through 100-μm cell strainers with RPMI to produce single cell suspensions, before being spun and resuspended in Red Blood Cell Lysis Buffer (Sigma, Gillingham, UK) for 5 minutes on ice. Splenocytes were washed with RPMI, spun down, and resuspended in RPMI–Glutamax-1 with 10% FCS and antibiotics and plated out in triplicate for each mouse in 96-well round-bottomed tissue culture plates (2.5 × 105 cells/well). Culture medium was added to the negative control wells and 2.5 lg/ml concanavilin A (Sigma, Gillingham, UK) was added to the positive control wells. Recombinant enhanced green fluorescent protein (rEGFP; BioVision Research Products, Mountain View, California, USA) was added to test wells (2 μg and 10 μg/ml). All wells were incubated for 48 hours at 37 °C before cells were pulsed with H3–thymidine and incubated at 37 °C for a further 16 hours. The cells were then harvested on to glass fiber paper (Minimash 2000 Plate Harvester, Dynatech, Laboratories, Alexandria, VA), scintillant added, and the samples read (Liquid Scintillation Analyzer 1600TR), with the radioactivity of each vial recorded as counts per minute.
Flow Cytometry
Cervical lymph nodes were carefully dissected out and passed through 100-μm cell strainers with RPMI to produce single cell suspensions. Cells were spun down and resuspended in RPMI at 1 × 106 cells/ml. A total of 106 cells/sample were spun down and resuspended in blocking solution (BSA 1% in PBS), for 1 hour on ice. The cells were washed in PBS and stained for 1 hour on ice in the dark. A FITC-conjugated antimouse CD3e (clone 145-2C11, BD Biosciences, Oxford, U.K.) antibody was used, at a 1:20 dilution in blocking solution. Cell-only and isotype controls (anti-rat-conjugated antibodies) were also prepared. The cells were washed twice with PBS, fixed with 1% PFA (15 minutes) on ice in the dark, then washed and resuspended in blocking solution, and stored at 4 °C in the dark. Flow cytometry was performed using a FACSCalibur cytometer (Becton Dickinson, Oxford, U.K.) and analyzed using WinMDI Version 2.8 software.
Cryosections
Mice were sacrificed at various time points post-transplantation and eyes were fixed in 4% PFA in PBS prior to cryosectioning (18-μm sections). All sections were collected for analysis.
Immunohistochemistry
Sections were air dried, rinsed in TBS, and blocked (5% NGS, 1% BSA in TBS) for 2 hours before being incubated with primary antibody overnight at 4 °C. After washing, sections were incubated with secondary antibody for 2 hours at room temperature, washed, and counter-stained with Hoechst 33,342 (Molecular Probes Inc., Paisley, U.K.). Negative controls omitted the primary antibody. Antibodies used: rat anti-CD4, rat anti-CD8a (1:50; BD Pharmingen, Oxford, U.K.), biotin-conjugated rat anti-CD68 (1:250; AbD Serotec, Kidlington, U.K.), rat anti-F4/80 (1:250; Abcam, Cambridge, U.K.), and anti-rat and streptavidin Alexa Fluor 546 (1:500; Molecular Probes, Paisley, U.K.).
Confocal Microscopy
Retinal sections were imaged using a Zeiss LSM510 confocal microscope. XY optical sections, ~0.5-μm apart, were taken throughout the depth of the section and built into a stack to give an XY projection image.
Cell Counts
Nrl.gfp+ve photoreceptors were counted as integrated if the whole cell body was correctly located within the ONL and at least one of spherule synapse, inner/outer processes and/or inner segments was visible [2]. Cell counts for individual eyes were only excluded if there were cells in the vitreous, indicative of accidental intravitreal transplantation of the cells.
Macrophage Grading
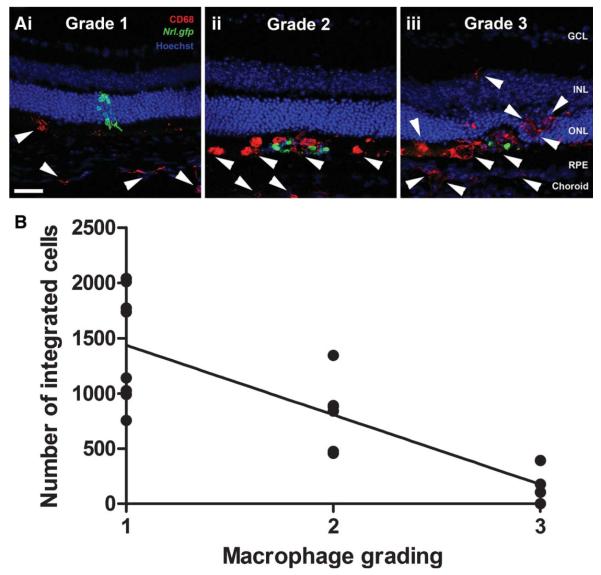
Macrophage recruitment to the site of transplantation was graded on a scale of 1–3. Grade 1 demonstrated none to occasional macrophages in the subretinal space, with more present in the choroid, at the site of transplantation only. Grade 2 represented greater numbers of macrophages in the subretinal space, especially present around the cell mass and ramified macrophages present in the retina, around the site of transplantation only. Grade 3 demonstrated many amoeboid macrophages in the subretinal space and the retina, combined with decreased Nrl.gfp expression in the cell mass and the presence of autofluorescent cell debris. Examples of each grade are shown in Figure 2A, i-iii. Integrated photoreceptor cell counts for individual eyes were not excluded on the basis of macrophage grading for any of the time points examined.
Figure 2.
Assessment of macrophage activation and photoreceptor cell integration. (A): Projection confocal images showing the grading scheme used to assess macrophage activation and infiltration in transplanted eyes (CD68, an activated macrophage marker, red; white arrow heads). (B): Line graph showing a negative correlation between the number of integrated cells and level of macrophage activation (Pearson correlation, p < .0001, n = 18). Scale bar = 50 μm. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
ONL Thickness
Standardized images (>3) were taken of the ONL at both the superior (injection site) and inferior retina and ONL thickness measured using ImageJ (http://rsbweb.nih.gov/ij/). The mean ONL thickness at the site of transplantation was calculated for each group and compared with wild-type data by an analysis of variance (ANOVA) and Bonferroni multiple comparisons test. The % ONL thickness of eyes receiving transplants was calculated by normalizing to the inferior retina (away from the injection site) for individual eyes and to control for age-related effects.
Statistics
All means are presented ± SEM (standard error of the mean), unless otherwise stated; N, number of animals; n, number of eyes or sections examined, where appropriate. Graphpad Prism 5 (GraphPad, La Jolla, CA, USA) was used for all data and regression analysis. In figures, statistical significance is represented by *, p < .05, **, p < .01, ***, p < .001.
RESULTS
Time Course of Integrated Photoreceptor Cell Survival
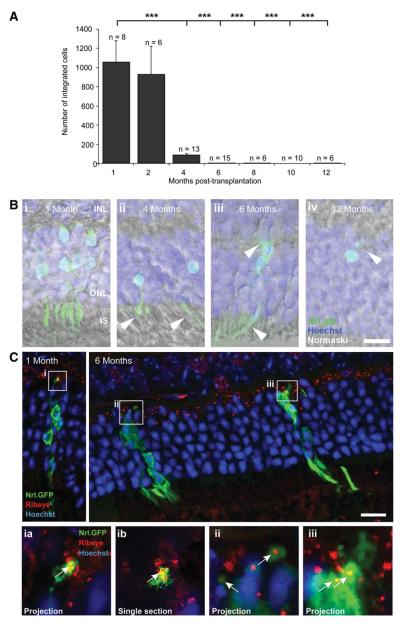
Previous studies investigating the integration of Nrl.gfp+ve photoreceptor precursor cells have examined short-term-integrated cell survival (<1-month) [2, 3, 5]. To assess the long-term survival of these cells, recipients received subretinal transplants, as previously described [2], and the total number of integrated Nrl.gfp+ve cells was quantified at 1, 2, 4, 6, 8, 10, and 12 months (Fig. 1A). Numerous integrated photoreceptors were observed at 2 months post-transplantation and there was no significant difference compared with 1-month (930 ± 291 vs. 1,057 ± 223 cells per eye; p > .05, ANOVA). However, we observed a significant loss of integrated photoreceptors with time. At 4 months only 85 ± 21 cells per eye remained (p < .001, ANOVA) and by 6 months integrated cells were observed only occasionally (3 ± 1 cells per eye; p < .001, ANOVA). The majority of surviving integrated cells presented appropriate rod photoreceptor morphology including inner/outer segments (Fig. 1B, i–iv) and expressed rod synaptic markers [23], until at least 6 months post-transplantation (numbers at 12 months were too few to test; Fig. 1C). Thus, correctly integrated photoreceptors can survive for extended periods of time in the recipient retina albeit at significantly reduced numbers. This prompted us to examine how integrated Nrl.gfp+ve photoreceptors are lost over time.
Figure 1.
Investigation of integrated photoreceptor cell survival in the adult retina. (A): Integrated Nrl.gfp+ve photoreceptor number as a function of time post-transplantation (***, p < .001, ANOVA). (B): Projection confocal images of surviving transplanted photoreceptors (Nrl.gfp; green) at 1, 4, 6, and 12 months (i–iv). (C): Integrated Nrl.gfp+ve cells express markers of mature rod spherule synapses including RIBEYE (CtBP2) at 1 (top left) and 6 (top right) months post-transplantation. Insets, projection (ia, ii, iii) or single section (ib) images of regions of interest highlighted. Note colocalization of GFP (green) and RIBEYE (red; arrows). Scale bar = 20 μm. Abbreviations: GFP, green fluorescent protein; INL, inner nuclear layer; IS, inner segments; ONL, outer nuclear layer.
Acute Loss of Integrated Photoreceptor Cells at 1 Month
Integrated photoreceptor survival may be affected by nonimmunological mechanisms, such as intrinsic developmental programs and synaptic connectivity, and/or host immune responses including acute innate and adaptive immune responses to allogeneic grafts. Although the majority of retinae recovered quickly following transplantation, a proportion (22%) exhibited macrophages at the site of transplantation, together with decreased green fluorescent protein (GFP) expression in the cell mass and the presence of autofluorescent cell debris within the subretinal space and, occasionally, the retina. We also observed significantly reduced numbers (169 ± 83 cells per eye) of integrated Nrl.gfp+ve photoreceptors in these eyes, compared with the majority of unaffected eyes (1,435 ± 180 cells per eye; p < .001, ANOVA, n = 18) from the same cohort of transplantations. These observations suggest that an early inflammatory response, possibly due to increased mechanical/surgical trauma at the time of transplantation, resulted in reduced photoreceptor integration and/or their subsequent rejection in a subset of eyes. Such effects are most likely mediated by blood-derived macrophages or resident microglia. We therefore assessed whether or not there is a significant association between the presence of macrophages and the early reduction in integrated photoreceptors by staining retinal sections for CD68 [24]. CD68, a member of the lysosomal-associated membrane protein family, is predominantly expressed by activated tissue macrophages [25]. Retinal sections were graded on a scale of 1–3, with three representing the highest level of activated macrophage infiltration (see “Materials and Methods” and Fig. 2A, i–iii). A Pearson correlation test demonstrated a significant negative correlation between the number of integrated photoreceptors and the extent of macrophage infiltration (Fig. 2B; p < .01, n = 8). These data suggest that within a subset of transplants an early acute inflammatory response leads to increased macrophage recruitment and reduced integrated photoreceptor number by 1 month post-transplantation. This relatively acute loss of photoreceptors occurs in a minority of eyes (22%) and can be considered a consistent variable between all later time points examined, as cell counts were not excluded on the basis of macrophage grading.
Chronic Loss of Integrated Photoreceptors over Time
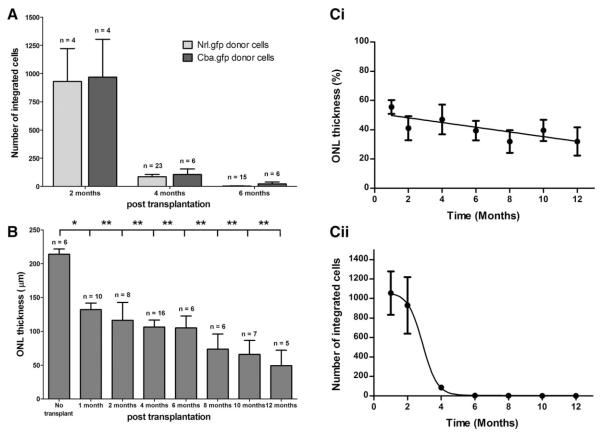
A possible cause of apparent integrated Nrl.gfp+ve cell loss is a downregulation of the Nrl promoter, leading to reduced GFP expression in these cells. To examine this possibility, Cba.gfp+ve donor cells were transplanted and integrated cells quantified at 2, 4, and 6 months post-transplantation. No significant difference was found in the number of surviving integrated photoreceptors using Cba.gfp+ve donor cells compared with Nrl.gfp+ve donor cells (Fig. 3A; p > .05, ANOVA). Therefore, downregulation of Nrl expression is unlikely to explain the reduction in the number of integrated GFP+ve photoreceptors.
Figure 3.
Integrated photoreceptor cell loss and outer nuclear layer thinning. (A): Integrated Cba.gfp+ve photoreceptor number as a function of time post-transplantation, compared with Nrl.gfp+ve-integrated photoreceptors (mean ± SEM; p > .05, ANOVA). (B): ONL thickness of a wild-type retina compared with retinas receiving cell transplants, at various time points post-transplantation (p < .05, ANOVA). (C): i, Host ONL thinning followed a linear kinetic (p < .05, F test), whereas transplanted integrated photoreceptor loss followed a sigmoidal model (p < .0001, F test; mean ± SEM, n > 6). Abbreviation: ONL, outer nuclear layer.
Previously, we have observed ONL thinning and decreased retinal function in aged wild-type mice [26], while prolonged retinal detachment can also lead to ONL degeneration [27, 28]. It is possible that integrated photoreceptors are lost as a secondary consequence of the normal ONL loss over time. Therefore, ONL thickness was measured for all time points examined and compared with unprocedured (no transplant) wild-type retinae (Fig. 3B) or the inferior (away from the site of transplantation) retina (Fig. 3C, i). In the transplanted retinae, ONL thickness was significantly reduced at 1 month post-transplantation, compared with unprocedured controls (Fig. 3B; 132 ± 9 and 214 ± 8 μm, respectively; p < .05, ANOVA). This was maintained at all other time points examined (Fig. 3B; p < .01, ANOVA). However, by controlling the expected age-related thinning observed in nondegenerate wild-type retinae [26], there was no significant difference in ONL thickness after 1 month post-transplantation (Fig. 3C, i; p > .05, ANOVA). The ONL thinning seen at 1 month post-transplantation is most likely a consequence of the detachment caused by the large number of donor cells transplanted. We have subsequently developed optimized protocols that do not result in ONL thinning (manuscript in preparation). Regression analysis demonstrates that the agerelated ONL thinning seen between 1 month and 12 months follows a linear kinetic (Fig. 3C, i; p < .05, F test). In contrast, the loss of transplanted integrated cells around 4 months fits a sigmoidal (Fig. 3C, ii; p < .0001, F test), not a linear (p > .05, F test), model. Together, these results suggest that ONL thinning and integrated photoreceptor loss follow very different kinetics and as such the loss of integrated cells is due to a different and specific underlying biological process, such as an immune response by the host.
Chronic Immune Response Following Cell Transplantation
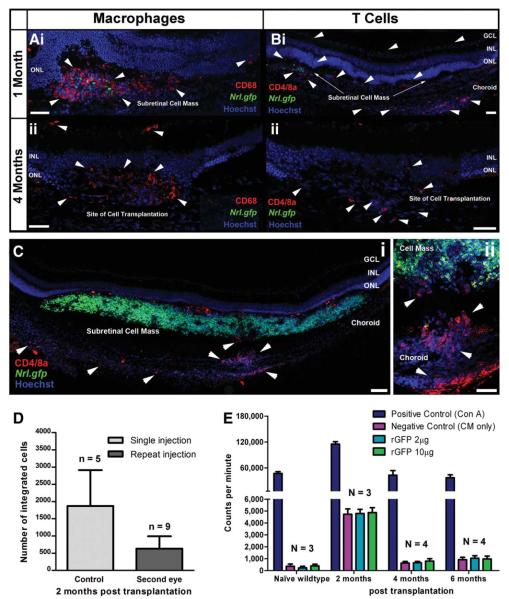
To determine whether or not the delayed loss of transplanted cells was a specific cell-mediated host immune response, we examined the types of immune cells present shortly after transplantation, compared with a later time point when survival is significantly reduced; CD68 labels activated macrophages and microglia [24] and CD4 and 8a are predominantly expressed by T cells. Both CD68+ve macrophages and CD4 and 8a+ve T cells were observed at 1 and 4 months post-transplantation (Fig. 4A, i, ii; 4B, i, ii). At 1 month, activated macrophages were observed in the subretinal space, predominantly around the transplanted cell mass (Fig. 4A, i). At 4 months, activated macrophages were similarly distributed despite the absence of the cell mass in some eyes (Fig. 4A, ii). T cells were observed 1 month post-transplantation in the neural retina, subretinal space, and, more extensively, in the choroid around the transplantation site (Fig. 4B, i). By 4 months, fewer T cells were observed in the choroid although some remained in the retina and subretinal space, around the transplantation site (Fig. 4B, ii). The recruitment of T cells specifically to the site of transplantation (Fig. 4C, i–ii) and their presence near surviving integrated photoreceptors at 1 and 4 months post-transplantation is indicative of an adaptive immune response against the donor cell population.
Figure 4.
Immune cell types at the site of cell transplantation. Projection confocal images of the site of transplantation at 1 month ([A], i; [B], i) and 4 months ([A], ii; [B], ii) post-transplantation. (A): i, Subretinal cell mass 1 month post-transplantation shows extensive macrophage activation and infiltration (CD68+ve; red; white arrow heads). (A): ii, Site of transplantation demonstrating the persistent macrophage presence (CD68+ve; red; white arrow heads) after 4 months. (B): i, A substantial increase in the presence of T cells (CD4/8a+ve; red; white arrow heads) was observed in the retina, subretinal space and the choroid at the site of transplantation after 1 month. (B): ii, T cells were still present at 4 months post-transplantation (CD4/8a+ve; red; white arrow heads). (C): i and ii, Projection confocal images of the transplanted cell mass (Nrl.gfp; green) at 1 month, illustrating in more detail the increased presence (i) and infiltration (ii) of T cells (red; white arrow heads). (D): Number of integrated cells 2 months post-transplantation in control (both eyes injected at a single time point) and the second eye of “primed” recipients (first eye injected 3 months prior to the second). Fewer integrated cells were present 2 months post-transplantation in the primed eye (p = .06, t test). (E): Rate of proliferation of splenocytes after culture with rGFP. Scale bar = 50 μm, 100 μm ([C], i only). Abbreviations: CM, culture medium; GCL, ganglion cell layer; inner nuclear layer; ONL, outer nuclear layer; rGFP, recombinant green fluorescent protein.
To further investigate whether an adaptive immune response may be the cause of integrated cell loss, we “primed” the recipient immune system by transplanting cells into one eye only. After 3 months, we transplanted cells into the contralateral eye and waited for further 2 months before sacrificing and quantifying the number of integrated cells in the second eye. Control recipients had transplants to both eyes at the same time and were examined 2 months post-transplantation. If the loss of integrated cells occurring by 4 months post-transplantation (Fig. 1A) is due to systemic antigen-specific T cells, it is likely that “priming” the host immune system with the initial transplantation would cause integrated cells in the second eye to be rejected more rapidly. The number of integrated cells in the primed recipients was markedly reduced (636 ± 356 cells per eye) compared with the control group (1,872 ± 1,044) 2 months post-transplantation (Fig. 4D; p = .06, t test), suggesting that integrated cells are lost as the result of a specific immune response against the donor population, rather than a nonspecific, innate immune response-mediated loss.
We therefore examined whether integrated cell loss was due to an adaptive immune response against the obvious candidate antigen, GFP, which is only present in the donor cells. As phagocytosis of transplanted cells by macrophages results in the production of cell debris and the release of intracellular GFP, it is possible that a delayed adaptive immune response could be initiated toward GFP. We performed an assay to examine T-cell proliferation from transplanted recipients in response to increasing concentrations of rEGFP (Fig. 4E). We observed no increase in proliferation above background following rEGFP stimulation of splenocytes isolated from naïve (non-transplanted) wild-type or transplanted animals at 2, 4, or 6 months post procedure. Of note, splenocytes isolated at 2 months post-transplantation demonstrated increased proliferation compared with the other time points and wild-type controls, a finding replicated in other assays using different populations of splenocytes, which maybe due to a residual T-cell activation at this time point, post-procedure. Thus, there was no specific cell-mediated immune response toward GFP in transplanted animals, and it is unlikely to be the cause of integrated photoreceptor loss.
Effects of Immune Suppression on Integrated Photoreceptor Cell Survival
To determine whether an adaptive immune response against the donor population itself is responsible for the delayed loss of integrated photoreceptors, donor cells were transplanted into immunesuppressed recipients. Mice received CsA, a potent immunosuppressant that does not affect the acute inflammatory response [29] but reduces T-cell activation [30, 31], lessens cytotoxic CD8+ve cell induction, and reduces Th1 cell function [29].
CsA administration had no adverse effects on photoreceptor integration; similar numbers of integrated photoreceptors were present in normal and immunesuppressed recipients at 1 month post-transplantation (Fig. 5A; 1,057 ± 223 and 1,059 ± 449 cells per eye, respectively). As before (Fig. 2), we assessed macrophage recruitment and found a similar proportion of transplants underwent an acute early cell loss and that there was a significant negative correlation between integrated photoreceptor number and the extent of macrophage infiltration in immunesuppressed recipients at 1 month post-transplantation (p = .01, Pearson correlation; Data not shown). Both ramified mature resident macrophages (Fig. 5B, i) and activated, rounded phagocytic macrophages were present around the transplant site in this minority of eyes (Fig. 5B, i). T cells were also present and had a similar distribution (Fig. 5B, ii). These data suggest that the acute innate immune responses are largely unaffected by CsA up to 1 month post-transplantation, as previously reported [29].
Figure 5.
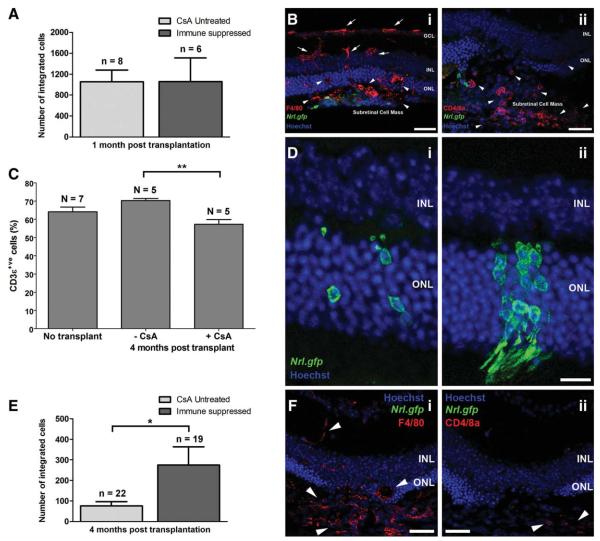
The effects of immune suppression on the number of surviving integrated photoreceptors. (A): Number of integrated cells present in untreated and immunesuppressed recipients, 1 month post-transplantation (mean ± SEM). (B): Projection confocal images of the site of transplantation in immunesuppressed recipients stained for (i) macrophages and (ii) T cells. (B): i, Both ramified (red; white arrows) and active macrophages (red; white arrow heads) were observed in the retina and subretinal space. (B): ii, Numerous T cells were also observed by the transplanted cell mass (red; white arrow heads). (C): Percentage of CD3ε+ve T cells in the cervical lymph nodes in naïve wild-type (no transplant) controls, CsA-untreated recipients, and CsA-treated (immunesuppressed) recipients, 4-months post-transplantation (mean ± SEM; p < .01, ANOVA). (D): Representative images of surviving integrated photoreceptors (Nrl.gfp; green) present in normal (i) and immunesuppressed (ii) recipients, at 4-months post-transplantation. (E): Number of integrated photoreceptors in untreated-recipients and immunesuppressed recipients, 4-months post-transplantation (mean ± SEM; *, p < .05, Mann Whitney U test). (F): Projection confocal images at the site of cell transplantation in immunesuppressed recipients, 4-months post-transplantation. Both macrophages (i; red; white arrow heads) and T cells (ii; red; white arrow heads) were still present, although the latter were reduced in number. Scale bar = 50 μm ([B] and [F] only), 20 μm ([D] only). Abbreviations: CsA, cyclosporin A; INL, inner nuclear layer; ONL, outer nuclear layer.
To confirm that the dosage of CsA used was sufficient to impair systemic T-cell proliferation, cervical lymph nodes were examined by flow cytometry. We observed no significant difference in the percentage of CD3ε+ve T cells present in naïve wild-type mice and recipient mice not pretreated with CsA (Fig. 5C; 64.1% ± 2.6% and 70.3% ± 1.2% CD3ε+ve cells, respectively; p > .05, ANOVA). Conversely, there were significantly fewer CD3ε+ve cells in CsA-treated immunesuppressed recipient mice (Fig. 5C; 57.3% ± 2.5% CD3ε+ve cells; p < .01, ANOVA), showing that the dose of CsA used was sufficient to suppress T cell-mediated immune responses in mice receiving retinal transplants.
We next examined integrated photoreceptor survival in immunesuppressed recipients, 4 months post-transplantation. In all animals (suppressed and unsuppressed), integrated photoreceptors cells assumed normal morphology (Fig. 5D, i, ii) [2]. As in Figure 1, we observed significantly fewer integrated cells in normal, unsuppressed recipients at 4 months, compared with 1 month, post-transplantation (Fig. 5E; 108 ± 39 and 1,057 ± 223 cells per eye, respectively; p < .001, ANOVA). However, significantly greater numbers of integrated cells were present in immunesuppressed animals, compared with unsuppressed recipients, 4 months post-transplantation (Fig. 5E; 275 ± 89 and 76 ± 20 cells per eye, respectively; p < .05, t test). Despite the significant variation that can occur between individuals regarding the extent of immune suppression following CsA treatment [32], we observed a marked difference between the two cohorts. In 6 of 12 of immunesuppressed recipients, the number of surviving integrated photoreceptors was similar to that seen at 1 month post-transplantation, compared with only 1 of 12 in the untreated group. In immunesuppressed recipients, macrophages, and, occasionally, T cells were still present around the site of transplantation 4 months post-transplantation (Fig. 5F, i and ii, respectively; red; white arrow heads), although were rarely observed in the neural retina near surviving integrated photoreceptors.
Together, these results demonstrate that T cell-mediated immune responses play a significant role in the loss of integrated photoreceptors, but that immune suppression with CsA increases their survival.
DISCUSSION
Photoreceptor transplantation represents a potential strategy for the repair of retinal degeneration [2, 5]. However, the long-term survival of correctly integrated cells has not been investigated. Here, we show that integrated rod photoreceptors can survive for several months following subretinal transplantation. These cells maintain normal rod morphology, including inner/outer segments and spherule synapses and express components of the synaptic machinery. Although the numbers are too few to test, these observations provide circumstantial evidence that surviving cells may remain appropriately and functionally connected within the recipient retina, similar to those previously described [2]. However, we also observed two significant patterns of cell loss. First, in a minority of eyes, we observed a relatively acute reduction in the number of integrated cells at 1 month post-transplantation, which was associated with an innate inflammatory reaction. Second, in the remaining and majority of eyes, we observed an adaptive immune response-based cell loss that could be significantly reduced by immune suppression with CsA. These results suggest that appropriately integrated photoreceptors from nonautologous sources can survive for extended periods of time in the adult host retina, provided immune responses are modulated.
We observed a relatively acute inflammatory response and concomitant reduction in integrated photoreceptor number in a small number of eyes at 1 month after transplantation. This maybe similar to the situation observed in an acute injury-induced model of retinal degeneration [33], where the increased presence of activated macrophages shortly after transplantation was correlated with reduced numbers of integrated cells. It is unclear if macrophages prevent photoreceptor precursors integrating or cause their destruction after integration. The presence of both macrophages and T cells in the CsA-treated group 1 month post-transplantation suggests that this is an inflammatory immune response, potentially induced by inadvertent trauma during the transplantation procedure. In axotomy-induced models of ganglion cell degeneration, injury-induced microglia have been shown to impair axonal regeneration in rats [34].
The majority of transplantation paradigms face the challenge of immune rejection. Although the eye is often described as immune privileged, we consider an active immune response to be the most likely explanation for the loss of integrated photoreceptors around 4 months post-transplantation. We could rule out the possible immunogenicity of GFP (used to label the donor cell population). However, minor differences in genetic background of the donor and recipient mice used may play a role. MHCs, present on all nucleated cells, are genetically variable between individuals and responsible for the allogeneicity of transplanted tissue. In mice, the MHC classes are known as H-2 haplotypes. Here, donor cells came from either neonatal Agouti B6SJL/J Nrl.gfp+/+ (H-2s/b) mice or C57Bl/6J Cba.gfp± (H-2d/b) mice and were transplanted into C57Bl/6J recipients (H-2b), constituting a partial mismatch of haplotypes. Despite this, we observed a consistent loss of integrated photoreceptors at 4 months post-transplantation. Other studies [14] observed a more rapid rejection of transplanted retinal fragments, using BALB/cJ (H-2d) donor and C57Bl/6J (H-2b) recipient mice. The delay in graft rejection observed in our study may be attributable to the partial match between the donor and recipient strains used. Although not technically possible at present, it will be very interesting to examine the survival of rod precursors derived from C57Bl/6J donors in C57Bl/6J recipients, that is, syngeneic, haplotypematched.
We observed a significantly extended survival of integrated photoreceptors following immune modulation. However, this improvement was not observed in all mice receiving immune suppression. This may be due to insufficient CsA dosage in some animals. Given the experimental constraints, oral CsA administration was most appropriate. However, this method is limited by the variable amounts of CsA ingested and absorbed in different animals. In addition, it is unclear which eyes may have undergone acute integrated cell loss due to early inflammatory responses 1 month post-transplantation, as this is indistinguishable by 4 months, the time point examined in these mice.
In previous studies, grafts of immature neural retina were rejected ~5 weeks post-transplantation [10, 35, 14], a deterioration thought to coincide with the onset of donor-specific delayed hypersensitivity [13]. Typically, donor-specific delayed hypersensitivity is a Th1 cell-mediated response to largely unknown chronic allograft rejection mechanisms [13]. Alloantigen-specific suppressor T cells have been found in the spleens of mice bearing subretinal or intravitreal neonatal retinal allografts by 12 days post-transplantation [10]. Antigens introduced into the anterior chamber of the eye can induce ACAID, an active form of immune tolerance [8, 36]. Immune deviation has also been shown for antigens delivered to the subretinal space and the vitreous cavity, although not as robustly as that observed in ACAID [11, 37]. When such active immune tolerance is lost, it can result in a delayed rejection of the grafted tissue [13]. Given the ability of CsA to significantly improve integrated cell survival, it seems likely that the loss of integrated Nrl.gfp photoreceptors occurring around 4 months post-transplantation is due to a similar mechanism. Other studies have shown a delayed (5 weeks) rejection of neonatal retinal allografts and reported no effect of CsA [38]. As with these reports, we observed the continued although reduced presence of T cells in immunesuppressed recipients. Nonetheless, we observed a significant prosurvival effect of CsA, supporting the hypothesis that T cell-mediated immune responses contribute significantly to the delayed loss of integrated photoreceptors.
Although immune suppression significantly improved integrated photoreceptor cell survival in the current study other mechanisms may also be involved in the loss of a proportion of the integrated cells. It is beyond the scope of this study to identify what these factors are but nonimmune-based causes for transplanted photoreceptor loss may include incomplete synaptic connectivity between the host retina and integrated cells, intrinsic developmental apoptosis mechanisms and metabolic stress and subsequent autophagy. Although integrated cells at all stages examined (up to 6 months post-transplantation) showed evidence of synaptic formation, it is possible that a proportion of the cells lost did not form correct connections. However, the time course of cell loss observed here does not fit readily with this explanation as models of photoreceptor degeneration resulting from synaptic abnormalities, such as the tulp1−/− mouse, undergo early onset photoreceptor cell loss, by postnatal week 3 [39, 40]. The donor cells used here are from the early postnatal retina and may be subject to intrinsic developmental apoptosis mechanisms, although again normal programed cell death mechanisms occurring throughout retinal development are usually complete by 3 weeks of age. Assuming transplanted photoreceptors follow the normal developmental time course following transplantation into the adult retina, this is shorter than the delayed loss observed here.
This study used freshly isolated retinal precursors as they are known to appropriately integrate following transplantation. However, various stem cell types are being investigated in the hope that they will provide a scalable source of differentiated photoreceptor precursors. In light of the findings presented here, it is reasonable to propose that autologous cells, such as induced pluripotent stem (iPS) cell- or other adult stem cell-derived differentiated photoreceptor precursors, would be optimal sources of donor cells. Several recent studies have demonstrated the differentiation of iPS cells into retinal phenotypes [41-43]. It will be important to ascertain whether such cells are able to functionally integrate into the adult neural retina and to compare their survival to the freshly isolated precursors investigated here.
These experiments were performed in wild-type recipients to enable examination of cell survival in the healthy retina. However, it is important to consider the impact of degeneration on the immune status of the recipient retina. One consequence of retinal degeneration is the activation of resident microglia [44-47]. The difference in inflammatory status between normal and degenerate retinas may have a significant impact on integrated photoreceptor cell survival and is an area that warrants further investigation.
CONCLUSION
We have shown that transplanted rod precursor cells can survive for extended periods of time in the adult murine retina and display all the morphological characteristics of correctly integrated rod photoreceptors. We also observed a host-mediated immune response and subsequent loss of these cells over time, an effect that could be countered by immune suppression. Together, these findings suggest that although autologous donor cells are likely to be optimal for therapeutic approaches to repair the neural retina, nonautologous cells may also be effective with appropriate immune modulation.
ACKNOWLEDGMENTS
We thank the Department of Genetics for helpful discussions. This work was supported by grants from the Wellcome Trust (082217), Medical Research Council, U.K. (G03000341), and Royal Society (RG080398). R.A.P is a Royal Society University Research Fellow. R.E.M is a Health Foundation Clinical Scientist Fellow. R.R.A is partially funded by the Department of Health’s National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital. R.E.M. is currently affiliated with the Nuffield Laboratory of Ophthalmology, University of Oxford, John Radcliffe Hospital, Oxford, U.K.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author contributions: E.L.W.: conception and design; collection and/or assembly of data; data analysis and interpretation; manuscript writing; R.A.P.: conception and design; financial support; collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript; S.E.B.: collection and/or assembly of data; data analysis and interpretation; U.F.O.L.: collection and/or assembly of data; data analysis and interpretation; manuscript writing; R.E.M.: financial support; collection and/or assembly of data; A.C.B.: collection and/or assembly of data; Y.D.: collection and/or assembly of data; A.J.S.: data analysis and interpretation; J.C.S.: conception and design; financial support; manuscript writing; R.R.A.: conception and design; financial support; data analysis and interpretation; manuscript writing. E.L.W. and R.A.P. are the joint first authors of this article.
REFERENCES
- 1.Akimoto M, Cheng H, Zhu D, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci USA. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 3.West EL, Pearson RA, Tschernutter M, et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86:601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartsch U, Oriyakhel W, Kenna PF, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after sub-retinal transplantation into adult mice. Exp Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Pearson R, Barber A, West E, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wildtype and degenerating retina. Cell Transplant. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streilein JW, Niederkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol. 1985;134:1381–1387. [PubMed] [Google Scholar]
- 7.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor. Cell Popul Immunol. 1990;71:383–389. [PMC free article] [PubMed] [Google Scholar]
- 8.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): Regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 9.D’Orazio TJ, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID) Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang LQ, Jorquera M, Streilein JW. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Invest Ophthalmol Vis Sci. 1993;34:3347–3354. [PubMed] [Google Scholar]
- 11.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998;39:1823–1834. [PubMed] [Google Scholar]
- 12.Ghosh F, Larsson J, Wilke K. MHC expression in fragment and full-thickness allogeneic embryonic retinal transplants. Graefes Arch Clin Exp Ophthalmol. 2000;238:589–598. doi: 10.1007/s004170000138. [DOI] [PubMed] [Google Scholar]
- 13.Jiang LQ, Jorquera M, Streilein JW, et al. Unconventional rejection of neural retinal allografts implanted into the immunologically privileged site of the eye. Transplantation. 1995;59:1201–1207. [PubMed] [Google Scholar]
- 14.Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vis Res. 2002;42:487–495. doi: 10.1016/s0042-6989(01)00185-7. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh F, Arner K. Transplantation of full-thickness retina in the normal porcine eye: Surgical and morphologic aspects. Retina. 2002;22:478–486. doi: 10.1097/00006982-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh F, Bruun A, Ehinger B. Graft-host connections in long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:126–132. [PubMed] [Google Scholar]
- 17.Ghosh F, Engelsberg K, English RV, et al. Long-term neuroretinal full-thickness transplants in a large animal model of severe retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2007;245:835–846. doi: 10.1007/s00417-006-0437-9. [DOI] [PubMed] [Google Scholar]
- 18.Mizumoto H, Mizumoto K, Shatos MA, et al. Retinal transplantation of neural progenitor cells derived from the brain of GFP transgenic mice. Vis Res. 2003;43:1699–1708. doi: 10.1016/s0042-6989(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 19.Klassen H, Schwartz PH, Ziaeian B, et al. Neural precursors isolated from the developing cat brain show retinal integration following transplantation to the retina of the dystrophic cat. Vet Ophthalmol. 2007;10:245–253. doi: 10.1111/j.1463-5224.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Hoffelen SJ, Young MJ, Shatos MA, et al. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003;44:426–434. doi: 10.1167/iovs.02-0269. [DOI] [PubMed] [Google Scholar]
- 21.Warfvinge K, Kiilgaard JF, Klassen H, et al. Retinal progenitor cell xenografts to the pig retina: Immunological reactions. Cell Transplant. 2006;15:603–612. doi: 10.3727/000000006783981594. [DOI] [PubMed] [Google Scholar]
- 22.Wojciechowski AB, Englund U, Lundberg C, et al. Long-term survival and glial differentiation of the brain-derived precursor cell line RN33B after subretinal transplantation to adult normal rats. Stem Cells. 2002;20:163–173. doi: 10.1634/stemcells.20-2-163. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- 25.Gordon S. Macrophage-restricted molecules: Role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 26.Luhmann UF, Robbie S, Munro PM, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50:5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mervin K, Valter K, Maslim J, et al. Limiting photoreceptor death and deconstruction during experimental retinal detachment: The value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164. doi: 10.1016/s0002-9394(99)00104-x. [DOI] [PubMed] [Google Scholar]
- 28.Fisher SK, Lewis GP, Linberg KA, et al. Cellular remodeling in mammalian retina: Results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Borel JF, Baumann G, Chapman I, et al. In vivo pharmacological effects of ciclosporin and some analogues. Adv Pharmacol. 1996;35:115–246. doi: 10.1016/s1054-3589(08)60276-8. [DOI] [PubMed] [Google Scholar]
- 30.Snyder SH, Lai MM, Burnett PE. Immunophilins in the nervous system. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 31.Snyder SH, Sabatini DM, Lai MM, et al. Neural actions of immunophilin ligands. Trends Pharmacol Sci. 1998;19:21–26. doi: 10.1016/s0165-6147(97)01146-2. [DOI] [PubMed] [Google Scholar]
- 32.Lu X, Dawson J, Borel JF. Effect of cyclosporine and some derivatives on chronic rejection. Transplant Proc. 1996;28:3152–3153. [PubMed] [Google Scholar]
- 33.Harada T, Harada C, Kohsaka S, et al. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J Neurosci. 2002;22:9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma N, Streilein JW. Contribution of microglia as passenger leukocytes to the fate of intraocular neuronal retinal grafts. Invest Ophthalmol Vis Sci. 1998;39:2384–2393. [PubMed] [Google Scholar]
- 36.Streilein JW, Niederkorn JY, Shadduck JA. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980;152:1121–1125. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenkel H, Chen PW, Ksander BR, et al. Immune privilege is extended, then withdrawn, from allogeneic tumor cell grafts placed in the subretinal space. Invest Ophthalmol Vis Sci. 1999;40:3202–3208. [PubMed] [Google Scholar]
- 38.Ishioka M, Okamoto S, Streilein JW, et al. Effect of cyclosporine on anterior chamber-associated immune deviation with retinal transplantation. Invest Ophthalmol Vis Sci. 1997;38:2152–2160. [PubMed] [Google Scholar]
- 39.Grossman GH, Pauer GJ, Narendra U, et al. Early synaptic defects in tulp1 −/− mice. Invest Ophthalmol Vis Sci. 2009;50:3074–3083. doi: 10.1167/iovs.08-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagstrom SA, Duyao M, North MA, et al. Retinal degeneration in tulp1 −/− mice: Vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2795–2802. [PubMed] [Google Scholar]
- 41.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 42.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. Plos One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes EH, Schlichtenbrede FC, Murphy CC, et al. Generation of activated sialoadhesin-positive microglia during retinal degeneration. Invest Ophthalmol Vis Sci. 2003;44:2229–2234. doi: 10.1167/iovs.02-0824. [DOI] [PubMed] [Google Scholar]
- 45.Hose S, Zigler JS, Jr, Sinha DA. Novel rat model to study the functions of macrophages during normal development and pathophysiology of the eye. Immunol Lett. 2005;96:299–302. doi: 10.1016/j.imlet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Lam TT, Tso MO. Heterogeneous populations of microglia/macrophages in the retina and their activation after retinal ischemia and reperfusion injury. Exp Eye Res. 2005;81:700–709. doi: 10.1016/j.exer.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Roque RS, Imperial CJ, Caldwell RB. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1996;37:196–203. [PubMed] [Google Scholar]